Abstract
The blood–brain barrier (BBB) is a kind of filter, highly selective in relation to various types of substances. The BBB supports the immune status of the brain and is an important regulator of neuroimmune interactions. Some of the molecular and cellular features of the BBB, as well as the five main pathways of neuroimmune communication mediated by the BBB, are analyzed in this article. The functions of the BBB in neuroimmune interactions in various diseases are discussed: multiple sclerosis and Alzheimer’s and Parkinson’s diseases. The latest data on BBB dysfunction in COVID-19 coronavirus infection caused by the SARS-CoV-2 virus are considered.
Keywords: blood−brain barrier, neuroimmune interactions, neurodegenerative diseases, COVID-19
The interaction of the nervous and immune systems provides the possibility of an adaptive response of the latter to the entry of foreign agents—bacteria, viruses, fungi, etc.—into the body. The study of the involvement of the blood–brain barrier (BBB) in this process is of particular interest. The BBB maintains homeostasis of the central nervous system (CNS) and performs a protective function [1]. This is a kind of filter that allows oxygen, ions, and other vital molecules to pass into the central nervous system but restricts the passage of many other substances. The high selectivity of the BBB in relation to substances of various types is explained by its histological structure.
When speaking of the blood–brain barrier, its endothelial component is usually meant; in the literature, however, epithelial cells of the choroid plexuses are distinguished as forming a barrier that serves to preserve the constancy of the composition of the cerebrospinal fluid. In 2012, the concept of the glymphatic system was formulated, which is a single brain cleansing system that includes some components of the BBB [2]. This review presents data on the vascular part of the BBB, but not on the features of the cerebrospinal fluid barrier or the glymphatic system.
Endothelial capillaries of the BBB constitute a microvascular network, the basis of which, as in other vessels of the body, is endothelial cells. CNS capillaries are continuous nonfenestrated [3]; that is, their basement membrane is continuous, without intercellular gaps and pores (fenestrations) in the plasma membrane. Thus, the endothelial lining of brain capillaries is continuous.
In addition to the basement membrane and endothelial cells, an important component of the BBB is glial and mural cells, as well as neurons, immune cells of the CNS, and peripheral blood, which together form the neurovascular unit. The functions of the BBB are mainly provided by endothelial cells interacting with other components of the neurovascular unit [4].
BBB COMPONENTS AND FUNCTIONS
The molecular and cellular composition of the BBB determines its functions. The BBB consists not only of the endothelial cells of the CNS capillaries and the basement membrane of the capillaries, but also of several other types of cells—mural, including smooth muscle cells and pericyte cells, as well as neurons, astrocytes, perivascular macrophages and microglia, and in some cases immune peripheral blood cells.
The endothelial cells of the CNS capillaries have unique properties that distinguish them from the endothelial cells of the capillaries of other organs. The absence of pores in the plasma membrane of endotheliocytes causes a low rate of transcytosis. These cells are connected to each other by continuous complexes of proteins—tight junctions, which also sharply reduces the likelihood of paracellular transport and makes it possible to regulate the movement of ions, molecules, and cells between the blood and the brain. The molecular basis of tight junctions consists of a number of proteins, including cadherins; catenins; occludin; claudins; and scaffold proteins ZO-1, ZO-2, and ZO-3 [5]. Claudins are necessary for the formation of the paracellular barrier, while the scaffold proteins ZO-1, -2, and -3 stabilize the structure and bind tight junctions with the cytoskeleton. In addition, tight junctions are associated with basal adherent junctions connecting all endothelial cells, which enhances the structural strength. Tight junctions are a kind of filter with a bandwidth of less than 4 nm: as a physical barrier, they allow only small gaseous and lipophilic molecules to penetrate freely into and out of the brain, while the transfer of large molecules is carried out through special transport systems [6].
The efflux transporters are polarized to the luminal surface of the endothelial cell. They are of the greatest interest for study since they prevent the penetration of pharmacological drugs into the CNS. Efflux transporters move potentially harmful compounds, such as glutamate, out of the brain, even against the concentration gradient, which requires ATP as an energy source. These include the superfamily of ABC transporters—proteins with a common domain organization (the presence of transmembrane and ATP-binding domains, which are also called ABC domains). The most studied member of this superfamily in the BBB is P-glycoprotein (Pgp), which has a wide substrate specificity and actively transports various substances. It is believed that the main physiological role of Pgp is the excretion of xenobiotics. Endotheliocytes of the BBB and astrocytes contain more Pgp than other cells of the body [7].
The second type of transporters are highly specific nutrient transporters that facilitate the transport of certain molecules across the BBB into the CNS (influx transporters). Influx transporters facilitate the delivery of drugs to the CNS [8]. A large number of mitochondria in the endothelial cells of the CNS capillaries is probably of decisive importance for the regulation of active transport.
As a rule, large hydrophilic molecules cannot be transported through the BBB, except in cases of specific receptor or adsorption-mediated transcytosis. This is how transferrin, low-density lipoproteins, insulin, and other peptide hormones are transferred to the extracellular space of the brain [9].
The combination of the above properties of endothelial cells allows the BBB to regulate tightly the transport of substances from the bloodstream to the CNS and vice versa, maintaining homeostasis in the brain tissue.
Mural cells are smooth muscle cells that surround large vessels, and pericyte cells cover the endothelial walls of smaller vessels. Pericytes are located on the abluminal surface and are immersed in the basement membrane of endothelial cells. A feature of pericytes is the absence of specific markers, which makes it difficult to identify them among other cell types. Molecules that could theoretically be pericyte markers include PDGFRβ, NG2, CD13, and CD146, but NG2 and CD13 are also expressed by smooth muscle cells. Pericytes contain constrictor proteins, which allows them to contract and makes it possible to control the capillary diameter [10]. Pericytes do not adjoin endothelial cells but form adherens junctions with them according to the plug–socket type, as well as other types of junctions: adhesion plaques and tight and gap junctions [11]. The main differences between CNS pericytes and peripheral pericytes include their origin (neural crest, not mesoderm), as well as the ratio of the number of endotheliocytes and pericytes: in the CNS, this figure reaches 1 : 1, while in the periphery, it is 100 : 1 [12]. The functions of pericytes are diverse, including regulation of angiogenesis, synthesis of extracellular matrix (ECM) components, participation in wound healing, and regulation of the degree of infiltration of the CNS by immune cells [13].
The basement membrane (BM) lining the capillaries is divided into two types: internal vascular and external parenchymal. The vascular BM is an ECM synthesized by endotheliocytes and pericytes, while components of the parenchymal BM are synthesized by astrocytes [14]. The molecular compositions of the vascular and parenchymal basement membranes are somewhat different, although they predominantly consist of type IV collagen, laminin, nidogen, proteoglycans, and glycoproteins. The main function of the BM is that of a barrier, and the destructive effect of matrix metalloproteinases (MMPs) on it is one of the links in the pathogenesis of various neurological disorders [15].
Astrocytes are macroglial cells, the processes of which approach the blood vessels and form the dystroglycan–dystrophin complex necessary for proper integration of aquaporin-4 into the membrane. Astrocytes regulate the flow of water into the CNS and provide cellular communication between neuronal circuits and blood vessels. These neurovascular connections allow astrocytes to regulate blood flow in response to signals from nerve cells, such as regulating the contraction and relaxation of smooth muscle cells and pericytes surrounding blood vessels. Presumably, during embryonic development, astrocytes are not a necessary condition for the formation of the BBB, but the normal development and maintenance of BBB functions are impossible in the absence of astrocytes [16].
Cells of the immune system of the brain are divided into two main types—microglial cells and perivascular macrophages. Microglial cells migrate into the CNS during embryogenesis. They are formed from the hematopoietic precursors of the yolk sac, while perivascular macrophages are of monocytic origin and pass through the BBB, settling in the perivascular Virchow–Robin space [17]. Perivascular macrophages provide the first line of defense for innate immunity. The functions of microglial cells are not limited to participation in immune defense: they promote wound healing, regulate neuronal development in embryogenesis, and act as antigen-presenting cells in adaptive immunity [18].
Other types of cells—neutrophils, macrophages, T-cells—in various forms of pathology are involved in the regulation of the function of the BBB. Reactive oxygen species synthesized by these cells increase vascular permeability, which may underlie BBB disruption in various neurological diseases [19].
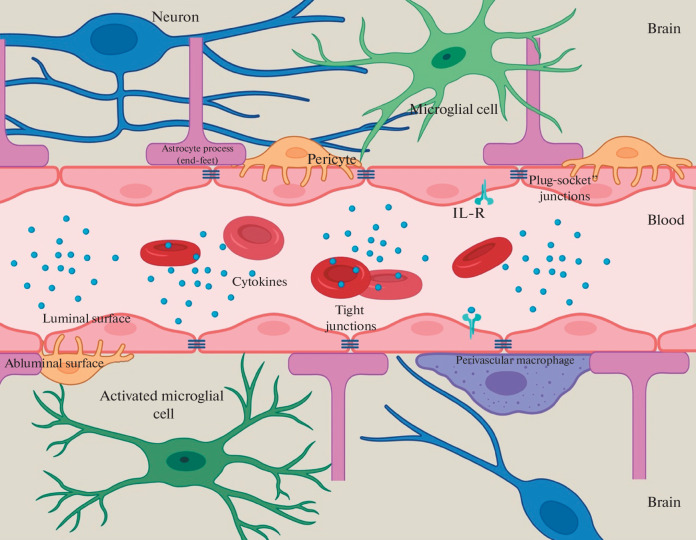
Therefore, the BBB neurovascular unit consists of brain capillary endotheliocytes, pericytes, astrocytes, microglial cells, as well as neurons, the ECM, and the glycocalyx (Fig. 1).
Fig. 1.
Schematic representation of the neurovascular unit.
There are special areas in the BBB that delimit the so-called circumventricular organs adjacent to the walls of the III and IV ventricles of the brain. These include the subfornical organ, the area postrema in the medulla oblongata, the pineal gland, and the median eminence of the neurohypophysis. This is the most permeable part of the blood−brain barrier, which is due to a different type of capillaries—continuous fenestrated, forming a dense network that penetrates the circumventricular organs. Due to increased blood supply and BBB permeability, circumventricular organs are an important component of the neuroendocrine regulation system, controlling the level of cytokines and body temperature, the salt and water balance, and blood pressure and regulating the activity of the GI tract [20].
BBB PARTICIPATION IN NEUROIMMUNE INTERACTIONS
The BBB and cells of the neurovascular unit serve as an interface in the interaction between the CNS and the periphery and are important regulators of neuroimmune communication. There are five BBB-mediated neuroimmune axes: BBB permeability modulation; modulation of BBB transporters; capture and transport of immunoactive substances by BBB cells; transfer of immune cells through the BBB into the brain tissue; and secretion of immunoactive substances by cells of the BBB and the neurovascular unit [21]. These axes can function independently of each other, but are more often activated together, providing different ways of transmitting signals from the immune system to the brain.
BBB permeability disorders can be caused by several factors, including the activity of cytokines and chemokines, bacteria and their components, complement proteins, acute phase proteins, etc.
Strictly speaking, the term destruction is usually applied to pathological processes; however, even under physiological conditions, the protective function of the BBB can vary slightly. The degree of its permeability depends on the location: small arterioles of the brain have many caveolar vesicles, which are practically absent in the capillaries of the brain. It was shown that these caveolae in arterioles mediate the neurovascular link and physiological functions with greater BBB permeability [22].
Permeability disturbances can be the result of damaging and nondamaging changes, reflecting the presence or absence of physical damage, respectively. The former imply changes at the histological level: damage to endothelial cells, tight junctions, etc. Nondamaging changes affect the molecular level of the implementation of the BBB functions.
An increase in the permeability of the BBB can occur through an increase in para- and transcellular transport due to a decrease in the functions of tight and intercellular junction proteins and an increase in vesicular mechanisms. Destruction of tight junctions between BBB endotheliocytes occurs when the synthesis of tight junction proteins is reduced if they are incorrectly localized or posttranslationally modified [23]. It has been shown that inflammatory mediators modulate the synthesis and activity of tight junction proteins. For example, injection of IL-1β into the brain parenchyma results in loss of occludin and ZO-1 expression in endothelial cells. In the experiment, this led to an increase in the paracellular transport of phosphotyrosine, which is used as a marker, and the recruitment of neutrophils into vessels where there were no tight junctions. TGF-β1 suppresses the expression of claudin-5 [1]. Inflammatory mediators that increase paracellular transport through the BBB include bradykinin, histamine, serotonin, arachidonic acid, and ATP [24].
One of the mechanisms underlying the modulation of tight junction proteins by inflammatory mediators is the action of calpain, an intracellular calcium-dependent protease that regulates cell adhesion. Calpain inhibition has been shown to prevent IL-1β-induced loss of the ZO-1 protein in tight junctions of endotheliocytes and changes in the assembly of the F-actin cytoskeleton [25].
Protective factors supporting the synthesis of endotheliocyte tight junction proteins include IL-25, netrin-1, annexin A1 (synthesized by endotheliocytes), and SHH family proteins (synthesized by astrocytes). IL-1β reduces SHH expression [26].
Enzymatic degradation of tight junction proteins can also occur during the development of neuroinflammation. Neutrophil proteases such as MMP9 and elastase contribute to the destruction of the ECM and endothelium during ischemia and reperfusion. Intracerebral injection of neutrophil elastase causes endothelial edema and focal necrosis of blood vessels in rats [27]. Inhibition or knockout of matrix metalloproteases prevents degradation of tight junctions and disruption of the BBB in the acute phase after cerebral ischemia and reperfusion [28].
Another possible mechanism for BBB permeability impairment is the physical destruction of endothelial cells, resulting in the formation of vesicular channels, through which large molecules pass. This mechanism has been demonstrated in cerebral edema, traumatic brain injury, and sepsis. Disturbances in the synthesis of the plasmalemma vesicle protein PLVAP in endotheliocytes are associated with Alzheimer’s disease and multiple sclerosis [29]. Therefore, pathological fenestration of the BBB may be an important factor in the violation of its permeability.
Modulation of BBB functions by immunoactive substances. As was mentioned above, BBB endotheliocytes have many transporter systems on their surface, among which there are both active transport systems and those operating according to the principle of facilitated diffusion. The functions of some of them change during neuroinflammation and are modulated by signaling molecules, for example, the Pgp protein. Its ligands include protease inhibitors, opiates, antiepileptics, cyclosporins, glucocorticoids, aldosterone, dexamethasone, and calcium channel blockers. Pgp activity explains why certain substances do not accumulate in the brain in sufficient quantities to affect the CNS. The function changes during inflammation, and the main effect in vivo is the suppression of its transport activity. Thus, induction by proinflammatory cytokines (TNFα, IL-1β, IL-6, IL-2, IFNγ) leads to a decrease in Pgp mRNA expression in CNS endotheliocytes and the synthesis and activity [30].
Inflammation also affects influx transporters. Thus, the interleukin-6 (IL-6) protein secreted by astrocytes stimulates the activity of the Na–K–Cl cotransporter and its excessive activation leads to the development of cerebral edema [31].
Of special interest are the effects of lipopolysaccharide (LPS) on BBB functions. It is known that BBB endotheliocytes express the TLR4 receptor, which directly mediates the LPS effects. In the primary culture of endotheliocytes, LPS causes a disturbance of the blood–brain barrier associated with the dysfunction of tight junction proteins and in high doses induces their apoptosis [32]. The effects of LPS on the destruction of the BBB are mitigated by the cyclooxygenase inhibitor indomethacin [33], which means that cyclooxygenases are involved in the mechanisms of LPS-induced destruction of the BBB.
Contradictory data were obtained on the effect of mural and glial cells of the neurovascular unit on the response of endotheliocytes to LPS. Cultivation of bovine brain endotheliocytes together with rat astrocytes had a protective effect on LPS-induced destruction of endotheliocytes. This effect was not observed in cocultivation of mouse endotheliocytes with astrocytes and pericytes [34].
Do the BBB and endotheliocytes respond to baseline levels of LPS in the bloodstream? As is known, the physiological concentration of LPS in the blood (detected using the LAL test) is up to 1 EU/mL, and LPS may play a role in the physiological regulation of BBB functions. According to the authors of [35], who analyzed the results of 74 studies on the effects of LPS on the BBB, 60% of cases reported a damaging effect. The effects of LPS on the BBB depend on the dose of lipopolysaccharide, the frequency of its administration, the type of experimental animal, its sex, age, etc. The authors built a logistical regression model that accounted for these experimental factors and found that a significant predictor among them is only the type of experimental animal. Thus, damaging changes in the BBB in mice are four times more likely than in rats. The dose of LPS, in contrast, was not a significant factor. Most of the studies used a septic dose of LPS, making it difficult to generalize the results to less severe infections in humans.
Transport of immunoactive substances through the BBB. The main function of the BBB is to prevent the penetration of substances that can have neurotoxic effects from the blood into the brain. These molecules include cytokines and chemokines, but many of them are transported through the BBB in a controlled manner from the blood to the brain. The differences between the entry of neuroactive substances into the CNS through unregulated “leakage” and through regulated transport in this case is a key issue: in the first case, BBB dysfunction occurs, which can lead to neurotoxicity; in the second, the CNS apparently regulates the transport properties of the BBB in accordance with its needs.
Little is known about the mechanisms of cytokine transport through the BBB and the factors affecting this transport. For example, the cytokine-induced neutrophil chemoattractant CINC1 enters the brain through an unsaturable mechanism, presumably by transcellular diffusion. The CCL2 chemokine is transported through a caveolae-dependent pathway. The TNF transporter protein in the BBB is probably its receptor since, in mice with a knockout of this receptor, TNF transport through the BBB does not occur, but the transport of the epidermal growth factor, IL-1, and its receptors is not impaired [36]. Most studies of cytokine transport have been carried out in mice, but it has been demonstrated that transport of IL-1α and IL-6 through the BBB occurs in embryogenesis in rats, while that of IL-1β and IL-6 occurs in sheep embryos [37], which indicates the expression of cytokine transporters in the BBB at early stages of development and in different animal species.
TNF-α, transported through the BBB during systemic inflammation, stimulates microglial cells, promoting the development of neuroinflammation and increased secretion of TNF, which leads to apoptosis of dopaminergic cells in the substantia nigra. This model of dual action of peripheral blood cytokines and CNS cells can generally be applied to cytokines that pass through the BBB during inflammation [38].
Interestingly, the rate of cytokine transport is not uniform throughout the brain. Thus, the cells of the circumventricular organs transport about seven times more cytokines than the neighboring regions [39]. Such a rapid exchange of information between the immune and nervous systems makes it possible to realize the regulatory function of these organs.
Transport of cells of the immune system through the BBB. The CNS is presumably an immunologically privileged organ; therefore, under physiological conditions, the transport of immune system cells through the BBB is minimal and strictly regulated. Normally, mononuclear cells enter the brain during embryonic development and become resident immunocompetent cells—microglia. However, during pathological processes—infection, trauma—peripheral blood leukocytes are recruited to the brain under the action of cytokine signals and the BBB has special mechanisms to monitor and recruit leukocytes into the brain tissue.
The entry of leukocytes into the cerebrospinal fluid and brain parenchyma through the BBB depends on a multistep extravasation process, which varies depending on the basal expression of adhesion molecules. A typical process of extravasation of leukocytes into tissues includes the initial capture of circulating leukocytes, their “anchoring,” “rolling” along the endothelium, and subsequent diapedesis. Attachment of leukocytes to endothelial cells is provided by increased expression of adhesion molecules VCAM, ICAM, and integrins. The initial stages of leukocyte uptake are mediated by interactions of selectins on the surface of endothelial cells with glycoproteins on the surface of leukocytes [40]. Peripheral and meningeal endothelial cells, as well as endothelial cells of the choroid plexus, express selectins, which are stored in Weibel–Palade bodies. In response to inflammatory mediators, Weibel–Palade bodies migrate to the luminal surface of endotheliocytes, providing rapid expression of selectins on the luminal membrane. Endothelial cells of the brain parenchyma do not store selectins in Weibel–Palade bodies and require synthesizing selectins de novo to ensure the uptake and migration of leukocytes. Diapedesis of leukocytes can be carried out both by paracellular and transcellular transport, affecting mainly proteins of tight junctions and the endotheliocyte cytoskeleton. Activated microglia secrete inflammatory factors, accelerate the expression of adhesion molecules in endotheliocytes, and participate in the recruitment of CNS leukocytes, intensifying the inflammatory process [41].
One of the factors stimulating the penetration of leukocytes into the CNS is the proinflammatory cytokine IL-1β. The mechanism of penetration in this case is probably receptor based: endotheliocytes express the receptor for type 1 IL-1 (IL-1R1) in the CNS. Knockdown of IL-1R1 in the endothelium abolishes the influx of leukocytes into the CNS induced by intracerebroventricularly administered IL-1β. Interestingly, the effect of IL-1β on leukocyte migration can be inhibited in systemic inflammation. Systemic administration of LPS within two hours after intracerebroventricular injection of IL-1β inhibits the recruitment of leukocytes into the CNS, preventing the activation of selectins on endotheliocytes [42].
Presumably, the transfer of leukocytes to the CNS in pathology occurs mainly in the vascular plexuses. When the spinal cord is injured, macrophages are recruited to the injury site through the choroid plexus by increasing the expression of adhesion molecules on the epithelial cells of the plexus. Using a model of middle cerebral artery occlusion, it was shown that about two-thirds of the T cells that infiltrate the peri-infarct zone are recruited from the choroid plexus stroma. The same study notes that the apical region of the choroid plexus stroma is physically connected to the cerebral parenchymal vessels. It is assumed that the rapid migration of T cells from the stroma of the choroid plexus to the corpus callosum and the peri-infarct zone is associated with the presence of a direct route for the delivery of T cells to the CNS tissues, bypassing the cerebrospinal fluid and cerebral barriers [43].
A number of studies devoted to the mechanisms of the onset and development of experimental autoimmune encephalomyelitis (EAE) in mice demonstrated the existence of “neural gateways,” including sympathetic noradrenergic innervation of local blood vessels and regulation of the penetration of autoreactive CD4+ T cells into the CNS. These studies showed that noradrenaline released by sympathetic endings in response to various stimuli enhances NFkB-mediated transcription of proinflammatory genes in endothelial cells of certain vessels, thereby providing a gateway for immune cells to enter the CNS. For example, the so-called “gravity−gateway” reflex promotes the penetration of autoreactive CD4+ cells into the dorsal blood vessels corresponding to the fifth lumbar (L5) vertebra. This reflex is somatosympathetic, mediated by proprioceptive signals triggered by muscle contractions in the hind limbs. Norepinephrine, released by sympathetic endings at the L5 level, causes vasodilation and enhances IL-6 signaling and CCL20 chemokine expression, facilitating the entry of activated CD4+ cells into the CNS. The “pain–gateway” reflex, a pain reflex induced by stimulation of hindlimb nociceptors, triggers the release of norepinephrine and increases the expression of CX3CL1 and the activation of resident monocytes in the ventral vessels innervated by sympathetic nerves at the L5 level, which leads to a relapse of experimental allergic encephalomyelitis (EAE) [44].
These studies also confirmed the presence of a stress−gateway reflex, which involves the paraventricular nucleus of the hypothalamus and sympathetic nerve fibers innervating vessels in the border regions of the third ventricle, thalamus, and dentate gyrus. It is hypothesized that this reflex may induce CCL5-mediated accumulation of autoreactive pathogenic CD4+ T cells in these structures and indirectly trigger vagal fiber responses, contributing to gut damage in mice with EAE [45].
Using in vivo two-photon microscopy on the EAE model, H. Wekerle’s research team traced the process of invasion of myelin basic protein-specific T-cells (TMBP-cells) into the CNS. Experimental animals were injected with 5 × 103 TMBP cells to induce EAE. It was shown that a small number of single TMBP cells penetrate leptomeningeal vessels on the first day after cell injection. In subsequent hours, more TMBP cells enter the meningeal vessels; they actively move within the blood vessels, often against the flow of blood. This crawl-like movement predominates in the vessels of the meninges, while the classic T-cell “rolling” is observed in the vessels of peripheral organs. After “crawling” inside the vessels of the membranes, T cells penetrate the walls of the vessels and continue to migrate along their abluminal surface. A significant part of T cells forms junctions with local antigen presenting cells, as a result of which effector T cells are stimulated to produce proinflammatory mediators, followed by tissue invasion and the formation of inflammatory infiltrates [46]. These observations describe in detail the initial stages of immunological reactions in the CNS.
Secretion of immunoactive substances by BBB cells. The cells that form the BBB and choroid plexuses constantly interact with other CNS cells. The concept of cross interactions is embodied in the term neurovascular unit. Cross interactions serve to inform the BBB cells about the needs of the CNS and the adaptation of its functions in a particular situation. Many of the substances that mediate this “cross-talk” are neuroimmune mediators, including cytokines, prostaglandins, nitric oxide, and so on. Thus, neuroimmune mediators are directly involved in the cross interactions of the neurovascular unit and the formation of the functional activity of the BBB.
BBB endotheliocytes secrete various cytokines. The constitutive secretion of cytokines can be modulated by immune stimulants such as LPS, viruses, viral proteins, and bacteria. Endotheliocytes can respond to these signals coming from the CNS or blood. Cocultivation of endotheliocytes with other components of the neurovascular unit, such as astrocytes and pericytes, modulates the intensity of cytokine secretion. The secretion of cytokines is bipolar: they can be secreted from the luminal surface of the endotheliocyte into the blood or from the abluminal surface of the endotheliocyte into the CNS [47].
The ability to combine several neuroimmune axes means that the endotheliocyte can receive a signal (for example, from the luminal surface) and release a cytokine from the other side (from the abluminal side to the brain). In this case, information from one side of the BBB is transmitted to the other. For example, the authors of [48] discovered the mechanism that provides immune regulation of iron transport through the BBB using astrocytic ceruloplasmin. LPS, acting on the luminal surface of endotheliocytes, causes the secretion of IL-6 and IL-1β from their abluminal surface, which, in turn, stimulates astrocytic secretion of ceruloplasmin, acting on the abluminal surface of endotheliocytes, which facilitates the transfer of iron through the BBB [48].
Thus, the BBB is a multicomponent system in which each molecular and cellular link is involved in the formation of a protective barrier to maintain CNS homeostasis. Disturbances in the work of any of these links threaten the permeability of the barrier, which, as a result, can lead to the development of a pathological process. BBB dysfunctions were found in many diseases: multiple sclerosis, hypoxic and ischemic stroke, Parkinson’s and Alzheimer’s diseases, epilepsy, and brain tumors. The observed dysfunction of the barrier can vary from mild and temporary changes in its permeability to the chronic destruction of the barrier associated with the rearrangement of the functional activity of transporters and degradation of the basement membrane. In most cases, it is impossible to determine whether a breach of the barrier is the cause of the disease or the result of its progression. Nevertheless, the violation of the barrier often contributes to the development of pathology and its intensification.
BBB dysfunction is observed in Alzheimer’s disease: there is a hypothesis of two-stage vascular disorders, according to which vascular dysfunction plays a leading role in the initial stages of the disease, leading to ischemia−hypoxia and damage to endotheliocytes at the first stage. During the second stage, damage to endothelial cells and the BBB leads to the deposition of neurotoxic substances in the CNS, oxidative stress, and neuroinflammation. Neuroinflammation and oxidative stress increase the activity of β- and γ-secretases, which in turn contribute to the formation and gradual accumulation of amyloid Aβ. In addition, a correlation is known between BBB dysfunction and accumulation of neurofibrillary tangles from the hyperphosphorylated τ protein. Therefore, the BBB may be a new therapeutic target for the treatment of Alzheimer’s disease [49].
In Parkinson’s disease, an increased permeability of the BBB is also observed. It was demonstrated that excessive accumulation of alpha-synuclein contributes to the suppression of the expression of tight junction proteins ZO-1 and ccluding. In patients with Parkinson’s disease, a decrease in the expression and functional activity of BBB transporters, such as GLUT-1 and Pgp, was revealed. Dysfunction of transporters in Parkinson’s disease causes a decrease in the clearance of neurotoxic substances, which contributes to the progression of the disease [50].
The amount of angiogenesis factors is increased in the cerebrospinal fluid of patients with Parkinson’s disease [51]. Aberrant angiogenesis leads to the formation of an immature vasculature with a lack of tight junctions, which results in the penetration of neurotoxic substances into the CNS and the loss of dopaminergic neurons. Thus, damage to the BBB accelerates the development of neurodegenerative diseases.
BBB disruption and the transendothelial migration of activated leukocytes are among the earliest cerebrovascular anomalies observed in the brains of patients with multiple sclerosis. In people with a genetic predisposition, viral infections and environmental toxins reduce immune tolerance and stimulate the release of proinflammatory factors such as IL-6 and NF-kB. The latter contribute to the destruction of tight junctions of endotheliocytes, violate the integrity of the BBB, and stimulate the transmigration of leukocytes. Expression of the Pgp transporter also increases and enhances the migration of CD4+ and CD8+ T cells, which enhances neuroinflammation [51]. Under conditions of inflammation, the expression of endotheliocyte selectins also increases, which promotes adhesion of leukocytes. Cytokines and chemokines activate endothelial adhesion receptors and enhance subsequent leukocyte infiltration. CD4+ T cells identify myelin sheath proteins and activate the release of proinflammatory factors (including IFN-γ, TNF-α, nitric oxide, and free radicals), leading to demyelination. Neutrophils, monocytes, and microglia infiltrate the brain parenchyma and release extracellular glutamate, causing excitotoxicity and amplifying BBB dysfunction, which accelerates disease progression [53].
Research on the BBB in COVID-19 disease, caused by the SARS-CoV-2 virus, is in its early stages. It appears that the virus infects the vasculature of many systems and organs, including the brain. The consequences of COVID-19 include neurological symptoms such as headache, nausea, dizziness, microthrombi formation, and, in rare cases, encephalitis. The study of the interaction of SARS-CoV-2 with the cells that form the BBB seems to be very relevant to understand the mechanisms of occurrence of such consequences. Using postmortem materials from the brains of patients with COVID-19, it was shown that the ACE2 protein, known as the binding target of the SARS-CoV-2 spike protein, is ubiquitously expressed in vessels of various calibers of the frontal cortex. Note that ACE2 expression is increased in patients with dementia and hypertension. The cell culture of human primary brain endothelial cells (hBMVEC) also shows ACE2 expression. Under the conditions of BBB modeling in vitro, it was demonstrated that the S1 spike protein contributes to the loss of barrier integrity, causing a proinflammatory reaction on brain endothelial cells, which can contribute to a change in the state of the BBB function [54].
Pellegrini et al. found expression of the ACE2 receptor in mature cells of the choroid plexus but not in neurons or other cell types. They proved the tropism of SARS-CoV-2 to the epithelial cells of the choroid plexus and found that the virus hardly infects neurons or glia. It is argued that, when the epithelium is damaged by SARS-CoV-2, the protective function of the BBB is impaired, which can lead to neuroinflammation [55]. Radioactively labeled spike protein S1 was also shown to cross the BBB in mice both intravenously and intranasally. Further studies confirmed that S1 penetrates through the BBB with the participation of the ACE2 enzyme with the help of adsorption receptor-mediated transcytosis [56]. The complex of data demonstrates the susceptibility of the BBB to SARS-CoV-2, which is necessary to understand the mechanisms of both neurological and psychiatric manifestations of this viral infection.
* * *
Thus, it is obvious that disturbances in the functions of the blood−brain barrier play a significant role in the mechanisms of development of neuroinflammation and neurodegeneration. The combination of the barrier function with the ability to respond to and secrete immunoactive substances allows the BBB to interact with the nervous and immune systems, transmitting signals in two directions. These interactions are not constant and are modulated by the BBB microenvironment. Such modulation requires cross interactions between the components of the barrier and the neurovascular unit. The BBB is a dynamic regulatory system that is involved both in the transformation of the CNS into an immunologically privileged organ and in the provision of bidirectional communication between the immune system and the brain.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Footnotes
Anastasiya Sergeevna Dyatlova is a Junior Researcher in the Laboratory of Immunopathophysiology in the IEM Department of General Pathology and Pathological Physiology. Nataliya Sergeevna Novikova, Cand. Sci. (Biol.), is a Senior Researcher at the same laboratory. RAS Corresponding Member Boris Germanovich Yushkov is a Laboratory Head at the IIP, RAS Ural Branch. RAS Academician Elena Andreevna Korneva is Chief Researcher of the Laboratory of Immunopathophysiology in the IEM Department of General Pathology and Pathological Physiology. RAS Academician Valerii Aleksandrovich Chereshnev is Director for Science of the IIP, RAS Ural Branch.
Translated by B. Alekseev
Contributor Information
A. S. Dyatlova, Email: anst.diatlova@gmail.com
N. S. Novikova, Email: novikiem@gmail.com
B. G. Yushkov, Email: b.yushkov@iip.uran.ru
E. A. Korneva, Email: korneva_helen@mail.ru
V. A. Chereshnev, Email: v.chereshnev@mail.ru
REFERENCES
- 1.Bolton S. J., Anthony D. C., Perry V. H. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood–brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/S0306-4522(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 2.A. N. Kondrat’ev and L. M. Tsentsiper, “Glymphatic system of the brain: Structure and practical significance,” Russ. J. Anaesthesiol. Reanimatol., No. 6, 72–80 (2019) [in Russian].
- 3.Muoio V., Persson P. B., Sendeski M. M. The neurovascular unit—Concept review. Acta Physiol. (Oxf.) 2014;210:790–798. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 4.Tietz S., Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.R. Daneman and A. Prat, “The blood–brain barrier,” Cold Spring Harb. Perspect. Biol. 7 (1), a020412 (2015). [DOI] [PMC free article] [PubMed]
- 6.Löscher W., Potschka H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Covarrubias L., Slosky L. M., Thompson B. J. Transporters at CNS barrier sites: Obstacles or opportunities for drug delivery? Curr. Pharm. Des. 2014;20:1422–1449. doi: 10.2174/13816128113199990463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayloo S., Gu C. Transcytosis at the blood–brain barrier. Curr. Opin. Neurobiol. 2019;57:32–38. doi: 10.1016/j.conb.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Methner C., Mishra A., Golgotiu K. Pericyte constriction underlies capillary derecruitment during hyperemia in the setting of arterial stenosis. Am. J. Physiol. Heart Circ. Physiol. 2019;317:H255–H263. doi: 10.1152/ajpheart.00097.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney M., Foldes G. It takes two: Endothelial-perivascular cell cross-talk in vascular development and disease. Front. Cardiovasc. Med. 2018;5:154. doi: 10.3389/fcvm.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepro D., Morel N. M. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 12.Muramatsu R., Yamashita T. Pericyte function in the physiological central nervous system. Neurosci. Res. 2014;81–82:38–41. doi: 10.1016/j.neures.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Sorokin L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen M. S., Routhe L. J., Moos T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017;37:3300–3317. doi: 10.1177/0271678X17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolburg H., Wolburg-Buchholz K., Fallier-Becker P. Structure and functions of aquaporin-4-based orthogonal arrays of particles. Int. Rev. Cell Mol. Biol. 2011;287:1–41. doi: 10.1016/B978-0-12-386043-9.00001-3. [DOI] [PubMed] [Google Scholar]
- 16.Abbott N. J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 17.Lapenna A., De Palma M., Lewis C. E. Perivascular macrophages in health and disease. Nat. Rev. Immunol. 2018;18:689–702. doi: 10.1038/s41577-018-0056-9. [DOI] [PubMed] [Google Scholar]
- 18.Ajami B., Bennett J. L., Krieger C. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 19.Pun P. B. L., Lu J., Moochhala S. Involvement of ROS in BBB dysfunction. Free Rad. Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- 20.Mimee A., Smith P. M., Ferguson A. V. Circumventricular organs: Targets for integration of circulating fluid and energy balance signals? Physiol. Behav. 2013;121:96–102. doi: 10.1016/j.physbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Erickson M. A., Banks W. A. Neuroimmune axes of the blood–brain barriers and blood–brain interfaces: Bases for physiological regulation, disease states, and pharmacological interventions. Pharm. Rev. 2018;70:278–314. doi: 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow B. W., Kaplan L., Granger A. J. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020;579:106–110. doi: 10.1038/s41586-020-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luissint A.-C., Artus C., Glacial F. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott N. J. Inflammatory mediators and modulation of blood–brain barrier permeability. Cell. Mol. Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alluri H., Grimsley M., Anasooya Shaji C. Attenuation of blood–brain barrier breakdown and hyperpermeability by calpain inhibition. J. Biol. Chem. 2016;291:26958–26969. doi: 10.1074/jbc.M116.735365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podjaski C., Alvarez J. I., Bourbonniere L. Netrin 1 regulates blood–brain barrier function and neuroinflammation. Brain. 2015;138:1598–1612. doi: 10.1093/brain/awv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armao D., Kornfeld M., Estrada E. Y. Neutral proteases and disruption of the blood–brain barrier in rat. Brain Res. 1997;767:259–264. doi: 10.1016/S0006-8993(97)00567-2. [DOI] [PubMed] [Google Scholar]
- 28.Rempe R. G., Hartz A. M., Bauer B. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016;36:1481–1507. doi: 10.1177/0271678X16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo L., Zhang H., Hou Y. Plasmalemma vesicle-associated protein: A crucial component of vascular homeostasis. Exp. Ther. Med. 2016;12:1639–1644. doi: 10.3892/etm.2016.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez C., Buyse M., German-Fattal M., Gimenez F. Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J. Pharm. Pharm. Sci. 2004;7:359–371. [PubMed] [Google Scholar]
- 31.Sun D., Lytle C., O’Donnell M. E. IL-6 secreted by astroglial cells regulates Na–K–Cl cotransport in brain microvessel endothelial cells. Am. J. Physiol. 1997;272:C1829–1835. doi: 10.1152/ajpcell.1997.272.6.C1829. [DOI] [PubMed] [Google Scholar]
- 32.F. L. Cardoso, A. Kittel, S. Veszelka, et al., “Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells,” PloS One 7 (5), e35919 (2012). [DOI] [PMC free article] [PubMed]
- 33.Wang H., Sun J., Goldstein H. Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood–brain barrier into the brain and the in vivo sensitivity of the blood–brain barrier to disruption by lipopolysaccharide. J. Virol. 2008;82:7591–7600. doi: 10.1128/JVI.00768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks W. A., Gray A. M., Erickson M. A. Lipopolysaccharide-induced blood–brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation. 2015;12:223. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varatharaj A., Galea I. The blood–brain barrier in systemic inflammation. Brain Behav. Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Pan W., Kastin A. J. TNFalpha transport across the blood–brain barrier is abolished in receptor knockout mice. Exp. Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- 37.Y. Wang, S. Jin, Y. Sonobe, et al., “Interleukin-1β induces blood–brain barrier disruption by downregulating Sonic hedgehog in astrocytes,” PloS One 9 (10), e110024 (2014). [DOI] [PMC free article] [PubMed]
- 38.Joshi G., Aluise C. D., Cole M. P. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: Implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166:796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarlagadda A., Alfson E., Clayton A. H. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009;6:18–22. [PMC free article] [PubMed] [Google Scholar]
- 40.Vestweber D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 41.Reale M., Iarlori C., Thomas A. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Ching S., Zhang H., Lai W. Peripheral injection of lipopolysaccharide prevents brain recruitment of leukocytes induced by central injection of interleukin-1. Neuroscience. 2006;137:717–726. doi: 10.1016/j.neuroscience.2005.08.087. [DOI] [PubMed] [Google Scholar]
- 43.Llovera G., Benakis C., Enzmann G. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 2017;134:851–868. doi: 10.1007/s00401-017-1758-y. [DOI] [PubMed] [Google Scholar]
- 44.Kamimura D., Ohki T., Arima Y., Murakami M. Gateway reflex: Neural activation-mediated immune cell gateways in the central nervous system. Int. Immunol. 2018;30:281–289. doi: 10.1093/intimm/dxy034. [DOI] [PubMed] [Google Scholar]
- 45.Arima Y., Harada M., Kamimura D. Regional neural activation defines a gateway for autoreactive T cells to cross the blood–brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Bartholomäus I., Kawakami N., Odoardi F. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 47.M. A. Erickson, W. A. Banks Neuroimmune Axes of the Blood–Brain Barriers and Blood–Brain Interfaces: Base for Physiological Regulation, Disease States, and Pharmacological Interventions // Pharmacological Reviews. 2018. № 2 (70). P. 278–314. [DOI] [PMC free article] [PubMed]
- 48.McCarthy R. C., Kosman D. J. Activation of C6 glioblastoma cell ceruloplasmin expression by neighboring human brain endothelia-derived interleukins in an in vitro blood–brain barrier model system. Cell Commun. Signal. 2014;12:65. doi: 10.1186/s12964-014-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai Z., Qiao P. F., Wan C. Q. Role of blood–brain barrier in Alzheimer’s disease. J. Alzheimer’s Dis. 2018;63:1223–1234. doi: 10.3233/JAD-180098. [DOI] [PubMed] [Google Scholar]
- 50.Pan Y., Nicolazzo J. A. Impact of aging, Alzheimer’s disease, and Parkinson’s disease on the blood–brain barrier transport of therapeutics. Adv. Drug Deliv. Rev. 2018;135:62–74. doi: 10.1016/j.addr.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Janelidze S., Lindqvist D., Francardo V. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology. 2015;85:1834–1842. doi: 10.1212/WNL.0000000000002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kooij G., Kroon J., Paul D. P-glycoprotein regulates trafficking of CD8(+) T cells to the brain parenchyma. Acta Neuropathol. 2014;127:699–711. doi: 10.1007/s00401-014-1244-8. [DOI] [PubMed] [Google Scholar]
- 53.Macrez R., Stys P. K., Vivien D. Mechanisms of glutamate toxicity in multiple sclerosis: Biomarker and therapeutic opportunities. Lancet Neurol. 2016;15:1089–1102. doi: 10.1016/S1474-4422(16)30165-X. [DOI] [PubMed] [Google Scholar]
- 54.T. P. Buzhdygan, B. J. DeOre, A. Baldwin-Leclair, et al., “The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier,” Neurobiol Dis. 146, 105131 (2020). 10.1016/j.nbd.2020.105131 [DOI] [PMC free article] [PubMed]
- 55.Pellegrini L., Albecka A., Mallery D. L. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood–CSF barrier in human brain organoids. Cell Stem Cell. 2020;27:951–961. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhea E. M., Logsdon A. F., Hansen K. M. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]