Abstract
Objective
To describe the feasibility and outcomes of endovascular repair of distal aortic arch aneurysms using a patient-specific stent graft with a pre-loaded single retrograde left subclavian artery (LSA) branch stent graft.
Methods
We reviewed the clinical data and outcomes of consecutive patients enrolled in an ongoing prospective, non-randomized physician-sponsored investigational device exemption study to evaluate the outcomes of endovascular aortic arch repair using patient-specific arch branch stent grafts (William Cook Europe, Bjaeverskov, Denmark) between 2019 and 2022. All patients received a design with triple-wide scallop and a single retrograde LSA branch with a pre-loaded catheter.
Results
There were five male patients with median age of 77 years old (72–80) treated using the single LSA branch stent graft. Technical success was achieved in all patients. Median operating time, fluoroscopy time, and total radiation dose area product were 103 (78–134) minutes, 26 (19–39) minutes, and 123 (71–270) mGy.cm2, respectively. There were no 30-day or in-hospital mortality, neurological or other major adverse events (MAEs). During median follow-up of 21 (20–27) months, all patients were alive with patent LSA branches, except for one who died of COVID-19 complications. There was no branch instability or secondary interventions.
Conclusion
This early feasibility study demonstrates successful endovascular repair of distal aortic arch aneurysms using a patient-specific stent graft with single retrograde LSA branch without technical failures, mortality or neurological events. Larger clinical experience and longer follow-up are needed to determined effectiveness of this approach in patients who need endovascular repair with proximal extension into Zone 2.
Keywords: Endovascular arch aortic repair, Arch, Chronic dissection, Aneurysm, Inner branch stent graft
Background
Thoracic endovascular aortic repair (TEVAR) is considered the first line of treatment in most patients with degenerative descending thoracic aneurysms and complicated acute type B aortic dissections [1]. It is estimated that 30 to 60% of patients treated by TEVAR require proximal extension of the repair into the distal aortic arch because of insufficient landing zone [2–4] . In these patients, revascularization of the left subclavian artery (LSA) has been shown to reduce risk of upper extremity ischemia, stroke and spinal cord injury [5, 6] . Although cervical debranching procedures have been well established, a recent study suggests the risk of phrenic nerve injury has been underreported, occurring in 25% of patients [7]. In addition, LSA bypass or transposition is associated with risk of cervical hematoma, lymphatic leak, infection and vagus nerve injury [7].
Several thoracic stent graft manufacturers are investigating designs to address the distal aortic arch with fenestrations, directional branches, or a wider scallop [8–11]. The third-generation arch branch endovascular graft designed by Cook Medical (William Cook Europe, Bjaeverskov, Denmark) incorporates a retrograde LSA branch into the design with a pre-loaded catheter to facilitate access into the branch. The single LSA branch device was designed to include the retrograde LSA branch and a triple-wide scallop, allowing placement of the stent graft in the mid-segment of the aortic arch with preservation of flow into the left common carotid artery (LCCA) and innominate artery (IA) [9]. Clinical experience with this design has been limited to a few centers [12]. The aim of this study is to evaluate the early feasibility of endovascular repair of distal aortic arch lesions using the single retrograde LSA inner branch with pre-loaded catheter.
Methods
Study Design
This is a prospective, non-randomized study approved by the Mayo Clinic and the University of Texas Health Science at Houston Institutional Review Boards under a physician-sponsored investigational device exemption (IDE) protocol (G130266) registered under clinicaltrials.gov (NCT02089607). All patients provided written informed consent and received manufactured fenestrated and branched stent grafts (Cook Medical, Brisbane, Australia and William Cook Europe, Bjaeverskov, Denmark). For this subgroup analysis, we included the clinical data and outcomes of all consecutive patients treated by endovascular repair of distal aortic arch lesions using patient-specific inner branch stent graft with a single retrograde LSA branch between 2019 and 2022.
Demographics, cardiovascular risk factors, imaging, procedural data and follow-up were recorded prospectively in case report forms and stored in an electronic iMedidata database (Medidata Solutions Inc., Boston MA). Technical success was defined by successful implantation of the arch device and intended LSA branch stent graft. Early outcomes were defined as the first 30 days or within the hospital stay if longer than 30 days. Outcome measurements included 30-day mortality, hemispheric or cerebellar neurological events (e.g., stroke or transient ischemic attack) and any major adverse event (MAEs) [13]. Target vessel instability included any complication affecting one of the three supra-aortic trunks and leading to aneurysm rupture, death, vessel occlusion or branch-related endoleak, component separation or secondary intervention. Follow-up included clinical examination, laboratory studies and computed tomography angiography (CTA) 2 months, 6 months, 12 months and annually after the index procedure for up to five years. Categorical variables were presented as numbers and percentages. Continuous variables were presented as median with interquartile ranges (median, 25th–75th interquartile [IQ] range).
Device Design
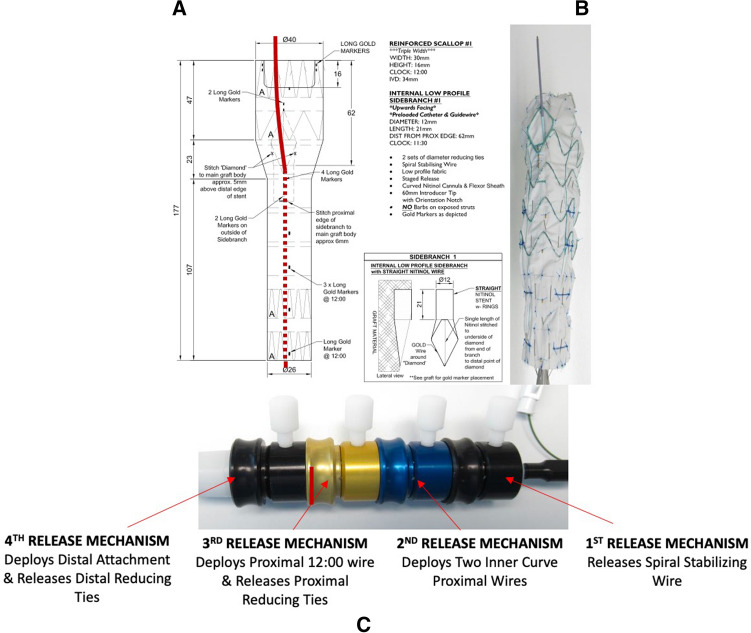
Devices were designed at the Cook planning center using centerline of flow analysis based on pre-operative CTA. All patients received a patient-specific arch branch endovascular graft with triple-wide scallop to accommodate the LCCA and IA and single retrograde LSA inner branch with pre-loaded catheter. The location of the LSA branch was positioned at the 12:00 o’clock orientation (Fig. 1).
Fig. 1.
A Schematic planning of the one-vessel inner branch stent graft showing the diameter and position of inner branch in the graft. B The one-vessel inner branch stent graft with the pre-loaded catheter. C The one-vessel inner branch stent graft has a 4-step release mechanism
Implantation
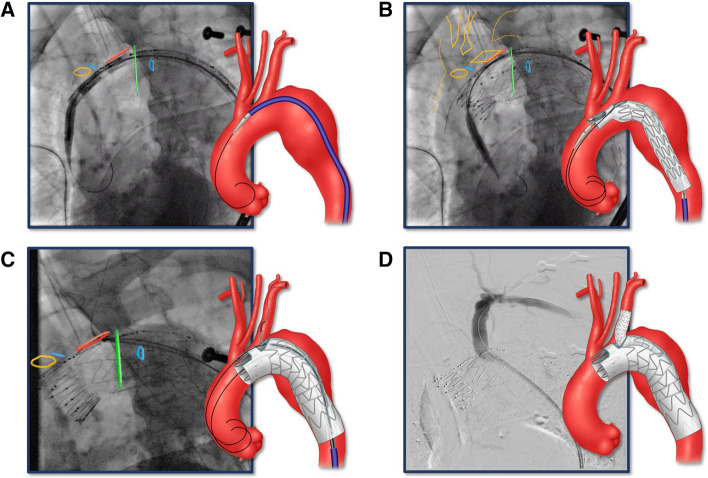
All procedures were performed under general endotracheal anesthesia in a hybrid operating room with advanced imaging including on-lay fusion and high-definition cone beam computed tomography (CBCT). The description of the device implantation is shown in Fig. 2.
Fig. 2.
Bilateral percutaneous femoral approach using pre-closure technique was established using duplex ultrasound guidance. Systemic heparinization was performed to achieve an activated clotting time (ACT) > 250 s. A 0.035-inch double curve Lunderquist wire (Cook Medical, Bloomington, Indiana, USA) was positioned in the proximal ascending aorta. Angiography was performed to identify the supra-aortic trunks and calibrate the on-lay fusion. The arch branch stent graft was flushed with carbon dioxide and subsequently with heparinized saline [19]. The stent graft was introduced over the Lunderquist wire and advanced into position. Systolic blood pressure was decreased to approximately 90 mmHg prior to device deployment. A Stent graft was introduced over Lunderquist wire and advanced into position using radiopaque markers and on-lay fusion. B Arch branch stent graft was deployed. C A second Lunderquist wire was advanced via the pre-loaded catheter through the retrograde LSA branch up to the ascending aorta. The aortic stent graft delivery system and the main aortic Lunderquist wire were removed and a 22 to 24 Fr Dryseal sheath (WL Gore, Flagstaff AZ) was introduced over the second LSA inner branch Lunderquist wire. A 10-Fr 80 cm long Flexor® Ansel sheath (Cook Medical, Bloomington, Indiana, the USA) was advanced into the LSA branch. Using a “buddy” 5Fr VanSchie 3 catheter (Cook Medical Inc., Bloomington IN), the LSA was selectively anterogradely catheterized with a glidewire, which was exchanged for a 1-cm tip Amplatz wire (Cook Medical, Bloomington, IN). Limited angiography was performed via the sheath to identify the origin of the LSA. The repair was extended into the LSA by placement of self-expandable or balloon-expandable Viabahn stent graft (WL Gore, Flagstaff AZ). D Completion LSA angiography was performed to demonstrate patency and the absence of dissection, endoleak or embolization. Final rotational digital subtraction angiography and high-definition CBCT were performed to evaluate technical success, vessel patency and the absence of endoleaks, dissections or embolization
Results
Patient characteristics and procedure details are summarized in Table 1. There were no early mortalities or hemispheric/cerebellar neurological events. None of the patients had MAEs within first 30 days or hospital stay. One patient died of COVID-19 at 21 months. The median hospital length of hospital stay was 4 days (2–8 days). The median follow-up was 21 months (20–27 months). There were no mortalities, aneurysm ruptures, conversions to open surgical repair, neurological events or secondary interventions during follow-up. Analysis of CTA obtained following the procedure revealed the absence of type I or III endoleak and widely patent LSA branches in all patients.
Table 1.
Demographics, clinical and anatomical characteristics, and procedure details of 5 patients treated by endovascular aortic arch repair using an LSA branch stent grafts for aneurysms and chronic dissections
| n = number of patients | Overall |
|---|---|
| n or median, IQR (25th–75th) | |
| Demographics | |
| Age (years old) | 77, 72–80 |
| Age > 80 years old | 2 |
| Male gender | 5 |
| Cardiovascular risk factors | |
| Hypertension | 5 |
| Hypercholesterolemia | 4 |
| Coronary Artery Disease | 3 |
| Chronic obstructive pulmonary disease | 2 |
| Chronic kidney disease Stage III-V | 4 |
| Prior aortic repair* | 2 |
| Intentional first stage of FB-EVAR | 4 |
| Risk assessment | |
| ASA Score | |
| Class 2 | 3 |
| Class 4 | 2 |
| Anatomical Characteristics | |
| Max aortic diameter | 58, 57–59 |
| Arch Type III | 4 |
| Bovine arch | 1 |
| Prior aortic dissection Stanford B | 2 |
| Procedure details | |
| General Anesthesia | 5 |
| Hypotension during deployment (Pharmacologic) | 4 |
| Amount of contrast used (ml) | 124, 112–150 |
| Total operating time (min) | 103, 78–134 |
| Total fluoroscopy time (min) | 26, 19–39 |
| Total air kerma (Gy) | 0.47, 0.32–1.5 |
| Dose Area Product (Gy.cm2) | 0.12, 0.071–0.27 |
| Estimated blood loss (ml) | 100, 50–100 |
| Hospital stay (days) | 4, 2–8 |
| Technical success | 5 |
| Number of target vessel incorporated | 5 |
| Left subclavian inner branch bridging stent | 5 |
| Viabahn stent graft | 4 |
| VBX stent graft | 1 |
| More than one bridging stent | 4 |
AAA abdominal aortic aneurysm; TAAA thoracoabdominal aortic aneurysm; EVAR endovascular aortic repair; TEVAR thoracic endovascular aortic repair; FB-EVAR fenestrated and branched endovascular aortic repair; eGFR estimated glomerular filtration rate; ASA American Society of Anesthesiologist
*No open ascending or arch aorta replacement
Discussion
This small early feasibility study demonstrates successful implantation of the single LSA retrograde branch stent graft in patients with distal aortic lesions with high technical success and no early mortality or neurological events. The use of a pre-loaded catheter facilitated immediate access to the retrograde branch. There was no difficulty in gaining access into the LSA following deployment of the aortic device, nor in the advancement and deployment of the bridging stent via total femoral approach. Although the stent graft design was considered patient-specific, the anatomic location of the LSA is predictable with low variation, allowing an off-the-shelf concept to be utilized in future studies.
Evidence supporting the recommendation for routine revascularization of the LSA during TEVAR is based on large single-center and multi-center studies that demonstrate potential benefits in patients with extensive thoracic or thoracoabdominal disease or poor collateral networks to the upper extremity, brain and spinal cord [6, 14]. Patients with left internal mammary grafts and those with dominant left vertebral artery flow or isolated posterior inferior cerebellar arteries originating from the LSA comprise absolute indications [15]. The Society of Vascular Surgery (SVS) recommends revascularization during elective TEVAR whenever possible, but coverage without revascularization is an acceptable alternative in emergency scenarios such as ruptured aneurysms, complicated dissections and transections [5, 15]. Although the standard for comparison is a hybrid approach with LSA bypass or transposition, the risk of phrenic nerve palsy is high affecting one in four patients who undergo this procedure [7]. Other complications such as vagal nerve injury, cervical hematomas and lymphatic leaks are less frequent, but undermine potential benefits of endovascular approaches [7, 16].
A few aspects of the design and technique should be emphasized as compared to other alternative LSA branch stent grafts. Anatomical suitability is dependent upon the presence of sealing in Zone 2 and adequacy of a patent LSA without dissection or thrombus precluding successful stent placement. The triple-wide scallop is intended to allow placement of the stent graft in the mid-segment of the aortic arch (Zone 1), which typically has a straight configuration and normal diameter. This may increase utilization of the device and provide a durable seal as compared to Zone 2 landing, which affords a relatively short seal zone. The pre-shaped curved delivery system and a spiral fixation wire provide orientation of the device to the outer aortic curvature, minimizing the need for stent manipulation during deployment and providing reliable access to the LSA via the diamond-shaped fenestration [17, 18]. Finally, advancement of the sheath into the inner branch using the pre-loaded catheter simplifies cannulation and catheterization of the LSA. It is possible that a simplified deployment technique with fewer endovascular maneuvers may reduce the risk of embolization and stroke.
Although this is a small series of five patients, the purpose of early feasibility studies is proof of concept allowing evolution of the design into feasibility and pivotal studies. The small cohort and short follow-up interval limit the ability to assess time-dependent outcomes such as target patency, instability and secondary interventions. Therefore, there is a need to expand use of the LSA branch stent graft to better understand its feasibility, limitations, complications, and long-term efficacy.
Conclusion
This study demonstrates the early feasibility of the LSA arch branch stent graft with pre-loaded catheters for treatment of distal aortic arch lesions requiring revascularization of the LSA. Although the early outcomes are auspicious, the small study sample and short follow-up interval warrant continued investigation to assess long-term safety and reliability.
Acknowledgments
Not applicable
Abbreviations
- TEVAR
Thoracic endovascular aortic repair
- LSA
Left subclavian artery
- LCCA
Left common carotid artery
- IA
Innominate artery
- IDE
Investigational device exemption
- MAEs
Major adverse events
- AKI
Acute kidney injury
- CTA
Computed tomography angiography
- CBCT
Cone beam computed tomography
- ACT
Activated clotting time
- ASA
American Society of Anesthesiology
- SVS
Society of vascular surgery
Author’s Contributions
JW, ET, BM, and GO designed current study. GL, MD, AB collected data. JK, LO, TM, and GO analyzed and interpreted patient data. JW and ET were a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
This study was not supported by any funding.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Gustavo Oderich MD has received consulting fees and grants from Cook Medical, W. L. Gore, and GE Healthcare (all paid to Mayo Clinic with no personal income). Other co-authors declare that they have no conflict of interest.
Consent for Publication
For this type of study, consent for publication is not required.
Consent to Participate
Committee For the Protection of Human Subjects (CPHS) approved the study with all patients providing written informed consent.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This is a retrospective review of a prospective maintained database of patients treated by FB-EVAR conducted under the US Food and Drug Administration (FDA) PS-IDE G130030 and G130266 and registered under ClinicalTrial.gov NCT01937949 and NCT02089607. The study was approved by the Institutional Review Board at University of Texas Health Science Center at Houston and Mayo Clinic Rochester.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
The original online version of this article was revised: The last name of the fifth author is misspelled. The correct spelling is Baghbani-Oskouei (without “a” between “h” and “b”).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/22/2022
A Correction to this paper has been published: 10.1007/s00270-022-03318-5
References
- 1.Uchida T, Sadahiro M. Thoracic endovascular aortic repair for acute aortic dissection. Ann Vasc Dis. 2018;ra. 18–00127. [DOI] [PMC free article] [PubMed]
- 2.Lombardi JV, Cambria RP, Nienaber CA, Chiesa R, Teebken O, Lee A, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55(3):629–40. E2. doi: 10.1016/j.jvs.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Maldonado TS, Dexter D, Rockman CB, Veith FJ, Garg K, Arko F, et al. Left subclavian artery coverage during thoracic endovascular aortic aneurysm repair does not mandate revascularization. J Vasc Surg. 2013;57(1):116–124. doi: 10.1016/j.jvs.2012.06.101. [DOI] [PubMed] [Google Scholar]
- 4.Patterson BO, Holt PJ, Nienaber C, Fairman RM, Heijmen RH, Thompson MM. Management of the left subclavian artery and neurologic complications after thoracic endovascular aortic repair. J Vasc Surg. 2014;60(6):1491–8. E1. doi: 10.1016/j.jvs.2014.08.114. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura JS, Rizvi AZ. Left subclavian artery revascularization: society for vascular surgery® practice guidelines. J Vasc Surg. 2010;52(4):65S–70S. doi: 10.1016/j.jvs.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Holt PJ, Johnson C, Hinchliffe RJ, Morgan R, Jahingiri M, Loftus IM, et al. Outcomes of the endovascular management of aortic arch aneurysm: implications for management of the left subclavian artery. J Vasc Surg. 2010;51(6):1329–1338. doi: 10.1016/j.jvs.2009.10.131. [DOI] [PubMed] [Google Scholar]
- 7.Voigt SL, Bishawi M, McCann RL, Hughes GC. Long-term outcomes of carotid-subclavian bypass in the setting of thoracic endovascular aortic repair. J Vasc Surg. 2018;67(1):e16. doi: 10.1016/j.jvs.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Tenorio ER, Oderich GS, Kölbel T, Dias NV, Sonesson B, Karelis A, et al. Multicenter global early feasibility study to evaluate total endovascular arch repair using three-vessel inner branch stent-grafts for aneurysms and dissections. J Vasc Surg. 2021;74(4):1055–65. e4. doi: 10.1016/j.jvs.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Haulon S, Kratzberg J, Guihaire J, Fabre D. Current status of arch branch technology. Endovasc Today. 2018.
- 10.Kashef E, Aldin Z, Jenkins MP, Gibbs R, Bicknell CD, Cheshire NJ, et al. Scalloped thoracic stent-graft for treatment of aortic arch aneurysms with unfavourable landing zones. Cardiovasc Intervent Radiol. 2011;34(4):845–851. doi: 10.1007/s00270-011-0099-9. [DOI] [PubMed] [Google Scholar]
- 11.Hanna L, Abdullah A, Kashef E, Riga C, Jenkins M, Bicknell C, et al. Four-year results of the Bolton relay proximal scallop endograft in the management of thoracic and thoracoabdominal aortic pathology with unfavorable proximal landing zone. J Vasc Surg. 2021;74(5):1447–1455. doi: 10.1016/j.jvs.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Haulon S, Greenberg RK, Spear R, Eagleton M, Abraham C, Lioupis C, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;148(4):1709–1716. doi: 10.1016/j.jtcvs.2014.02.072. [DOI] [PubMed] [Google Scholar]
- 13.Oderich GS, Forbes TL, Chaer R, Davies MG, Lindsay TF, Mastracci T, et al. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg. 2021;73(1):4S–52S. doi: 10.1016/j.jvs.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Hage A, Ginty O, Power A, Dubois L, Dagenais F, Appoo JJ, et al. Management of the difficult left subclavian artery during aortic arch repair. Ann Cardiothorac Surg. 2018;7(3):414. doi: 10.21037/acs.2018.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoglio L, Salvati S, Fittipaldi A, Melloni A, Kahlberg A, Cambiaghi T, et al. Carotid to subclavian bypass and Amplatzer vascular plug subclavian endovascular occlusion before thoracic open or endovascular repair. J Vasc Surg. 2020;71(5):1480–8. e1. doi: 10.1016/j.jvs.2019.08.237. [DOI] [PubMed] [Google Scholar]
- 16.Christensen JD, Seaman DM, Washington L. Imaging of complications of thoracic and cardiovascular surgery. Radiol Clin. 2014;52(5):929–959. doi: 10.1016/j.rcl.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Mougin J, Sobocinski J, Kratzberg J, Fabre D, Haulon S. Applicability of a standardized thoracic endograft with a single branch for the left subclavian artery to treat aortic disease involving the distal arch. J Vasc Surg. 2020;72(5):1516–1523. doi: 10.1016/j.jvs.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Haulon S, Soler R, Watkins AC, Amabile P, Fadel E, Fabre D. Endovascular arch replacement with an endoprosthesis with three inner branches. Ann Cardiothorac Surg. 2018;7(3):431. doi: 10.21037/acs.2018.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kölbel T, Rohlffs F, Wipper S, Carpenter SW, Debus ES, Tsilimparis N. Carbon dioxide flushing technique to prevent cerebral arterial air embolism and stroke during TEVAR. J Endovasc Ther. 2016;23(2):393–395. doi: 10.1177/1526602816633705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.