Abstract
Purpose
To report the occurrence of posterior ocular adverse events following the administration of the BNT162b2 mRNA vaccine against SARS-CoV-2.
Methods
A retrospective consecutive case series, in which the medical files of patients presenting with ocular adverse events within 30 days of the vaccine inoculation, were analyzed.
Results
Four patients (2 females) were included in the study. The diagnoses included: posterior scleritis, paracentral acute middle maculopathy, herpes panuveitis, and Vogt–Koyanagi–Harada (VKH)-like uveitis. Three of the patients had no relevant ocular history, but the patient who developed scleritis was in remission without medical therapy for four years, until the flare-up, which occurred one day after the vaccine. All patients improved with treatment.
Conclusion
Though a causal relationship cannot be definitively established, the temporal relationship suggests a possible link between the COVID-19 vaccine and the posterior ocular complications. The benefits of vaccination clearly outweigh the potential adverse effects; however, ophthalmologists should be aware of the potential for vaccine-associated uveitis.
Keywords: SARS-CoV-2, COVID-19, Vaccine, Uveitis, Scleritis, PAMM, Herpes, Ocular inflammation
Introduction
The coronavirus disease-2019 (COVID-19) pandemic is a continuing cause of large-scale morbidity and mortality worldwide. The urgency to find a solution for this global concern led to the development of multiple vaccines against SARS-CoV-2. Several vaccines have been approved after the demonstration of safety and efficacy, the most prevalent of which in Israel is the BNT162b2 mRNA vaccine (BNT162b2, Pfizer/BioN-Tech) [1]. Following the widespread vaccinations of the population, suspicion of associated ocular adverse events arose [2–5]. We report a case series of patients presenting with posterior ocular manifestations at a tertiary referral center within the first four weeks after receiving the BNT162b2 vaccine.
Methods
A retrospective study of consecutive patients presenting at the ophthalmology department of the Hadassah Medical Organization, between February and November 2021, was performed in accordance with the ethical standards of the Declaration of Helsinki. The institutional review board of the hospital approved the study, including waiver of informed consent for this chart review study. The main inclusion criterion was the development of posterior ocular inflammation within 30 days following the administration of the BNT162b2 vaccine. Each patient underwent Snellen best-corrected visual acuity (BCVA) examination, as well as biomicroscopic examination and ocular imaging per requirement.
Case 1
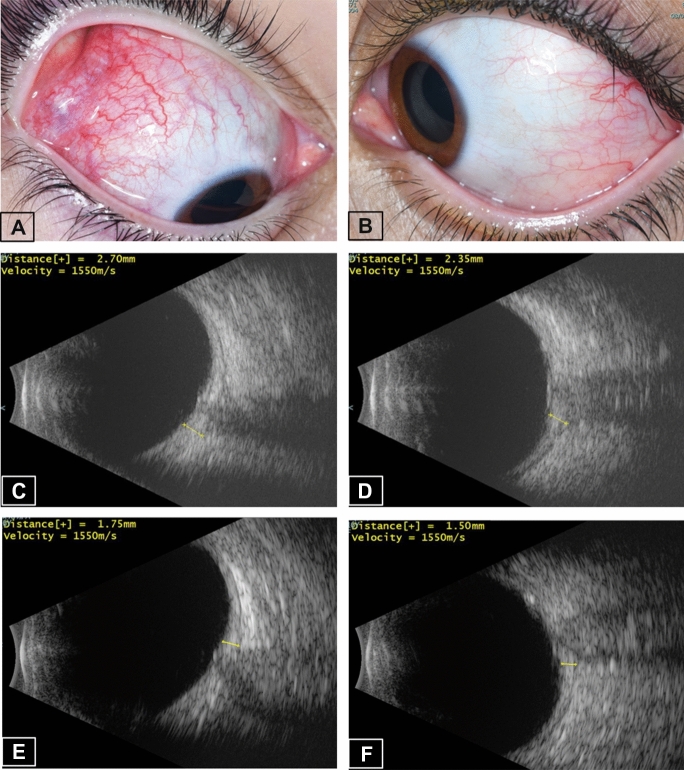
A 16-year-old female started complaining of left eye (LE) deep-seated pain and redness one day following the first dose of the BNT162b2 vaccine. Two weeks later, the pain and redness involved the right eye (RE) as well, at which point she presented to the emergency room (ER). Her past ocular history revealed that she suffered from idiopathic bilateral posterior scleritis 11 years prior, which was treated with systemic steroids and methotrexate. She had been in remission without medical treatment for the past 4 years. Past medical history included hypothyroidism and periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA), for which she did not receive treatment. At the ER, visual acuity (VA) was 6/6 in each eye, and she was diagnosed to have bilateral (BE) anterior scleritis (Fig. 1a, b), more marked in the RE. B-scan ultrasound (US) revealed thickening of posterior sclera and the classical T-sign resulting from fluid in the posterior subtenon space, indicative of posterior scleritis (Fig. 1c, d). Spectral-domain optical coherence tomography (SD-OCT) detected several vitreal hyperreflective opacities bilaterally, as well as fine irregularities of retinal outer layers surrounding the optic disk. The patient was treated with prednisone (1 mg/kg/day) and anterior scleritis resolved within 2 weeks. She was then lost to follow-up, and returned 6 months later (6 weeks after cessation of steroids). At that time, she had BE active posterior scleritis and papillitis and prednisone was therefore reinstituted. At the last follow-up, 9 months after first presentation to the ER, there were no signs of active scleritis in either eye, while the patient was treated with prednisone 5 mg daily. Posterior scleral thickening had resolved (Fig. 1e, f), and the fluid in posterior subtenon space was absorbed, as was revealed by US.
Fig. 1.
Patient #1—bilateral anterior and posterior scleritis. External eye photos at presentation showing anterior scleritis more pronounced in the right eye (a) than in the left (b). Ultrasound B-scan at presentation showing thickened sclera and choroid with a positive “T” sign bilaterally, with scleral thickness of 2.70 mm in the RE (c), and 2.35 mm in the LE (d). After 9 months of follow-up, scleral thickening had resolved, as measured by Ultrasound B-scan to be 1.75 mm in the RE (e) and 1.50 mm in the LE (f)
Case 2
An 18-year-old healthy female presented to the ER with an abrupt onset of a faintly colored, LE visual field defect of one-day duration. The symptoms occurred 13 days after receiving the third BNT162b2 vaccine (she denied any adverse events after either of the first 2 doses). She had no previous ocular or systemic history. On examination, VA was 6/6 in each eye, and there was no relative afferent pupillary defect. RE anterior and posterior segments were normal, and in the LE she had mild anterior uveitis. No obvious signs were seen on funduscopy (Fig. 2a), but on SD-OCT a hyperreflective band was detected at the junction of the outer plexiform layer (OPL) and inner nuclear layer (INL) supero-temporal to the fovea (Fig. 2c), with corresponding infrared hyporeflectance (Fig. 2b). Findings were deemed consistent with paracentral acute middle maculopathy (PAMM). The lesion’s location correlated to that of the scotoma, which was infero-nasal to the center of vision, as per the patient’s description, as well as was apparent from visual field testing. She was treated with topical and oral steroids (1 mg/kg/day), which were later tapered and stopped after 6 weeks. On last follow-up, 6 weeks after presentation, the scotoma had improved, though not resolved, and SD-OCT findings of thinning of the involved OPL and INL were consistent with the residual sequelae of PAMM (Fig. 2d).
Fig. 2.
Patient #2—paracentral acute middle maculopathy. a Fundus photo of the left eye at presentation showing no obvious pathology. b Infrared image of the left eye at presentation showing a hyporeflectant area (red arrow) superotemporally to the fovea. c SD-OCT of the left eye at presentation section through the affected area reveals a hyperreflective band (yellow brackets) at the junction of the outer plexiform layer and inner nuclear layer. d After 6 weeks of follow-up, OCT revealed characteristic thinning of the area
Case 3
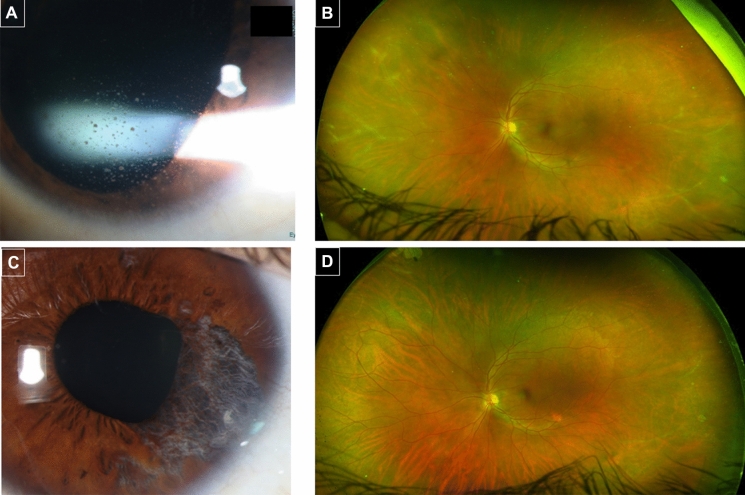
A 38-year-old healthy male presented to the ER because of LE pain, redness, blurred vision and photophobia, that started 26 days after the second BNT162b2 vaccine (the patient denied any adverse events after the first dose). He underwent LE retinal detachment repair with gas retinopexy 14 years earlier. VA was 6/6 in each eye and exam revealed LE mild non-granulomatous iritis and intraocular pressure (IOP) of 40 mmHg. He was treated with topical dexamethasone and a timolol-dorzolamide combination for possible Posner–Schlossman syndrome. On presentation to uveitis clinic 3 weeks later, large keratic precipitates were noted in LE (Fig. 3a), as well as mild vitritis, peripheral perivascular sheathing (Fig. 3b) and sectoral iris atrophy. He was thus diagnosed as herpetic panuveitis. Fluorescein angiography demonstrated diffuse vascular leakage and areas of peripheral capillary non-perfusion. Oral acyclovir 400 mg*5/day and prednisone 60 mg*1/day were instituted, in addition to topical treatment. The patient improved and inflammation had resolved, which allowed for slow tapering of systemic and topical treatment. However, upon lowering the dosage of medications, IOP spikes occurred, and iris atrophy expanded (Fig. 3c). On last follow-up, 8 months after initial presentation, he was still on acyclovir, prednisone and topical steroids. Vascular sheathing had markedly subsided (Fig. 3d).
Fig. 3.
Patient #3—herpetic panuveitis in the left eye. a Slit lamp image of large keratic precipitates at presentation to uveitis clinic, 3 weeks after onset of symptoms. b Ultra-wide field fundus imaging showing extensive peripheral perivascular sheathing. c External photograph showing sectoral iris atrophy five months after initial presentation. d 8 months after initial presentation vascular sheathing is markedly reduced
Case 4
A 24-year-old immunocompetent male presented with headache, bilateral eye pain and blurred vision three weeks after the first dose of BNT162b2 Vaccine. VA was 6/7.5 in each eye. He had bilateral ciliary injection, mild non-granulomatous iritis, mild vitritis, hyperemic optic disks, small white choroidal lesions in superior and inferior peripheral fundi, subretinal fluid (SRF) along the inferotemporal arcade of right fundus and subfoveal SRF bilaterally (Fig. 4). Fluorescein angiography revealed the presence of diffusely scattered hypofluorescent lesions in the early phase with optic disk leakage subsequently. Choroidal thickening with multiple foci of choriocapillaris flow void were identified on swept-source optical coherence tomography (SS-OCT) and OCT angiography (Fig. 5). Extensive investigations ruled out infectious (syphilis, tuberculosis) or a systemic inflammatory condition (normal chest radiograph and angiotensin converting enzyme, negative antinuclear antibody and c-reactive protein and normal C3, C4 and erythrocyte sedimentation rate). He was thus diagnosed with acute Vogt–Koyanagi–Harada (VKH)-like disease.
Fig. 4.
Patient #4—Vogt–Koyanagi–Harada-like uveitis—Ultra-wide field fundus imaging of the right eye. Imaging shows clear vitreous, hyperemic optic disk, a localized area of exudative retinal detachment along the inferotemporal arcade (arrows) (a) and fine grayish-white choroidal lesions in superior peripheral retina (b)
Fig. 5.
Patient #4—Vogt–Koyanagi–Harada-like uveitis—optical coherence tomography (OCT). Swept-source OCT (Topcon DRI OCT Triton) of the right macula: a At presentation, showing subretinal fluid in the fovea, irregularities along the ellipsoid zone and marked choroidal thickening (arrow). b Two weeks later, showing resolution of subretinal fluid and better demarcation of thickened choroid. c Eight weeks after presentation, showing restoration of the ellipsoid zone and resolution of the choroidal thickening (arrow). d OCT angiography (Topcon DRI OCT Triton) of the right macula shows at presentation at the level of the choriocapillaris (arrow) multiple hyporeflective round-to-oval lesions, representing areas of flow void or choriocapillaris hypoperfusion corresponding to the hypofluorescent lesions observed in the early phase of fluorescein angiogram. e Flow void areas resolved 8 weeks later (arrow)
Oral corticosteroids were initiated at 1 mg/kg/day with subsequent taper. Rapid SRF resorption occurred within one week of prednisone administration. Gradual and persistent resolution of choroidal thickening was observed in sequential SS-OCT images (Fig. 5). Visual acuity recovered to 6/6 in each eye four weeks after first presentation. Flow void areas resolved eight weeks (Fig. 5) after starting treatment and VA remained 6/6 in each eye.
There was no recurrence of intraocular inflammation and integumentary signs like vitiligo, poliosis and alopecia did not develop over a 12-week-follow-up period while continuing prednisone taper. The patient declined receiving the 2nd dose of the vaccine.
Discussion
Worldwide, the year 2020 was dominated by the health and economic harm caused by the Covid-19 pandemic. That year ended with a glimmer of hope, as regulators began to approve Covid-19 vaccines and governments around the world began to administer them. The FDA issued an emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine on December 11, 2020.
BNT162b2 is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein [1].
In the current case series, we report four patients presenting with posterior ocular manifestations within 1 month of inoculation with the BNT162b2 vaccine.
Two cases of scleritis and one of episcleritis were reported in patients after COVID-19 vaccines, at a mean of 5 days [6]. Details are given regarding only one of the scleritis cases, in which the patient had rheumatoid arthritis and no previous ocular history. The patient was inoculated with an inactivated COVID-19 vaccine, and one week later developed bilateral anterior scleritis, which resolved within one week of systemic steroid treatment. In our series, we describe a reactivation of anterior and posterior scleritis after the BNT162b2 vaccine, in a patient who had been free of inflammation for years.
Previous reports of episcleritis and scleritis following administration of live, attenuated viruses are rare but not unprecedented [7, 8]. Those cases have been described to be mild with a good response to therapy. To date, no previous descriptions of posterior scleritis have been reported in association with COVID-19 vaccines. Our case, however, was severe, bilateral, and involving both anterior and posterior sclera. It improved with systemic steroids but relapsed upon tapering thus requiring prolonged treatment over the course of months.
PAMM was first described by Sarraf et al. in 2013 in five patients who presented with paracentral scotomas, referring to a hyperreflective parafoveal band at the level of the inner nuclear layer (INL) on OCT, that co-localizes with the intermediate and deep capillary plexuses [9]. The development of subsequent INL thinning corresponding to the original lesion is hypothesized to indicate an etiology of ischemia of the intermediate and deep capillary systems.
In the original case series, one of the patients was reported to suffer from a flu-like illness [9]. It has since been described in association with various other conditions, including the H1N1 vaccine [10]. Virgo and Mohamed [11] described a patient with PAMM induced by COVID-19 infection. The authors gave a detailed description of the patient’s symptoms they refer to a “faintly colored paracentral scotoma,” which was the exact characterization given by our patient. Recently, cases of PAMM have also been described following the COVID-19 vaccines Sinopharm [6] and Covishield [12]. To our knowledge, ours is the first report of PAMM in a patient following the Pfizer BNT162b2 vaccine.
Herpetic infections following COVID-19 vaccines are a matter of growing debate [13–15]. The cutaneous reactions are generally mild and self-limiting. Herpes simplex keratitis reactivation has been described in two patients after the BNT162b2 vaccine [16]. Rehman et al. [17] described two patients who presented with herpes zoster ophthalmicus (HZO) after receiving a live COVID-19 vaccine. Also reported is a case of reactivation of varicella zoster infection, presenting as acute retinal necrosis in a patient 2 days after a Covishield vaccination [18]. In our series, we report a case of herpetic panuveitis occurring for the first time in a patient few weeks after the COVID vaccine.
Vaccines were shown in few case reports to trigger the development of VKH, namely, influenza, yellow fever, hepatitis B and BCG vaccines [19–24]. Recently, Papasavvas and Herbort [25] described a patient who suffered from VKH reactivation 6 weeks after the second dose of a BNT162b2 COVID-19 vaccine, while treated with infliximab infusions every 10 weeks. Two other reports suggested new onset of VKH shortly after receiving the BNT162b2 and after receiving the Oxford-AstraZeneca AZD1222 COVID-19 vaccines [26, 27]. Table 1 summarizes the clinical features of patients who presented with post-vaccine VKH. Ocular manifestations developed at a median of 2.5 weeks after receiving the vaccine (range: 1 day–4 weeks). It developed bilaterally and was associated with excellent prognosis in all reported cases. All patients were treated with systemic corticosteroids as a first-line therapy and steroid-sparing agents were added in some cases. Patients were usually healthy with unremarkable medical history and most presented with a prodrome of headache.
Table 1.
Summary of post-vaccination VKH cases: Demographic features, systemic and ocular characteristics, vaccine type, dose and mode of administration, treatment, visual outcome, and follow-up time
| Dogan et al. [19] | Sood et al. [20] | Campos et al. [21] | Pereima et al. [23] | Kim [22] | Gallagher et al. [24] | Papasavvas et al. [25] | Saraceno et al. [27] | Koong et al. [26] | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 39 | 43 | 34 | 45 | 52 | 44 | 43 | 62 | 54 |
| Gender | Male | Male | Male | Male | Female | Female | Female | Female | Male |
| Ethnicity | Caucasian | Caucasian and Cherokee Native American ancestors | Latino | Latino | Indian | Not mentioned | Not mentioned | Not mentioned | Chinese |
| Type of Vaccine | Bacille Calmette-Guérin (BCG) | Single-antigen hepatitis B vaccine | 17 D yellow fever vaccine | 17 D yellow fever vaccine | Influenza vaccine | Influenza vaccine | Pfizer BNT162b2 COVID-19 vaccine | Oxford-AstraZeneca AZD1222 COVID-19 vaccine | Pfizer BNT162b2 COVID-19 vaccine |
| Dose of vaccine | Fourth dose | First dose | Booster dose | Not mentioned | Not mentioned | Not mentioned | Second dose | Not mentioned | First dose |
| Mode of Administration | Intravesical | Not mentioned, but usually administered IM | Not mentioned, but it is administered SC or IM | Not mentioned, but it is administered either SC or IM | Not mentioned, but usually administered IM | Not mentioned, but usually administered IM | Not mentioned, but usually administered IM | Not mentioned, but usually administered IM | Not mentioned, but it is usually administered IM |
| Time interval between vaccine and VKH | 4 weeks after the 1st dose and shortly after 4th dose | 3 days | 10 days | 2 weeks | 4 weeks | 4 weeks | 6 weeks | 2 days | 1 day |
| Treatment | Prednisone | Prednisone and Methotrexate subsequently | Pulse IVMP, followed by prednisone and azathioprine | Pulse IVMP, followed by prednisone and azathioprine | Pulse IVMP followed by oral corticosteroids | Pulse IVMP, followed by prednisone, azathioprine and cyclosporine | Infliximab- loading dose | Prednisone | Pulse IVMP, followed by prednisone |
| Duration of treatment | 6 months | Not mentioned | Not mentioned | 9 months | 2 months | Not mentioned | Not mentioned | Not mentioned | planned for 6 to 12 months |
| Presenting Visual acuity |
RE 20/25 LE 20/50 |
RE 20/800 LE 20/40 |
RE 20/100 LE 20/80 |
RE CF LE CF |
RE 20/30 LE 20/100 |
RE CF LE CF |
RE 20/20 LE 20/20 |
RE 20/600 LE 20/200 |
RE 20/80 LE 20/150 |
| Laterality of uveitis | BE | BE | BE | BE | BE | BE | BE | BE | BE |
| Visual outcome |
RE 20/20 LE 20/20 |
RE 20/30 LE 20/30 |
RE 20/20 LE 20/20 |
RE 20/20 LE 20/20 |
RE 20/20 LE 20/25 |
RE 20/25 LE 20/30 |
Not mentioned |
RE 20/20 LE 20/20 |
RE 20/40 LE 20/40 |
| Time to full visual acuity recovery | One month | 2 months | 3 weeks | One month | 2 months | Not mentioned | Not mentioned | 3 weeks | 13 days |
| Ocular Complications | None | Peripapillary choroidal neovascular membrane | None | Sunset glow fundus | None | Not mentioned | Not mentioned | None | None |
| Systemic Manifestations | Prodrome of headache, malaise | After ocular manifestations, patient developed hearing loss, tinnitus, and integumentary changes | Prodrome of headache, tinnitus, CSF pleocytosis | Prodrome of headache, tinnitus, CSF pleocytosis | Prodrome of tinnitus | Prodrome of tinnitus | Not mentioned | Severe headache and tinnitus | None |
| Follow-up time | 6 months | Not mentioned | 2 years | 30 months | 2 months | Not mentioned | Not mentioned | 2 months | 13 days |
| Associated morbidities | Superficial TCC of bladder | Type II diabetes mellitus | None | None | None | None | None | None | Type II Diabetes Mellitus and hyperlipidemia |
SC subcutaneous, IM intramuscular, RE right eye, LE left eye, BE both eyes, CF counting fingers, IVMP intravenous methylprednisolone, CSF cerebrospinal fluid, TCC transitional cell carcinoma
A recent publication [28] assessed the risk of vaccine-associated uveitis (VAU) following SARS-CoV-2 vaccination using the Centers for Disease Control and Prevention (CDC) Vaccine Adverse Events Reporting System (VAERS). The study found an estimated crude reporting rate (per million doses) of 0.57, 0.44, and 0.35 for BNT162b2, mRNA-1273, and Ad26.COV2.S, respectively, i.e., the three vaccines rarely caused VAU. Most of the patients had anterior uveitis (44.88%). Similarly, Tomkins-Netzer et al. [29] and Wang et al. [30] reported that 90.96 and 74% of their cohort, respectively, had anterior uveitis. Analysis of the largest adverse event global database suggested that VAU was primarily diagnosed after first dose and within first week following vaccination.
The criteria described by Naranjo et al. [31] and World Health Organization [32] may assist in the attempt to determine whether a causal relationship exists between the vaccine and uveitis. It seems an association would be considered “possible” in the four cases presented, though the criteria are more fitting for use with chronic treatment, as opposed to a vaccine given as an isolated incident.
In conclusion, we report four cases of posterior uveitic manifestations after COVID-19 vaccines. All patients still exhibited some signs and symptoms after substantial follow-up. While such associations continue to surface, it is important to emphasize that causality has not yet been established. It is vital to identify uveitic entities presenting soon after vaccinations, as ophthalmologists attempt to ascertain reliable correlations. Large, controlled studies are needed to verify whether a true causal relationship exists.
The benefits of vaccination clearly outweigh the potential adverse effects; however, ophthalmologists should be aware of the potential for vaccine-associated uveitis.
Acknowledgements
None
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Radgonde Amer and Shani Pillar. The first draft of the manuscript was written by Shani Pillar and Radgonde Amer and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This is an observational study. The HMO Research Ethics Committee has confirmed that no ethical approval is required.
Informed Consent
This type of study does not require an informed consent.
Consent for Publications
All authors thoroughly reviewed the manuscript and consented for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(6):1216–1224. doi: 10.1080/09273948.2021.1976221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, et al. Uveitis after the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association. Retina Phila Pa. 2021;41:2462–2471. doi: 10.1097/IAE.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 4.Neri P, Pichi F. SARS-CoV-2 and the eye: the Pandora's box of ocular immunology. J Ocul Pharmacol Ther. 2021;37(9):502–509. doi: 10.1089/jop.2021.0058. [DOI] [PubMed] [Google Scholar]
- 5.Neri P, Pichi F. COVID-19 and the eye immunity: lesson learned from the past and possible new therapeutic insights. Int Ophthalmol. 2020;40(5):1057–1060. doi: 10.1007/s10792-020-01389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichi F, Aljneibi S, Neri P, et al. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139(10):1131–1135. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moorthy RS, Moorthy MS, Cunningham ET. Drug-induced uveitis. Curr Opin Ophthalmol. 2018;29:588–603. doi: 10.1097/ICU.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 8.Thurairajan G, Hope-Ross MW, Situnayake RD, et al. Polyarthropathy, orbital myositis and posterior scleritis: an unusual adverse reaction to influenza vaccine. Br J Rheumatol. 1997;36:120–123. doi: 10.1093/rheumatology/36.1.120. [DOI] [PubMed] [Google Scholar]
- 9.Sarraf D, Rahimy E, Fawzi AA, et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131:1275–1287. doi: 10.1001/jamaophthalmol.2013.4056. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Rahimy E, Sergott RC, et al. Spectrum of retinal vascular diseases associated with paracentral acute middle maculopathy. Am J Ophthalmol. 2015;160:26–34.e1. doi: 10.1016/j.ajo.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye Lond Engl. 2020;34:2352–2353. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinzamuri S, Pradeep TG, Kotian R. Bilateral paracentral acute middle maculopathy and acute macular neuroretinopathy following COVID-19 vaccination. Indian J Ophthalmol. 2021;69:2862–2864. doi: 10.4103/ijo.IJO_1333_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi: 10.3390/jcm10225344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluger N, Klimenko T, Bosonnet S. Herpes simplex, herpes zoster and periorbital erythema flares after SARS-CoV-2 vaccination: 4 cases. Ann Dermatol Venereol. 2022;149(1):58–60. doi: 10.1016/j.annder.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birabaharan M, Kaelber DC, Karris MY. Risk of herpes zoster reactivation after mRNA COVID-19 vaccination: a cohort study. J Am Acad Dermatol. 2021;S0190–9622(21):02892–2899. doi: 10.1016/j.jaad.2021.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alkhalifah MI, Alsobki HE, Alwael HM, et al. Herpes simplex virus keratitis reactivation after SARS-CoV-2 BNT162b2 mRNA Vaccination: a report of two cases. Ocul Immunol Inflamm. 2021;29(6):1238–1240. doi: 10.1080/09273948.2021.1986548. [DOI] [PubMed] [Google Scholar]
- 17.Rehman O, Ichhpujani P, Nayyar S, et al. COVID-19 pandemic and lockdown: changing trends in ophthalmology for in-patient and emergency services. Indian J Ophthalmol. 2021;69:701–705. doi: 10.4103/ijo.IJO_3009_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra SB, Mahendradas P, Kawali A et al (2021) Reactivation of varicella zoster infection presenting as acute retinal necrosis post COVID 19 vaccination in an Asian Indian male. Eur J Ophthalmol 11206721211046484 [DOI] [PubMed]
- 19.Dogan B, Erol MK, Cengiz A. Vogt–Koyanagi–Harada disease following BCG vaccination and tuberculosis. Springerplus. 2016;5:603. doi: 10.1186/s40064-016-2223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sood AB, O’Keefe G, Bui D, et al. Vogt–Koyanagi–Harada disease associated with hepatitis B vaccination. Ocul Immunol Inflamm. 2019;27:524–527. doi: 10.1080/09273948.2018.1483520. [DOI] [PubMed] [Google Scholar]
- 21.Campos WR, Cenachi SPF, Soares MS, et al. Vogt–Koyanagi–Harada-like disease following yellow fever vaccination. Ocul Immunol Inflamm. 2021;29:124–127. doi: 10.1080/09273948.2019.1661498. [DOI] [PubMed] [Google Scholar]
- 22.Kim M. Vogt–Koyanagi–Harada syndrome following influenza vaccination. Indian J Ophthalmol. 2016;64:98. doi: 10.4103/0301-4738.178141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereima RR, Bonatti R, Crotti F et al (2021) Ocular Adverse Events following yellow fever vaccination: a case series. Ocul Immunol Inflamm 1–5 [DOI] [PubMed]
- 24.Gallagher MJ, Yilmaz T, Foster CS (2009) Vogt–Koyanagi–Harada syndrome associated with bilateral serous macular detachments responsive to immunomodulatory therapy. Ophthalmic Surg Lasers Imaging Off J Int Soc Imaging Eye 40:345–347 [DOI] [PubMed]
- 25.Papasavvas I, Herbort CP. Reactivation of Vogt–Koyanagi–Harada disease under control for more than 6 years, following anti-SARS-CoV-2 vaccination. J Ophthalmic Inflamm Infect. 2021;11:21. doi: 10.1186/s12348-021-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koong LR, Chee WK, Toh ZH, et al. Vogt–Koyanagi–Harada disease associated with COVID-19 mRNA vaccine. Ocul Immunol Inflamm. 2021;29(6):1212–1215. doi: 10.1080/09273948.2021.1974492. [DOI] [PubMed] [Google Scholar]
- 27.Saraceno JJF, Souza GM, Dos Santos Finamor LP, et al. Vogt–Koyanagi–Harada syndrome following COVID-19 and ChAdOx1 nCoV-19 (AZD1222) vaccine. Int J Retina Vitr. 2021;7:49. doi: 10.1186/s40942-021-00319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh RB, Singh Parmar UP, Kahale F, et al. Vaccine-associated uveitis following SARS-CoV-2 vaccination: a CDC-VAERS database analysis. Ophthalmology. 2022;S0161–6420(22):00672–678. [Google Scholar]
- 29.Tomkins-Netzer O, Sar S, Barnett-Griness O, et al. Association between vaccination with the BNT162b2 mRNA COVID-19 vaccine and non-infectious uveitis: a population-based study. Ophthalmology. 2022;129(10):1087–1095. doi: 10.1016/j.ophtha.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang MTM, Niederer RL, McGhee CNJ, et al. COVID-19 vaccination and the eye. Am J Ophthalmol. 2022;240:79–98. doi: 10.1016/j.ajo.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 32.Holloway K (editor) and Green T (2003) World Health Organization. (2003). Drug and therapeutics committees: a practical guide. https://apps.who.int/iris/handle/10665/68553