Abstract
Colonization of the human nasopharyngeal region by Neisseria meningitidis is believed to lead to natural immunity. Although the presence of bactericidal antibody in serum has been correlated with immunity to meningococcal disease, mucosal immunity at the portal of entry may also play an important role. This study was undertaken to examine in mice the possibility of safely using native outer membrane vesicles (NOMV) not exposed to detergent as an intranasal (i.n.) vaccine. The mucosal and systemic responses of mice to intranasal and intraperitoneal (i.p.) vaccination with NOMV were compared over a range of doses from 0.1 to 20 μg. Intranasal vaccination of mice with NOMV induced a strong systemic bactericidal antibody response, as well as a strong local immunoglobulin A immune response in the lung as determined by assay of lung lavage fluid by enzyme-linked immunosorbent assay and lung antibody secreting cells by enzyme-linked immunospot assay. However, 8- to 10-fold-higher doses of NOMV were required i.n. compared to i.p. to elicit an equivalent bactericidal antibody response in serum. Some NOMV vaccine was aspirated into the lungs of mice during i.n. immunization and resulted in an acute inflammatory response that peaked at 1 to 2 days postimmunization and was cleared by day 7. These results indicate that i.n. delivery of meningococcal NOMV in mice is highly effective in eliciting the production of both a mucosal immune response and a systemic bactericidal antibody response.
Neisseria meningitidis frequently colonizes the human nasopharynx, which is its sole natural habitat, leading to the induction of natural immunity (11). In some cases, this colonization also initiates the pathogenic process that leads to invasive meningococcal disease. Serum bactericidal antibody, which develops after exposure to meningococcal antigen (10, 11), has been correlated with immunity to meningococcal disease, but mucosal immunity at the portal of entry may also play an important role.
There is currently much interest in the mucosal route of immunization to protect against various pathogens that gain entry into the host via mucosal tissues. Recent studies have shown that intranasal immunization can protect mice against challenge with a variety of organisms, including the bacterial pathogens Bordetella pertussis (4), Borrelia burgdorferi (17), Chlamydia trachomatis (27), streptococci (7), and Helicobacter felis (38). Importantly, meningococcal outer membrane proteins (OMPs) used as proteosomes have been successfully used as a mucosal delivery system in mice to present antigens such as staphylococcal enterotoxin B toxoid (18) and Shigella lipopolysaccharide (19, 26).
Several OMP-based vaccines for group B meningococcal disease have shown 50 to 80% efficacy in older children (43). However, efficacy in young children receiving the same vaccines was much lower, despite the induction of high levels of antibody (2, 22). This may have occurred because the immune system of the young children had not previously been exposed to meningococci or other neisseriae by natural colonization, and the initial exposure to detergent-extracted OMPs led to the production of antibodies that did not recognize epitopes expressed or exposed on the native organism. This suggests that a more native antigen may be needed to direct the immune response toward the bactericidal epitopes.
Native outer membrane vesicles (NOMV) that bleb off the meningococcal cell surface during growth (40) are highly immunogenic and would be an excellent vaccine candidate except for their high lipooligosaccharide (LOS) content and consequent toxicity. The toxicity of the NOMV can be reduced by one of several methods that typically involve the use of detergents to remove most of the LOS and phospholipids. These procedures, however, result in exposure of OMP epitopes to the immune system that are highly immunogenic but predominantly induce antibodies that are not bactericidal, possibly because the epitopes are not fully exposed at the cell surface of the native organism. In addition, the detergent extraction may cause the OMPs to undergo subtle changes in conformation even though the porin trimeric structure and in some cases the overall membrane vesicle morphology appear to remain intact. Although NOMV would probably be too toxic for use as a parenteral vaccine, such a vaccine would likely be safe to use via the intranasal (i.n.) route.
The purpose of this study was to examine the immunogenicity of meningococcal NOMV delivered i.n. to mice. Mice were given this vaccine either i.n. or intraperitoneally (i.p.) or by a combination of the two routes. These studies showed that i.n. immunization induced high levels of bactericidal antibody in serum and localized immunoglobulin A (IgA) in the lungs but that i.p. immunization only induced a systemic antibody response.
MATERIALS AND METHODS
Mice.
CD-1 outbred mice (Charles Rivers Laboratories, Wilmington, Maine) were used in all experiments. Mice were immunized at days 0 and 28 with NOMV with 1 μg of protein i.p. in a 100-μl volume or 20 μg of protein i.n. in a 25-μl volume. Dose-response experiments were conducted in which NOMV was given at i.p. or i.n. doses ranging from 0.01 to 10 μg or from 0.03 to 20 μg, respectively. The i.n. immunizations were given to unanesthetized (histology experiment) or anesthetized mice (0.30 mg ketamine HCl and 1.0 mg of xylazine administered intramuscularly) by using a micropipette. Bleeds were done via the retroorbital plexus at days 0 and 28 on anesthetized mice and by cardiac puncture at day 42 following euthanasia by CO2 overdose. Lung lavage fluid was obtained at day 42 by first euthanizing mice with an overdose of CO2, then inserting and tying off a 21-gauge needle in the needle in the trachea, and finally injecting and aspirating 1 ml of sterile phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 10 μg of gentamicin per ml. Typical recovery was 0.6 to 0.7 ml. Specimens contaminated with blood were discarded. Samples were centrifuged at 16,700 × g for 10 min to remove cellular debris.
Bacterial strains and vaccines.
The vaccine seed strain was derived from N. meningitidis 9162 (B:15:P1.3), which was a case isolate obtained from a patient in Iquique, Chile, in October 1990. This isolate was representative of the epidemic strain prevalent in northern Chile from 1985 to 1994. This parent strain was genetically modified by partial deletion of synX (unpublished data) and replacement by a kanamycin resistance cassette. SynX, also called siaA (8), is essential for sialic acid biosynthesis (34), and the resulting mutant, 9162synX(−), is capsule negative and unable to sialylate its LOS. The encapsulated parent strain 9162 was used as the target strain in bactericidal assays and in production of NOMV to use in enzyme-linked immunosorbent assays (ELISA) and enzyme-linked immunospot-forming assays (ELISPOT).
The vaccine consisted of NOMV, referred to in previous publications as native cell wall complex or outer membrane complex (40, 41). The vesicles were extracted from whole cells of strain 9162synX(−) without the use of detergents or denaturing agents (41). Briefly, packed cells were suspended in a buffer containing 0.15M NaCl, 0.05 M Tris-HCl, and 0.01 M EDTA at pH 7.5. The suspension was warmed at 56°C for 30 min and sheared in a Waring blender for 3 min. The resulting suspension was centrifuged at 23,500 × g for 20 min to remove cells and cell debris. The supernatant was retained, and the cells were extracted a second time with a volume of distilled water equal to half the volume of buffer used in the first extraction and with the heating step omitted. Combined supernatants were recentrifuged at 23,500 × g for 20 min, and the supernatants were centrifuged at 200,000 × g for 1 h to pellet the outer membrane vesicles. The pellets of NOMV were washed once by repelleting from distilled water. A lot of NOMV vaccine for clinical use was prepared under current good manufacturing practice conditions at the Walter Reed Army Institute of Research Pilot Vaccine Production Facility (Forest Glen, Md.). The vaccine was prepared from cells grown under iron-limiting conditions in order to induce the iron-regulated proteins. This lot contained about 25% LOS relative to protein and is essentially pure outer membrane material. This purity was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, UV spectrophotometry, and analysis by negative-stain electron microscopy. The vaccine was bottled in sterile normal saline at 0.8 mg of protein/ml and stored at 4°C prior to use.
Serum bactericidal assay.
A standard bactericidal assay was performed as previously described (24). Briefly, meningococci were grown overnight on solid GC agar, transferred to Mueller-Hinton broth, grown to an optical density at 650 nm of 0.28, washed, resuspended, and diluted to a final concentration of 104 organisms/ml. Test sera were diluted in Gey’s buffered salt solution with 0.2% gelatin. Then, 50 μl of a twofold dilution series of serum were combined with 25 μl of extrinsic human complement, and 25 μl of bacterial suspension in a 96-well plate. The mixture was incubated with shaking for 1 h at 37°C, after which two 20-μl aliquots were plated onto GC agar along with appropriate controls. The number of colonies formed after 16 h of incubation was counted, and the endpoint titer was determined as the highest dilution of serum that killed ≥50% of the meningococci.
ELISA procedures.
An ELISA was performed as previously described (31) except that mouse antibodies were detected. Briefly, flat-bottom high-binding 96-well plates (Costar Corp., Cambridge, Mass.) were coated with NOMV for 2 h at 37°C, incubated with 200 μl of blocking solution for 1 h at 37°C, and washed twice with PBS buffer. Serial twofold dilutions made in separate plates were added and incubated overnight at room temperature (RT). Plates were washed with PBS, and alkaline phosphatase-labeled goat anti-mouse IgG, IgA, or IgM (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was added. The plates were incubated overnight at RT. Plates were washed with PBS and developed for 30 min with p-nitrophenylphosphate (Sigma 104; Sigma, St. Louis, Mo.). The development reaction was stopped by the addition of 3 N NaOH. Absorbance values were read at 405 nm. Quantitation of immunoglobulin values in serum was done by running standard plates with mouse IgG (Organon Teknika-Cappel, Durham, N.C.), IgM (Rockland, Inc., Gilbertsville, Pa.), or IgA (Calbiochem, La Jolla, Calif.) standard captured by anti-mouse IgG, IgM, or IgA (Kirkegaard & Perry) bound to the plate (41, 42).
ELISPOT procedures.
ELISPOT was done with modifications from the original descriptions of this method (5, 32). All steps up to the development of spots were done aseptically. Meningococcal NOMV was diluted to 25 μg/ml in carbonate buffer, and 100 μl was added to each well of 96-well flat-bottom, high-binding microtiter plates (Costar) and allowed to incubate in a humidified chamber overnight at RT. Plates were washed twice with sterile PBS and incubated with 100 μl of 10% fetal calf serum in PBS per well for at least 1 h in a humidified chamber at 37°C. Mice were euthanized by cervical dislocation, and spleens and lungs were collected aseptically. Single-cell suspensions were made from spleens by grinding between the frosted ends of two glass microscope slides. Lungs were cut up into 1-mm pieces and pressed through a 60-mesh screen with the plunger from a 3-ml syringe. Cells were suspended in RPMI 1640 (Life Technologies, Inc., Gaithersburg, Md.) and pelleted by centrifugation at 200 × g for 10 min. Lysis of erythrocytes was accomplished by resuspending cells in RT NH4 Cl-Tris (1 ml per 0.1 ml of packed cells; Sigma) for 3 min, resuspending them in 12 ml of RPMI 1640, and washing them four times with RPMI 1640 (200 × g for 10 min). The number of viable cells was ascertained by trypan blue dye exclusion. Cells were resuspended in culture medium (RPMI 1640 with 10% heat-inactivated fetal bovine serum, 50 μg of gentamicin per ml, and 2 mM l-glutamine) and counted. Cells were added to 8 to 12 wells of the antigen-coated plates at the desired concentration and then incubated overnight at 37°C in 5% CO2. Plates were washed with PBS five times, and 100 μl of a 1-μg/ml concentration of phosphatase-labeled antibody (Kirkegaard & Perry) of the appropriate specificity was added to each well. Plates were incubated for 1 h at 37°C in a humidified chamber and washed four times with PBS. Development was accomplished by adding 2 ml of 5-bromo-4-chloro-3-indolylphosphate toluidinium reagent (BCIP; Kirkegaard & Perry) to 10 ml of 0.7% agarose in 0.1 M Tris and adding 100 μl per well. Once the agarose solidified, the plates were wrapped in plastic wrap and stored at 4°C until spots were counted with a stereomicroscope. The number of isotype-specific antibody-secreting cells (ASCs) was then calculated and expressed in terms of the number of ASCs per 106 lymphocytes plated.
Histopathology.
Unanesthetized mice were immunized i.n. with 20 μg of NOMV in a 25-μl volume (n = 12) or sham immunized with 25 μl of saline at day 0 (n = 12). Three mice from each group were sacrificed at days 1, 2, 4, and 7, and the lungs were inflated with and fixed in 10% phosphate-buffered formalin, then sectioned, stained with hematoxylin and eosin, and examined by light microscopy.
Analysis of data.
Results are expressed in tabular format as the mean ± the standard error of the mean (SEM), or as boxplots showing the median, minimum, maximum, and 25th and 75th percentiles. Statistical analyses were performed with the software package Minitab version 11 (Minitab, Inc., State College, Pa.). Differences in ELISA and bactericidal results across groups were examined for by using Student’s t test or one-way analysis of variance followed by multiple comparisons with Tukey’s pairwise comparison. P values of <0.05 were considered statistically significant.
RESULTS
NOMV stimulation of systemic and mucosal antibody responses.
High bactericidal antibody levels in serum were observed at day 42 for mice immunized with NOMV regardless of the immunization scheme (Fig. 1A), although significantly higher levels were observed in mice immunized i.p./i.p. compared to mice immunized i.p./i.n. (P < 0.01) or i.n./i.n. (P < 0.01). An initial i.n. immunization at day 0 induced bactericidal levels in serum at day 28 comparable to those with the i.p. immunization. However, the i.n. boost at day 28 only increased the day 42 bactericidal titer slightly (≤2-fold) for the i.p./i.n. and i.n./i.n. groups, whereas the i.p. boost at day 28 increased the day 42 bactericidal titer by almost 14-fold for the i.p./i.p. group.
FIG. 1.
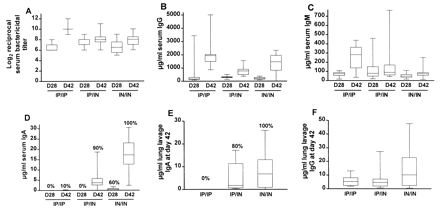
Antibody levels in serum and lung lavage fluid for mice immunized with meningococcal NOMV. Animals were immunized in groups of 10 (i.p. [IP] dosage = 1 μg; i.n. [IN] dosage = 20 μg) (i) i.n. twice (IN/IN), (ii) i.p. at day 0 and i.n. at day 28 (IP/IN), or i.p. twice (IP/IP). Serum was collected on days 28 and 42, and lung lavage fluid was collected on day 42. A fourth group was sham immunized i.n. and i.p. with saline; all values for this group were negligible and are not shown. Boxplots show median, minimum, and maximum values and the 25th and 75th percentiles. Panels: A, serum bactericidal antibody; B, serum IgG; C, serum IgM; D, serum IgA; E, lung lavage IgA; F, lung lavage IgG. Percentages above the maximum values in panel D represent percentage of mice with detectable specific IgA antibody.
Isotype-specific immunoglobulin responses as measured by ELISA showed the mean day 42 IgG level in serum for the i.p./i.p. group was significantly higher than for the i.p./i.n. group (P < 0.05) but was not significantly different from the i.n./i.n. group (Fig. 1B). No significant differences in IgM levels in serum were observed between groups at day 28 or 42 (Fig. 1C). No detectable meningococcus-specific serum IgA was found 28 days after an initial i.p. immunization, and only 1 of 10 mice immunized i.p./i.p. had a detectable serum IgA response at day 42. In contrast, two i.n. immunizations induced specific serum IgA in 100% of mice at day 42 (Fig. 1D), and a significantly higher response was observed in the i.n./i.n. group compared to the i.p./i.n. group (P < 0.01).
Lung lavage IgA was detected on day 42 in 80% of the mice immunized i.p./i.n., and 100% of the mice immunized i.n./i.n., but none was detected in mice immunized i.p./i.p. (Fig. 1E). Measurable lung lavage IgM at day 42 was only detected at low levels in 20 to 40% of mice across the groups (data not shown). Levels of lung lavage IgG were slightly higher but not significantly different (P = 0.15) in the i.n./i.n. group compared to the i.p./i.p. or i.n./i.p. groups (Fig. 1F).
Stimulation of local ASCs.
Mice were immunized at days 0 and 28, and lung and spleen tissues were harvested at day 33 and used in an ELISPOT assay to determine the number of meningococcus-specific IgA, IgG, and IgM ASCs present. Two i.n. immunizations induced a strong mucosal response, as evidenced by the more than 500 IgA ASCs per 106 lymphocytes in the lungs, whereas no IgA ASCs were observed in the lungs of mice immunized i.p./i.p. (Table 1). A threefold-higher number of IgG ASCs was present in the lungs of mice immunized i.n./i.n. than in those immunized i.p./i.p. Virtually no IgM ASCs were observed in the lungs from any group.
TABLE 1.
Results of ELISPOT assay on mouse spleen and lung lymphocytes after immunization with meningococcal NOMVa
| Source of lymphocytes | Route of immunizationa | Mean ASCs/106 lymphocytes ± SEM
|
||
|---|---|---|---|---|
| IgG | IgA | IgM | ||
| Spleen | i.p./i.p. | 104.7 ± 21.0 | 30.1 ± 18.3 | 16.2 ± 3.3 |
| i.p./i.n. | 42.3 ± 9.9 | 42.7 ± 19.9 | 11.7 ± 3.2 | |
| i.n./i.n. | 91.4 ± 9.4 | 190.3 ± 44.1 | 5.0 ± 1.6 | |
| Saline i.n. and i.p. | 0.1 ± 0.1 | 0.0 ± 0.0 | 1.9 ± 1.1 | |
| Lungb | i.p./i.p. | 75.1 | 0.0 | 8.3 |
| i.p./i.n. | 133.3 | 90.0 | 1.7 | |
| i.n./i.n. | 311.7 | 525.0 | 5.0 | |
| Saline i.n. and i.p. | 0.0 | 0.0 | 4.5 | |
Mice (five per group) were immunized at days 0 and 28 with either 1 μg of NOMV i.p. (i.p./i.p.), 20 μg of NOMV i.n. (i.n./i.n.), or 1 μg of NOMV i.p. at day 0 followed by 20 μg of NOMV i.n. at day 28. Lungs and spleens were harvested at day 33; lymphocytes were plated onto NOMV-coated microtiter plates, and ELISPOT assays were conducted.
Lung lymphocytes were pooled from five mice prior to plating.
Mice immunized i.n./i.n. had significantly higher numbers of splenic IgA ASCs (Table 1) than the i.p./i.p. or the i.p./i.n. group (P < 0.05). Similar levels of splenic IgG ASCs were observed in both the i.p./i.p. and the i.n./i.n. groups, although significantly higher numbers of splenic IgG ASCs were observed in i.p./i.p. compared to i.p./i.n. mice (P < 0.05).
Dose-response testing.
Mean bactericidal activity in serum and serum IgG and IgM levels generally increased with increasing dosage (Fig. 2A to F). The i.p. immunization resulted in bactericidal titers in serum that were two- to ninefold higher than with the same dose given i.n. Similarly, immunization with the same dose of NOMV generally resulted in 8- to 10-fold higher IgG and IgM levels in serum for mice immunized i.p. in contrast to the i.n. immunization.
FIG. 2.
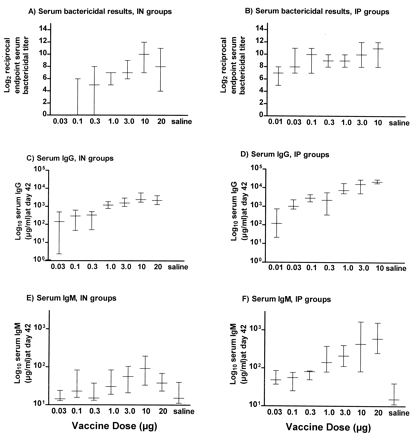
Antibody responses of 15 groups of five mice each immunized i.n. (IN) or i.p. (IP) with meningococcal NOMV. Seven groups received the following doses of NOMV vaccine i.n., under anesthesia, in a volume of 25 μl, at days 0 and 28: 0.03, 0.10, 0.30, 1.0, 3.0, 10, and 20 μg. Seven additional groups received the following doses of NOMV vaccine i.p. at days 0 and 28: 0.01, 0.03, 0.10, 0.30, 1.0, 3.0, and 10 μg. Mice were bled at days 28 and 42. One group of five mice served as negative controls; these animals were sham immunized either i.n. or i.p. with saline. Median, minimum, and maximum values are represented by the vertical bars.
Serum IgA was induced in all mice receiving an i.n. dose of ≥0.3 μg (Table 2), whereas i.p. immunization did not induce a consistent serum IgA response. This was as expected, since IgA is typically associated with mucosal stimulation, and was in accordance with results from the ELISPOT study that showed the i.n./i.n. route induced a sixfold-higher number of splenic IgA ASCs than did i.p./i.p. immunization. Lung lavage IgA was also induced in a general dose-dependent fashion (Table 2) for i.n. immunized mice. Lung lavages were not taken for mice immunized i.p. since previous experiments have shown IgA is not induced in the lungs with i.p. immunization.
TABLE 2.
Serum and lung lavage IgA values at day 42 for mice immunized i.n. or i.p.a with meningococcal NOMV at days 0 and 28
| Dose (μg) | i.n. groups
|
i.p. Groups
|
||||
|---|---|---|---|---|---|---|
| No. with detectable serum IgA (%) | Mean serum IgA (μg/ml)b ± SEM | No. with detectable lung lavage IgA (%) | Mean lung lavage IgA (μg/ml)b ± SEM | No. with detectable serum IgA (%) | Mean serum IgA (μg/ml)b ± SEM | |
| 0.01 | 1/5 (20) | 2.3 | ||||
| 0.03 | 3/5 (60) | 3.7 ± 1.2 | 0/5 (0) | 1/5 (20) | 6.9 | |
| 0.10 | 4/5 (80) | 6.3 ± 3.1 | 3/5 (60) | 0.1 ± 0.1 | 0/5 (0) | |
| 0.30 | 5/5 (100) | 25.4 ± 20.3 | 4/5 (80) | 0.2 ± 0.1 | 1/5 (20) | 15.5 |
| 1.0 | 5/5 (100) | 13.1 ± 6 | 5/5 (100) | 0.4 ± 0.1 | 3/5 (60) | 32.7 ± 11.8 |
| 3.0 | 5/5 (100) | 24.0 ± 5.9 | 5/5 (100) | 1.0 ± 0.3 | 1/5 (20) | 6.3 |
| 10 | 5/5 (100) | 64.9 ± 18.9 | 5/5 (100) | 6.5 ± 3.1 | 2/5 (40) | 16.2 ± 11.5 |
| 20 | 5/5 (100) | 33.1 ± 19.1 | 5/5 (100) | 5.2 ± 1.7 | ||
| Saline | 0/5 (0) | 0/5 | 0/5 (0) | |||
Lung lavages were not taken from mice immunized i.p. in this experiment; however, in an identical experiment with mice immunized i.p. with meningococcal NOMV, no IgA was detected in lung lavage samples.
Calculated for just those mice with detectable IgA.
Mice immunized i.n. with 10 and 20 μg of NOMV experienced substantial weight loss by day 3 postimmunization (PI) but had recovered to preimmunization weights by day 7 PI (Fig. 3). Mice immunized i.p. had some minor (<0.35 g) weight loss at days 1 and 3 but had recovered preimmunization weights by day 5 (data not shown).
FIG. 3.
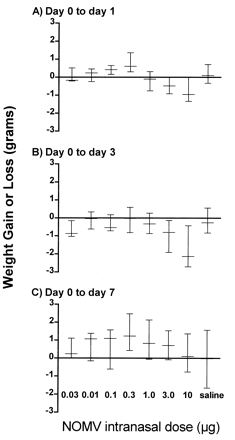
Body weight differences (median, minimum, and maximum values) from day 0 of mice (five per group) at day 1 (A), day 3 (B), day 7 (C) after i.n. immunization with various doses of meningococcal NOMV.
Histopathology.
Light microscopic examination of the lungs of the saline control mice were within normal limits at all time points (Fig. 4a). The lungs of mice immunized i.n. with meningococcal NOMV and sacrificed on days 1 and 2 PI revealed an acute inflammatory response characterized by neutrophils centered around airways and extending into the surrounding alveoli (Fig. 4b). The neutrophilic response was decreased in severity on day 4 (Fig. 4c) and was absent by day 7 (Fig. 4d). Two of the three mice immunized with NOMV had evidence of active antigenic stimulation distinguished by mononuclear infiltration and lymphocytic aggregates (interpreted as bronchiolar associated lymphoid tissue) which contained morphologically activated lymphocytes and plasma cells. The remaining day 7 mouse had essentially normal lung tissue.
FIG. 4.
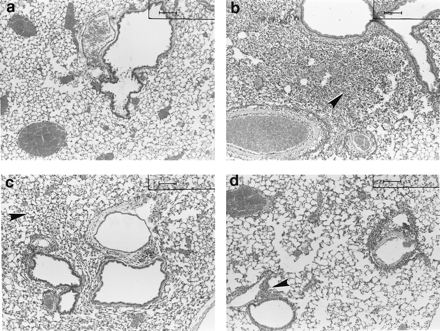
Unanesthetized mice were immunized i.n. with 20 μg of meningococcal NOMV (n = 12) in a 25-μl volume or with 25 μl of saline (n = 12). Three mice from each group were euthanized at days 1, 2, 4, and 7 PI, lungs were inflated with 1 ml of formalin, and sections were cut and stained with hematoxylin and eosin. (A) Representative lung sections from a saline control mouse at day 2. Note the clear alveolar spaces and the paucity of peribronchiolar and perivascular infiltrates. (B to D) Mice immunized with NOMV. (B) At day 2 PI, note the severe filling of the alveoli with neutrophils that obscure the normal architecture. (C) At day 4 PI, relative clearing of neutrophils from the alveolar spaces can be seen. (D) At day 7 PI, the neutrophils are essentially gone from the alveoli, and the inflammatory component has become mononuclear with peribronchiolar lymphocytic aggregates. Bar = 100 mm.
DISCUSSION
The importance of mucosal IgA in preventing group B meningococcal disease is unknown, but it may be a factor in preventing colonization (1) or in preventing the progression of carriage to invasive disease (12). Nasopharyngeal carriage of meningococci typically results in natural immunization in people by inducing bactericidal antibodies in serum which are believed to be required for protection (10). Meningococcal and influenza vaccines delivered i.n. have been shown to be immunogenic in humans (14, 23, 25). Further, human challenge studies following i.n. immunization with a purified group A Streptococcus M protein vaccine have shown a reduction in colonization, as well as protection from clinical disease among the vaccinees (6, 29).
Meningococcal NOMV is a good mucosal immunogen, as evidenced by the results presented in this study. Mice immunized i.n. with NOMV responded with bactericidal antibody titers in serum at levels approaching those in mice immunized i.p. The level of the mucosal and systemic response obtained by immunization with NOMV was significant, particularly when compared to that obtained with a vaccine consisting of OMPs purified by using the detergent Empigen BB and complexed to alkaline-detoxified LOS. Mice immunized i.n. with NOMV responded with bactericidal antibody titers in serum approximately 2 logs higher than mice immunized i.n. with the OMP-LOS vaccine (unpublished data). Leaving the phospholipid and LOS intact in the NOMV ensures that the OMPs have surface exposure and conformation similar to that on the whole organism. This serves to direct the immune response toward those epitopes that can induce protective antibodies. The native conformation of NOMV proteins, as well as the adjuvant effect of the LOS present, may be responsible for the higher antibody responses that NOMV elicits in animals. It has been previously noted that purified group B OMPs have adjuvant activity in the mucosal immunization of both mice and guinea pigs (36).
The i.n. route of immunization has several desirable characteristics over intramuscular immunization, not the least being the fact that the i.n. route of delivery was preferred by human volunteers over intramuscular injection for an influenza vaccine (13). Furthermore, meningococcus-specific IgA has been detected in the nasal secretions of patients convalescent from the disease (39) and in volunteers who have been immunized i.n. with meningococcal antigens (14; unpublished data). It has been suggested that host IgA is disadvantageous to the survival of meningococci and that a specific IgA1 protease produced by pathogenic Neisseria species may be a virulence factor (16, 21). However, antibody to IgA1 protease also develops after nasopharyngeal carriage and disease, but whether this is important in preventing meningococcal disease is unknown (3).
The vaccine strain used for the production of the NOMV vaccine was chosen in part because it had good expression of Opa proteins that could potentially mediate the binding of the NOMV to the mucosal surfaces of the nose and throat and possibly induce antibodies that could block adherence (37). The vaccine strain was genetically modified to block sialic acid synthesis, which resulted in the lack of a capsule and the lack of sialylation of the LOS (unpublished data). Use of a sialic acid-negative mutant was thought to have several advantages for a mucosal vaccine. First, several studies have shown that the sialic acid capsule inhibits adherence of meningococci to epithelial cells (33, 37). Thus, capsular polysaccharide present on the NOMV might reduce interaction of the vaccine with the epithelial cells and result in reduced uptake (immunogenicity) of the vaccine. Second, both the group B capsule and sialylated LOS have been shown to be molecular mimics and are therefore both undesirable as vaccine components and are poor immunogens (9, 20). Third, both the capsule and the sialylated LOS appear to be virulence factors (15, 21). Eliminating them results in a much safer vaccine strain with which to work.
A potential drawback in using NOMV as a vaccine is its high native endotoxin content, which might lead to unacceptably high reactogenicity in humans. However, we have given high doses (>400 μg of protein and 90 μg of LOS) of NOMV i.n. to both rabbits and humans without eliciting a pyrogenic response (unpublished data), indicating that relatively large amounts of antigen containing native endotoxin can be safely administered by the i.n. route. In mice, NOMV (20 μg of protein and 5 μg of LOS) given i.n. appeared to be more toxic, as evidenced by acute weight loss, than when given i.p.
We performed an experiment with unanesthetized mice to determine if alert, responsive mice could successfully swallow excess vaccine, thus avoiding the aspiration of endotoxin. We observed the aspiration of the vaccine regardless of anesthesia, as determined by histopathology and by the gasping response of some mice after vaccination. We believe this was due to the relatively large volume (25 μl) of NOMV used to immunize the mice, which was aspirated into the lungs, resulting in an acute inflammatory response, the timing of which coincided with weight loss. The acute inflammatory response we observed in the lungs is similar to a previous report of acute lung injury in mice after i.n. administration of lipopolysaccharide, where peak lung injury occurred at 24 to 48 h (35). The administration of a smaller volume of vaccine would eliminate the involvement of the lungs and bronchus-associated lymphoid tissue (38). We do not believe it would be necessary or desirable for an i.n. NOMV vaccine to reach the lungs in humans since the palatine tonsils and adenoids serve as mucosal induction sites (30). Further investigation with a larger animal model, the rabbit, to exclude lung involvement has been undertaken in our laboratory.
The goal of this study was to examine the local and systemic antibody response of mice given meningococcal antigens i.n. We found this route of presentation effective in both respects. Since the meningococcus is strictly a human pathogen, a good animal challenge model that correlates with efficacy in humans does not exist. The anti-meningococcal response in mice is not highly predictive of the human response, but the development of bactericidal antibodies and protection in animal models is the best available correlate of the potential of inducing a protective response in humans (28). Further investigations of the immune response in the mouse model will be used to compare modifications in NOMV production, immunization schedules, and dose responsiveness.
ACKNOWLEDGMENT
We thank Fonzie Quance-Fitch for assistance with preparation of the photomicrographs.
REFERENCES
- 1.Anderson E L, Bowers T, Mink C M, Kennedy D J, Belshe R B, Harakeh H, Pais L, Holder P, Carlone G M. Safety and immunogenicity of meningococcal A and C polysaccharide conjugate vaccine in adults. Infect Immun. 1994;62:3391–3395. doi: 10.1128/iai.62.8.3391-3395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W, Moran E, Hankins W, Gilly J, Mays J the Chilean National Committee for Meningococcal Disease. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine. 1995;13:821–829. doi: 10.1016/0264-410x(94)00037-n. [DOI] [PubMed] [Google Scholar]
- 3.Brooks G F, Lammel C J, Blake M S, Kusecek B, Achtman M. Antibodies against IgA1 protease are stimulated both by clinical disease asymptomatic carriage of serogroup A Neisseria meningitidis. J Infect Dis. 1992;166:1316–1321. doi: 10.1093/infdis/166.6.1316. [DOI] [PubMed] [Google Scholar]
- 4.Cahill E S, O’Hagan D T, Illum L, Redhead K. Mice are protected against Bordetella pertussis infection by intra-nasal immunization with filamentous haemagglutinin. FEMS Microbiol Lett. 1993;107:211–216. doi: 10.1111/j.1574-6968.1993.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky C C, Nilsson L-A, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 6.Dale J B, Chiang E C. Intranasal immunization with recombinant group A streptococcal M protein fragment fused to the B subunit of Escherichia coli labile toxin protects mice against systemic challenge infections. J Infect Dis. 1995;171:1038–1041. doi: 10.1093/infdis/171.4.1038. [DOI] [PubMed] [Google Scholar]
- 7.D’Alessandri R, Plotkin G, Kluge R M, Wittner M K, Fox E N, Dorfman A, Waldman R H. Protective studies with group A streptococcal M protein vaccine. III. Challenge of volunteers after systemic or intranasal immunization with Type 3 or Type 12 group A Streptococcus. J Infect Dis. 1978;138:712–718. doi: 10.1093/infdis/138.6.712. [DOI] [PubMed] [Google Scholar]
- 8.Edwards U, Muller A, Hammerschmidt S, Gerardy-Schahn R, Frosch M. Molecular analysis of the biosynthesis pathway of the α-2,8 polysialic acid capsule by Neisseria meningitidis serogroup B. Mol Microbiol. 1994;14:141–149. doi: 10.1111/j.1365-2958.1994.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 9.Finne J, Leinonen M, Makela P H. Antigenic similarities between brain components and bacteria causing meningitis: implications for vaccine development and pathogenesis. Lancet. 1983;ii:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 10.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorringe A R, Borrow R, Fox A J, Robinson A. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine. 1995;13:1207–1212. doi: 10.1016/0264-410x(95)00055-6. [DOI] [PubMed] [Google Scholar]
- 13.Gruber W C, Hinson H P, Holland K L, Thompson J M, Reed G W, Wright P F. Comparative trial of large-particle aerosol and nose drop administration live attenuated influenza vaccines. J Infect Dis. 1993;168:1282–1285. doi: 10.1093/infdis/168.5.1282. [DOI] [PubMed] [Google Scholar]
- 14.Haneberg B, Dalseg R, Wedege E, Hoiby E A, Haugen I L, Oftung F, Andersen S R, Naess L M, Aase A, Michaelsen T E, Holst J. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect Immun. 1998;77:1334–1341. doi: 10.1128/iai.66.4.1334-1341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones D M, Borrow R, Fox A J, Gray S, Cartwright K A, Poolman J T. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathog. 1992;13:219–224. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- 16.Kilian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langermann S, Palaszynski S, Sadziene A, Stover C K, Koenig S. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature. 1994;372:552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 18.Lowell G H, Kaminski R W, Grate S, Hunt R E, Charney C, Zimmer S, Colleton C. Intranasal and intramuscular proteosome-staphylococcal enterotoxin B (SEB) toxoid vaccines: immunogenicity and efficacy against lethal SEB intoxication in mice. Infect Immun. 1996;64:1706–1713. doi: 10.1128/iai.64.5.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallett C P, Hale T L, Kaminski R W, Larsen T, Orr N, Cohen D, Lowell G H. Intranasal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–2386. doi: 10.1128/iai.63.6.2382-2386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandrell R E, Griffiss J M, Macher B A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. J Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer T F, Frosch M, Gibbs C P, Haas R, Halter R, Pohlner J, van Putten J P M. Virulence functions and antigen variation in pathogenic neisseriae. Antonie Leeuwenhoek. 1988;54:421–430. doi: 10.1007/BF00461860. [DOI] [PubMed] [Google Scholar]
- 22.Milagres L G, Ramos S R, Sacchi C T, Melles C E A, Viera V S D, Sato H, Brito G S, Moraes J C, Frasch C E. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect Immun. 1994;62:4419–4424. doi: 10.1128/iai.62.10.4419-4424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moldoveanu Z, Clements M L, Prince S J, Murphy B R, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 24.Moran E E, Brandt B L, Zollinger W D. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect Immun. 1994;62:5290–5295. doi: 10.1128/iai.62.12.5290-5295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh Y, Ohta K, Kuno-Sakai H, Kim R, Kimura M. Local and systemic influenza haemagglutinin-specific antibody responses following aerosol and subcutaneous administration of inactivated split influenza vaccine. Vaccine. 1992;10:506–511. doi: 10.1016/0264-410x(92)90348-n. [DOI] [PubMed] [Google Scholar]
- 26.Orr N, Robin G, Cohen D, Arnon R, Lowell G H. Immunogenicity and efficacy of oral or intranasal Shigella flexneri 2a and Shigella sonnei proteosome-lipopolysaccharide vaccines in animal models. Infect Immun. 1993;61:2390–2395. doi: 10.1128/iai.61.6.2390-2395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal S, Fielder T J, Peterson E M, de la Maza L M. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkins B A, Jonsdottir K, Briem H, Griffiths E, Plikaytis B D, Hoiby E A, Rosenqvist E, Holst J, Nokleby H, Sotolongo F, Sierra G, Campa H C, Carlone G M, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger J D, Broome C V. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;177:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 29.Polly S M, Waldman R H, High P, Wittner M K, Dorfman A, Fox E N. Protective studies with a group A streptococcal M protein vaccine. II. Challenge of volunteers after local immunization in the upper respiratory tract. J Infect Dis. 1975;131:217–224. doi: 10.1093/infdis/131.3.217. [DOI] [PubMed] [Google Scholar]
- 30.Quiding-Jarbrink M, Granstrom G, Nordstrom I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders N B, Shoemaker D R, Brandt B L, Zollinger W D. Confirmation of suspicious cases of meningococcal meningitis by PCR and enzyme-linked immunosorbent assay. J Clin Microbiol. 1997;35:3215–3219. doi: 10.1128/jcm.35.12.3215-3219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sedgwick J D, Holt P G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;57:301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- 33.Stephens D S, McGee Z A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981;143:525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- 34.Swartley J S, Stephens D S. Identification of a genetic locus involved in the biosynthesis of N-acetyl-d-mannosamine, a precursor of the (α-2-8)-linked polysialic acid capsule of serogroup B Neisseria meningitidis. J Bacteriol. 1994;176:1530–1534. doi: 10.1128/jb.176.5.1530-1534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szarka R J, Wang N, Gordon L, Nation P N, Smith R H. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997;202:49–57. doi: 10.1016/s0022-1759(96)00236-0. [DOI] [PubMed] [Google Scholar]
- 36.Van de Verg L L, Hartman A B, Bhattacharjee A K, Tall B D, Yuan L, Sasala K, Hadfield T L, Zollinger W D, Hoover D L, Warren R L. Outer membrane protein of Neisseria meningitidis as a mucosal adjuvant for lipopolysaccharide of Brucella melitensis in mouse and guinea pig intranasal immunization models. Infect Immun. 1996;64:5263–5268. doi: 10.1128/iai.64.12.5263-5268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virji M, Makepeace K, Ferguson D J P, Achtman M, Moxon E R. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 38.Weltzin R, Kleanthous H, Guirakhoo F, Monath T P, Lee C K. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–376. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 39.Wenzel R P, Mitzel J R, Davies J A, Beam W E., Jr Serum and nasal secretion immune response in meningococcal disease. Infect Immun. 1972;5:627–629. doi: 10.1128/iai.5.4.627-629.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zollinger W D, Kasper D L, Veltri B J, Artenstein M S. Isolation and characterization of a native cell wall complex from Neisseria meningitidis. Infect Immun. 1972;6:835–851. doi: 10.1128/iai.6.5.835-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zollinger W D, Mandrell R E, Griffiss J M, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979;63:836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zollinger W D, Boslego J W. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J Immunol Methods. 1981;46:129–140. doi: 10.1016/0022-1759(81)90130-7. [DOI] [PubMed] [Google Scholar]
- 43.Zollinger W D. New and improved vaccines against meningococcal disease. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 469–488. [Google Scholar]


