Abstract
Dissemination of robotic surgical technology for robot-assisted laparoscopic prostatectomy (RALP) has yielded advancements including the Retzius-sparing (RS) approach and the single-port (SP) platform. The safety and feasibility of each individual advancement have been evaluated, yet there is a lack of literature comparing SP RS-RALP to conventional multi-port (MP) RS-RALP. All patients who underwent RS-RALP at our institution between January 2019 and February 2021 were retrospectively reviewed. Data regarding baseline patient and tumor characteristics, operative characteristics, and surgical outcomes were collected and analyzed using the Fisher’s exact test and two-tailed unpaired t tests. 62 patients were evaluated: 31 received SP RS-RALP and 31 received MP RS-RALP. Differences in patient age, BMI, and initial PSA were not observed. Lower median lymph node yield (SP: 4 vs MP: 12, p < 0.01), lower estimated blood loss (SP: 111.2 vs. MP 157.8 mL, p < 0.01), shorter operative time (SP: 207.7 vs. MP: 255.9 min, p < 0.01) and decreased length of stay (SP: 0.39 vs. MP: 1.23 days, p < 0.01) were observed in the SP RS-RALP cohort. No differences in positive surgical margins, complications, or biochemical recurrence rates were observed. SP RS-RALP is non-inferior to MP RS-RALP in terms of both perioperative and early oncologic outcomes. Despite the small sample size, the SP platform is a safe and feasible option for RS-RALP and confers potential benefits in the form of shorter operative time and reduced length of stay.
Keywords: Retzius-sparing, Single-port, Multiport, Prostate cancer, Outcomes
Introduction
Robot-assisted laparoscopic prostatectomy (RALP) has become the primary surgical approach to prostate cancer in North America with over 85% of all prostatectomies performed with robotic assistance [1]. While providing patients with the benefits of minimally invasive surgery, RALP has also been demonstrated to be non-inferior from an oncologic standpoint when compared to open or laparoscopic approaches [2–4]. With the widespread adoption of robotics, new surgical techniques as well as platforms have been developed.
One such technique is the Retzius-Sparing (RS) approach. First described by the Bocciardi group in 2010, the RS approach entails the development of an intrafascial plane without compromising neurovascular bundles or ligaments of the anterior compartment [5]. Preservation of these structures is believed to play a role in improving post-operative continence and potency. This approach is a safe and feasible alternative to the standard retropubic RALP [6]. RS affords patients with a more expedient recovery of continence with comparable rates of long-term function and oncologic outcomes [7–9]. Although the RS approach is not particularly pervasive, the learning curve has been demonstrated to be manageable and can be performed safely by relatively inexperienced surgeons [10].
The widespread uptake of the da Vinci® robotic surgery systems (Intuitive Surgical, Inc; Sunnyvale, CA) have resulted in subsequent developments such as the single-port (SP) platform. The SP platform allows surgeons to operate through a single 25 mm port. This has previously been described within the context of RALP as an alternative to the standard multi-port (MP) platform, with comparable perioperative outcomes [11, 12]. The SP platform confers possible benefits in the form of reduced post-operative pain and increased likelihood of same-day discharge [12]. Furthermore, the learning curve for SP systems has been demonstrated to be similar to that of MP [13].
Although the advantages of the RS approach and the SP platform for RALP have been investigated independently, there is a lack of data on the combination of both. With SP showing promise as an alternative to the standard MP, we sought to compare RS-RALP performed on the SP platform versus those performed with the traditional MP systems (MP RS-RALP). We hypothesized that the SP platform would be non-inferior to MP regarding both oncologic and perioperative outcomes.
Methods
Subjects
After Institutional Review Board approval was granted, we performed a retrospective chart review of RS-RALP performed utilizing either SP or MP da Vinci® surgical platforms at our institution from January 2019 to February 2021. Our comparison included consecutive cases from multiple surgeons. All cases were performed at the start of each surgeon’s learning curve for each respective platform.
Retzius-sparing Technique
Our approach to MP RS-RALP was performed as originally described by the Bocciardi group [5, 14]. First, the patient was placed in Trendelenburg position to 20–25 degrees. Trocars were placed in the standard pelvic configuration with the assistant port on the left (Fig. 1A). The parietal peritoneum was incised at the anterior surface of the recto-vesical pouch. The seminal vesicles were dissected and vas deferens transected. The dissection was then carried antegrade over the posterior and posterolateral surface of the prostate until the apex was reached. Full nerve sparing was performed when oncologically safe. The vesico-prostatic junction was then identified and divided posteriorly, the catheter was identified, and the anterior bladder neck divided. The anterior surface of the prostate was bluntly dissected up to the apex and the urethra was divided. The anastomosis was performed with a double arm 2–0 Quill™ barbed suture (Corza Medical, Westwood, MA). Pelvic lymph nodes were dissected with the external iliac vein, obturator nerve, and the inguinal ligament as boundaries of dissection.
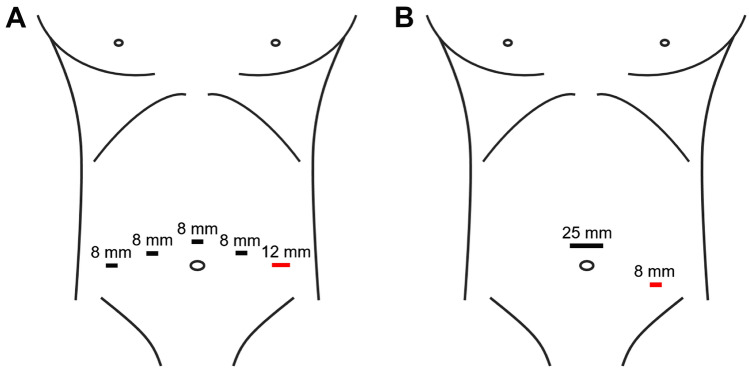
Fig. 1.
Port placement locations for both MP (A) and SP (B) RS-RALP approaches. Red = assistant port, mm = millimeters
SP RS-RALP was performed with the da Vinci® SP system. Our approach with the SP system has been previously described [15, 16]. Key intraoperative images are provided in Figs. 2 and 3. This co-linear single entry device with flexible camera and three robotic arms allows surgery to be accomplished through a single 2.5 cm cannula (Fig. 1B). The Hasson technique was used to place the periumbilical SP trocar. The same operative steps were performed using the SP platform as described above for MP. The camera was placed in the 6 o’clock (down) position, Cadiere forceps in the 12 o’clock, monopolar scissors in the 3 o’clock, and Maryland bipolar instrument in the 9 o’clock position during the extirpative portion of the procedure. The monopolar scissors were replaced with a needle driver for the anastomosis. A standard template lymph node dissection was performed in all cases. Briefly, the anterior peritoneum was opened just lateral to the medial umbilical ligament from the crossing of the vas inferiorly to the level of the inguinal canal. The vas was dissected laterally, and the lymph node packet approached. This was repeated for the contralateral dissection.
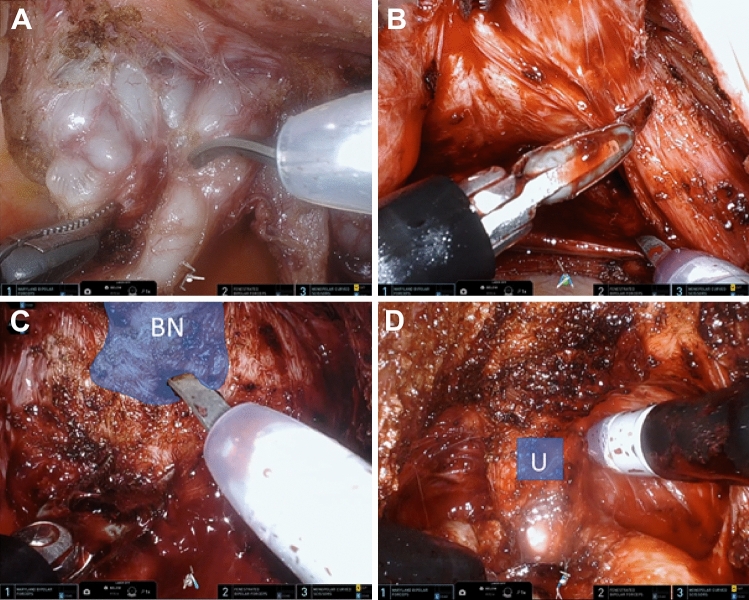
Fig. 2.
Intraoperative images during dissection portion of SP RS-RALP. A Exposure of seminal vesicles and vas deferens bilaterally. B Transection of right prostatic pedicle with electrocautery. C Dissection and exposure of bladder neck. D Direct visualization of urethra and foley catheter is identified
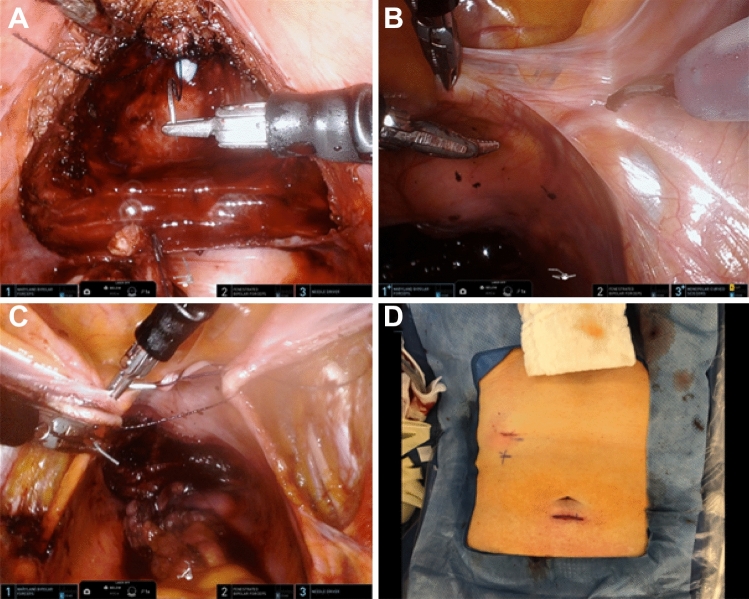
Fig. 3.
Intraoperative images during SP RS-RALP A Anterior anastomosis is performed. B Beginning of right pelvic lymph node dissection. C Holding stitch placed. D Postoperative patient abdomen
In both MP and SP RS-RALP, we performed a peritoneal holding suture (Fig. 3C) following our lymph node dissection to facilitate intraperitoneal drainage of any fluid in the dissection bed [15].
Outcome measures
The following variables were recorded: positive surgical margin rate (≥ 3 mm), lymph node yield, post-operative length of stay (LOS), pain control/milligram morphine equivalents (MME), estimated blood loss (EBL), operative time, postoperative complication rate (Clavien-Dindo ≥ 3 within 90 days of surgery), and time to biochemical recurrence (BCR) [17]. A prostate-specific antigen (PSA) threshold value of 0.2 ng/mL was used to evaluate for BCR.
Statistical analysis
Statistical comparisons were performed using Fisher’s exact test for categorical data and two-tailed unpaired t-tests for continuous data. Statistical significance was determined using a p-value cutoff of < 0.05. All statistics were performed using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline patient demographics and clinical characteristics are summarized in Table 1. No significant differences were seen in any patient demographics assessed, including age, body-mass index (BMI), and preoperative PSA. Significant differences were detected in the final pathology T stages between the groups (SP: ≥ T3 12.9% (4/31) vs. MP: ≥ T3 45.2% (14/31), p = 0.01).
Table 1.
Patient demographics and tumor characteristics
| Patient and tumor characteristics | SP (n = 31) |
MP (n = 31) |
p-value |
|---|---|---|---|
| Age, years (SD) | 62.1 (7.8) | 62.3 (6.2) | 0.90 |
| BMI, kg/m2 (SD) | 28.7 (3.9) | 31.0 (5.3) | 0.053 |
| Preoperative PSA, ng/mL (SD) | 7.13 (5.6) | 8.66 (6.0) | 0.32 |
| T Stage | 0.0106 | ||
| T2 (%) | 27 (87.1) | 17 (54.8) | |
| T3 (%) | 4 (12.9) | 14 (45.2) | |
| Gleason Grade Group | 0.185 | ||
| Grade Group 1 (%) | 1 | 0 | |
| Grade Group 2 (%) | 19 | 11 | |
| Grade Group 3 (%) | 6 | 14 | |
| Grade Group 4 (%) | 2 | 4 | |
| Grade Group 5 (%) | 3 | 2 |
Surgical outcomes and operative characteristics for SP vs MP approaches to RS-RALP are summarized in Table 2. The SP platform was associated with a shorter operative time (SP: 207.7 vs. MP: 255.9 min, p < 0.01), lower median lymph node yield (SP: 4 vs MP: 12, p < 0.01), shorter average LOS (SP: 0.39 vs. MP: 1.23 days, p < 0.01), and lower EBL (SP: 111.2 vs. MP 157.8 mL, p < 0.01). There were no significant differences in rates of positive surgical margins (SP: 38.7 vs. MP: 38.7%, p = 1.00), Clavien–Dindo ≥ 3 complications (SP: 19.4 vs. MP: 22.6%, p = 1.00), or BCR (SP: 6.5 vs. MP: 6.5%, p = 1.00) at most recent follow-up. Mean follow-up time was significantly lower for the SP group, as these cases were performed more recently (SP: 117.8 vs. MP: 355.0 days, p < 0.01).
Table 2.
Comparison of outcomes and operative characteristics between patients that underwent single-port vs. multiport RALP
| Outcomes and operative | SP | MP | |
|---|---|---|---|
| Characteristics | (n = 31) | (n = 31) | p-value |
| EBL, mL (SD) | 112.4 (46.5) | 157.8 (69.7) | < 0.001 |
| Operative time, minutes (SD) | 207.7 (40.2) | 255.9 (73.5) | < 0.001 |
| Median lymph node yield, n (IQR) | 4 (4) | 12 (6) | < 0.001 |
| Complications, n (%) † | 6 (19.4) | 7 (22.6) | 1.00 |
| Lymphocele | 4 | 5 | |
| Bladder neck disruption (partial) | 2 | 1 | |
| Rectal perforation | 0 | 1 | |
| MME, mg (SD) | 7.62 (9.4) | 10.77 (22.6) | 0.48 |
| Length of Stay | 0.39 (0.5) | 1.23 (0.76) | < 0.001 |
| Positive margins, n (%) | 12 (38.7) | 12 (38.7) | 1.00 |
| BCR, n (%) | 2 (6.5) | 2 (6.5) | 1.00 |
| Mean follow-up time, days, (SD) | 122.5 (85.1) | 358.0 (170.0) | < 0.001 |
Discussion
This study represents the largest-known SP RS-RALP series. We performed a direct comparison between consecutive SP and MP RS-RALP performed at our institution. LOS and operative time were both noted to be lower for the SP cohort. Furthermore, our data also demonstrate that SP RS-RALP were non-inferior to MP in terms of both perioperative and early oncologic outcomes. This further validates that it is a feasible alternative to MP in the context of RS-RALP.
Patients that underwent SP RS-RALP had a significantly lower average LOS when compared to those operated on with an MP system. This corresponds with previous literature that has reported increased rates of patients both qualifying and opting for same-day discharge post-SP RALP [18]. This reduction in LOS associated with the SP platform is not preserved in the context of larger surgeries such as radical cystectomies [19]. Interestingly, despite the decreased LOS, there were no significant differences detected in patient post-operative analgesia when assessed via MME. This could potentially be due to more aggressive initial pain control for patients that were anticipated to be discharged the same day. SP systems are associated with increased costs in certain domains. Specifically, there is a high financial burden in the form of up-front investments as well as well as a greater utilization of disposables during surgeries. However, Lenfant et al. demonstrated that this increased cost of disposables (SP: $2445 vs. MP: $763) is offset by a decreased LOS, resulting in comparable average total costs for patients undergoing RALP (SP: $13,512 vs. MP: $13,284) [20].
Our data demonstrate that SP RS-RALP are non-inferior to MP from both a perioperative as well as an early oncologic standpoint. Similar findings have previously been reported when comparing SP and MP during standard retropubic RALP [11]. There were no significant differences in the rates of positive surgical margins, BCR, or post-operative complications. The rates of positive surgical margins observed in this study was equal between both platforms at 38.7%. This falls in line with previous literature that has reported rates ranging from 20 to 55% for SP RALP [11, 21, 22]. As for RS-RALP, positive surgical margin rates have been reported at 22.4% and 10%, depending on which point in the surgeon’s learning curve they took place [14]. Despite the rates of positive surgical margins, only two patients in each cohort (6.5%) met our criteria for BCR. This value is on the lower end of previously published BCR and may reflect our relatively early follow-up for this cohort of patients [2, 23].
Notably, cases performed with the SP platform were associated with decreased median lymph node yields. Both values are lower than previously reported lymph node yields for both SP as well as RS-RALP with median values of 19 and 15 lymph nodes, respectively [7, 24]. A potential reason for this is that our group detected high rates of symptomatic lymphoceles early on in our experience with RS-RALP [15]. Symptomatic lymphoceles presented at an average of 34 days post-operatively and were typically managed via IR drain placement. Our detection of the high lymphocele rate likely resulted in more cautious subsequent lymph node dissections and, consequently, lower yields. Furthermore, the cohort that underwent SP RS-RALP had a significantly lower proportion of T3 + disease. Therefore, within this clinical context, it is likely that less aggressive lymph node dissections were performed. It is worth noting that less aggressive dissections could potentially skew the complication rates in favor of the SP platform.
SP RS-RALP were, on average, nearly 50 min shorter than those performed with an MP system. Importantly, this reduced operative time likely enhanced the feasibility of patients receiving a same day discharge. The reason for difference is likely multifactorial. One explanation for the decreased operative time is that of an expedited port placement and closure for cases on the SP platform. Additionally, the altered LN dissection and decreased yield addressed above likely also contributed to shorter operative times. Lastly, the SP RS-RALP examined in this study took place early on during our institution’s adoption of the platform. Therefore, there was likely less trainee time on the robot console, which could have further contributed to shorter overall case lengths. EBL was also found to be lower for our SP RS-RALP. This is also likely directly related to the differences in operative time discussed above.
This study is not without limitations. A majority of the SP RS-RALP were performed during the height of the COVID-19 pandemic. Previous research has indicated an increased patient preference for same-day discharge following urologic surgery while governmental restrictions were in place [25]. However, as discussed previously, other studies have also reported increased feasibility of same-day discharge without such external pressures [18]. The relatively shorter follow-up period for patients within the SP group is also a limiting factor, particularly when discussing BCR. However, this is expected as the SP platform represents a more recent series of patients at our institution. Our group felt that it was important to capture cases at the beginning of each surgeon’s learning curve in order for a more valid comparison. This validity is further enhanced by previous literature, which has demonstrated comparable prostatectomy learning curves between the SP and MP platforms [13]. Lastly, there were significant differences in the T-stages of tumors between the two groups studies. This is likely due to a more prudent selection of patients to receive SP RS-RALP, a relatively pioneering procedure. While the selection bias may contribute to the differences observed in operative time and EBL, it does not detract from our findings that the SP platform a safe and feasible option for RS-RALP.
Conclusions
In the context of RS-RALP, the SP platform is non-inferior to MP in terms of both perioperative and early oncologic outcomes. Within this setting, the SP system may in fact confer certain advantages over MP in the form of decreased LOS, operative time, and EBL. This proof-of-concept study will ultimately aid in setting the stage for future multi-institutional prospective trials with the potential to shape the standard of care.
Funding
None.
Declarations
Conflicts of interest
No conflicts of interest, competing conflicts, or personal financial interests for any author.
Ethics approval
Approved by Washington University IRB.
Consent for publication
All authors have provided consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexander L. Shiang, Email: shianga@wustl.edu
Joshua K. Palka, Email: palka@wustl.edu
Shiva Balasubramanian, Email: bshiva@wustl.edu.
R. Sherburne Figenshau, Email: figenshaur@wustl.edu.
Zachary L. Smith, Email: smithzl@wustl.edu
Eric H. Kim, Email: ehkim@wustl.edu
References
- 1.Faiena I, Dombrovskiy VY, Modi PK, et al. Regional cost variations of robot-assisted radical prostatectomy compared with open prostatectomy. Clin Genitourin Cancer. 2015;13:447. doi: 10.1016/j.clgc.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyberg M, Hugosson J, Wiklund P, LAPPRO group et al. Functional and oncologic outcomes between open and robotic radical prostatectomy at 24-month follow-up in the Swedish LAPPRO trial. Eur Urol Oncol. 2018;1(5):353. doi: 10.1016/j.euo.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basiri A, De La Rosette JJ, Tabatabaei S, et al. Comparison of retropubic, laparoscopic and robotic radical prostatectomy: who is the winner? World J Urol. 2018;36:609. doi: 10.1007/s00345-018-2174-1. [DOI] [PubMed] [Google Scholar]
- 4.Pearce SM, Pariser JJ, Karrison T, et al. Comparison of perioperative and early oncologic outcomes between open and robotic assisted laparoscopic prostatectomy in a contemporary population based cohort. J Urol. 2016;196:76. doi: 10.1016/j.juro.2016.01.105. [DOI] [PubMed] [Google Scholar]
- 5.Galfano A, Ascione A, Grimaldi S, et al. A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol. 2010;58:457. doi: 10.1016/j.eururo.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Checcucci E, Veccia A, Fiori C, et al. Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: a systematic review and analysis of comparative outcomes. BJU Int. 2020;125:8. doi: 10.1111/bju.14887. [DOI] [PubMed] [Google Scholar]
- 7.Nyarangi-Dix JN, Gortz M, Gradinarov G, et al. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: functional and early oncologic results in aggressive and locally advanced prostate cancer. BMC Urol. 2019;19:113. doi: 10.1186/s12894-019-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umari P, Eden C, Cahill D, et al. Retzius-sparing versus standard robot-assisted radical prostatectomy: a comparative prospective study of nearly 500 patients. J Urol. 2021;205:780. doi: 10.1097/JU.0000000000001435. [DOI] [PubMed] [Google Scholar]
- 9.Egan J, Marhamati S, Carvalho FL, et al. Retzius-sparing robot-assisted radical prostatectomy leads to durable improvement in urinary function and quality of life versus standard robot-assisted radical prostatectomy without compromise on oncologic efficacy: single-surgeon series and step-by-step guide. Eur Urol. 2021;79:839. doi: 10.1016/j.eururo.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Olivero A, Galfano A, Piccinelli M, et al. Retzius-sparing robotic radical prostatectomy for surgeons in the learning curve: a propensity score-matching analysis. Eur Urol. 2020;S2405–4569(20):30073. doi: 10.1016/j.euf.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Saidian A, Fang AM, Hakim O, et al. Perioperative outcomes of single vs multi-port robotic assisted radical prostatectomy: a single institutional experience. J Urol. 2020;204:490. doi: 10.1097/JU.0000000000000811. [DOI] [PubMed] [Google Scholar]
- 12.Kaouk J, Valero R, Sawczyn G, et al. Extraperitoneal single-port robot-assisted radical prostatectomy: initial experience and description of technique. BJU Int. 2020;125:182. doi: 10.1111/bju.14885. [DOI] [PubMed] [Google Scholar]
- 13.Talamini S, Halgrimson WR, Dobbs RW, et al. Single port robotic radical prostatectomy versus multi-port robotic radical prostatectomy: a human factor analysis during the initial learning curve. Int J Med Robot. 2021;17:2209. doi: 10.1002/rcs.2209. [DOI] [PubMed] [Google Scholar]
- 14.Galfano A, Di Trapani D, Sozzi F, et al. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of the first 200 patients with >/= 1 year of follow-up. Eur Urol. 2013;64:974. doi: 10.1016/j.eururo.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Wong D, Rincon J, Henning G, et al. Retzius sparing prostatectomy effect on symptomatic lymphocele rates. Urology. 2021;149:129. doi: 10.1016/j.urology.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Palka, J, Shiang, A, Henning, G, Figenshau, RS, Kim, E. Retzius-Sparing Robotic Assisted Laparoscopic Prostatectomy with DaVinci SP Robot. Videourology. [In press].
- 17.Dindo D, Demartines N, Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abaza R, Murphy C, Bsatee A, et al. Single-port robotic surgery allows same-day discharge in majority of cases. Urology. 2021;148:165. doi: 10.1016/j.urology.2020.08.094. [DOI] [PubMed] [Google Scholar]
- 19.Gross JT, Vetter JM, Sands KG et al: Initial experience with single-port robot-assisted radical cystectomy: comparison of perioperative outcomes between single-port and conventional multiport approaches. J Endourol 2021. [DOI] [PubMed]
- 20.Lenfant L, Sawczyn G, Kim S, et al. Single-institution cost comparison: single-port versus multiport robotic prostatectomy. Eur Urol Focus. 2020;7:532. doi: 10.1016/j.euf.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Lai A, Dobbs RW, Talamini S, et al. Single port robotic radical prostatectomy: a systematic review. Transl Androl Urol. 2020;9:898. doi: 10.21037/tau.2019.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng CF, Teoh JY, Chiu PK, et al. Robot-assisted single-port radical prostatectomy: a phase 1 clinical study. Int J Urol. 2019;26:878. doi: 10.1111/iju.14044. [DOI] [PubMed] [Google Scholar]
- 23.Kaouk JH, Haber GP, Autorino R, et al. A novel robotic system for single-port urologic surgery: first clinical investigation. Eur Urol. 2014;66:1033. doi: 10.1016/j.eururo.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Kim KH, Song W, Yoon H, et al. Single-port robot-assisted radical prostatectomy with the da Vinci SP system: a single surgeon's experience. Investig Clin Urol. 2020;61:173. doi: 10.4111/icu.2020.61.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abaza R, Kogan P, Martinez O. Impact of the COVID-19 crisis on same-day discharge after robotic urologic surgery. Urology. 2021;149:40. doi: 10.1016/j.urology.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]