Abstract
Chylous ascites is the accumulation of lymphatic fluid in the peritoneal cavity due to disruption of lymphatic drainage caused due to obstruction or trauma. We report a man in his 60s who was previously treated for diffuse large B cell lymphoma with radiation to bulky abdominal/mesenteric lymphadenopathy. He was later found to have recurrent chylous ascites several years later, requiring multiple paracentesis. Recurrent lymphoma was ruled out with negative cytology of peritoneal fluid as well as lymph node biopsy with no evidence of malignancy. We believe that the patient had obstruction of lymphatic drainage due to previous radiation therapy causing fibrosis. The patient underwent lymphangiography which did not visualise the central lymphatic duct within the abdomen raising suspicion for obstruction of the ducts secondary to previous radiation.
Keywords: radiotherapy, interventional radiology
Background
Chylous ascites is a rare condition usually associated with liver cirrhosis or malignancy. Development of chylous ascites postradiation treatment is rare but has been reported in literature. We describe an interesting case of a patient with diffuse large B-cell lymphoma who was treated with chemotherapy and involved field radiotherapy to bulky abdominal lymphadenopathy, and later developed recurrent chylous ascites several years later, thought to be secondary to previous radiation to retroperitoneum causing fibrosis and obstruction of the lymphatic ducts.
Case presentation
A man in his 60s presented with abdominal discomfort, increasing distension and constipation ongoing for a month. Symptoms were progressively worsening and now associated with significant fatigue and poor appetite. The patient’s medical history is significant for diagnosis of diffuse large B-cell lymphoma more than 5 years ago. The patient had stage IIB disease with bulk of disease in the retroperitoneal and mesenteric lymph nodes. Molecular studies revealed t(14;18) suggestive of transformation from underlying follicular lymphoma but no evidence of master regulator of cell cycle entry and proliferative metabolism/B-cell lymphoma 6 rearrangement. The patient was treated with chemotherapy with rituximab, cyclophosphamide, doxorubicin, oncovin, prednisone, with initial plans for total of six cycles. Treatment course was complicated by significant cytopenia requiring prolonged hospitalisation for pneumonia and renal failure after four cycles of chemotherapy. Clinical decision was made to discontinue further chemotherapy. He received involved field radiation therapy to the abdominal and retroperitoneal lymph node regions over 32 calendar days with total dose of 40.0 Gy delivered to the involved region in 20 fractions of 2.0 Gy daily. The patient had reasonably good tolerance to radiation treatment with periodic nausea that was treated with antiemetics. In addition, the patient has no known history of coronary artery disease, chronic lung disease or liver disease.
Investigations
Given this new presentation, systemic imaging was obtained which revealed large volume abdomino-pelvic ascites and few mesenteric lymph nodes (figure 1). Paracentesis was done with removal of 2.5 L of chylous fluid, cytology was negative for malignancy. Positron emission tomography/computed tomography scan showed hypermetabolic mesenteric lymph node 2.3 cm with standardized uptake value 12.7 along with a couple of other non-hypermetabolic abdominal lymph nodes. The patient underwent laparoscopic biopsy of the mesenteric lymph node which was negative for malignancy. He also simultaneously underwent drainage of 3.6 L of reaccumulated chylous ascitic fluid. The patient had yet another paracentesis and drainage of 5 L of milky ascitic fluid 10 weeks later. Further analysis of ascitic fluid showed elevated triglycerides >2000 mg/dL. Serum-ascites albumin gradient was 1.0 g/dL. Amylase <10, elevated cell count of 745 /μL with 37% lymphocytes. Ascitic fluid culture was negative for infection.
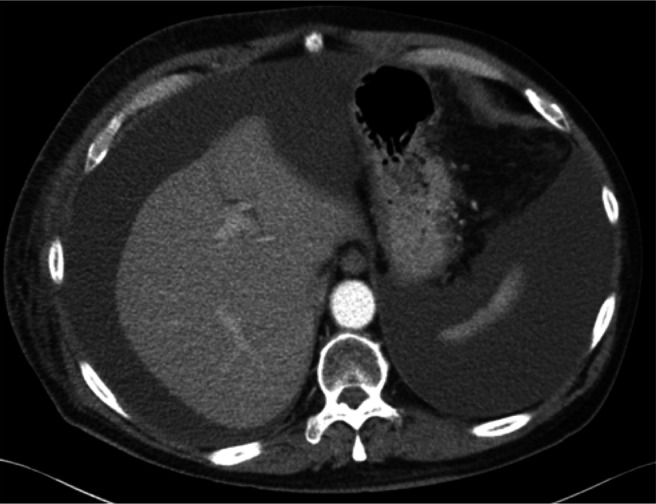
Figure 1.
Pretreatment CT scan showing significant ascitic fluid accumulation.
Differential diagnosis
Chylous ascites is an uncommon condition with an incidence of 1 in 50 000–1 in 100 000 hospital admissions.1 Chylous ascites is a term used to describe ascitic chyle which is a milky fluid with high content of triglycerides.
The common causes of chylous ascites include primary disorders of the lymphatic system, infections such as tuberculosis and liver cirrhosis. Malignancy is the most common cause in adults among which lymphoma accounts for at least one-third of the cases.2 Several other benign and malignant tumours can present in the retroperitoneum. Common benign tumours include schwannomas, neurofibromas and paragangliomas. Malignant tumours include soft tissue sarcomas, commonly liposarcoma and leiomyosarcoma, angiosarcoma and gastrointestinal stromal tumour. Immunohistochemical markers play an important role in distinguishing the sarcomas.3 Other malignancies include carcinomas such as primary mucinous neoplasms of the retroperitoneum and disseminated carcinomas originating from primary colon, ovarian, pancreatic or testicular malignancies. Given the anatomical location, these tumours are often present in close relation to important vascular structures which pose therapeutic challenges.4 Correct diagnosis using image-guided retroperitoneal biopsy is important for determining the treatment approach.5
Few less common causes are retroperitoneal fibrosis and post-radiation treatment of retroperitoneum.6 The primary pathophysiology of accumulation of chylous fluid is the obstruction of lymphatic flow from the abdomen to the cisterna chili which then causes increased pressure within the lymphatic system and subsequent extravasation of the lymph and development of chylous ascites.7 8 In malignancy-associated conditions, the obstruction is caused by tumour infiltrating and blocking the lymphatic system. Similarly postradiation treatment fibrosis causes obstruction and blockage of the ducts.9
In our patient, there was no primary malignancy causing blockage of the lymphatic ducts, however, he did receive radiation therapy in the past. So we postulate that radiation-induced fibrosis of the lymphatic channels caused obstruction and subsequent extravasation of the chylous fluid.
Treatment
Interventional radiology was consulted and the patient underwent lymphangiography, which showed normal bilateral lymphatic channels from the groin entering into pelvis and abdomen by uptake of the lipoidal dye (figure 2). Central lymphatic duct within abdomen was not well visualised (figure 3). The patient underwent embolisation of the cisterna chyli.
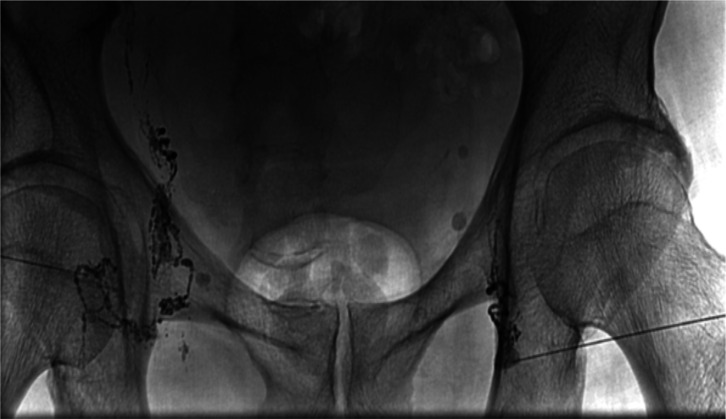
Figure 2.
Lymphangiography demonstrates normal bilateral lymphatic channels with uptake of lipoidal dye.
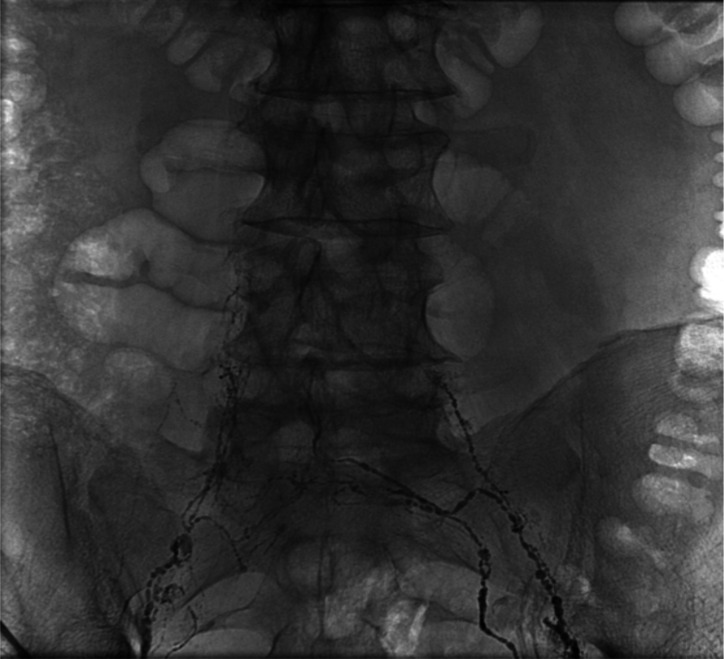
Figure 3.
Lymphangiography shows bilateral lymphatic channels in pelvis, however central lymphatic duct is not visualised.
Outcome and follow-up
The patient tolerated the procedure well. On the follow-up, the patient has not required any repeat paracentesis so far more than 4 months since the procedure (figure 4).
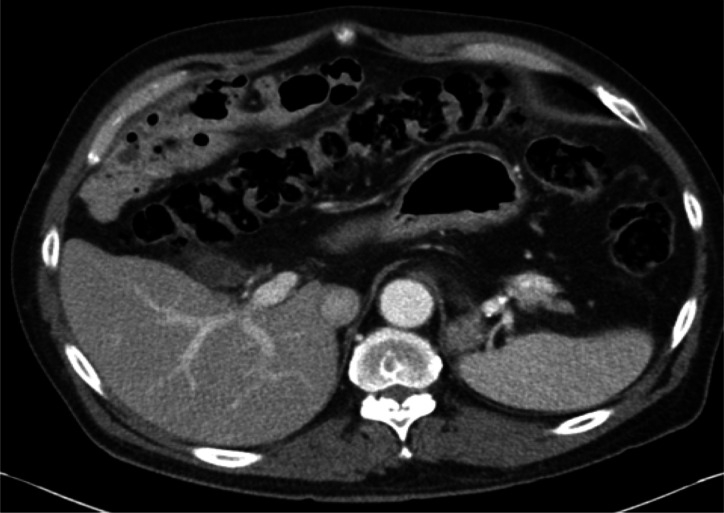
Figure 4.
Postembolisation follow-up CT scan showing no evidence of ascitic fluid.
Discussion
In a review of 207 patients who received whole abdomen irradiation for gynaecological malignancies at the Mayo Clinic, a 3% incidence of chylous ascites was observed.10 Mean cumulative radiation doses given in 150 cGy daily fractions were 2925 and 5122 cGy to the abdomen and pelvis, respectively. Mean time from completion of irradiation and development of ascites was 12 months (ranging from 6 to 18 months). They were treated conservatively with two out of eight patients requiring frequent paracentesis. Clinical resolution of ascites was seen in all eight patients by 18 months since development of ascites. Similarly it has been reported in a case series of three patients with ovarian carcinoma after radiation therapy.11
Our patient got a much smaller dose of radiation treatment (40 Gy) as compared with the high doses reported in the gynaecological malignancies. Also in our patient’s case, the proposed fibrosis of the lymphatic channels and the resultant chylous ascites developed more than 5 years later, although literature review shows that it is typically observed after a mean of 12 months after radiation therapy.12
Diagnosis of chylous ascites is by analysis of fluid which has a milky turbid appearance along with elevated triglycerides, high leucocyte count, low cholesterol content and varying protein content.7 Imaging modalities such as CT scan, lymphangiogram, lymph node biopsy, exploratory laparotomy can be used to determine aetiology of chylous ascites as clinically indicated.8 If lymphatic obstruction is suspected, lymphangiography is the gold standard.13 It is helpful in detecting leakage from dilated lymphatics, fistulisation and patency of thoracic duct. Contrast hypersensitivity, tissue necrosis and fat embolism are commonly associated complications.14 Lymphoscintigraphy is another modality that is used to detect abnormal lymphatic drainage.
Treatment of chylous ascites is aimed towards treating the underlying cause. In patients with liver disease and cirrhosis, conservative measures such as fluid and salt restriction is advised. Low fat diet supplemented with medium chain triglycerides is the recommended approach which helps direct intestinal absorption and decrease in production of chyle.13 If ascites is caused by obstruction of the lymphatic channel, surgical procedures to remove the obstructing lesion or ligation of the leaking lymphatic vessels may be required. Lymphangiography helps in identifying the site of leak. Also, peritoneovenous shunting surgery has also shown benefit especially in postoperative chylous ascites and is associated with less surgical complications.15 Case reports have shown that shunt procedures lead to resolution of recurrent ascites but does have complications of shunt occlusion, superior vena cava/subclavian thrombosis and infection.16 Surgical excision of the affected bowel segment may be required if there is chyle leakage from the submucosal lymphatics on the bowel wall.17 Some promising new techniques (eg, use of octreotide, etilefrine or angiography) may play a role in post-operative chylous ascites.18
Learning points.
Chylous ascites is a rare condition with malignancies and liver cirrhosis being the leading causes.
Certain rare aetiologies such as fibrosis caused by previous radiation therapy need to be considered in the appropriate clinical setting.
Elevated triglycerides in the ascitic fluid analysis is diagnostic. Lymphangiography can help in identifying the leakage in the lymphatic system. Treatment is based on underlying cause of the chylous ascites.
Frequent paracentesis may provide palliative relief of symptoms. Other treatment options for resistant cases include surgical exploration and peritoneovenous shunt.
New techniques such as use of etilefrine or percutaneous embolisation of cisterna chyli are waiting further evaluation.
Footnotes
Contributors: PS: contributed in conception and design of idea, data acquisition, interpretation of data, drafting. KC: contributed in conception and design of idea, data acquisition, interpretation of data, drafting, revising and final approval.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Vasko JS, Tapper RI. The surgical significance of chylous ascites. Arch Surg 1967;95:355–68. 10.1001/archsurg.1967.01330150031006 [DOI] [PubMed] [Google Scholar]
- 2.Almakdisi T, Massoud S, Makdisi G. Lymphomas and chylous ascites: review of the literature. Oncologist 2005;10:632–5. 10.1634/theoncologist.10-8-632 [DOI] [PubMed] [Google Scholar]
- 3.Bratu OG, Marcu RD, Socea B, et al. Immunohistochemistry Particularities of retroperitoneal tumors. Rev Chim 2018;69:1813–6. 10.37358/RC.18.7.6422 [DOI] [Google Scholar]
- 4.Marcu DR, Ionita-Radu F, Iorga LD, et al. Vascular involvement in primary retroperitoneal tumors. Rev Chim 2019;70:445–8. 10.37358/RC.19.2.6932 [DOI] [Google Scholar]
- 5.Marcu R, Diaconu C, Constantin T, et al. Minimally invasive biopsy in retroperitoneal tumors (review). Exp Ther Med 2019;4:5016–20. 10.3892/etm.2019.8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma PV, Jobanputra YB, Perdomo Miquel T, et al. Primary intratracheal schwannoma resected during bronchoscopy using argon plasma coagulation. BMJ Case Rep 2018;2018:bcr2018225140. 10.1136/bcr-2018-225140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ablan CJ, Littooy FN, Freeark RJ. Postoperative chylous ascites: diagnosis and treatment. A series report and literature review. Arch Surg 1990;125:270–3. 10.1001/archsurg.1990.01410140148027 [DOI] [PubMed] [Google Scholar]
- 8.Laterre PF, Dugernier T, Reynaert MS. Chylous ascites: diagnosis, causes and treatment. Acta Gastroenterol Belg 2000;63:260–3. [PubMed] [Google Scholar]
- 9.Sanchez RE, Mahour GH, Brennan LP, et al. Chylous ascites in children. Surgery 1971;69:183–8. [PubMed] [Google Scholar]
- 10.Lentz SS, Schray MF, Wilson TO. Chylous ascites after whole-abdomen irradiation for gynecologic malignancy. Int J Radiat Oncol Biol Phys 1990;19:435–8. 10.1016/0360-3016(90)90554-W [DOI] [PubMed] [Google Scholar]
- 11.Murray JM, Massey FM. Chylous ascites after radiation therapy for ovarian cancer. Obstet Gynecol 1974;44:749–51. [PubMed] [Google Scholar]
- 12.Keung Y-K, Whitehead RP, Cobos E. Chemotherapy treatment of chyloperitoneum and peritoneal carcinomatosis due to cervical Cancer—Review of literature. Gynecol Oncol 1996;61:448–50. 10.1006/gyno.1996.0173 [DOI] [PubMed] [Google Scholar]
- 13.Al-Busafi SA, Ghali P, Deschênes M, et al. Chylous ascites: evaluation and management. ISRN Hepatol 2014;2014:1–10. 10.1155/2014/240473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto T, Yamagami T, Kato T, et al. The effectiveness of lymphangiography as a treatment method for various chyle leakages. Br J Radiol 2009;82:286–90. 10.1259/bjr/64849421 [DOI] [PubMed] [Google Scholar]
- 15.Ohri SK, Patel T, Desa LA, et al. The management of postoperative chylous ascites. A case report and literature review. J Clin Gastroenterol 1990;12:693–7. 10.1097/00004836-199012000-00021 [DOI] [PubMed] [Google Scholar]
- 16.Shelat VG, Pandya GJ, Shabbir A, et al. Post radiation chylous ascites: a case report. Cases J 2009;2:9393. 10.1186/1757-1626-2-9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst PA, Edwards JM. Chylous ascites and obstructive lymphoedema of the small bowel following abdominal radiotherapy. Br J Surg 2005;66:780–1. 10.1002/bjs.1800661108 [DOI] [PubMed] [Google Scholar]
- 18.Benedix F, Lippert H, Meyer F. [Post-surgical lymphocutaneous fistula, chylous ascites and chylothorax--infrequent but serious complications: etiology, diagnosis and therapeutic options]. Zentralbl Chir 2007;132:529–38. 10.1055/s-2007-981364 [DOI] [PubMed] [Google Scholar]