Abstract
Despite a growing body of evidence, the distinct contributions of biological sex and the sociocultural dimension of gender to the manifestations and outcomes of ischaemic heart disease and heart failure remain unknown. The intertwining of sex-based differences in genetic and hormonal mechanisms with the complex dimension of gender and its different components and determinants that result in different disease phenotypes in women and men needs to be elucidated. The relative contribution of purely biological factors, such as genes and hormones, to cardiovascular phenotypes and outcomes is not yet fully understood. Increasing awareness of the effects of gender has led to efforts to measure gender in retrospective and prospective clinical studies and the development of gender scores. However, the synergistic or opposing effects of sex and gender on cardiovascular traits and on ischaemic heart disease and heart failure mechanisms have not yet been systematically described. Furthermore, specific considerations of sex-related and gender-related factors in gender dysphoria or in heart–brain interactions and their association with cardiovascular disease are still lacking. In this Review, we summarize contemporary evidence on the distinct effects of sex and gender as well as of their interactions on cardiovascular disease and how they favourably or unfavourably influence the pathogenesis, clinical manifestations and treatment responses in patients with ischaemic heart disease or heart failure.
Subject terms: Cardiovascular biology, Cardiovascular diseases, Public health
In this Review, the authors summarize the evidence on the different effects of sex and gender on the pathogenesis, clinical manifestations and treatment responses of patients with ischaemic heart disease or heart failure. In addition, they highlight unexplored areas of sex-related and gender-related factors in cardiovascular disease such as in individuals with gender dysphoria.
Key points
Sex-related and gender-related factors often have opposite effects on the clinical manifestations and outcomes of cardiovascular disease.
Some sex-related differences in the human cardiovascular system already exist at birth and are due to purely biological mechanisms, that is, genes and sex hormones.
Gender-related variables or scores allow for the characterization of individuals beyond their biological sex, and the effects of gender might even oppose the effects of biological sex on clinical outcomes.
The predominantly male leadership and workforce in clinical cardiology is a disadvantage for women as patients.
Cardiovascular disease risk factors related to female biological sex or feminine gender include, among others, pregnancy complications, breast cancer therapy, autoimmune and rheumatic diseases, depression, and household-related stress.
Despite a more favourable biology, gender-related factors impair outcomes in women with coronary artery disease or heart failure compared with those in men.
Introduction
The term ‘gender medicine’ was first introduced in the late 1990s1. Gender medicine is the study of how diseases differ between men and women in terms of prevention, clinical manifestation, diagnostic and therapeutic approaches, prognosis, psychosocial effects, and interactions with the health-care system. The World Health Organization defines gender medicine as the study of how (sex-based) biological and (gender-based) socioeconomic and cultural differences influence an individual’s health2,3. Biological differences between females and males comprise genetic differences and differences in hormonal status. By contrast, sociocultural gender refers to socially constructed norms that impose and determine roles, relationships and positional power for individuals in a specific society and time4 (Box 1). Biological differences between females and males were the focus of the Organization for the Study of Sex Differences, founded in 2006 in the USA. The International Society for Gender Medicine, founded in 2007 in Berlin, Germany, by Vera Regitz-Zagrosek and colleagues, focused on the integration of both biological sex and sociocultural gender5 on the basis of the concept that sex and gender exist together in an individual and closely interact6. The International Society for Gender Medicine founders used gender medicine as a synonym for sex-sensitive and gender-sensitive medicine comprising both biological and sociocultural aspects. This concept is also shared by the project ‘Gendered Innovations’ implemented by Londa Schiebinger at Stanford University, USA, as well as by the Canadian Office for Women’s Health7–10. Both organizations provided definitions and contributed substantially to a better understanding of the roles of biological sex and sociocultural gender in health and disease. However, despite these efforts and agreements, the knowledge of how biological sex and sociocultural gender modify disease phenotypes is still limited. In particular, we have a very limited understanding of the concept that sex and gender can exert opposite effects on disease outcomes. This opposite effect can be attributed, at least in part, to the complexity and multidimensionality of sex and gender as well as to a lack of specific research on their interaction. Indeed, sex and gender overlap, interact, influence and even oppose each other in a dynamic way (Fig. 1). However, research in the field of gender medicine has been criticized for the lack of clear definitions of sex and gender and the arbitrary use of both terms, thereby limiting their application11. Therefore, in this Review, our aim is to provide clear definitions and examples of sex-related and gender-related mechanisms in health and disease. Moreover, to dissect the mechanisms and causalities of diseases, which is essential to advance research in gender medicine, it is important to analyse the contribution of each of these two dimensions and their components. Thus, we discuss the distinct effects of sex-related and gender-related mechanisms in ischaemic heart disease (IHD) and heart failure (HF) by describing purely sex-dependent mechanisms, the effects of gender and their combined effect on disease management, risk factors and phenotypes. Finally, we highlight currently unexplored areas of sex and gender interaction such as cardiovascular disease (CVD) in individuals with gender dysphoria.
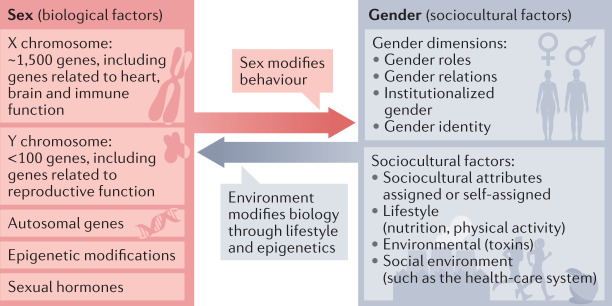
Fig. 1. Concepts of sex and gender in medicine.
Health and disease are influenced by biological sex factors (genes and sex hormones) and sociocultural gender factors that interact with and modify each other.
Box 1 Common terms used in gender medicine.
Biological sex. Either of the two main categories (male and female) into which humans and most other species are classified on the basis of their reproductive functions, sex chromosomes, sex hormones, gonads and genitals.
Intersex. Describes individuals born with biological sex characteristics, including chromosome patterns, gonads or genitals, that do not fit typical binary notions of male or female bodies.
Gender. Refers to the socially constructed roles, behaviours, expressions and identities of girls, boys, women, men and gender-diverse people. Gender is neither binary nor fixed. Gender has four dimensions,
Gender roles: behavioural norms that a society or culture designates as typically masculine or feminine.
Gender identity: a person’s inner sense of self as a woman, man or as a diverse gender.
Gender relations: refer to how we interact with or are treated by people in the world around us on the basis of our ascribed gender.
Institutionalized gender: reflects the distribution of power between genders in the political, educational and social institutions in society.
Cisgender. Refers to people whose gender identity corresponds to the sex they were assigned at birth.
Gender dysphoria. The feeling of discomfort or distress occurring when gender identity differs from biological sex.
Transgender. Describes individuals with a gender identity that does not match the sex they were assigned at birth.
Cross-sex hormone therapy or gender-affirming hormone therapy. Hormone therapy involving the administration of sex hormones and other hormonal medications in transgender or gender non-conforming individuals for the purpose of more closely aligning their secondary sexual characteristics with their gender identity.
Biological sex in CVD
Classification of sex
In most experimental studies, sex is defined by a static and binary approach that is based on genes, hormones and reproductive organs. In cell culture systems, the derivation of cells from a female or male organism or the presence of sex chromosomes is decisive for the definition of sex. In animal models, all three criteria can be used to categorize sex according to the binary system and to exclude animals that do not fit into this system. In humans, however, the binary understanding of biological sex (female or male) is limited because the varying degrees of expression of genes and hormones that cause intersex phenotypes can lead to a disagreement between the different categorizations (that is, between sex chromosomes, hormones and genital organs at birth). This limitation led to the inclusion of a ‘diverse’ category in German birth certificates. The ‘diverse’ category has generally not yet been used in clinical studies because the number of intersex people is low (<1% of the population), the information is frequently difficult to assess and appropriate investigation of these individuals would require specific studies. Intersex must be clearly distinguished from gender dysphoria or transgender (see below).
Therefore, a binary classification of biological sex, independent of gender, is presently maintained in experimental and clinical cardiovascular research even though solid evidence indicates that this conceptualization is imperfect and can only be a proxy for a more nuanced biological reality. However, including a binary definition of sex has clear advantages over sex-blind investigations that still exist12. Future improved approaches should aim to include information on modifiers of sexual status such as sex chromosome variants in cells, the hormonal status of cells, animal models or humans, and environmental conditions13. Efforts should also be made to disentangle the effects of sex and gender, for example, by excluding the effects of gender when sex is analysed (examples are provided below and in Box 2).
Box 2 Examples of biological sex-related differences in cardiovascular disease.
Examples of studies that have investigated differences in cardiovascular disease mechanisms according to biological sex:
Sex-specific effects caused by the X chromosome14
Sex-specific effects caused by 15% of the single nucleotide polymorphisms in autosomes that were assessed in the study15
Oestradiol-induced, sex-specific gene regulation in cardiac biopsy samples from female and male individuals24
Oestradiol-induced, sex-specific collagen regulation in human cardiac fibroblasts in vitro25
Sex-specific gene expression in human endothelial cells26
Sex-specific regulatory networks in the arterial wall27
Presence of sex-specific differences in cardiomyocytes before gonads are developed29
Blood lipid levels in transgender individuals receiving cross-sex hormone treatment23
Sex-specific genetic determinants of cardiovascular phenotypes
Genetic mechanisms are a purely biological disease pathway in CVD, independent of gender. Studies have highlighted the role of the X chromosome in sex-specific effects in human CVD13,14. Furthermore, using data from the UK Biobank, investigators have assessed the degree to which genetic background contributes to sex-specific differences in a large number of traits that are partially associated with the risk of CVD15. Approximately 50% of binary traits had significant differences in genetic heritability between the sexes, indicating the presence of sex-specific underlying genetic variants and genetic architecture. Most sex-specific markers were located on autosomes. This study is an important step towards the inclusion of sex-specific genetic markers in research to understand disease susceptibility in human diseases15,16. In agreement with these results, sex-linked genetic mechanisms that might influence the sex-biased propensity for coronary artery disease (CAD) and atrial fibrillation have been reported17,18. Indeed, a genome-wide association study (GWAS) combined with targeted metabolomics identified a sex-specific association between CAD and the mitochondrial enzyme carbamoyl-phosphate synthase 1, suggesting a potential novel target for sex-specific treatment approaches in CAD17. The mechanisms underlying sex-related differences in autosomal gene expression are not fully understood but might be explained by differences in the levels of transcription factors resulting from sex-specific imprinting. Taken together, these studies provide convincing evidence that genetic mechanisms resulting from sex-related differences in chromosomes lead to sex-specific differences in gene expression and CVD phenotypes.
Sex steroids in CVD
Numerous studies have described the effects of sex hormones on cardiovascular cells, organs and disease phenotypes (reviewed previously19–21). However, the effects of the physical or sociocultural environment, nutrition, or stress (that is, the effects of gender) were not always excluded in these reports. Only a few studies describing the effects of sex steroids, such as oestradiol and testosterone, on gene regulation in adipose tissue, the liver transcriptome and gene networks associated with metabolic, immune and vascular regulation have overcome this limitation by excluding environmental effects22. The affected genes were associated with human disease traits such as CAD, diabetes mellitus and inflammatory bowel disease, suggesting that these disease traits arise as a result of biological mechanisms. Moreover, the association between serum lipid levels and sex steroids was investigated in transgender individuals receiving cross-sex hormone therapy, which allowed the differentiation between sex-related and gender-related mechanisms23. The study found that cisgender women had higher plasma HDL levels and lower plasma VLDL and LDL levels than cisgender men, that these sex-related differences were not evident in prepubertal children, and that a significant increase in HDL levels occurred in trans women (assigned male at birth) receiving cross-sex hormone therapy, indicating that sex hormones, not gender, regulate lipid metabolism in vivo23. These data further support the assumption of hormonal control of cholesterol metabolism, which could contribute to the sex dimorphism observed in CVD risk after menarche23.
Sex-specific interaction of genes and hormones
Increasing evidence suggests that sex hormones have different actions in the cardiovascular cells of females and males. Accordingly, an ex vivo study of myocardial samples obtained from patients with aortic valve stenosis at the time of surgery demonstrated that a number of genes in cardiomyocytes are regulated in a sex-specific manner after treatment with oestradiol24. For example, MYLIP was upregulated by oestradiol treatment in heart samples and cardiomyocytes from male individuals but not in samples from female individuals24. In cardiac fibroblasts, oestradiol treatment for 24 h resulted in significant downregulation in the expression of collagen I and III in female rats, whereas both collagens were upregulated in cardiac fibroblasts from male rats25. Oestradiol-induced, sex-specific collagen regulation was also detected in human cardiac fibroblasts, indicating that this regulation is conserved across species25. In the same study, sex-specific phosphorylation at different serines of the oestrogen receptor-α and oestrogen receptor-β and binding of these receptors to different sites of the promoters of the genes encoding collagen I and collagen III caused a sex-related difference in the physiological response to oestradiol, further supporting the concept that sex hormones exert different physiological effects in the male and female cardiovascular system.
Intrinsic versus acquired sex differences
The distinction between intrinsic and acquired sex differences is essential given that we can assume that intrinsic or innate sex differences (those that are present at birth and are frequently maintained throughout life) are almost exclusively due to biological sex, whereas acquired differences can also arise from environmental conditions such as gender. To disentangle these mechanisms, investigators analysed endothelial cells obtained from opposite-sex twins at birth and from non-related female and male patients at different stages of their life26. The study demonstrated that 14–25% of the endothelial cell transcriptome is sex biased. The researchers identified both innate and acquired sex differences. Furthermore, genes showing an acquired sex-related difference in expression were more likely to be targets of sex steroids26. Annotating both gene sets with data from multiple GWAS revealed that, in endothelial cells, genes with intrinsic sex-specific differences in expression were enriched for CAD-related GWAS hits. Therefore, genetic markers for CAD might have a sex-specific effect in endothelial cells already at birth and might contribute to the development of sex-specific phenotypes. During the lifetime, further differences are acquired due to the influence of sex hormones or environmental modifiers.
A second study from the same research group identified sex-stratified gene regulatory networks and female-specific key driver genes of atherosclerosis in atherosclerotic tissue samples from patients with CAD27. Sex-specific gene regulatory networks of the atherosclerotic arterial wall were generated in 160 age-matched female and male patients. By comparing sex-specific gene regulatory networks, the researchers found that pro-atherosclerotic genes that were more active in females were associated with mesenchymal cells and endothelial cells, whereas pro-atherosclerotic genes that were more active in males were associated with the immune system27. The study underscores the relevance of biological sex differences in endothelial cell physiology as potential targets for the prevention and treatment of CAD.
In myocardial diseases, sex differences have been described at the tissue level20,28. However, in cells obtained from unrelated adults, it is difficult to distinguish genetically determined sex differences that exist at birth from sex differences that develop during the disease course and are the result of hormones or the environment. A study published in 2021 showed that sex-specific differences in cardiomyocytes exist even before gonads are activated in the embryo29. This finding confirms that cardiac sex-related disparities can occur at the earliest stages of heart formation, before gonad formation, and are therefore independent of the influence of sex hormones or the environment29.
Summary
Taken together, there is solid evidence demonstrating that some sex-related differences in the cardiovascular system already exist at birth and are due to purely biological mechanisms, that is, genes and sex steroids (Box 2). Studies investigating biological sex differences in humans must exclude environmental influences during pregnancy. The assessment of opposite-sex twins at birth allows for the control of environmental influences. These studies agree that purely biological mechanisms contribute to sex-related differences in CVD and thereby emphasize the importance of sex-specific experimental research on human diseases.
Sociocultural gender and CVD
The challenge of measuring gender
Gender is a multidimensional and dynamic construct that refers to the sociocultural dimension of being a woman, a man or a gender-diverse person in a given society30,31. Gender comprises four dimensions, including gender roles, gender relations, institutionalized gender and gender identity30,31. Gender is not identical to the sex of the individual but is strongly associated with sex. In health care, gender comprises the interaction of patients with their social environment (for example, health-care staff) and physical environment (such as environmental stimuli and toxins) as well as access to and use of the health-care system. Gender influences health and disease differently than biological sex8,32,33 and acts during the lifetime through several mechanisms34 (Fig. 2). Current efforts aim to make gender ‘measurable’ to include this variable in multivariate statistical models.
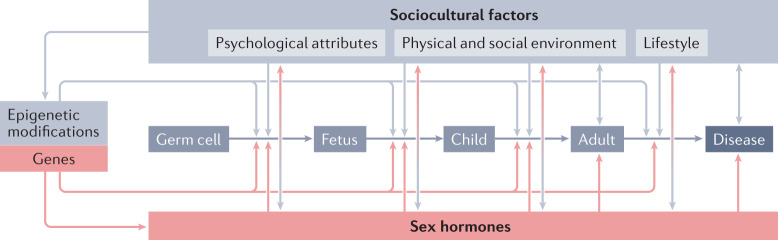
Fig. 2. Interactions between sex and gender in health and disease throughout the life cycle.
Sex chromosomes and sex hormones are active throughout the entire life cycle, starting in germ cells and continuing in the embryo, fetus, child, adult, and aged and diseased individual. Biological factors interact with the sociocultural dimension of gender (including psychological attributes, the physical and social environment, and lifestyle) at all stages of the life course. Adapted with permission from ref.34.
To achieve this goal, several researchers have developed instruments to measure gender32,33,35–39. Gender indices or scores incorporate the multidimensional aspects of gender in a single variable and, therefore, cannot cover the full complexity of gender. Nevertheless, a large number of sociocultural attributes that characterize women and men in Western societies has been integrated into these scores, and several scores have been successfully validated and applied in clinical studies32,37. The first gender score used in clinical studies on CVD incorporated >50 variables representing gender into a single, continuous variable, ranging from 0 (masculine gender) to 100 (feminine gender)32,33. The successful implementation of this gender score in the analysis enabled the researchers to investigate the influence of gender on clinical end points in >1,000 patients with acute coronary syndrome (ACS) in the GENESIS-PRAXY cohort. The study demonstrated a strong and independent association between gender, CVD risk factors and clinical end points33. Gender scores provide the opportunity to include gender as a single, continuous variable in logistic regression models or other statistical approaches and thereby add a further dimension to the currently oversimplified classification of being male, intersex or female in clinical research37,40,41. In addition, the consideration of individual gender-related effects on clinical end points allows the identification of novel CVD risk factors for individualized preventive strategies39. To date, several methods have become available (and coexist) to reliably apply the sex and gender lens to prospective and retrospective research approaches35,39,42–44. There is also a general agreement that male individuals score lower (have more masculine characteristics) on the gender score whereas female individuals score higher (have more feminine characteristics) yet both sexes have masculine and feminine traits (Fig. 3).
Fig. 3. Gender score distribution in females and males in an aged German population.
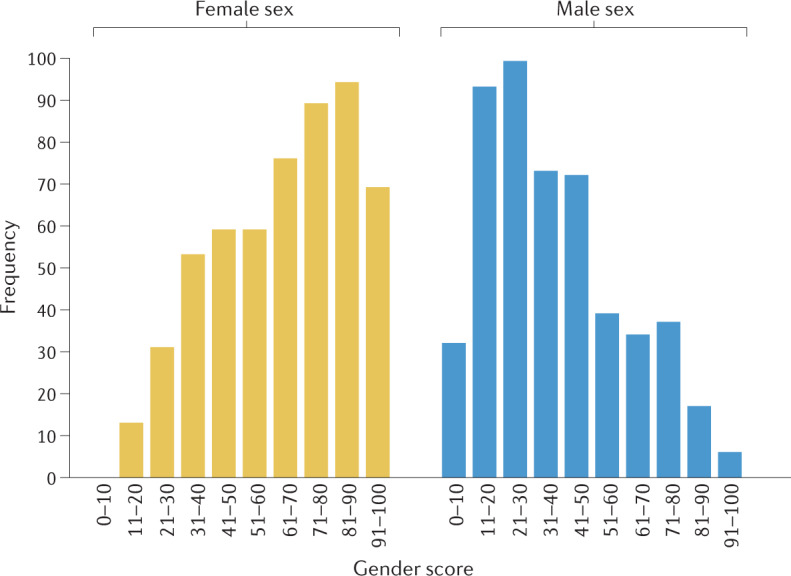
The graph shows the distribution of a retrospective gender score, which included gender-related factors, such as education, income, household activity and stress, according to biological sex in a population of German individuals with a mean age of 75.6 years35. A score of 0 represents individuals who have only masculine characteristics, whereas 100 represents an individual with only feminine characteristics. The data show that male individuals have a lower gender score (have more masculine characteristics) whereas female individuals have higher scores (have more feminine characteristics) yet both have masculine and feminine traits. Adapted with permission from ref.35.
Gender-related versus sex-related effects
The GENESIS-PRAXY study analysed the effect of sex-related and gender-related variables on long-term health-related quality of life (HRQoL) and major adverse events (MACE) among young adults with ACS38. Women had an overall worse HRQoL than men and reported worse physical and mental functions. Gender-related factors, such as femininity score, social support and housework responsibility, had a closer association with HRQoL than sex-based variables38. In an additional study, the same authors demonstrated that female biological sex reduced the risk of MACE by 50%, whereas feminine gender increased the risk fivefold33. The same gender score, adapted for the German societal system, was applied in the German GendAge study in 1,100 elderly individuals45. Differences in some clinical variables, such as grip strength and metabolic syndrome, were mainly due to biological sex, whereas others (systolic blood pressure level, pulse wave velocity, body mass index and depression) could be predominantly attributed to gender. Most importantly, cognitive performance was positively associated with female biological sex but negatively associated with feminine gender: multiple regression models that included both gender and sex as competing co-variates revealed a regression coefficient of +2.47 for biological sex and –3.58 for gender for the dependent variable cognitive performance45. Both studies show that each of these two variables influences clinical end points in a distinct way and can sometimes even have opposite effects on outcomes as was shown for cognitive performance or MACE.
Our group applied a gender score that was based on seven sociocultural items in the Swiss COGEN cohort of 3,000 individuals infected with SARS-CoV-2 (ref.46). We found that biological and gender-related factors contribute differently to the course of coronavirus disease 2019 (COVID-19) and should be included in risk prediction models46. The inclusion of gender-related factors is of particular importance in the era of precision medicine and artificial intelligence because we will not succeed in obtaining equitable risk prediction models for all individuals without the consideration of sex-related and gender-related variables47.
Summary
Taken together, these studies — conducted in different societies — indicate that gender depends on the sociocultural environment of the cohort and on the variables that are available for defining gender in that specific cohort. Solid evidence indicates that gender-related variables or scores allow us to characterize individuals beyond biological sex and might even reveal opposing effects of gender and biological sex on clinical outcomes32,33,37–39,45. Although the discussion about the most optimal method to measure gender is still ongoing, it is evident that gender has to be considered in clinical studies for a better understanding of human disease development.
Combined influence of sex and gender on CVD
Cardiovascular health care
Western health-care systems frequently neglect sociocultural determinants of health (SDOH) in CVD48. Sociocultural factors, such as low socioeconomic status, limited formal education, stress levels, low health literacy and limited access to high-quality health care, some of which are over-represented in women and characteristic of a feminine gender, are major confounders when patient groups with CVD are compared48. Low levels of income and social support as well as a lack of diversity among cardiology clinicians and within clinical trial cohorts also contribute to inequalities in CVD health care49. In our study, old age, low socioeconomic status and poor health literacy correlated with an underestimation of one’s own CVD risk as well as with poor outcomes in women50. These data emphasize the importance of developing gender-specific concepts for the implementation of SDOH into patient management48.
Examples of gender-specific effects on CVD health care include persistent treatment delays observed in women with ACS as well as underdiagnosis and undertreatment of women with CVD51. A nationwide analysis of 450,000 patients with CVD in Switzerland demonstrated that women were less likely to be admitted to an intensive care unit than age-matched men despite being similarly or more severely ill52. Even though these gender-related disparities in CVD health care have been known for decades, they remain essentially unchanged in contemporary medicine and have a disadvantageous effect on women11 (Fig. 4). The development of heart centres specifically for women might help to improve this situation because these institutions might implement gender-sensitive concepts to counteract the female disadvantage in SDOH in cardiovascular medicine53.
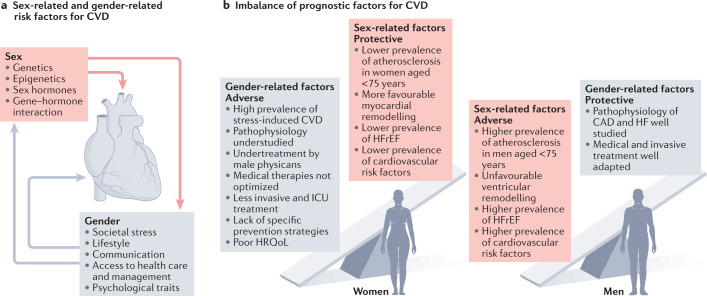
Fig. 4. Sex-related and gender-related disparities in CVD risk and outcomes.
a, Factors contributing to sex-related and gender-related modulation of cardiovascular disease (CVD) risk and outcomes in women and men. b, Factors associated with positive or negative CVD outcomes in women and men. CAD, coronary artery disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HRQoL, health-related quality of life; ICU, intensive care unit.
Patient–physician interaction
Gender-related factors in patient–physician communication might also contribute to differences in CVD outcomes. In a US study, female patients with myocardial infarction (MI) who were treated by a female physician survived better than those treated by a male physician54. However, if male physicians had more female colleagues in their departments, their performance with women as patients improved54. The influence of physician–patient sex concordance on treatment outcomes in women was confirmed in a large, population-based study in Ontario, Canada, published in 2022 (ref.55). Female patients treated by male surgeons more often encountered adverse outcomes than female patients treated by female surgeons. No difference in outcomes according to the sex of the surgeon was found in male patients. The differences were most pronounced in cardiovascular surgery. Therefore, the predominantly male leadership and workforce in cardiology might represent another disadvantage for female patients.
Risk factors and prevention
The bulk of clinical manifestations and events in patients with CAD is due to obstructive CAD, which is mainly caused by atherosclerosis and accounts for 90–95% of clinical ACS cases56. The high rate of obstructive CAD is in part due to a higher accumulation of atherosclerotic risk factors in men than in women57. Moreover, male sex is a stronger cardiovascular risk factor than smoking, diabetes, high plasma lipid levels or high blood pressure58,59. However, a stricter preventative management plan in men than in women since the late 1990s, which includes prescription of indicated medications and risk factor monitoring, has put women at greater risk of CVD60. Women still receive less therapy for their CVD risk factors than men and their cardiovascular protection is incomplete61. According to several large-scale prevention studies, CVD risk factors, in particular dyslipidaemia, were not sufficiently treated in women62–64. More than a decade after the publication of those studies, inferior control of LDL-cholesterol levels in women is still the primary sex-related difference in modifiable CVD risk, as documented in a large-scale, cross-sectional, primary care study published in 2021 (ref.65). Undertreatment of women has also been described in diabetes, in particular for CVD prevention66,67. Therefore, men seem to have a stronger biological risk of CVD but women still receive less therapy for their CVD risk factors than men, and CVD prevention in women is incomplete (Fig. 4).
Several female-specific CVD risk factors have been identified during the past 20 years; however, although some of these variables have been mentioned in recent guidelines68, these risk factors have yet to be implemented into the most frequently applied CVD risk calculators69. Female-specific and women-specific CVD risk factors include pregnancy complications, breast cancer therapy, autoimmune and rheumatic diseases, depression, and household-related stress. The relationship between reproductive factors and incident CVD has been demonstrated in several studies, including in a 2018 analysis of the UK Biobank showing that early menarche, early menopause, earlier age at first pregnancy, and a history of miscarriage, stillbirth or hysterectomy were each independently associated with a higher risk of CVD later in life70. Finally, the increased relative risk of CVD in women with diabetes is not adequately addressed in the most recent guidelines71.
Ischaemic heart disease
The particularities of IHD and HF in women were described in 2021 by The Lancet Women and Cardiovascular Disease Commission11 and, therefore, will not be reviewed here. This section focuses instead on the disentanglement of sex-related and gender-related mechanisms in these conditions. The observation that IHD — and its best-known manifestation, CAD — occurs 6–8 years earlier in men than in women might point to biological factors driving these differences such as earlier atherosclerosis in men owing to differences in lipid metabolism or immune responses72. However, sociocultural differences between men and women might also influence the epidemiology and manifestation of CAD, given that lifestyle factors and mental stress are well-known modifiers of cardiovascular risk73. Undertreatment and higher mortality in women with ST-segment elevation MI, non-ST-segment elevation MI or unstable angina have repeatedly been described during the past 20 years72. Despite increasing awareness of gender inequalities in the management of patients with ACS in Europe, a study published in 2022 using country-level data from four European countries confirmed that women still receive guideline-recommended therapies less often than men and have a higher mortality that persists after multivariate adjustment for known cardiovascular risk factors, including age74. Outcomes, including mortality, are worst among women aged <50 years, who are less likely to have ACS than men or older women72,75. Although these disparities were first described 20 years ago across different geographical regions, they have generally remained unchanged over time76,77. We can argue that the lack of knowledge on female-specific pathophysiology might contribute to this lack of reduction in gender-related disparities given that women more frequently have ACS from non-atherosclerotic causes, which is less well studied78.
In contrast to obstructive CAD, IHD comprises all disease manifestations arising from myocardial ischaemia both in women and men, such as microvascular dysfunction (a supply–demand mismatch of the myocardium) or ischaemia with non-obstructive coronary arteries (INOCA) and MI with non-obstructive coronary arteries (MINOCA) resulting from non-obstructive CAD, which has a higher prevalence in women than in men73. Patients with INOCA report a poor quality of life and recurrent symptoms of angina, require frequent hospital admission and diagnostic interventions, and are a major burden to the health-care system73,79,80. Although the pathophysiology of INOCA is not fully understood, this condition is associated with endothelial dysfunction as reflected by impaired vasodilatation in response to adenosine, acetylcholine and nitroglycerin affecting mainly the microcirculation and, therefore, has a biological basis81. In women with MINOCA, multimodality imaging with coronary optical coherence tomography and cardiac MRI identified potential mechanisms of MINOCA in 84.5% of patients, of which 75.5% had myocardial ischaemia and 24.5% had no ischaemia, pointing equally to a biological basis for this condition82. Of note, perceived stress might also contribute to INOCA development81. Indeed, mental stress is a greater risk factor for CVD in women than in men, particularly in younger women, which most probably relates to sociocultural triggers83,84. In summary, the pathophysiology, presentation and clinical manifestation of IHD differ between women and men.
As discussed above, gender seems to be more important than sex in predicting long-term HRQoL or MACE after an ACS33. Interestingly, sex and gender drive the results in opposite directions, with female biological sex showing a trend towards improved survival whereas feminine gender has been associated with adverse outcomes.
In addition, women continue to be at disadvantage in drug development because they remain severely under-represented in CVD clinical trials. For example, in studies on repurposing colchicine as a treatment for CAD only <20% of participants were women and the authors did not discuss the finding that risk reduction was 33% in men compared with 1% in women85. Women also have a disadvantage in cardiac surgery outcomes. A study published in 2004 showed that women with CAD often have worse outcomes after coronary artery bypass graft surgery than men, with biological and sociocultural factors identified as underlying causes86. Age, physical function and postoperative complications were key mediators of the excess mortality of women after coronary artery bypass graft surgery87. The investigators concluded that self-assessed physical function should be more seriously considered in preoperative risk assessment, particularly in women87. Notably, as previously described, studies have linked the poorer outcome in women after cardiac surgery with sex-specific patient–physician interactions55.
In conclusion, although women develop obstructive CAD later in life than men, the underestimation of women-specific IHD pathophysiology, including biological and sociocultural components, the lack of early recognition and the lack of women-specific treatments increase the risk of CVD in women (Fig. 4). The higher mortality in young women with CAD than in young men despite their lower burden of atherosclerotic risk factors reflects a disadvantage of women based on behavioural, treatment or societal aspects regardless of the advantage of biological female sex in CVD risk.
Heart failure
HF is classified into HF with reduced (HFrEF), mid-range (HFmrEF) or preserved (HFpEF) left ventricular ejection fraction (LVEF)57, and the sex-specific distributions of these phenotypes have been previously reviewed elsewhere88.
Female individuals without heart disease have a higher LVEF than male individuals, with the lower cut-off value of normal being 61% in females and 55% in males89,90. Given that current 2021 ESC guidelines do not account for these sex-related differences, women with an LVEF of 55–61% might be miscategorized to the HFpEF phenotype in clinical studies although they should instead be classified into the HFrEF or HFmrEF groups91. Hearts from female patients with HFpEF are smaller and stiffer than hearts from male patients with HFpEF and more frequently develop concentric remodelling92. Instead, women with HFrEF have a more favourable adaption of the myocardium to stress conditions (with less ventricular dilatation, less downregulation of mitochondrially encoded genes and less fibrosis) and have a lower risk of ventricular tachycardia, sudden cardiac death and atrial fibrillation compared with men88,93. Therefore, sex-related factors in the pathophysiology of HF seem to be more favourable to females. However, women with HF were more likely to have lower HRQoL, more symptoms and depression than men48. The pathophysiological basis of this gender-related difference is understudied. Nevertheless, clinical outcomes in women with HFpEF or HFrEF have always been better compared with those of men, even before the most recent neurohormonal modulators were introduced into HF therapy94.
Trends favouring a better outcome in women with HF have been observed with several neurohormonal modulators. The PARAGON-HF investigators demonstrated a positive effect on end points in women with HFpEF treated with sacubitril–valsartan, whereas the compound did not improve outcomes in men90,95. In addition, several retrospective studies investigating the effect of mineralocorticoid receptor antagonists on HF end points reported trends towards a better efficacy of this drug in women than in men, resulting in better outcomes in women96. Unfortunately, despite these observations, mechanistic studies explaining these sex-related and gender-related differences in HF treatment are lacking. Notably, although the sodium–glucose co-transporter 2 inhibitor empagliflozin has been shown to reduce the risk of cardiovascular death or hospitalization in patients with HFpEF, women, who predominantly have HFmrEF or HFpEF, are still left with less treatment options than men. In addition, increasing evidence suggests that women with HFrEF are more likely to experience adverse effects from HF drugs (such as digoxin) than men and their risk of being overdosed with the guideline-recommended drugs angiotensin-converting enzyme inhibitors and β-blockers is higher than in men97,98. The latter clearly indicates that pharmacological treatment strategies in HF are less adapted to women than to men99,100.
With regard to non-pharmacological HF treatment, women respond well to cardiac resynchronization therapy, apparently better than men, but less often receive cardiac resynchronization therapy devices101,102. In addition, although the risk of a life-threatening arrhythmia is lower in women than in men, women might still be undertreated with implantable cardioverter–defibrillators because recommendations for implantation of these devices rely on data mainly derived from male populations61,103. Similarly, women with end-stage HF undergo heart transplantation less often than men although they experience similar benefits from heart transplantation. Indeed, data from one of the largest heart transplantation centres in Europe indicate that only 15.5% of 698 patients with dilated cardiomyopathy undergoing heart transplantation between 1995 and 2008 were women104. In this study, women were more frequently classified as having NYHA class III–IV HF and had a lower exercise tolerance, worse pulmonary function, and poorer kidney function than men but less frequently had diabetes. Therefore, women were referred with more severe HF but fewer relative contraindications for heart transplantation such as diabetes. The option of heart transplantation was less intensely considered in women, particularly in those with comorbidities104. A 2022 report from a heart transplantation centre in the UK found a persisting gender bias regarding the referral for heart transplantation and left ventricular assist device implantation: women accounted for only 32% of total referrals and were less likely to receive a left ventricular assist device (13%)105.
In summary, despite a more favourable biology, gender-related and treatment-related factors impair outcomes in women with HF compared with men (Fig. 4). Only a few mechanistic studies have been performed for HF classes that predominantly affect women such as HFpEF. Anatomical and pathophysiological differences between females and males and women and men, such as different normal ranges of LVEF and differences in neurohormonal activation or in symptom profiles, are insufficiently considered in contemporary research and current guidelines. Consequently, treatment strategies in HF are less well adapted to women and a persisting referral bias exists against women with regard to advanced HF treatments.
Heart–brain interactions
A growing body of evidence suggests that (patho)physiological interactions between the heart and the brain have important roles in cardiovascular and neurovascular conditions and that these interactions are modified by sex and gender106–111. Both organs share common risk factors, such as hypertension, diabetes, smoking or dyslipidaemia, and both are affected by systemic inflammation, atherosclerosis and dysfunction of the neuroendocrine system106. One of the most obvious examples of sex-specific and gender-specific heart–brain interactions in CVD is Takotsubo syndrome. Gender-specific factors predominantly affect postmenopausal women in whom a stressful life event translates into temporary dysfunction of the left ventricle112. Current evidence suggests that sex-related and gender-related differences in emotional stress perception and processing via heart–brain interactions might contribute to the higher prevalence of Takotsubo syndrome in women113–116. Indeed, women perceive greater and prolonged episodes of mental stress during their lifespan than men117. The limbic system118, in particular the amygdala, which regulates peripheral sympathetic activity in response to emotions and fear, has been suggested to have a key role in Takotsubo syndrome110,119. Although mechanistic data are sparse, several reports conclude that the link between metabolic activity in the amygdala and abnormal cardiac function is particularly pronounced in women107,120–122. In addition, sympathetic nervous system activation, a potential mediator of heart–brain interactions, shows a clear sex dimorphism because sympathetic activity is attenuated by oestrogen123 and is therefore higher in men under physiological conditions124, increases with age in women113 and is an independent predictor of MACE in women but not in men125,126. These sex differences in autonomic function seem to translate into differential treatment responses to β-blockers in males and females97,127,128. In addition, increasing evidence suggests that the immune system and inflammatory responses might be a potential pathway mediating heart–brain interactions due to their capacity to alter tissue perfusion and neurohumoral activation120. Clinical data point to substantial sex-specific differences in inflammatory and innate immune responses, with females showing higher baseline levels of circulating inflammatory markers129 and more pronounced production of pro-inflammatory cytokines in response to different myocardial injuries than males120,130–132. Given that both sympathetic activity and inflammation are pharmacological targets, further investigation of the molecular mechanisms that regulate the heart–brain axis is needed. Investigating the heart–brain axis is crucial given that sex-specific differences in heart–brain interactions do not only exist in Takotsubo syndrome but also in CAD, with women being at a greater risk of mental stress-induced endothelial dysfunction and myocardial ischaemia than men133,134, and in HF, in which sex steroids, excessive stimulation of the sympathetic nervous system and neurohumoral activation drive both disease development and sex-related differences90,95,97,135,136. Notably, sex and gender differences in heart–brain interactions have also been reported in primary brain diseases, such as stroke, dementia or depression; however, this topic is beyond the scope of this article and has been reviewed elsewhere106. Despite many efforts to better understand the influence of sex and gender on heart–brain interactions, most previous work has focused on biological mechanisms only. Therefore, future research will have to assess the effect of gender-specific behaviour and cardiovascular health inequalities between men and women on the brain’s stress network and its cardiovascular consequences.
Gender dysphoria and CVD
Transgender people and gender-diverse minorities (such as non-binary gender identity or genderqueer individuals) now comprise an estimated 0.3–0.5% (25 million) of the global population137. Transgender is an umbrella term that describes individuals with a gender identity that does not match the sex they were assigned at birth. Gender dysphoria is defined as the feeling of discomfort or distress occurring when gender identity differs from biological sex.
Transgender and non-binary individuals undergo discrimination, experience psychological distress and suffer adverse childhood experiences — these stressors are associated with increased odds of CVD. Furthermore, transgender individuals have specific health-care needs related to gender-affirmative treatments, which include social, psychological, behavioural or medical (hormonal treatment or surgery) interventions designed to support an individual’s gender identity. Furthermore, despite increasing awareness of their specific health-care needs, transgender individuals remain a marginalized group and face unique intrapersonal (such as self-stigma), interpersonal (for example, discrimination, transphobia and bias-motivated violence) and structural (for example, laws that codify discrimination) stressors that are associated with reduced access to health care and an increased risk of mental health disorders and CVD across the lifespan138–141. However, despite their higher risk of CVD, transgender individuals remain an under-served group in clinical cardiovascular research. Indeed, common conditions, such as diabetes, metabolic syndrome, malignancies and CVD, are the least researched aspects of the global disease burden in transgender individuals142. In addition, although growing evidence emphasizes the importance of gender-affirming hormone treatments in transgender individuals to improve gender dysphoria and promote well-being, knowledge on the cardiovascular effects of these treatments is scarce. Most of the available literature consists of retrospective cohort studies that are limited by insufficient follow-up time, a focus on younger age groups, lack of appropriate control populations, cross-sectional designs and a small sample size143. Accordingly, although most studies describe a worsening cardiovascular risk profile in transgender individuals receiving cross-sex hormone therapy, inconsistent data in transgender men have been reported on whether these risk alterations are associated with increased cardiovascular morbidity and mortality144–146. In transgender women receiving ethinyloestradiol and cyproterone acetate treatment, an increased risk of thromboembolic events compared with cisgender women has consistently been observed across studies147–149. Likewise, several epidemiological studies suggest that the use of oestrogens in transgender women is associated with an increased risk of MI and ischaemic stroke compared with cisgender women150. However, large-scale, prospective studies are required to confirm these findings and elucidate the underlying mechanisms. Overall, a multifaceted approach considering both gender-related (social factors such as general life stressors and gender minority stressors) and sex-related (clinical biological factors such as effects of cross-sex hormone treatment) risk determinants of CVD is needed to better understand the specific risk exposures of transgender and gender-diverse individuals. This knowledge will advance our understanding of the role of sex and gender in CVD and will be critical to reducing cardiovascular health disparities and identifying targets for behavioural and medical interventions in this population.
Conclusions
Clear evidence indicates that sex-related and gender-related factors interact in generating differences in CVD outcomes in women and men, and might even have opposite effects on clinical manifestations and outcomes. The influence of biological sex on CVD manifestations frequently favours females, such as the relative protection from obstructive CAD in premenopausal females or the more favourable left ventricular remodelling observed in females with HFrEF compared with males. Conversely, gender-related factors, including a higher prevalence of anxiety in women with CVD, a stronger association between mental stress and disease manifestations in women, poorer communication with health-care representatives, a lack of consideration of sex-specific and gender-specific pathophysiology in medical research, and the underrepresentation of the female population in drug development, more adversely affect women than men. Therefore, studies that investigate the influence of biological and sociocultural differences between women and men on CVD outcomes, and which also consider that these two variables might drive outcomes in opposite directions, are urgently needed. A better understanding of these factors will subsequently result in optimized treatments and improved health care for all patients.
Acknowledgements
The authors thank Susan Bengs (University Hospital Zurich, Zurich, Switzerland) for preparing the illustrations for initial submission and Nicola Lott (University Hospital Zurich, Zurich, Switzerland) for proofreading. V.R.-Z. has received research funding from GE Academy H2020-SwafS-2018-2020/EU: 824585; Gendage (01GL1716A), Bundesministerium für Bildung und Forschung (BMBF), Germany; and Gender/Sex in Multiple Sclerosis (ZMV I 1 - 25 20 FSB 431), Bundesministerium für Gesundheit (BMG), Germany. C.G. has received research funding from the Swiss National Science Foundation (SNSF); the Olga Mayenfisch Foundation, Switzerland; the OPO Foundation, Switzerland; the Novartis Foundation, Switzerland; the Swiss Heart Foundation; the Olten Heart Foundation, Switzerland, the Helmut Horten Foundation, Switzerland; the EMDO Foundation, Switzerland; the Iten-Kohaut Foundation, Switzerland, the University Zurich (UZH) Foundation, Switzerland; the University Hospital Zurich (USZ) Foundation, Switzerland; and the LOOP Zurich, Switzerland.
Author contributions
V.R.-Z. researched data for the article. Both authors wrote the article and reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Cardiology thanks Sanne Peters, Valeria Raparelli and Eva Swahn for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Gendered Innovations: https://genderedinnovations.stanford.edu
International Society for Gender Medicine: http://www.isogem.eu/
Organization for the Study of Sex Differences: https://www.ossdweb.org/
References
- 1.Legato MJ. Gender-specific physiology: how real is it? How important is it? Int. J. Fertil. Womens Med. 1997;42:19–29. [PubMed] [Google Scholar]
- 2.World Health Organization. Gender and Healthhttps://www.who.int/health-topics/gendertab=tab_1 (2022).
- 3.Baggio G, Corsini A, Floreani A, Giannini S, Zagonel V. Gender medicine: a task for the third millennium. Clin. Chem. Lab. Med. 2013;51:713–727. doi: 10.1515/cclm-2012-0849. [DOI] [PubMed] [Google Scholar]
- 4.Shannon G, et al. Gender equality in science, medicine, and global health: where are we at and why does it matter. Lancet. 2019;393:560–569. doi: 10.1016/S0140-6736(18)33135-0. [DOI] [PubMed] [Google Scholar]
- 5.International Society of Gender Medicine. Aims of the IGMhttp://www.isogem.eu/IGM/Aims-of-IGM/ (2022).
- 6.Krieger N. Genders, sexes, and health: what are the connections — and why does it matter? Int. J. Epidemiol. 2003;32:652–657. doi: 10.1093/ije/dyg156. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Institutes of Health Research. Institute of Gender and Healthhttps://cihr-irsc.gc.ca/e/8673.html (2022).
- 8.Day S, Mason R, Tannenbaum C, Rochon PA. Essential metrics for assessing sex & gender integration in health research proposals involving human participants. PLoS One. 2017;12:e0182812. doi: 10.1371/journal.pone.0182812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiebinger L, Klinge I. Gendered innovation in health and medicine. Adv. Exp. Med. Biol. 2018;1065:643–654. doi: 10.1007/978-3-319-77932-4_39. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum C, Ellis RP, Eyssel F, Zou J, Schiebinger L. Sex and gender analysis improves science and engineering. Nature. 2019;575:137–146. doi: 10.1038/s41586-019-1657-6. [DOI] [PubMed] [Google Scholar]
- 11.Vogel B, et al. The Lancet Women and Cardiovascular Disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. doi: 10.1016/S0140-6736(21)00684-X. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez FD, et al. Sex bias is increasingly prevalent in preclinical cardiovascular research: implications for translational medicine and health equity for women: a systematic assessment of leading cardiovascular journals over a 10-year period. Circulation. 2017;135:625–626. doi: 10.1161/CIRCULATIONAHA.116.026668. [DOI] [PubMed] [Google Scholar]
- 13.Ventura-Clapier R, et al. Sex in basic research: concepts in the cardiovascular field. Cardiovasc. Res. 2017;113:711–724. doi: 10.1093/cvr/cvx066. [DOI] [PubMed] [Google Scholar]
- 14.Konig IR, Loley C, Erdmann J, Ziegler A. How to include chromosome X in your genome-wide association study. Genet. Epidemiol. 2014;38:97–103. doi: 10.1002/gepi.21782. [DOI] [PubMed] [Google Scholar]
- 15.Bernabeu E, et al. Sex differences in genetic architecture in the UK Biobank. Nat. Genet. 2021;53:1283–1289. doi: 10.1038/s41588-021-00912-0. [DOI] [PubMed] [Google Scholar]
- 16.Barc J, Erdmann J. Sex matters? Sex matters! Cardiovasc. Res. 2022;118:e1–e3. doi: 10.1093/cvr/cvab356. [DOI] [PubMed] [Google Scholar]
- 17.Hartiala JA, et al. Genome-wide association study and targeted metabolomics identifies sex-specific association of CPS1 with coronary artery disease. Nat. Commun. 2016;7:10558. doi: 10.1038/ncomms10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wren G, Davies W. Sex-linked genetic mechanisms and atrial fibrillation risk. Eur. J. Med. Genet. 2022;65:104459. doi: 10.1016/j.ejmg.2022.104459. [DOI] [PubMed] [Google Scholar]
- 19.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 20.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2017;97:1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 21.Arosio B, et al. Sex differences in cardiovascular diseases: a matter of estrogens, ceramides, and sphingosine 1-phosphate. Int. J. Mol. Sci. 2022 doi: 10.3390/ijms23074009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blencowe M, et al. Relative contributions of sex hormones, sex chromosomes, and gonads to sex differences in tissue gene regulation. Genome Res. 2022 doi: 10.1101/gr.275965.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson GA, et al. Sex hormones drive changes in lipoprotein metabolism. iScience. 2021;24:103257. doi: 10.1016/j.isci.2021.103257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kararigas G, et al. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J. Am. Coll. Cardiol. 2012;59:410–417. doi: 10.1016/j.jacc.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Dworatzek E, et al. Sex-specific regulation of collagen I and III expression by 17β-estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovasc. Res. 2019;115:315–327. doi: 10.1093/cvr/cvy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartman RJG, et al. Intrinsic transcriptomic sex differences in human endothelial cells at birth and in adults are associated with coronary artery disease targets. Sci. Rep. 2020;10:12367. doi: 10.1038/s41598-020-69451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman RJG, et al. Sex-stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation. 2021;143:713–726. doi: 10.1161/CIRCULATIONAHA.120.051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kararigas G, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur. J. Heart Fail. 2014;16:1160–1167. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 29.Shi W, et al. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Dev. Cell. 2021;56:3019–3034 e3017. doi: 10.1016/j.devcel.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips SP. Defining and measuring gender: a social determinant of health whose time has come. Int. J. Equity Health. 2005;4:11. doi: 10.1186/1475-9276-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson JL, Greaves L, Repta R. Better science with sex and gender: facilitating the use of a sex and gender-based analysis in health research. Int. J. Equity Health. 2009;8:14. doi: 10.1186/1475-9276-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelletier R, Ditto B, Pilote L. A composite measure of gender and its association with risk factors in patients with premature acute coronary syndrome. Psychosom. Med. 2015;77:517–526. doi: 10.1097/PSY.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier R, et al. Sex versus gender-related characteristics: which predicts outcome after acute coronary syndrome in the young? J. Am. Coll. Cardiol. 2016;67:127–135. doi: 10.1016/j.jacc.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 34.Regitz-Zagrosek V. Sex and gender differences in health. Science & society series on sex and science. EMBO Rep. 2012;13:596–603. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauman AT, et al. Gender score development in the Berlin Aging Study II: a retrospective approach. Biol. Sex Differ. 2021;12:15. doi: 10.1186/s13293-020-00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PM, Koehoorn M. Measuring gender when you don’t have a gender measure: constructing a gender index using survey data. Int. J. Equity Health. 2016;15:82. doi: 10.1186/s12939-016-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacasse A, et al. Conducting gender-based analysis of existing databases when self-reported gender data are unavailable: the GENDER Index in a working population. Can. J. Public Health. 2020;111:155–168. doi: 10.17269/s41997-019-00277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung Yinko SS, et al. Health-related quality of life in premature acute coronary syndrome: does patient sex or gender really matter? J. Am. Heart Assoc. 2014 doi: 10.1161/JAHA.114.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen MW, et al. Gender-related variables for health research. Biol. Sex Differ. 2021;12:23. doi: 10.1186/s13293-021-00366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song MK, Lin FC, Ward SE, Fine JP. Composite variables: when and how. Nurs. Res. 2013;62:45–49. doi: 10.1097/NNR.0b013e3182741948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin. Pharmacol. Toxicol. 2006;98:253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azizi Z, et al. Sex, gender, and cardiovascular health in Canadian and Austrian populations. Can. J. Cardiol. 2021;37:1240–1247. doi: 10.1016/j.cjca.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Raparelli V, et al. Identification and inclusion of gender factors in retrospective cohort studies: the GOING-FWD framework. BMJ Glob. Health. 2021 doi: 10.1136/bmjgh-2021-005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tadiri CP, et al. Methods for prospectively incorporating gender into health sciences research. J. Clin. Epidemiol. 2021;129:191–197. doi: 10.1016/j.jclinepi.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Pohrt A, et al. Differentiating sex and gender among older men and women. Psychosom. Med. 2022;84:339–346. doi: 10.1097/PSY.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 46.Gebhard CE, et al. Sex versus gender-related characteristics: which predicts clinical outcomes of acute COVID-19? Intensive Care Med. 2022 doi: 10.1007/s00134-022-06836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cirillo D, et al. Sex and gender differences and biases in artificial intelligence for biomedicine and healthcare. NPJ Digit. Med. 2020;3:81. doi: 10.1038/s41746-020-0288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White-Williams C, et al. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141:e841–e863. doi: 10.1161/CIR.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 49.Lindley KJ, et al. Socioeconomic determinants of health and cardiovascular outcomes in women: JACC review topic of the week. J. Am. Coll. Cardiol. 2021;78:1919–1929. doi: 10.1016/j.jacc.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Oertelt-Prigione S, et al. Cardiovascular risk factor distribution and subjective risk estimation in urban women–the BEFRI study: a randomized cross-sectional study. BMC Med. 2015;13:52. doi: 10.1186/s12916-015-0304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarma AA, et al. Outcomes of women compared with men after non-ST-segment elevation acute coronary syndromes. J. Am. Coll. Cardiol. 2019;74:3013–3022. doi: 10.1016/j.jacc.2019.09.065. [DOI] [PubMed] [Google Scholar]
- 52.Todorov A, et al. Gender differences in the provision of intensive care: a Bayesian approach. Intensive Care Med. 2021;47:577–587. doi: 10.1007/s00134-021-06393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khandelwal A, et al. Managing ischemic heart disease in women: role of a women’s heart center. Curr. Atheroscler. Rep. 2021;23:56. doi: 10.1007/s11883-021-00956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenwood BN, Carnahan S, Huang L. Patient-physician gender concordance and increased mortality among female heart attack patients. Proc. Natl Acad. Sci. USA. 2018;115:8569–8574. doi: 10.1073/pnas.1800097115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallis CJD, et al. Association of surgeon-patient sex concordance with postoperative outcomes. JAMA Surg. 2022;157:146–156. doi: 10.1001/jamasurg.2021.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 57.McDonagh TA, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 58.Walli-Attaei M, et al. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;396:97–109. doi: 10.1016/S0140-6736(20)30543-2. [DOI] [PubMed] [Google Scholar]
- 59.Desai R, et al. Nationwide trends in prevalent cardiovascular risk factors and diseases in young adults: differences by sex and race and in-hospital outcomes. South. Med. J. 2020;113:311–319. doi: 10.14423/SMJ.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 60.Lee CMY, et al. Sex disparities in the management of coronary heart disease in general practices in Australia. Heart. 2019;105:1898–1904. doi: 10.1136/heartjnl-2019-315134. [DOI] [PubMed] [Google Scholar]
- 61.Narasimha D, Curtis AB. Sex differences in utilisation and response to implantable device therapy. Arrhythm. Electrophysiol. Rev. 2015;4:129–135. doi: 10.15420/AER.2015.04.02.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mosca L, Navar AM, Kass Wenger N. Reducing cardiovascular disease risk in women beyond statin therapy: new insights 2020. J. Womens Health. 2020 doi: 10.1089/jwh.2019.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cushman M, et al. Ten-year differences in women’s awareness related to coronary heart disease: results of the 2019 American Heart Association National Survey: a special report from the American Heart Association. Circulation. 2021;143:e239–e248. doi: 10.1161/CIR.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rachamin Y, Grischott T, Rosemann T, Meyer MR. Inferior control of low-density lipoprotein cholesterol in women is the primary sex difference in modifiable cardiovascular risk: a large-scale, cross-sectional study in primary care. Atherosclerosis. 2021;324:141–147. doi: 10.1016/j.atherosclerosis.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 66.Harreiter J, Kautzky-Willer A. Sex and gender differences in prevention of type 2 diabetes. Front. Endocrinol. 2018;9:220. doi: 10.3389/fendo.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kautzky-Willer A, Harreiter J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res. Clin. Pract. 2017;131:230–241. doi: 10.1016/j.diabres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Visseren FLJ, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 69.SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021;42:2439–2454. doi: 10.1093/eurheartj/ehab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104:1069–1075. doi: 10.1136/heartjnl-2017-312289. [DOI] [PubMed] [Google Scholar]
- 71.Xu G, et al. Risk of all-cause and CHD mortality in women versus men with type 2 diabetes: a systematic review and meta-analysis. Eur. J. Endocrinol. 2019;180:243–255. doi: 10.1530/EJE-18-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haider A, et al. Sex and gender in cardiovascular medicine: presentation and outcomes of acute coronary syndrome. Eur. Heart J. 2020;41:1328–1336. doi: 10.1093/eurheartj/ehz898. [DOI] [PubMed] [Google Scholar]
- 73.Merz NB, et al. Diagnostic, prognostic, and cost assessment of coronary artery disease in women. Am. J. Manag. Care. 2001;7:959–965. [PubMed] [Google Scholar]
- 74.Hellgren T, et al. Sex-related differences in the management and outcomes of patients hospitalized with ST-elevation myocardial infarction: a comparison within four European myocardial infarction registries. Eur. Heart J. Open. 2022;2:oeac042. doi: 10.1093/ehjopen/oeac042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosmidou I, et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: results from an individual patient-level pooled analysis of 10 randomized trials. Eur. Heart J. 2017;38:1656–1663. doi: 10.1093/eurheartj/ehx159. [DOI] [PubMed] [Google Scholar]
- 76.Vaccarino V, Abramson JL, Veledar E, Weintraub WS. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105:1176–1181. doi: 10.1161/hc1002.105133. [DOI] [PubMed] [Google Scholar]
- 77.Humphries KH, et al. Sex differences in cardiovascular disease — impact on care and outcomes. Front. Neuroendocrinol. 2017;46:46–70. doi: 10.1016/j.yfrne.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehilli J, Presbitero P. Coronary artery disease and acute coronary syndrome in women. Heart. 2020;106:487–492. doi: 10.1136/heartjnl-2019-315555. [DOI] [PubMed] [Google Scholar]
- 79.Jespersen L, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 80.Mehta PK, et al. Ischemia and no obstructive coronary arteries in patients with stable ischemic heart disease. Int. J. Cardiol. 2022;348:1–8. doi: 10.1016/j.ijcard.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tjoe B, et al. Coronary microvascular dysfunction: considerations for diagnosis and treatment. Cleve. Clin. J. Med. 2021;88:561–571. doi: 10.3949/ccjm.88a.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reynolds HR, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143:624–640. doi: 10.1161/CIRCULATIONAHA.120.052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaccarino V, et al. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J. Am. Heart Assoc. 2016 doi: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaccarino V, et al. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation. 2018;137:794–805. doi: 10.1161/CIRCULATIONAHA.117.030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gebhard C, Regitz-Zagrosek V. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 2021;384:776–777. doi: 10.1056/NEJMc2034992. [DOI] [PubMed] [Google Scholar]
- 86.Regitz-Zagrosek V, et al. Gender as a risk factor in young, not in old, women undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol. 2004;44:2413–2414. doi: 10.1016/j.jacc.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 87.Lehmkuhl E, et al. Gender-specific predictors of early mortality after coronary artery bypass graft surgery. Clin. Res. Cardiol. 2012;101:745–751. doi: 10.1007/s00392-012-0454-0. [DOI] [PubMed] [Google Scholar]
- 88.Lam CSP, et al. Sex differences in heart failure. Eur. Heart J. 2019;40:3859–3868c. doi: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 89.Chung AK, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113:1597–1604. doi: 10.1161/CIRCULATIONAHA.105.574400. [DOI] [PubMed] [Google Scholar]
- 90.Dewan P, et al. Interactions between left ventricular ejection fraction, sex and effect of neurohumoral modulators in heart failure. Eur. J. Heart Fail. 2020;22:898–901. doi: 10.1002/ejhf.1776. [DOI] [PubMed] [Google Scholar]
- 91.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205. doi: 10.1161/CIRCULATIONAHA.118.034271. [DOI] [PubMed] [Google Scholar]
- 92.Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog. Cardiovasc. Dis. 2007;49:241–251. doi: 10.1016/j.pcad.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat. Med. 2019;25:1657–1666. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- 94.Martinez-Selles M, et al. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur. J. Heart Fail. 2012;14:473–479. doi: 10.1093/eurjhf/hfs026. [DOI] [PubMed] [Google Scholar]
- 95.McMurray JJV, et al. Effects of Sacubitril-Valsartan versus Valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON-HF. Circulation. 2020;141:338–351. doi: 10.1161/CIRCULATIONAHA.119.044491. [DOI] [PubMed] [Google Scholar]
- 96.Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail. 2019;7:228–238. doi: 10.1016/j.jchf.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Santema BT, et al. Identifying optimal doses of heart failure medications in men compared with women: a prospective, observational, cohort study. Lancet. 2019;394:1254–1263. doi: 10.1016/S0140-6736(19)31792-1. [DOI] [PubMed] [Google Scholar]
- 98.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N. Engl. J. Med. 2002;347:1403–1411. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- 99.Tamargo J, et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017;3:163–182. doi: 10.1093/ehjcvp/pvw042. [DOI] [PubMed] [Google Scholar]
- 100.Watson S, Caster O, Rochon PA, den Ruijter H. Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine. 2019;17:100188. doi: 10.1016/j.eclinm.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varrias D, et al. Sex-specific differences in ventricular remodeling and response after cardiac resynchronization therapy. Am. J. Cardiol. 2022 doi: 10.1016/j.amjcard.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Narasimha D, Curtis AB. Arrhythmias. Cardiac resynchronization therapy in women. Nat. Rev. Cardiol. 2014;11:501–502. doi: 10.1038/nrcardio.2014.113. [DOI] [PubMed] [Google Scholar]
- 103.Costanzo MR. Cardiac resynchronization therapy in women. Heart Fail. Clin. 2017;13:165–178. doi: 10.1016/j.hfc.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Regitz-Zagrosek V, et al. Heart transplantation in women with dilated cardiomyopathy. Transplantation. 2010;89:236–244. doi: 10.1097/TP.0b013e3181c35255. [DOI] [PubMed] [Google Scholar]
- 105.MacGowan GA, et al. Gender differences in the assessment, decision making and outcomes for ventricular assist devices and heart transplantation: an analysis from a UK transplant centre. Clin. Transplant. 2022 doi: 10.1111/ctr.14666. [DOI] [PubMed] [Google Scholar]
- 106.Rossi A, et al. Heart-brain interactions in cardiac and brain diseases: why sex matters. Eur. Heart J. 2022 doi: 10.1093/eurheartj/ehac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fiechter M, et al. Association between resting amygdalar activity and abnormal cardiac function in women and men: a retrospective cohort study. Eur. Heart J. Cardiovasc. Imaging. 2019;20:625–632. doi: 10.1093/ehjci/jez047. [DOI] [PubMed] [Google Scholar]
- 108.Ishai A, et al. Amygdalar metabolic activity independently associates with progression of visceral adiposity. J. Clin. Endocrinol. Metab. 2019;104:1029–1038. doi: 10.1210/jc.2018-01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Osborne MT, et al. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology. 2019;100:32–40. doi: 10.1016/j.psyneuen.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Radfar A, et al. Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur. Heart J. 2021;42:1898–1908. doi: 10.1093/eurheartj/ehab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tawakol A, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Templin C, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 113.Burger IA, et al. Age- and sex-dependent changes in sympathetic activity of the left ventricular apex assessed by 18F-DOPA PET imaging. PLoS One. 2018;13:e0202302. doi: 10.1371/journal.pone.0202302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cammann VL, et al. Age-related variations in Takotsubo syndrome. J. Am. Coll. Cardiol. 2020;75:1869–1877. doi: 10.1016/j.jacc.2020.02.057. [DOI] [PubMed] [Google Scholar]
- 115.Hogarth AJ, Graham LN, Mary DA, Greenwood JP. Gender differences in sympathetic neural activation following uncomplicated acute myocardial infarction. Eur. Heart J. 2009;30:1764–1770. doi: 10.1093/eurheartj/ehp188. [DOI] [PubMed] [Google Scholar]
- 116.Wittstein IS. Why age matters in Takotsubo syndrome. J. Am. Coll. Cardiol. 2020;75:1878–1881. doi: 10.1016/j.jacc.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 117.Mazure CM, Weinberger AH, Pittman B, Sibon I, Swendsen J. Gender and stress in predicting depressive symptoms following stroke. Cerebrovasc. Dis. 2014;38:240–246. doi: 10.1159/000365838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Templin C, et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019;40:1183–1187. doi: 10.1093/eurheartj/ehz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hiestand T, et al. Takotsubo syndrome associated with structural brain alterations of the limbic system. J. Am. Coll. Cardiol. 2018;71:809–811. doi: 10.1016/j.jacc.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 120.Fiechter M, et al. Sex-dependent association between inflammation, neural stress responses, and impaired myocardial function. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:2010–2015. doi: 10.1007/s00259-019-04537-8. [DOI] [PubMed] [Google Scholar]
- 121.Mehta PK, Lima BB, Nelson MD, Bairey Merz CN. Adverse cardiovascular outcomes in women: blame the amygdala? Eur. Heart J. Cardiovasc. Imaging. 2019;20:633–635. doi: 10.1093/ehjci/jez086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gebhard C, Bengs S, Haider A, Fiechter M. The neuro-inflammatory-vascular circuit: evidence for a sex-dependent interrelation? Front. Neurosci. 2020;14:614345. doi: 10.3389/fnins.2020.614345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J. Clin. Endocrinol. Metab. 1999;84:606–610. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- 124.Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc. Res. 2002;53:678–687. doi: 10.1016/S0008-6363(01)00508-9. [DOI] [PubMed] [Google Scholar]
- 125.Maredziak M, et al. Microvascular dysfunction and sympathetic hyperactivity in women with supra-normal left ventricular ejection fraction (snLVEF) Eur. J. Nucl. Med. Mol. Imaging. 2020;47:3094–3106. doi: 10.1007/s00259-020-04892-x. [DOI] [PubMed] [Google Scholar]
- 126.Gebhard CE, et al. Heart rate reserve is a long-term risk predictor in women undergoing myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:2032–2041. doi: 10.1007/s00259-019-04344-1. [DOI] [PubMed] [Google Scholar]
- 127.Luzier AB, et al. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin. Pharmacol. Ther. 1999;66:594–601. doi: 10.1053/cp.1999.v66.103400001. [DOI] [PubMed] [Google Scholar]
- 128.Mauvais-Jarvis F, et al. Sex- and gender-based pharmacological response to drugs. Pharmacol. Rev. 2021;73:730–762. doi: 10.1124/pharmrev.120.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khera A, et al. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 130.Sullivan S, et al. Young women with coronary artery disease exhibit higher concentrations of interleukin-6 at baseline and in response to mental stress. J. Am. Heart Assoc. 2018;7:e010329. doi: 10.1161/JAHA.118.010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fiechter M, et al. Sex differences in the association between inflammation and ischemic heart disease. Thromb. Haemost. 2019;119:1471–1480. doi: 10.1055/s-0039-1692442. [DOI] [PubMed] [Google Scholar]
- 132.Diggelmann, F, et al. Potential impact of statins on neuronal stress responses in patients at risk for cardiovascular disease. J. Pers. Med. 2021;11:261. doi: 10.3390/jpm11040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martin EA, et al. Sex differences in vascular and endothelial responses to acute mental stress. Clin. Auton. Res. 2008;18:339–345. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vaccarino V, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom. Med. 2014;76:171–180. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Triposkiadis F, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 136.Tadic M, et al. Sex and heart failure with preserved ejection fraction: from pathophysiology to clinical studies. J. Clin. Med. 2019;8:792. doi: 10.3390/jcm8060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aranda G, et al. Cardiovascular risk associated with gender affirming hormone therapy in transgender population. Front. Endocrinol. 2021;12:718200. doi: 10.3389/fendo.2021.718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caceres BA, et al. Assessing and addressing cardiovascular health in LGBTQ adults: a scientific statement from the American Heart Association. Circulation. 2020;142:e321–e332. doi: 10.1161/CIR.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Azagba S, Latham K, Shan L. Cigarette, smokeless tobacco, and alcohol use among transgender adults in the United States. Int. J. Drug Policy. 2019;73:163–169. doi: 10.1016/j.drugpo.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 140.Alzahrani T, et al. Cardiovascular disease risk factors and myocardial infarction in the transgender population. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005597. doi: 10.1161/CIRCOUTCOMES.119.005597. [DOI] [PubMed] [Google Scholar]
- 141.de Blok CJ, et al. Mortality trends over five decades in adult transgender people receiving hormone treatment: a report from the Amsterdam cohort of gender dysphoria. Lancet Diabetes Endocrinol. 2021;9:663–670. doi: 10.1016/S2213-8587(21)00185-6. [DOI] [PubMed] [Google Scholar]
- 142.Reisner SL, et al. Global health burden and needs of transgender populations: a review. Lancet. 2016;388:412–436. doi: 10.1016/S0140-6736(16)00684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Defreyne J, Van de Bruaene LDL, Rietzschel E, Van Schuylenbergh J, T’Sjoen GGR. Effects of gender-affirming hormones on lipid, metabolic, and cardiac surrogate blood markers in transgender persons. Clin. Chem. 2019;65:119–134. doi: 10.1373/clinchem.2018.288241. [DOI] [PubMed] [Google Scholar]
- 144.Streed CG, Jr, et al. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann. Intern. Med. 2017;167:256–267. doi: 10.7326/M17-0577. [DOI] [PubMed] [Google Scholar]
- 145.Wierckx K, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur. J. Endocrinol. 2013;169:471–478. doi: 10.1530/EJE-13-0493. [DOI] [PubMed] [Google Scholar]
- 146.Maraka S, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2017;102:3914–3923. doi: 10.1210/jc.2017-01643. [DOI] [PubMed] [Google Scholar]
- 147.Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism. 1989;38:869–873. doi: 10.1016/0026-0495(89)90233-3. [DOI] [PubMed] [Google Scholar]
- 148.Wierckx K, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J. Sex. Med. 2014;11:1999–2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 149.Dekker MJ, et al. A European network for the investigation of gender incongruence: endocrine part. J. Sex. Med. 2016;13:994–999. doi: 10.1016/j.jsxm.2016.03.371. [DOI] [PubMed] [Google Scholar]
- 150.Connelly PJ, et al. Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension. 2019;74:1266–1274. doi: 10.1161/HYPERTENSIONAHA.119.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]