Abstract
Purpose of Review
To summarize recent evidence on mental stress-induced myocardial ischemia (MSIMI), its mechanisms, and clinical significance.
Recent Findings
MSIMI can occur in patients with normal cardiac stress testing, is only weakly related to severity of coronary artery disease (CAD), and it is often silent. Among patients with CAD, MSIMI is associated with a twofold increased risk of major adverse cardiovascular events compared to those who do not have MSIMI. Certain groups such as young women with myocardial infarction and those with psychological comorbidities are more susceptible to MSIMI. Abnormal microvascular vasoreactivity and inflammation are implicated mechanisms in MSIMI. Increased brain activity in regions that modulate autonomic reactivity to emotional stress and fear is associated with MSIMI.
Summary
MSIMI has important prognostic implications in patients with CAD. Stress can no longer be ignored as a risk factor in cardiology care. Clinical trials testing effective strategies to target MSIMI are needed.
Keywords: Mental stress, Cardiovascular reactivity, Microvascular endothelial dysfunction, Coronary vasospasm
Introduction
Coronary artery disease (CAD) remains a leading health threat in the USA [1]. Cardiometabolic risk factors such as hypertension, diabetes, and obesity are major contributors to CAD, but there is unexplained heterogeneity in the risk and outcome of CAD. It is increasingly recognized that psychological stress contributes to CAD risk and outcomes [2, 3], and such effects can be especially powerful among high-risk individuals with preexisting CAD [4]. Stressful exposures such as work stress, interpersonal conflict, low socioeconomic status, early life trauma, and care-giver strain play an important role in CAD in both men and women, but women may be more susceptible to these effects. More recently, mental stress and depression in the COVID-19 pandemic era have been linked to increased cardiovascular risk factors, especially in women [5, 6]. Psychosocial stressors contribute to cardiovascular risk factors by influencing health behaviors (i.e., poor dietary choices, lack of exercise, less medication compliance, and follow-up care) [3, 7], but stress also has direct adverse effects on cardiovascular physiology [8•]. Substantial prior evidence links mental stress to endothelial dysfunction [9], atheroma progression, ischemia, arrhythmias, and cardiovascular morbidity and mortality [10–12]. An important potential consequence of stress, among patients with preexisting CAD, is the provocation of myocardial ischemia. This phenomenon, known as mental stress-induced myocardial ischemia (MSIMI), has been recognized for decades, but it is only recently that its prognostic importance has been fully appreciated. In this review, we will summarize recent findings from our laboratory and others that have used mental stress provocation in patients with CAD to study the impact of acute mental stress on cardiovascular physiology, myocardial ischemia, and major adverse cardiovascular outcomes (MACE). We will also discuss neurobiological correlates of these stress-induced alterations.
Stress Physiology
Acute mental stress-related ischemia, infarction, and arrhythmias have been described during natural disasters and wars [12–15]. Unexpected enhanced emotional states such as intense anger, extreme fear, panic, bereavement, or even heightened excitement can trigger cardiac events or exacerbate angina in susceptible individuals [8•, 16, 17]. Studies have also linked early trauma and posttraumatic stress disorder (PTSD) to increased risk of cardiac events [17–21]. There are multiple mechanisms by which stress can adversely impact normal cardiovascular physiology (Fig. 1) [8•, 22]. This can be studied in the laboratory setting by exposing participants to various mental stress tasks such as anger recall, exposure to personalized traumatic scripts, mental arithmetic, or a stressful speech task. In response to a mental stress challenge, changes in hemodynamics, electrical conduction, vascular (coronary and peripheral) response, and inflammatory biomarkers are measured to assess stress responses (Table 1). Although assessing transient responses to mental stress remains a laboratory research technique and is not currently translated into clinical practice, this method provides a window as to what may be happening during repeated and chronic daily life stressors in a susceptible individual. Mechanisms of stress physiology in CAD have been recently published [8•]. Here, we summarize key concepts related to stress physiology, focusing on hemodynamic reactivity, vascular function, autonomic function, and inflammation, in patients with CAD [8•].
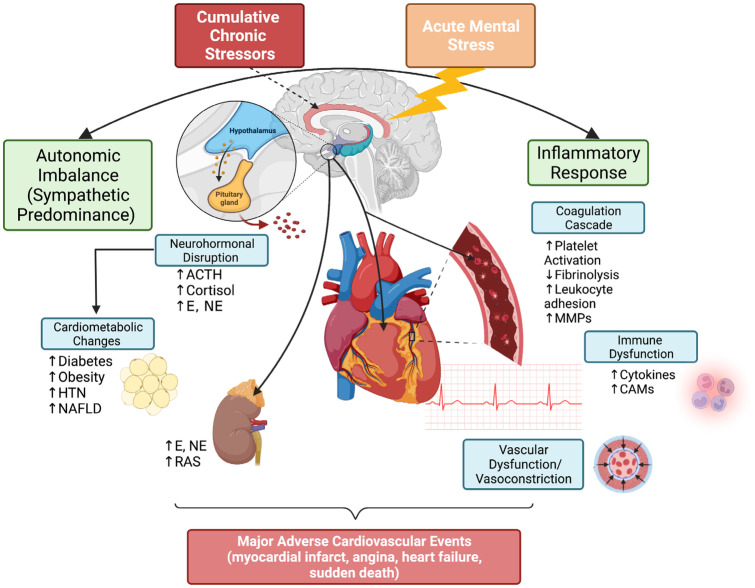
Fig. 1.
Mechanisms of stress on cardiovascular physiology. Chronic and acute stressors contribute to an increased inflammatory response and autonomic imbalance, which can lead to adverse cardiometabolic effects, endothelial dysfunction, abnormal vascular reactivity, and abnormal immune function. These factors ultimately can contribute to adverse cardiovascular events. ACTH, adrenocorticotrophic hormone; CAM, cell adhesion molecules; E, epinephrine; HTN, hypertension; MMP, matrix metalloproteinases; NAFLD, nonalcoholic fatty liver disease; NE, norepinephrine; RAS, renin-angiotensin system. Figure created using BioRender.com
Table 1.
Mental stress myocardial ischemia measures
| Measures of mental stress response | |
|---|---|
| Cardiovascular reactivity | ↑Heart rates and blood pressures during mental stress |
| Electrocardiogram and heart rate variability |
↑Heart rates during mental stress, arrhythmias, ST, and T-wave changes ↓Heart rate variability during mental stress |
| Cardiac impedance | ↓Pre-ejection period during mental stress |
| Macrovascular function |
↓Peripheral artery flow-mediated dilation during mental stress ↑Coronary vasoconstriction during mental stress |
| Peripheral microvascular function | ↑Peripheral microvascular vasoconstriction during mental stress |
| Electrodermal activity | ↑Galvanic skin response and sweat activity during mental stress |
| Myocardial imaging (nuclear imaging/echo wall motion) | ↑Myocardial ischemia or wall motion abnormality during mental stress |
| Neuroimaging | ↑Brain activation during mental stress (e.g., medial prefrontal cortex, anterior cingulate cortex, rostral medial prefrontal cortex, insula, amygdala, hippocampus, hypothalamus, periaqueductal gray) |
| Stress biomarkers (blood/urine) | ↑Circulating biomarkers during mental stress (e.g., cortisol, antidiuretic hormone, epinephrine, norepinephrine, copeptin) |
Cardiovascular Reactivity (CVR)
Typically, both heart rate and blood pressure increase in humans exposed to laboratory mental stress, although the increases are greater with exercise stress testing. Both elevated and blunted CVR responses to mental stress have been associated with outcomes [8•, 23]. Greater CVR, defined as changes in blood pressure and heart rate, was associated with myocardial ischemia and was predictive of outcomes in CAD patients [24, 25]. Greater CVR and poor recovery from acute mental stress were associated with an adverse effect on subsequent cardiovascular risk status with higher incident hypertension and increased carotid intima-media thickness [26]. An exaggerated mental stress-induced CVR may trigger acute ischemia in the setting of chronic coronary endothelial dysfunction [27, 28]. Even low CVR is associated with adverse health consequences in some studies [23] and may be a peripheral marker that signals abnormal neural activity and autonomic dysregulation in certain individuals [23].
Vascular Function
When exposed to acute mental stress, human studies demonstrate both coronary and peripheral endothelial dysfunction [8•, 29–32]. During mental stress, the normal coronary vasodilatory response was found to be blunted in patients with CAD compared to those with normal angiograms [31, 33]. A transient reduction in brachial artery flow-mediated dilation induced by mental stress was associated with adverse cardiovascular outcomes in patients with known CAD during a follow-up period of 3 years [30]. Of note, mental stress responses are highly variable, depending on underlying atherosclerosis and endothelial dysfunction [31–34]. Coronary artery diameter and flow responses to mental stress correlate with coronary diameter responses to acetylcholine, implicating an endothelium-dependent mechanism in abnormal mental stress vasoreactivity [31, 35]. Endothelin-1(ET-1), a potent vasoconstrictor, has also been implicated in contributing to enhanced vascular reactivity that is induced by sympathetic activation during mental stress stimulus in humans. In healthy participants with no cardiovascular risk factors, selective blockade of endothelin A receptors blocked mental stress-induced peripheral endothelial dysfunction [29]. In one exploratory study, the increase in ET-1 in response to mental stress was associated with parasympathetic withdrawal in CAD patients, but not with circulating catecholamines [36]. In addition to larger brachial artery and epicardial coronary artery reactivity to mental stress, abnormal microvascular reactivity is particularly implicated in mental stress response and discussed in this review.
Autonomic Function
The cardiac autonomic nervous system modulates heart rates, blood pressure, and vascular tone during day-to-day activities and controls physiologic responses to stimuli such as temperature changes, exercise, and stress. Mental stress increases sympathetic nervous system output with accompanying parasympathetic nervous system (vagal) withdrawal. These stress-related changes in autonomic function can be quantified by heart rate variability (HRV), which measures changes in time intervals between heart beats, and are a prognostic marker in cardiac populations [37]. Acute stress-induced sympathetic activation leads to changes in electrophysiology predisposing to abnormal HRV as well as arrhythmias [8•, 22]. Abnormal HRV is also associated with chronic stress factors such as PTSD and depression [8•]. Noninvasive measures such as cardiac impedance-derived pre-ejection period are also used to determine the impact of stress on myocardial physiology (Table 1) [38, 39]. A major area of emerging investigation is the role of neural and autonomic reactivity and triggering of the inflammatory process (see the “Brain Correlates of Angina and Mental Stress Ischemia” section below) [40].
Inflammation
Inflammation is central in CAD pathophysiology that triggers a cascade of events within the arterial wall and leads to development and progression of atherosclerosis [41]. Inflammation and immune dysfunction are key factors in stress response [8•, 42–44]. In addition to catecholamines and stress hormones, mental stress triggers the inflammatory cascade with increased levels of peripheral inflammatory biomarkers, such as interleukin-6 (IL-6) [8•], although timing of the sample collection is important to detect changes based on the biomarker of interest. Acute stress may trigger plaque rupture through a surge in inflammation but can also activate the coagulation cascade with altered fibrinolysis, resulting in an increased risk for thromboembolism [45, 46]. In a situation of chronic stress, inflammation over time may also predispose to progression of atherosclerosis.
Mental Stress-Induced Myocardial Ischemia (MSIMI)
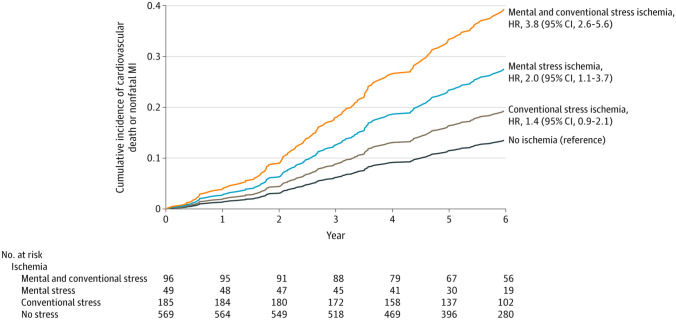
Using cardiac nuclear imaging methods, changes in myocardial blood flow and ischemia can be detected in response to mental stress. Ischemia that is detected with acute mental stress testing done in the laboratory appears to be a phenomenon that is quite distinct from the ischemia that is elicited with exercise or pharmacologic stress testing and is often silent [12, 47, 48]. MSIMI is also linked to factors such as depression as well as to angina and ischemia in daily life (Fig. 2) [24, 49–51]. While MSIMI is not necessarily related to atherosclerotic burden or severity of chest pain, it is emerging as a powerful prognostic marker that is associated with MACE [52••]. In a recent large study by Vaccarino et al. with a total of 918 participants, those who had myocardial ischemia from a standardized mental stress test (a public speech task) were compared to those who did not develop ischemia and also to those who had ischemia from a conventional stress test (exercise or pharmacological) using single-photon emission-computed tomography (SPECT) [52••]. Patients were pooled from two prospective cohort studies: the Mental Stress Ischemia Prognosis Study (MIPS) study and the Myocardial Infarction and Mental Stress Study 2 (MIMS2). The MIPS cohort included patients with CAD, defined based on a previous cardiac catheterization showing atherosclerosis, history of prior myocardial infarction, a history of percutaneous coronary intervention or coronary artery bypass grafting, or a positive nuclear stress test. The MIMS2 cohort included younger patients (ages 25 to 60, 50% women) with a prior history of myocardial infarction within 8 months. The primary endpoint was cardiovascular death or initial or recurrent nonfatal myocardial infarction, and a secondary outcome also included hospitalization for heart failure. In the combined cohort study, 16% of participants developed MSIMI, 31% developed conventional stress ischemia, and 10% were found to have both. MSIMI was significantly associated with elevated cardiovascular death or nonfatal myocardial infarction [52••]. Compared to patients with no ischemia, those with MSIMI in the absence of conventional stress ischemia had a twofold increased risk of subsequent adverse events. While conventional stress ischemia in the absence of MSIMI was not significantly associated with adverse outcomes, patients who developed both MSIMI and conventional stress ischemia had an almost fourfold increased risk. These patients also had an increased risk compared to patients with only conventional stress ischemia (Fig. 3).
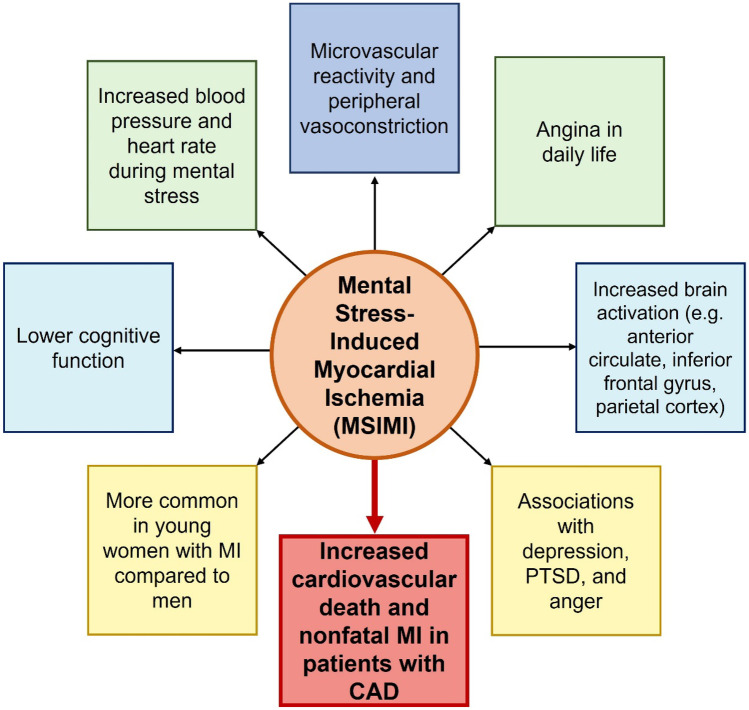
Fig. 2.
Correlates of mental stress-induced myocardial ischemia (MSIMI). MSIMI is associated with several factors and is a phenomenon that is not benign. MI, myocardial infarction; PTSD, post-traumatic stress disorder
Fig. 3.
Mental stress-induced myocardial ischemia and events in coronary artery disease. MSIMI is associated with twofold increased events in patients with CAD compared to those with no ischemia, and almost a fourfold increased risk when combined with conventional stress ischemia. MI, myocardial infarction. (Figure reprinted with permission from JAMA. 2021. 326(18): 1818–28. Copyright© (2021) American Medical Association. All rights reserved)
Potential Mechanisms of MSIMI
MSIMI is associated with CAD risk factors, but it is unclear why some individuals with CAD are more susceptible to MSIMI. MSIMI does not appear to be a phenomenon solely of hemodynamic demand triggering ischemia because while standardized mental stress testing typically leads to increases in blood pressure and heart rate, MSIMI occurs at lower levels of oxygen demand compared to that induced by physical stress. In addition to endothelial dysfunction, stress can also cause enhanced smooth muscle reactivity and trigger angina and ischemia episodes [8•, 49, 53–57]. There may be a genetic predisposition to develop MSIMI, as one study has shown a higher likelihood of MSIMI in patients with a β1-adrenergic receptor genetic polymorphism [58]. Although epicardial coronary vasoconstriction can lead to reduced myocardial blood flow, increased microcirculatory tone and resistance induced by mental stress are primarily implicated in MSIMI. Microcirculatory dysfunction (either limited dilation and/or enhanced constriction) is considered a leading mechanism of MSIMI and may trigger myocardial infarction even in the setting of no obstructive CAD, also known as MINOCA. Ersboll et al. reported that patients with MSIMI had left ventricular dysfunction based on impaired resting annular velocities (by echocardiography), even at rest, implicating microvascular dysfunction [59].
Studies have used a peripheral arterial tonometry (PAT) device (Itamar Medical®) to measure peripheral endothelial microvascular vasoconstriction in response to mental stress [32, 60–62]. The device measures changes in microvascular tone with stress as the ratio of pulse wave amplitude during mental stress compared with baseline (prestress). Peripheral vasoconstriction appears to be a correlate of abnormal coronary endothelial function and vasomotor responses [63]. In these studies, the peripheral arterial tonometry ratio during mental stress was consistently lower in patients who developed MSIMI compared with those who did not develop MSIMI, therefore confirming that MSIMI is associated with peripheral vasoconstriction. Furthermore, clear sex differences in hemodynamic and vascular mechanisms associated with MSIMI were found (discussed below) [53, 64]. Circulating norepinephrine (but not epinephrine) levels also predict peripheral vasoconstriction during mental stress [65].
While inflammation increases with mental stress, this increase is not associated with MSIMI. In a recent study, the inflammatory response to mental stress and its relationship to MSIMI was investigated in 607 patients (76% men) [66]. As expected, mental stress led to increases in several inflammatory biomarkers such as IL-6, chemoattractant protein-1 (MCP-1), and matrix metallopeptidase 9 (MMP-9) post stress [66]. However, the increase in these biomarkers with stress was not associated with MSIMI [66]. A biomarker of myocardial strain and injury, high-sensitivity cardiac troponin I (hs-cTnI), has also been investigated in relationship to MSIMI. In the MIPS, study levels were measured in 587 patients at rest and after both conventional and mental stress testing. Higher levels of hs-cTnI at rest were associated with MSIMI and conventional stress ischemia. However, there was no significant change in hs-TnI levels post mental stress (at 45 and 90-min post stress), which could be because the laboratory stressor is transient [67]. The association of MSIMI with resting troponin levels suggests that hs-cTnI elevation is an indicator of chronic ischemic burden experienced during everyday life.
Brain Correlates of Angina and Mental Stress Ischemia
In addition to cardiac imaging tools and techniques, investigations in recent years have increasingly used brain imaging techniques to understand mechanisms underlying cardiovascular stress physiology and adverse outcomes. In the MIPS study, CAD patients underwent high-resolution positron emission tomography (HR-PET) brain imaging during mental stress tasks [68]. Those with MSIMI compared to those without MSIMI had increased activation with mental stress in the medial prefrontal cortex/anterior cingulate cortex (mPFC/ACC) and related regions [68]. In particular, higher stress-induced activation in the rostromedial prefrontal cortex (rmPFC) was associated with MACE at a median follow-up of 3 years, after adjusting for cardiovascular risk factors [40]. MACE was defined as death, myocardial infarction, heart failure hospitalization, and revascularization. The rmPFC is an area of the brain that processes stress and regulates autonomic and immune function. The study also found that the association between higher rmPFC stress reactivity and adverse outcomes was mediated through vagal withdrawal and systemic inflammatory response pathways [40]. Sex differences were also found in neural responses to mental stress among those with and without MSIMI [69].
When those who reported angina in daily life and those without angina were compared, inferior frontal cortex (IFC) activation during mental stress was independently associated with angina frequency [70, 71]. This region is activated by negative stimuli related to decision-making and social behavior, fear, and emotional stress [71]. There was also increased activation of IFC in patients who had MSIMI [72, 73]. IFC activation is associated with activation in other pain processing regions (thalamus, insula, and amygdala) [70], a network that is activated with negative emotion and interoceptive awareness [71, 74–76]. Higher IFC activity was associated with worsening of angina at 2-year follow-up [70]. Prior work by Rosen et al. also found differences in brain activation patterns during angina in CAD patients compared to those with silent ischemia [77]. In the MIPS study, MSIMI was also found to be associated with cognitive impairment at baseline and with greater decline in cognitive abilities at 2-year follow-up [78].
Sex Differences
Based on emerging studies over the past decade that have included more women in mental stress studies, there are notable sex differences in MSIMI. In a sample of patients with stable CAD, Jiang et al. reported that there was a higher prevalence of MSIMI, assessed by echocardiography, in women than in men [79, 80]. In the MIPS study of 686 participants (191 women) with CAD who underwent mental stress testing followed by myocardial perfusion imaging, the incidence of MSIMI in young women (age ≤ 50) was over threefold higher than in age-matched men and older participants [81]. These sex differences in MSIMI were also reported in a prior study, where young women with a history of recent myocardial infarction were found to have more MSIMI detected by nuclear SPECT compared to age-matched men and older women [82]. In a subsequent larger study of 306 patients (150 women and 156 men) with history of myocardial infarction and a mean age of 50 years, MSIMI was twice as likely in women compared to men [83••]. Women in both groups showed greater microvascular constriction to mental stress and a lower reactive hyperemia index with stress. However, there were no sex differences in large artery flow-mediated dilation with mental stress, indicating that a microvascular mechanism is more prominent in women. Indeed, microvascular vasoconstrictive responses predict MSIMI in women [53, 83••]. Women, but not men, with MSIMI had more peripheral vasoconstriction compared to their counterparts who did not have MSIMI, whereas men, but not women, with MSIMI had a higher hemodynamic response (measured by rate pressure product change with mental stress) compared to those without MSIMI [53]. These sex differences in MSIMI point to microvascular reactivity being an important mechanism in women, especially since MSIMI was only weakly associated with CAD on coronary angiograms evaluated using the standardized Gensini score [64]. MSIMI was associated with CAD severity in men, but not in women [64].
There are also notable sex differences in the relationship between angina frequency and MSIMI. In the MIPS and MIMS2 samples, angina frequency (in the past month) was associated with more MSIMI (detected by nuclear imaging) in women but not in men, indicating that angina has important psychological origins among women [49, 84, 85].
It is unclear why young women with CAD are more susceptible to MSIMI. Women have a higher burden of psychosocial risk factors, especially young women, including depression, anxiety, early life adversity, and low socioeconomic status [17, 86, 87]. In the MIMS study, young women with a history of myocardial infarction had more psychosocial risk factors compared to men, although there were no differences in traditional cardiovascular risk factors [83••]. However, even after controlling for these psychosocial factors, the sex differences in MSIMI persisted. It is possible that women’s greater susceptibility towards microvascular dysfunction, which is heightened by sympathetic stimulation during mental stress, plays a role in the higher occurrence of MSIMI among women compared with men.
Inflammation is also actively being investigated as a potential mechanism to explain the differential susceptibility to MSIMI in young women. In a cohort of women (age ≤ 50) with a history of recent myocardial infarction, the levels of IL-6 were two times higher in women compared to men at rest and post mental stress, indicating a higher level of underlying inflammation in women [88]. In the larger combined MIPS and MIMS2 cohort, younger women with CAD had higher levels of inflammation (IL-6) at rest, at 90-min post mental stress, and in response to stress (change in IL-6) compared to young men [46, 88]. These sex differences in inflammatory response were not present in controls without CAD or among older patients. Interestingly, in 562 men and women with stable CAD who underwent a mental stress task, increased inflammation (IL-6 and monocyte chemoattractant protein-1) was associated with MACE (a combined endpoint of death, myocardial infarction, unstable angina with revascularization, and heart failure) among women at 3 years of follow-up, but not among men [42].
Ischemia with No Obstructive Coronary Arteries (INOCA)
Heart disease is the leading cause of death among women in the USA as it is in men, despite that women have less severity of obstructive CAD compared to men. A large proportion of women with angina and ischemia with no obstructive coronary arteries (INOCA) have coronary microvascular dysfunction, which contributes to adverse cardiovascular morbidity and mortality, despite the absence of flow-limiting epicardial stenosis [89–91]. Studies have reported a higher prevalence of anxiety and depression in patients with INOCA compared to those with obstructive CAD [92], although no difference in the prevalence of these factors was found in MI patients with and without obstructive CAD [93]. Given that MSIMI in women is not related to severity of CAD but is related to microvascular responses and to angina frequency, MSIMI could be a potential mechanism to explain chest pain and adverse outcomes in women with INOCA [53, 64].
Women with INOCA show greater peripheral microvascular constriction with mental arithmetic stress compared to age-matched asymptomatic controls [94]. A greater number of participants with INOCA had chest pain during mental stress testing compared to controls (41% vs. 10%, p = 0.01). Higher anxiety and frustration during mental stress correlated with vasoconstriction (anxious: r = −0.34, p = 0.04; frustrated: r = −0.37, p = 0.02). While there were no differences in emotional arousal among the groups at baseline (pre-mental arithmetic testing), those with INOCA reported more emotional arousal during the recovery period after mental stress and remained more anxious (p = 0.006), frustrated (p = 0.02), irritated (p = 0.03), and stressed (p = 0.002). Mental stress reactivity, vascular, and autonomic responses in relation to angina burden in women both with and without obstructive CAD are under investigation (NCT05401630).
Takotsubo Syndrome
Takotsubo syndrome (TTS) is thought to be an extreme manifestation of MSIMI. In this condition, acute stress exposure triggers a catecholamine storm leading to an exaggerated sympathetic response which in turn can cause acute coronary syndrome and heart failure [16]. In addition to acute increase in left ventricular afterload induced by the catecholamine surge, multivessel coronary vasospasm and coronary microvascular dysfunction contribute to acute myocardial ischemia in this condition [95, 96]. The most characteristic pattern of this syndrome is left ventricular apical stunning with basal hyperkinesis. This syndrome predominantly affects postmenopausal women that have experienced a physical or emotional stressor. In a study of hospitalized patients with TTS, women > 55 years had five times higher odds of TTS compared to women < 55 years old [97]. Approximately 50% of patients with TTS had a history of neurologic or psychiatric disorder compared to 25% of patients with acute coronary syndrome [16]. In a case–control study of patients with whole-body 18-FDG-PET/CT imaging (n = 104, 72% women), higher amygdalar activity was associated with developing TTS after adjustment for risk factors [98].
Potential Treatment
Evidence has mounted that mental stress contributes to myocardial ischemia and adverse outcomes. The next step is to investigate ways to attenuate MSIMI and to determine which strategies translate to improvement in both MACE and patient-centered outcomes of angina and quality of life. There have been a few studies that have used pharmacotherapy to improve MSIMI [99–101]. In the responses of mental stress induced myocardial ischemia to escitalopram treatment (REMIT) trial, the effects of selective serotonin reuptake inhibitor, escitalopram, were compared to placebo in stable patients with CAD and evidence of MSIMI over a period of 6 weeks [99]. MSIMI was assessed with three different mental stress tasks, and ischemia was detected by echocardiography or electrocardiography during mental stress. Escitalopram therapy led to a reduction in MSIMI (2.6-fold), and interestingly, no difference was noted with exercise-induced ischemia.
Autonomic nervous system modulation with beta-blockers in pilot studies of MSIMI has found variable results [100, 101]. Of note, newer generation vasodilating beta-blockers (i.e., carvedilol and nebivolol) were not tested in these MSIMI studies. In a study of 31 men with CAD who had ambulatory ischemia (using electrocardiographic monitoring) or MSIMI in the lab, those who were randomized to stress management training (1.5-h weekly class) had a lower number of events at 2 years compared to 26 patients randomized to exercise training (three times per week) [102]. This study, however, did not specifically measure the effects of treatment on MSIMI.
Targeting the autonomic nervous system with stress reduction in patients susceptible to MSIMI has not been adequately investigated and may be the next frontier, especially given the role of neural correlates in angina, MSIMI, and MACE. In addition to mind–body interventions and biofeedback techniques [103] that improve autonomic balance, one possible strategy for MSIMI may be vagal nerve stimulation, which was found to reduce sympathetic activity and inflammatory response [38, 104–107].
Conclusions and Future Directions
MSIMI is a unique phenomenon that is different from exercise/pharmacologic stress ischemia and is an independent predictor of adverse cardiovascular prognosis in CAD patients. Evidence thus far indicates that MSIMI is associated with abnormal microvascular reactivity and occurs at lower oxygen demand than conventional stress ischemia. There is substantial variability in responses to acute mental stress, but young women and patients with depression, PTSD, and high anger trait/scores appear to be especially vulnerable to MSIMI. Interdisciplinary mechanistic work combining neuropsychology, cardiovascular stress physiology, and advanced imaging is needed to improve our understanding of the stress pathways that may be playing a critical role in CAD. Research on sex differences in biological responses to psychological stressors (in the acute setting and in everyday life), and on the biological mechanisms that promote resilience, is needed. MSIMI could be used as a treatment target to design effective interventions in appropriate subgroups who may benefit from autonomic modulatory interventions that reduce sympathetic drive. Other important areas of research include assessing whether use of mental stress testing would improve risk stratification in routine clinical care and whether ameliorating MSIMI with interventions would improve outcomes. Even though much work needs to be done, psychological stress must be addressed to improve patients’ care and quality of life. A better integration of mental health and behavioral medicine with routine cardiology clinical care is needed.
Acknowledgements
We thank Esha Dave for assistance with figures. We also thank Mrs. Marcia Taylor and Mr. Jim Hills for their support.
Funding
This work was supported in part by grants from the NIH (R01HL157311, K23HL105787, P01HL101398, R01HL109413, K24HL077506, K24MH076955, and K24MH076955).
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
For the studies conducted by the co-authors that are discussed in this review, informed consent was obtained.
Footnotes
This article is part of the Topical Collection on Psychological Aspects of Cardiovascular Diseases
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 3.Sgoifo A, Montano N, Esler M, Vaccarino V. Stress, behavior and the heart. Neurosci Biobehav Rev. 2017;74(Pt B):257–259. doi: 10.1016/j.neubiorev.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kivimaki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15(4):215–229. doi: 10.1038/nrcardio.2017.189. [DOI] [PubMed] [Google Scholar]
- 5.Dhaibar HA, Cruz-Topete D. Predisposition of Women to cardiovascular diseases: a side-effect of increased glucocorticoid signaling during the COVID-19 pandemic? Front Glob Womens Health. 2021;2:606833. doi: 10.3389/fgwh.2021.606833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucciarelli V, Nasi M, Bianco F, Seferovic J, Ivkovic V, Gallina S, et al. Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: gender makes a difference. Trends Cardiovasc Med. 2022;32(1):12–17. doi: 10.1016/j.tcm.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremner JD, Moazzami K, Wittbrodt MT, Nye JA, Lima BB, Gillespie CF, et al. Diet, stress and mental health. Nutrients. 2020 doi: 10.3390/nu12082428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaccarino V, Shah AJ, Mehta PK, Pearce B, Raggi P, Bremner JD, et al. Brain-heart connections in stress and cardiovascular disease: implications for the cardiac patient. Atherosclerosis. 2021;328:74–82. doi: 10.1016/j.atherosclerosis.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 10.Lane RD, Laukes C, Marcus FI, Chesney MA, Sechrest L, Gear K, et al. Psychological stress preceding idiopathic ventricular fibrillation. Psychosom Med. 2005;67(3):359–365. doi: 10.1097/01.psy.0000160476.67536.41. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharyya MR, Steptoe A. Emotional triggers of acute coronary syndromes: strength of evidence, biological processes, and clinical implications. Prog Cardiovasc Dis. 2007;49(5):353–365. doi: 10.1016/j.pcad.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102(6):472–480. doi: 10.1136/heartjnl-2014-307306. [DOI] [PubMed] [Google Scholar]
- 13.Meisel SR, Kutz I, Dayan KI, Pauzner H, Chetboun I, Arbel Y, et al. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet. 1991;338(8768):660–661. doi: 10.1016/0140-6736(91)91234-l. [DOI] [PubMed] [Google Scholar]
- 14.Jiao Z, Kakoulides SV, Moscona J, Whittier J, Srivastav S, Delafontaine P, et al. Effect of Hurricane Katrina on incidence of acute myocardial infarction in New Orleans three years after the storm. Am J Cardiol. 2012;109(4):502–505. doi: 10.1016/j.amjcard.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Leor J, Kloner RA. The Northridge earthquake as a trigger for acute myocardial infarction. Am J Cardiol. 1996;77(14):1230–1232. doi: 10.1016/s0002-9149(96)00169-5. [DOI] [PubMed] [Google Scholar]
- 16.Lyon AR, Citro R, Schneider B, Morel O, Ghadri JR, Templin C, et al. Pathophysiology of Takotsubo syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(7):902–921. doi: 10.1016/j.jacc.2020.10.060. [DOI] [PubMed] [Google Scholar]
- 17.Vaccarino V, Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2017;74(Pt B):297–309. doi: 10.1016/j.neubiorev.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126(8):920–927. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bremner JD, Wittbrodt MT, Shah AJ, Pearce BD, Gurel NZ, Inan OT, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier's heart. J Nerv Ment Dis. 2020;208(3):171–180. doi: 10.1097/NMD.0000000000001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almuwaqqat Z, Wittbrodt M, Young A, Lima BB, Hammadah M, Garcia M, et al. Association of early-life trauma and risk of adverse cardiovascular outcomes in young and middle-aged individuals with a history of myocardial infarction. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampert R. Mental stress and ventricular arrhythmias. Curr Cardiol Rep. 2016;18(12):118. doi: 10.1007/s11886-016-0798-6. [DOI] [PubMed] [Google Scholar]
- 23.Whittaker AC, Ginty A, Hughes BM, Steptoe A, Lovallo WR. Cardiovascular stress reactivity and health: recent questions and future directions. Psychosom Med. 2021;83(7):756–766. doi: 10.1097/PSY.0000000000000973. [DOI] [PubMed] [Google Scholar]
- 24.Blumenthal JA, Jiang W, Waugh RA, Frid DJ, Morris JJ, Coleman RE, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation. 1995;92(8):2102–2108. doi: 10.1161/01.cir.92.8.2102. [DOI] [PubMed] [Google Scholar]
- 25.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84(11):1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 26.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 27.Sestito A, Maccallini A, Sgueglia GA, Infusino F, Larosa C, Aurigemma C, et al. Platelet reactivity in response to mental stress in syndrome X and in stable or unstable coronary artery disease. Thromb Res. 2005;116(1):25–31. doi: 10.1016/j.thromres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Markovitz JH, Matthews KA. Platelets and coronary heart disease: potential psychophysiologic mechanisms. Psychosom Med. 1991;53(6):643–668. doi: 10.1097/00006842-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105(24):2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 30.Lima BB, Hammadah M, Kim JH, Uphoff I, Shah A, Levantsevych O, et al. Association of transient endothelial dysfunction induced by mental stress with major adverse cardiovascular events in men and women with coronary artery disease. JAMA Cardiol. 2019;4(10):988–996. doi: 10.1001/jamacardio.2019.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammadah M, Kim JH, Al Mheid I, Samman Tahhan A, Wilmot K, Ramadan R, et al. Coronary and peripheral vasomotor responses to mental stress. J Am Heart Assoc. 2018 doi: 10.1161/JAHA.118.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, et al. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int J Cardiol. 2017;243:47–53. doi: 10.1016/j.ijcard.2017.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., III Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76(3):125–130. doi: 10.1016/S0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 34.Lacy CR, Contrada RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, et al. Coronary vasoconstriction induced by mental stress (simulated public speaking) Am J Cardiol. 1995;75(7):503–505. doi: 10.1016/S0002-9149(99)80590-6. [DOI] [PubMed] [Google Scholar]
- 35.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325(22):1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 36.Burg MM, Soufer A, Lampert R, Collins D, Soufer R. Autonomic contribution to endothelin-1 increase during laboratory anger-recall stress in patients with coronary artery disease. Mol Med. 2011;17(5–6):495–501. doi: 10.2119/molmed.2010.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 38.Gurel NZ, Huang M, Wittbrodt MT, Jung H, Ladd SL, Shandhi MMH, et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2020;13(1):47–59. doi: 10.1016/j.brs.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah AJ, Wittbrodt MT, Bremner JD, Vaccarino V. Cardiovascular pathophysiology from the cardioneural perspective and its clinical applications. Trends Cardiovasc Med. 2022;32(3):172–177. doi: 10.1016/j.tcm.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moazzami K, Wittbrodt MT, Lima BB, Nye JA, Mehta PK, Pearce BD, et al. Higher activation of the rostromedial prefrontal cortex during mental stress predicts major cardiovascular disease events in individuals with coronary artery disease. Circulation. 2020;142(5):455–465. doi: 10.1161/CIRCULATIONAHA.119.044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby P. Inflammation in atherosclerosis-no longer a theory. Clin Chem. 2021;67(1):131–142. doi: 10.1093/clinchem/hvaa275. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan S, Young A, Hammadah M, Lima BB, Levantsevych O, Ko YA, et al. Sex differences in the inflammatory response to stress and risk of adverse cardiovascular outcomes among patients with coronary heart disease. Brain Behav Immun. 2020;90:294–302. doi: 10.1016/j.bbi.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Moran CA, Collins LF, Beydoun N, Mehta PK, Fatade Y, Isiadinso I, et al. Cardiovascular implications of immune disorders in women. Circ Res. 2022;130(4):593–610. doi: 10.1161/CIRCRESAHA.121.319877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Kanel R. Acute mental stress and hemostasis: When physiology becomes vascular harm. Thromb Res. 2015;135(Suppl 1):S52–S55. doi: 10.1016/S0049-3848(15)50444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan S, Hammadah M, Wilmot K, Ramadan R, Pearce BD, Shah A, et al. Young women with coronary artery disease exhibit higher concentrations of interleukin-6 at baseline and in response to mental stress. J Am Heart Assoc. 2018;7(23):e010329. doi: 10.1161/JAHA.118.010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottdiener JS, Krantz DS, Howell RH, Hecht GM, Klein J, Falconer JJ, et al. Induction of silent myocardial ischemia with mental stress testing: relation to the triggers of ischemia during daily life activities and to ischemic functional severity. J Am Coll Cardiol. 1994;24(7):1645–1651. doi: 10.1016/0735-1097(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 48.Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318(16):1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 49.Pimple P, Hammadah M, Wilmot K, Ramadan R, Al Mheid I, Levantsevych O, et al. Chest pain and mental stress-induced myocardial ischemia: sex differences. Am J Med. 2018;131(5):540–7.e1. doi: 10.1016/j.amjmed.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burg MM, Meadows J, Shimbo D, Davidson KW, Schwartz JE, Soufer R. Confluence of depression and acute psychological stress among patients with stable coronary heart disease: effects on myocardial perfusion. J Am Heart Assoc. 2014 doi: 10.1161/JAHA.114.000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, et al. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PLoS ONE. 2014;9(7):e102986. doi: 10.1371/journal.pone.0102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaccarino V, Almuwaqqat Z, Kim JH, Hammadah M, Shah AJ, Ko YA, et al. Association of mental stress-induced myocardial ischemia with cardiovascular events in patients with coronary heart disease. JAMA. 2021;326(18):1818–1828. doi: 10.1001/jama.2021.17649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, et al. Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler Thromb Vasc Biol. 2018;38(2):473–480. doi: 10.1161/ATVBAHA.117.309535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol. 2010;56(22):1840–1846. doi: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin EA, Tan SL, MacBride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res. 2008;18(6):339–345. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al Mheid I, Hayek S, Quyyumi AA. Provoking coronary vasospasm for diagnosis of variant angina: outdated trick of the trade or a resurgent diagnostic modality? JACC Cardiovasc Interv. 2015;8(7):924–926. doi: 10.1016/j.jcin.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Mehta PK, Thobani A, Vaccarino V. Coronary artery spasm, coronary reactivity, and their psychological context. Psychosom Med. 2019;81(3):233–236. doi: 10.1097/PSY.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan M, York KM, Li H, Li Q, Gong Y, Langaee TY, et al. Association of beta1-adrenergic receptor genetic polymorphism with mental stress-induced myocardial ischemia in patients with coronary artery disease. Arch Intern Med. 2008;168(7):763–770. doi: 10.1001/archinte.168.7.763. [DOI] [PubMed] [Google Scholar]
- 59.Ersboll M, Al Enezi F, Samad Z, Sedberry B, Boyle SH, O'Connor C, et al. Impaired resting myocardial annular velocities are independently associated with mental stress-induced ischemia in coronary heart disease. JACC Cardiovasc Imaging. 2014;7(4):351–361. doi: 10.1016/j.jcmg.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burg MM, Graeber B, Vashist A, Collins D, Earley C, Liu J, et al. Noninvasive detection of risk for emotion-provoked myocardial ischemia. Psychosom Med. 2009;71(1):14–20. doi: 10.1097/PSY.0b013e318187c035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassan M, York KM, Li H, Li Q, Lucey DG, Fillingim RB, et al. Usefulness of peripheral arterial tonometry in the detection of mental stress-induced myocardial ischemia. Clin Cardiol. 2009;32(9):E1–6. doi: 10.1002/clc.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, et al. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2(5):e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Badri A, Kim JH, Liu C, Mehta PK, Quyyumi AA. Peripheral microvascular function reflects coronary vascular function. Arterioscler Thromb Vasc Biol. 2019;39(7):1492–1500. doi: 10.1161/ATVBAHA.119.312378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Almuwaqqat Z, Sullivan S, Hammadah M, Lima BB, Shah AJ, Abdelhadi N, et al. Sex-specific association between coronary artery disease severity and myocardial ischemia induced by mental stress. Psychosom Med. 2019;81(1):57–66. doi: 10.1097/PSY.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alkhoder A, Isakadze N, Obideen M, Vaccarino V, Bremner JD, Pearce BD, et al. Norepinephrine levels are associated with the magnitude of vasoconstriction during mental stress. Circulation. 2015;132(Suppl_3):17559. doi: 10.1161/circ.132.suppl_3.17559. [DOI] [Google Scholar]
- 66.Hammadah M, Sullivan S, Pearce B, Al Mheid I, Wilmot K, Ramadan R, et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun. 2018;68:90–97. doi: 10.1016/j.bbi.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Obideen M, et al. Association between high-sensitivity cardiac troponin levels and myocardial ischemia during mental stress and conventional stress. JACC Cardiovasc Imaging. 2018;11(4):603–611. doi: 10.1016/j.jcmg.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, et al. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med. 2018;80(6):515–525. doi: 10.1097/PSY.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasher N, Wittbrodt MT, Alam ZS, Lima BB, Nye JA, Campanella C, et al. Sex differences in brain activation patterns with mental stress in patients with coronary artery disease. Biol Sex Differ. 2019;10(1):35. doi: 10.1186/s13293-019-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moazzami K, Wittbrodt MT, Alkhalaf M, Lima BB, Nye JA, Mehta PK, et al. Association between mental stress-induced inferior frontal cortex activation and angina in coronary artery disease. Circ Cardiovasc Imaging. 2020;13(8):e010710. doi: 10.1161/CIRCIMAGING.120.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wittbrodt MT, Moazzami K, Shah AJ, Lima BB, Hammadah M, Mehta PK, et al. Neural responses during acute mental stress are associated with angina pectoris. J Psychosom Res. 2020;134:110110. doi: 10.1016/j.jpsychores.2020.110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, et al. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med. 2018;In Press. [DOI] [PMC free article] [PubMed]
- 73.Kogler L, Muller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, et al. Psychosocial versus physiological stress - meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci U S A. 2004;101(17):6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ligneul R, Obeso I, Ruff CC, Dreher JC. Dynamical representation of dominance relationships in the human rostromedial prefrontal cortex. Curr Biol. 2016;26(23):3107–3115. doi: 10.1016/j.cub.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Rosen SD, Camici PG. The brain-heart axis in the perception of cardiac pain: the elusive link between ischaemia and pain. Ann Med. 2000;32(5):350–364. doi: 10.3109/07853890008995938. [DOI] [PubMed] [Google Scholar]
- 78.Moazzami K, Sullivan S, Lima BB, Kim JH, Hammadah M, Almuwaqqat Z, et al. Mental stress-induced myocardial ischemia and cognitive impairment in coronary atherosclerosis. J Psychosom Res. 2021;141:110342. doi: 10.1016/j.jpsychores.2020.110342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang W, Samad Z, Boyle S, Becker RC, Williams R, Kuhn C, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol. 2013;61(7):714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, et al. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64(16):1669–1678. doi: 10.1016/j.jacc.2014.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, et al. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014;76(3):171–180. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, et al. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation. 2018;137(8):794–805. doi: 10.1161/CIRCULATIONAHA.117.030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pimple P, Shah AJ, Rooks C, Douglas Bremner J, Nye J, Ibeanu I, et al. Angina and mental stress-induced myocardial ischemia. J Psychosom Res. 2015;78(5):433–437. doi: 10.1016/j.jpsychores.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaccarino VAMI, Wilmot K, Ramadan R, Hammadah M, Abdelhadi N, Obideen M, et al. In Women, but not in men, angina is a stronger correlate of myocardial ischemia induced by psychological than by exercise/pharmacological stress. Circulation. 2016;134:A17005. [Google Scholar]
- 86.Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(20):2602–2618. doi: 10.1016/j.jacc.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, et al. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006;166(8):876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 88.Rooks CR, Ibeanu I, Shah A, Pimple P, Murrah N, Shallenberger L, et al. Young women post-MI have higher plasma concentrations of interleukin-6 before and after stress testing. Brain Behav Immun. 2016;51:92–98. doi: 10.1016/j.bbi.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pasupathy S, Tavella R, Beltrame JF. The what, when, who, why, how and where of myocardial infarction with non-obstructive coronary arteries (MINOCA) Circ J. 2016;80(1):11–16. doi: 10.1253/circj.CJ-15-1096. [DOI] [PubMed] [Google Scholar]
- 91.J. C. S. Joint Working Group Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2013) Circ J. 2014;78(11):2779–2801. doi: 10.1253/circj.CJ-66-0098. [DOI] [PubMed] [Google Scholar]
- 92.Asbury EA, Creed F, Collins P. Distinct psychosocial differences between women with coronary heart disease and cardiac syndrome X. Eur Heart J. 2004;25(19):1695–1701. doi: 10.1016/j.ehj.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 93.Daniel M, Agewall S, Berglund F, Caidahl K, Collste O, Ekenback C, et al. Prevalence of anxiety and depression symptoms in patients with myocardial infarction with non-obstructive coronary arteries. Am J Med. 2018;131(9):1118–1124. doi: 10.1016/j.amjmed.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 94.Mehta PK, Hermel M, Nelson MD, Cook-Wiens G, Martin EA, Alkhoder AA, et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol. 2018;251:8–13. doi: 10.1016/j.ijcard.2017.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel SM, Lerman A, Lennon RJ, Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy) Eur Heart J Acute Cardiovasc Care. 2013;2(2):147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scally C, Rudd A, Mezincescu A, Wilson H, Srivanasan J, Horgan G, et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (Takotsubo) cardiomyopathy. Circulation. 2018;137(10):1039–1048. doi: 10.1161/CIRCULATIONAHA.117.031841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J. 2012;164(1):66–71.e1. doi: 10.1016/j.ahj.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 98.Radfar A, Abohashem S, Osborne MT, Wang Y, Dar T, Hassan MZO, et al. Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur Heart J. 2021;42(19):1898–1908. doi: 10.1093/eurheartj/ehab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang W, Velazquez EJ, Kuchibhatla M, Samad Z, Boyle SH, Kuhn C, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139–2149. doi: 10.1001/jama.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bairey CN, Krantz DS, DeQuattro V, Berman DS, Rozanski A. Effect of beta-blockade on low heart rate-related ischemia during mental stress. J Am Coll Cardiol. 1991;17(6):1388–1395. doi: 10.1016/s0735-1097(10)80152-4. [DOI] [PubMed] [Google Scholar]
- 101.Andrews TC, Parker JD, Jacobs S, Friedman R, Cummings N, MacCallum G, et al. Effects of therapy with nifedipine GITS or atenolol on mental stress-induced ischemic left ventricular dysfunction. J Am Coll Cardiol. 1998;32(6):1680–1686. doi: 10.1016/s0735-1097(98)00445-8. [DOI] [PubMed] [Google Scholar]
- 102.Blumenthal JA, Babyak M, Wei J, O'Connor C, Waugh R, Eisenstein E, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164–168. doi: 10.1016/S0002-9149(01)02194-4. [DOI] [PubMed] [Google Scholar]
- 103.Paran E, Amir M, Yaniv N. Evaluating the response of mild hypertensives to biofeedback-assisted relaxation using a mental stress test. J Behav Ther Exp Psychiatry. 1996;27(2):157–167. doi: 10.1016/0005-7916(96)00020-1. [DOI] [PubMed] [Google Scholar]
- 104.Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrixal stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul. 2017;10:19–27. doi: 10.1016/j.brs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 105.Gurel NZ, Wittbrodt MT, Jung H, Ladd SL, Shah AJ, Vaccarino V, et al. Automatic detection of target engagement in transcutaneous cervical vagal nerve stimulation for traumatic stress triggers. IEEE J Biomed Health Inform. 2020;24(7):1917–1925. doi: 10.1109/JBHI.2020.2981116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi MMH, Huang M, et al. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimul. 2020;13(5):1333–1348. doi: 10.1016/j.brs.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yakunina N, Kim SS, Nam EC. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. 2017;20(3):290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]