Abstract
Introduction
The only curative treatment for most gastric cancer is radical gastrectomy with D2 lymphadenectomy (LAD). Minimally invasive total gastrectomy (MIG) aims to reduce postoperative morbidity, but its use has not yet been widely established in Western countries. Minimally invasivE versus open total GAstrectomy is the first Western multicentre randomised controlled trial (RCT) to compare postoperative morbidity following MIG vs open total gastrectomy (OG).
Methods and analysis
This superiority multicentre RCT compares MIG (intervention) to OG (control) for oncological total gastrectomy with D2 or D2+LAD. Recruitment is expected to last for 2 years. Inclusion criteria comprise age between 18 and 84 years and planned total gastrectomy after initial diagnosis of gastric carcinoma. Exclusion criteria include Eastern Co-operative Oncology Group (ECOG) performance status >2, tumours requiring extended gastrectomy or less than total gastrectomy, previous abdominal surgery or extensive adhesions seriously complicating MIG, other active oncological disease, advanced stages (T4 or M1), emergency setting and pregnancy.
The sample size was calculated at 80 participants per group. The primary endpoint is 30-day postoperative morbidity as measured by the Comprehensive Complications Index. Secondary endpoints include postoperative morbidity and mortality, adherence to a fast-track protocol and patient-reported quality of life (QoL) scores (QoR-15, EUROQOL EuroQol-5 Dimensions-5 Levels (EQ-5D), EORTC QLQ-C30, EORTC QLQ-STO22, activities of daily living and Body Image Scale). Oncological endpoints include rate of R0 resection, lymph node yield, disease-free survival and overall survival at 60-month follow-up.
Ethics and dissemination
Ethical approval has been received by the independent Ethics Committee of the Medical Faculty, University of Heidelberg (S-816/2021) and will be received from each responsible ethics committee for each individual participating centre prior to recruitment. Results will be published open access.
Trial registration number
DRKS00025765.
Keywords: surgery, oncology, gastrointestinal tumours
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Minimally invasivE versus open total GAstrectomy is the first Western multicentre randomised controlled trial to specifically compare open gastrectomy with minimally invasive gastrectomy in terms of postoperative morbidity using the Comprehensive Complication Index (CCI).
Usage of the CCI as a comprehensive outcome measure allows for objective comparisons with other trials.
Differentiation between robotic and laparoscopic total gastrectomy will be made in the explorative subgroup analysis only.
High levels of standardisation, intraoperative photo documentation, well-powered group sizes and risk-based monitoring by the Study Center of the German Society of Surgery will guarantee objective data acquisition, increase patients’ adherence to the protocol, and ultimately, lead to exceptional data quality.
Introduction
Gastric cancer is the sixth most common tumour disease in the world and causes the second most deaths.1 In 2018, approximately one million patients worldwide and approximately 15 000 patients in Germany were diagnosed with gastric cancer, of which an average of 76% die from the disease.1 Gastric cancer causes one of the highest oncological disease burdens as measured by lost disability-adjusted life-years (DALYs). This fact highlights the aggressiveness of the disease. Age-adjusted DALY rates per 100 000 reach 241 for men and 146 for women, ranking 4th after liver, lung and breast cancer.2 3
Currently, the only therapy that offers a chance of cure is gastrectomy, with a 5-year survival rate of 20%–30% and postoperative morbidity and mortality as high as 63%4 and 11%,5–10 even at experienced centres.4–18 Therefore, there is a great need to identify the optimal surgical approach using evidence from multicentre data in order to improve oncological outcome and to decrease postoperative complications.
The current gold standard is open gastrectomy (OG) with D2 lymphadenectomy (LAD) (online supplemental appendix 2), but its highly invasive nature leads to potentially high complication rates, especially in elderly and obese patients. These frequent postoperative complications result in higher mortality, lower QoL, a longer hospital stay and thus a higher burden on the healthcare system.6 19 In other fields of visceral surgery, such as appendectomy, cholecystectomy, obesity surgery and esophagectomy, minimally invasive surgery has already replaced the open approach as the standard of care.7 20–22 Several randomised controlled trials (RCTs) have demonstrated reduced postoperative complications following minimally invasive surgery compared with the open approach. This finding is due to the procedure’s resulting smaller wounds, reduced operative trauma, lower blood loss, shorter hospital stay and faster rehabilitation time.22–24
bmjopen-2022-064286supp001.pdf (83.7KB, pdf)
Postoperative complications, however, are not only important for the immediate postoperative course, which is usually secondary in relevance, but can also affect long-term oncological outcome.25–27 In a study of 432 patients with curative gastrectomy and D2 LAD for treatment of gastric cancer, the occurrence of postoperative in-hospital complications was an independent predictor of worse 5-year survival (22% vs 40%). This can be perceived as an indication that postoperative complications may lead to higher mortality in the long term.28 Therefore, the trend towards favouring minimally invasive gastrectomy (MIG) for gastric cancer is increasing.
Methods and analysis
Setting
The Minimally invasivE versus open total GAstrectomy (MEGA) trial is a prospective randomised, controlled, non-blinded, two-armed multicentre surgical superiority trial with a confirmatory character. It includes 14 surgical centres in Germany and Switzerland and is coordinated by the Department of General, Visceral and Transplantation Surgery at Heidelberg University Hospital, in Germany. Recruitment is planned for two consecutive years. The study protocol was accepted by the Independent Ethics Committee of the Medical Faculty, University of Heidelberg (registration number S-816/2021) prior to recruitment. The trial was registered at DRKS under the registration number DRKS00025765 on 22 December 2021.29 No secondary identifying numbers such as a Universal Trial Number have been assigned. Recommendations of the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) checklist were followed.30
Patient recruitment
Recruitment is planned to take place at 14 surgical centres in Germany and Switzerland. Certain eligibility criteria have to be met by the participating centres and surgeons in order to eliminate bias from inexperience or learning curves. Therefore, hospitals need to have a case load of ≥20 gastrectomies per year, and every trial surgeon has to provide evidence of at least 20 previously performed surgeries of the respective surgical procedure/s, he or she wants to contribute (OG, laparoscopic gastrectomy (LAG) or robotic-assisted gastrectomy (RAG)). Eligible patients will be screened consecutively to eliminate selection bias and will receive diagnostic staging laparoscopy prior to randomisation.
Inclusion criteria
Age between 18 and 84 years.
Planned total gastrectomy after first diagnosis of gastric carcinoma.
Ability of patient to understand character and consequences of the trial.
Written informed consent.
Exclusion criteria
ECOG performance status >2.
Planned extended gastrectomy or less than total gastrectomy (eg, adenocarcinoma of the esophagogastric junction (AEG) I and AEG II, or distal gastric tumours of an intestinal subtype).
Previous gastric surgery or extensive adhesions seriously complicating MIG.
Other active oncological disease or history of cancer limiting prognosis in comparison to the gastric cancer.
Emergency setting.
Language barriers rendering the patient unable to fill out patient-reported outcome questionnaires.
Participation in another intervention trial that might interfere with the intervention and/or outcome of this trial.
Pregnancy.
Exclusion criteria previously or during staging laparoscopy:
T4.
M1.
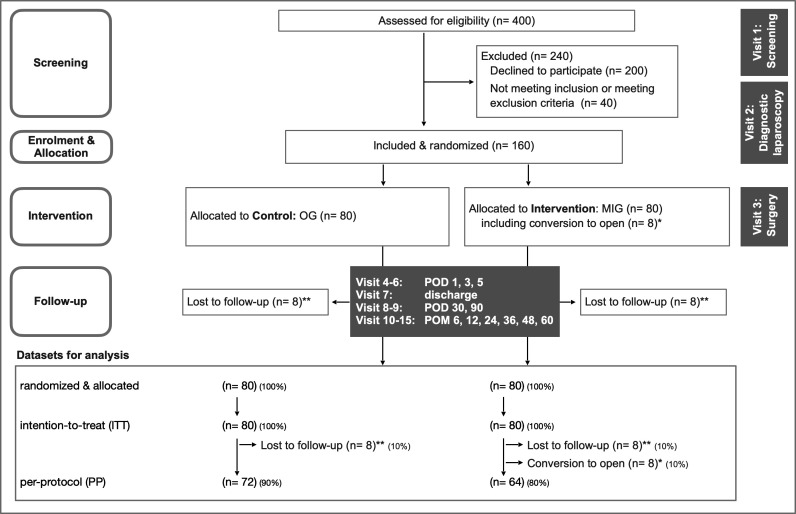
Neoadjuvant chemotherapy does explicitly not contribute to inclusion or exclusion criteria, but will of course be monitored. Inclusion takes place after the staging laparoscopy, and patients will be randomised to the intervention arm (MIG) or the control arm (OG) (figure 1).
Figure 1.
Trial design flow chart. *Intraoperative conversion from MIG to OG, for example, due to bleeding. **Lost to follow-up over 30 POD. MIG, minimally invasive gastrectomy; OG, open gastrectomy; POD, postoperative day; POM, postoperative month.
Trial duration and schedule
Recruitment is planned to take 24 months. The duration of the trial for each patient is expected to be 1 month for the primary endpoint and 60 months for the secondary endpoints with long-term follow-up. Consequently, the duration of data collection is expected to be 25 months for the primary endpoint and 84 months for the secondary endpoints (first-patient-in (FPI) to last-patient-out (LPO)). FPI is planned for September 2022 and last-patient-in is planned for September 2024. LPO is consequently planned for September 2029. Trial analysis will take an additional 6 months. The actual overall duration or recruitment time may differ. Recruitment is planned to be active until both arms contain at least 80 patients in the intention-to-treat (ITT) dataset.
Trial visits
Patients will be monitored intraoperatively, on postoperative days (POD) 1, 3 and 5, and on the day of discharge. Follow-up will be conducted on POD 30, 90 and after postoperative months 6, 12, 24, 36, 48 and 60 (table 1). Demographic and baseline clinical data, intraoperative findings and postoperative results will be recorded. During the follow-up, patients will complete established and validated questionnaires. To enhance participant retention and to avoid lost to follow-up, patients will be contacted for the completion of questionnaires and to collect missing data. Informed consent will be obtained and trial data will be collected by trained assessors using electronic case report forms (eCRFs).
Table 1.
Trial visits and overview over documented parameters and outcomes
| Activity and documentation | Visit 1 (screening) |
Visit 2 (laparosc.) |
Visit 3 (surgery) |
Visits 4–6 (POD 1, 3, 5) |
Visit 7 (discharge) |
Visits 8–9 (POD 30, 90) | Visits 10–15 (POM 6, 12, 24, 36, 48, 60) |
| Inclusion and exclusion criteria | X | ||||||
| Informed consent | X | ||||||
| Medical history and preoperative assessment* | X | ||||||
| Randomisation | X | ||||||
| Surgical and anaesthetic documentation† | X | ||||||
| Postoperative morbidity measured with CCI (primary endpoint) until POD 30 | X | X | X | X | X (V8) | ||
| Biological specimen retrieval | |||||||
| EDTA blood samples | X | ||||||
| Formalin and paraffin tissue samples | X | ||||||
|
Visit 1 (screening) |
Visit 2 (laparosc.) |
Visit 3 (surgery) |
Visits 4–6 (POD 1, 3, 5) |
Visit 7 (discharge) |
Visits 8–9 (POD 30, 90) | Visits 10–15 (POM 6, 12, 24, 36, 48, 60) | |
| Short-term clinical endpoints | |||||||
| Postoperative morbidity measured with the CCI until POD 90 | X | X | X | X | |||
| Major complications (Clavien-Dindo ≥3) unitl POD 90 | X | X | X | X | |||
| Conversion rate | X | ||||||
| Operation time | X | ||||||
| Blood loss | X | ||||||
| Length of stay in the ICU | X | X | |||||
| Length of hospital stay | X | ||||||
| Pain and postoperative analgesic required | X | X | |||||
| Laboratory parameters (CRP, leucocytes) |
X | X | |||||
| Mobilisation of the patient | X | ||||||
| Quality of the patient’s recovery (QoR-15) | X (V5) | ||||||
| Quality of life (EUROQOL EQ-5D-5L, EORTC QLQ-C30, EORTC QLQ-STO22, ADL) | X | X | X | X | |||
| Adherence to a fast-track gastrectomy SOP | X | X | X | ||||
| Objective evaluation of anastomoses | X | ||||||
| First bowl function | X | ||||||
| Wound healing deficits | X | X | X (V8) | ||||
| Vegetative function‡ | X | X | |||||
| Necessity of interventions due to complications | X | X | X | X | |||
| Oncological short-term data | |||||||
| No of lymph nodes removed and of tumour-positive lymph nodes | X | ||||||
| No of R0 resections | X | ||||||
| Development of tumour markers (CA 125, CA 19–9, CA 72–4, CEA) | X | ||||||
| Tumour histpathology§ | X | ||||||
| Long-term clinical data (5 year follow-up) |
|||||||
| Changes of body weight | X | X | X | ||||
| Quality of life (EUROQOL EQ-5D-5L, EORTC QLQ-C30, EORTC QLQ-STO22, ADL, BIS) | X | X | X | X | |||
| Incidence of incisional hernias | X | X | |||||
| Incidence of reoperations | X | X | X | X | |||
| Incidence of stenosis | X | X | |||||
| Cosmetic results and scar satisfaction (BIS) | X (V13) | ||||||
| Oncological long-term data (5-year follow-up) |
|||||||
| Oncological treatment (adjuvant and consecutive therapy) | X | X | |||||
| Disease-free survival recurrence-free survival, RFS | X (V9) | X | |||||
| Local recurrence | X (V9) | X | |||||
| RFS | X (V9) | X | |||||
| Progression-free survival | X (V9) | X | |||||
| Time to progression | X (V9) | X | |||||
| Overall survival | X (V9) | X |
*Includes body mass index, ASA status, preoperative oncological status, prior surgical treatment, drug use and comorbidities.
†Includes surgical documentation (surgeons, procedures, complications, drains) and anaesthesiology documentation.
‡Includes dysphagia, reflux and dumping syndromes.
§Includes entity, TNM, grading and resection status.
ADLs, activities of daily living; ASA, American Society of Anesthesiologists; BIS, Body Image Scale; CA, carbohydrate antigen; CCI, Comprehensive Complication Index; CEA, carcinoembryonic antigen; ECOG, Eastern Co-operative Oncology Group; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EORTC QLQ-STO22, EORTC Quality of Life Questionnaire for Gastric Cancer; EUROQOL EQ-5D-5L, EuroQol Group Questionnaire for Quality of Life with 5 dimensions and 5 levels; ICU, intensive care unit; POD, postoperative day; POM, postoperative month; QoR-15, Quality of Recovery 15; SOP, standard operating procedure.
Primary endpoint
The primary endpoint will be postoperative morbidity measured using the Comprehensive Complication Index (CCI) until POD 30.31 Usage of this index will enable a comparison of the severity and individual burden of postoperative complications with results from other trials.32 33 Postoperative morbidity is defined as any deviation from the normal postoperative course according to the Clavien-Dindo classification.34 This includes anastomotic insufficiency or loss of anastomotic integrity verified by either CT scan with detection of contrast agent external to the anastomosis, endoscopy, or the detection of methylene blue in a drain following oral intake.
Secondary endpoints
Secondary endpoints can be separated into short-term clinical and oncological endpoints as well as long-term clinical and oncological endpoints (at 5-year follow-up, as measured from the date of surgery) and can be found in table 1. Hyperspectral imaging of the surgical site intraoperatively (visit 3) will be performed in Heidelberg only.
Standardised therapy and trial interventions
Control: Total OG with D2/D2+LAD.
Intervention
Total MIG with D2/D2+LAD either as LAG or RAG. A minilaparotomy or a Pfannenstiel incision (≤8 cm incision in both the skin and fascia) may be performed for specimen removal.
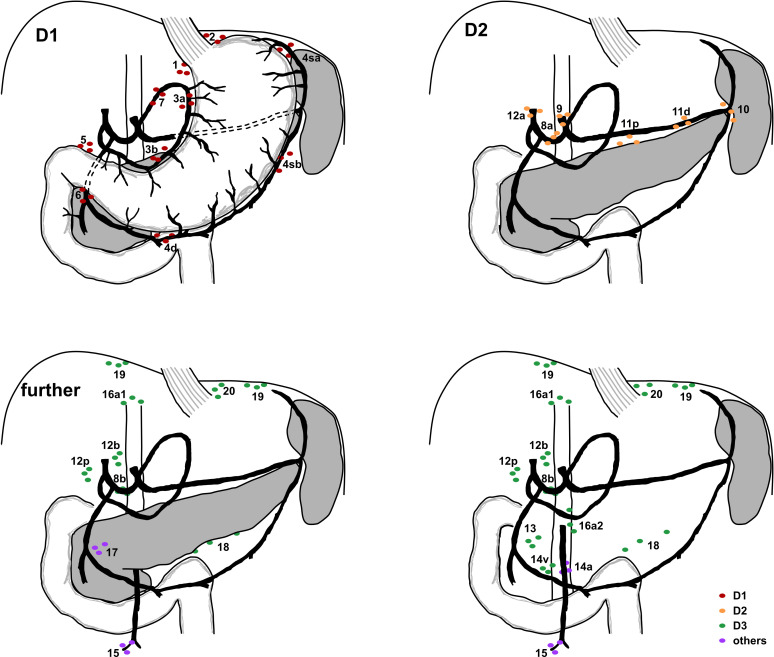
Modified cardia-preserving total gastrectomy (preservation of gastro-oesophageal junction) can also be accepted, but only if the short gastric vessels are dissected as well, and if LAD is the same as for total gastrectomy. Besides the open or minimally invasive approach, the remaining treatment is identical in both groups. Any other form of gastrectomy, explicitly conventional subtotal gastrectomy (preserved short gastric vessels and limited LAD of station 2 and 4sa), extended gastrectomy and distal gastrectomy with Billroth I or II reconstruction are not allowed. Reconstruction can be of any form including Roux-Y reconstruction, interposition or pouch reconstruction. Any other step of the procedure such as antibiotic prophylaxis, placement of abdominal drains and closure of the abdominal wall can be performed according to in-house standards. D2 LAD is defined according to the Japanese classification,35 with stations 1, 2, 3a, 3b, 4sa, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, 11d and 12a obligatory for the MEGA trial (figure 2). Station 10 is optional. Incomplete LAD is not allowed and has to be documented as a protocol deviation.
Figure 2.
Schematic lymphadenectomy. Stations for lymphadenectomy (LAD) as required for total gastrectomy according to the cited Japanese classification. Schemes are separated into D1 LAD, D2 LAD and further lymph node stations.
Removal of further stations (8b, 12b, 12p, 13, 14v, 14a, 15, 16a1, 16a2, 16b1, 16b2, 17, 18, 19, 20, 110, 111 and 112) is allowed when deemed appropriate, for example, in case of assumed tumour invasion, and has to be documented as D2+.
Postoperative management
Postoperative management should be performed in a fast-track approach with short durations until patient mobilisation, drainage removal and first oralisation of food. The patient should be extubated immediately after surgery and transferred to a normal ward, if possible. Further specifications for the postoperative course will be outlined in the provided standard operating procedure (SOP) for fast-track gastrectomy. The last in-hospital trial visit takes place on the day of discharge. Subsequent trial visits will be conducted via telephone. These will be questionnaire-based and focus on CCI (until POD 90), QoL and oncological outcome.
Randomisation and blinding
In order to ensure equal distribution of patient characteristics between both trial arms, randomisation will be performed using a web-based randomisation tool (www.randomizer.at). Randomisation will take place following diagnostic laparoscopy (visit 2). The allocation pattern is masked, block-randomised with variable block length, and stratified across centres. Due to the pragmatic character of the trial, blinding of the surgeon is not feasible.
Quality assurance and quality management
Clinical data monitoring
Clinical monitoring will be performed by independent monitors at the Study Center of the German Society of Surgery. The monitoring strategy will comprise a combination of centralised and onsite monitoring and will be described in a trial specific monitoring plan. To confirm site selection, prestudy visits will be performed. On-site monitoring will focus on patient informed consent, safety and surgical procedures as well as the correct recording and documentation of the primary and secondary endpoints by source data verification (SDV).
Surgical quality control
Several steps are necessary to ensure and evaluate surgical quality:
Trial surgeons must have performed 20 surgeries in the respective approach (OG, LAG or RAG), depending on the trial arm they will contribute to.
Each trial surgeon must provide photographic or video documentation of a former procedure.
-
Each trial surgeon has to provide photographic or video documentation of the trial procedures, which will be assessed by an expert. This photographic or video documentation is defined as follows:
Lhymph node station 7 (left gastric artery) after dissection.
Lymph node station 8 a (common hepatic artery) after dissection.
Lymph node station 9 (coeliac artery) after dissection.
Lymph node station 10 (splenic hilum) after dissection.
Lymph node station 11p (proximal splenic artery) after dissection.
Lymph node station 11d (distal splenic artery) after dissection.
Lymph node station 12a (hepatoduodenal ligament along the hepatic artery) after dissection.
Duodenal stump.
All anastomoses.
Incision for specimen retrieval in MIG.
Assessment of safety
Since the primary endpoint is postoperative complications as measured by the CCI, adverse (AE) and serious AEs (SAE) are already captured and no additional safety analysis will be performed (table 2).
Table 2.
Grading of adverse events (AEs)
| Clavien-Dindo | AE | Serious AE (SAE) | Minor complication | Major complication |
| Grade I complication | AE | - | Minor complication | - |
| Grade II complication | - | - | ||
| Grade III complication | - | - | Major complication | |
| Grade IV complication | SAE | - | ||
| Grade V complication | - |
Data management
The Institute of Medical Biometry (IMBI) is responsible for data management within this trial. An eCRF will be used for data collection. To assure safe and secure data use and storage, data transmission is encrypted with secure socket layer technology. Only authorised users are able to enter or edit data, and access is further restricted to data of the patients in that user’s respective centre only. All changes to data are logged with a computerised timestamp in an audit trail. All data will be pseudonymised. To guarantee high data quality, data validation rules will be defined in a data validation plan. Completeness, validity and plausibility of data will be checked at the time of data entry (edit checks) and using validating programmes, which will generate queries. If no further corrections are to be made in the database, eCRF data will be locked. Data will finally be downloaded and used for statistical analysis. All data management procedures will be conducted according to written defined SOPs of the IMBI that guarantee efficient conduct in compliance with Good Clinical Practice. At the end of the study, the data will be transformed into different data formats (eg, csv-files) for archiving and to ensure that it can be reused.
Statistical methods
Sample size
The sample size calculation is based on the primary endpoint ‘postoperative morbidity as measured with the CCI until POD 30’. A decrease of the CCI by 10 points between OG and MIG is considered relevant by patients and clinicians, and a conservative SD of 20 is assumed based on existing literature for upper GI surgery,36 leading to an effect size of d=0.5. Based on a t-test with a two-sided significance level of α=0.05, a sample size of n=128 patients (64 per group) has to be recruited to achieve a power of 80%. The primary endpoint will be analysed with a linear mixed regression model, which leads to equal or even increased power when compared with a two-sided t-test. To compensate for drop-outs and patients lost to follow-up, a further 20% of patients will be randomised, leading to a total sample size of n=160 (80 per group; 80×0.8 = 64.8). The number of patients to be screened (n=400 to be assessed for eligibility; 400×0.5×0.8=160) was calculated with an assumed 50% participation rate and an exclusion rate of 20%.
Randomised and allocated (n=160; 80 per group).
ITT dataset (n=160; 80 per group).
Per-protocol dataset (n=136; 72 and 64).
Statistical analysis
For the examination of the primary endpoint ‘postoperative morbidity measured with the CCI until POD 30,’ the hypotheses to be assessed in the primary analysis are as follows: H0: μ1 = μ2 vs H1: μ1 ≠ μ2, where μ1 and μ2 denote the mean CCI in the control and intervention groups, respectively. The significance level is set to a two-sided α=0.05. Therefore, the primary endpoint will be examined using a linear mixed model adjusting for the variables age and treatment group, as well as the surgical centre as a random effect (due to the stratified randomisation and relatively large number of centres in relation to the sample size, inclusion of centre as a random effect is recommended). Details of the primary model (eg, handling of missing values, sensitivity analyses) will be fully described in the statistical analysis plan.
The number of patients included in the primary analysis is determined as the full analysis set. Patients will be analysed in the group they were randomised to (converted patients remain in their group). This reflects an analysis according to the ITT principle. Specific events (eg, death) that can occur after randomisation will be handled within the primary endpoint definition, reflecting a composite strategy (according to the ICH E9 (R1) addendum). Other postrandomisation events will not be considered. This choice reflects our treatment policy approach.
In general, for the full analysis set, all baseline values and secondary outcomes will be evaluated descriptively, with p values reported alongside 95% CIs for the corresponding effects. Furthermore, secondary endpoints will be evaluated descriptively, using appropriate regression models. Time-to-event endpoints will be evaluated by methods of survival analysis including Kaplan-Meier methods and Cox proportional hazards models. In addition, subgroup analyses (including age, gender, tumour stage, tumour grade, histological tumour type, linear vs circular stapler for proximal anastomosis, linear vs hand-sewn for distal anastomosis, type of retrieval incision and intraoperative conversion) will be carried out. A detailed and comprehensive statistical analysis plan will be written shortly after the first patient is recruited. All analyses will be performed using SAS V.9.4 or higher.
Discussion
We performed a systematic literature search prior to planning this trial and identified 974 publications. Of those, 17 RCTs comparing LAG with OG7 37–55 and 2 RCTs comparing RAG with OG56 57 were found to be relevant. The studies showed comparable oncological and short-term postoperative outcomes for MIG and OG. However, 16 of the 19 studies were conducted in China, Koreaand Japan.7 38–50 56 57 These countries have a significantly higher incidence of gastric cancer, which consequently leads to significantly higher surgical volume and expertise among the participating centres.58 In addition, the body constitution of Asian patients is often different from that of Western patients, which limits the direct transferability of study results. Also, the incidence of gastric cancer is lower in Western populations and advanced disease stages are more frequently detected, because screening is less common. Therefore, it is unclear whether these results would be reproducible in a Western population.
Currently, there have only been three non-Asian RCTs directly comparing LAG and OG. The first RCT, by Huscher et al, focused exclusively on distal gastrectomy, did not define any specific primary or secondary endpoints, and included a total of 59 patients.37 Due to the missing differentiation between primary and secondary endpoints, the trial can be perceived as methodically limited and was most likely underpowered. However, no significant difference was found in perioperative outcome, oncological outcome or mortality (morbidity rates: 26.7% (LAG) and 27.6% (OG), lymph nodes harvested: 30.0±14.9 (LAG) and 33.4±17.4 (OG), operative mortality rates: 3.3% (LG) and 6.7% (OG), 5-year survival rate: 54.8% (LAG) and 55.7% (OG)).
The only two currently existing relevant Western multicentre RCTs comparing open versus minimally invasive oncological total gastrectomy are the laparoscopic versus open gastrectomy for gastric cancer (LOGICA) trial52 53 and the STOMACH trial,51 54 55 which were both puplished in 2021.
The LOGICA trial is a non-blinded, multicentre superiority trial with 227 patients with postoperative hospital stay as the primary endpoint. The study identified significant differences regarding blood loss (150 mL (LAG) and 300 mL (OG), p<0.001) and operating time (216 min (LAG) and 182 min (OG), p<0.001), but no significant differences in hospital stay (p=0.34), postoperative complications (44% (LAG) and 42% (OG), p=0.91), in-hospital mortality (4% (LAG) and 7% (OG), p=0.40), R0 resections (95% (LAG) and 95% (OG), p=1.00), median lymph node yield (29 (LAG) and 29 (OG), p=0.49), 1-year overall survival (76% (LAG) and 78% (OG), p=0.74), and health-related QoL (+1.5 (LAG) and +3.6 (OG) on a 1–100 scale).
The STOMACH trial is an observer-blinded, multicentre, non-inferiority trial with 96 patients following neoadjuvant chemotherapy with quality of oncological resection (radicality of surgery and number of retrieved lymph nodes) as the primary endpoint. Mean number of resected lymph nodes (41.7±16.1 (LAG) and 43.4±17.3 (OG), p=0.612), number of R0 resections (44/47 (LAG) and 48/49 (OG), p=0.617), 1-year survival (85.5% (LAG) and 90.4% (OG), p=0.701), postoperative complications (16/47 (LAG) and 21/49 (OG), p=0.408), and postoperative QoL (measured with EQ-5D, EORTC-QLQ-C30, and EORTC-QLQ-STO22) were not significantly different.
In a regular setting with a diagnosed carcinoma, patients should usually be advised to make their decision for or against a certain treatment option with regard to a combination of highest expected overall survival and simultaneous conservation of long-term QoL. Short-term postoperative complications should only be treated as secondary deciding factors. However, if postoperative complications might impair long-term QoL or even overall survival, they become equally relevant. In general, postoperative complications can have negative effects on QoL or overall survival; however, this is much more the case for gastric cancer, as time to continuation of peroperative chemotherapy can be prolonged and the prognosis therefore worsened.
The STOMACH trial provides evidence that MIG is non-inferior to OG in terms of oncological quality of resection, which is a necessary requirement for the MEGA trial, as postoperative morbidity and complications can only be decisive factors in the case of oncological non-inferiority for an oncological resection with curative intent.
While both the STOMACH and LOGICA trials suggest that postoperative complications might not be significantly different between both groups, a premature confirmative statement must be avoided as complications have only been investigated as secondary endpoints so far. Consequently, a multicentre RCT comparing total MIG and OG for gastric cancer in terms of postoperative complications is needed to decide whether MIG should be established as the new standard treatment for resectable gastric cancer in Europe.
The MEGA trial has strict quality control measures and will be conducted in line with all relevant guidelines. Therefore, it will provide the highest level of evidence on this very relevant clinical research question.
Ethics and dissemination
The MEGA trial conforms to the Declaration of Helsinki.59 The Independent Ethics Committee of the Medical Faculty, University of Heidelberg, approved the MEGA trial protocol (registration number S-816/2021). For other trial centres, recruitment will only be initiated after receiving approval from their respective local ethics committees. Online supplemental additional file 1 provides the SPIRIT checklist for interventional trials.60
Study objectives and procedures will be communitated clearly to all qualifying patients and written informed consent will be obtained from those who agree to participate. Results will be presented at scientific meetings and published in international peer-reviewed journals. Summaries will be provided to the funders of the study and results will be published in open-access journals.
Patient and public involvement
Patients are involved in the design and conduction of this trial. Priority of the research question, outcome measures and recruitment methods were discussed with patients during the initial planning stage. Patients have stated an uneventful postoperative course as a very notable feature, and every possible intervention contributing to lower postoperative morbidity was rated to be of great importance.
The chairman of one of Germany’s largest patient self-aid groups concerning minimally invasive surgery (SHG Frankfurt Sachsenhausen) will be a member of the data safety and monitoring board as a patient representative. Therefore, this study will continue to take the patient’s perspective into account.
Modification of the protocol
The current protocol version (1.2) will be used during trial initiation. In case of protocol amendments, these will be submitted to the relevant ethics committees for approval.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the Study Center of the German Society of Surgery (SDGC) in the Department of General, Visceral, and Transplantation Surgery at Heidelberg University Hospital for its assistance in coordinating this RCT. We would also like to thank the other centres that have committed to participating in the trial.
Footnotes
FN and AS-F contributed equally.
Contributors: FN and AS-F contributed equally as first authors. BPM-S, FN and AS-F developed the original concept of the trial and applied for funding. FN, AS-F, DH, CK, MF, SZ and BPM-S developed the design and methodology. BPM-S and FN recruited all participating trial centres. FN, AS-F, CK, MF, SZ and BPM-S performed initial statistical steps to develop the analysis plan. FN, AS-F, RK, SLV-A, ST, PP, AB and HN contributed to drafting the protocol. DH, CK, MF, SZ, BB, FB, CB, IG, SG, PG, CAG, JH, KL, LM, SM, DR, FS, DS, PP, TS and BPM-S contributed to the revision of the final protocol. All authors have read and approved of the final manuscript.
Funding: The MEGA trial is funded by the Federal Ministry of Education and Research (BMBF), funding number 01KG2029. All trial aspects will be performed independently from the funding source, including trial design and conduction, analysis and interpretation of data, as well as submission of the report for publication.
Disclaimer: The funder does not have any influence in study design or collection, management, analysis and interpretation of data.
Competing interests: The authors declare that they have no conflicts of interest or relevant financial ties to disclose. FN reports support for courses and travel from Johnson & Johnson, Medtronic, Intuitive Surgical, Cambridge Medical Robotics and KARL STORZ as well as consultancy fees from KARL STORZ.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 2012;380:1840–50. 10.1016/S0140-6736(12)60919-2 [DOI] [PubMed] [Google Scholar]
- 3., Fitzmaurice C, Allen C, et al. , Global Burden of Disease Cancer Collaboration . Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–48. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selby LV, Vertosick EA, Sjoberg DD, et al. Morbidity after total gastrectomy: analysis of 238 patients. J Am Coll Surg 2015;220:863–71. 10.1016/j.jamcollsurg.2015.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13. 10.1158/1055-9965.EPI-13-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64. 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- 7.Kim W, Kim H-H, Han S-U, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg 2016;263:28–35. 10.1097/SLA.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs H, et al. Operative Fallzahlen beeinflussen die Mortalität nACh Gastrektomie erheblich – eine analyse des U.S. Nationwide inpatient sample.
- 9.Pacelli F, Papa V, Rosa F, et al. Four hundred consecutive total gastrectomies for gastric cancer: a single-institution experience. Arch Surg 2008;143:769–75. 10.1001/archsurg.143.8.769 [DOI] [PubMed] [Google Scholar]
- 10.Bartlett EK, Roses RE, Kelz RR, et al. Morbidity and mortality after total gastrectomy for gastric malignancy using the American College of surgeons national surgical quality improvement program database. Surgery 2014;156:298–304. 10.1016/j.surg.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 11.Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol 2014;21:3008–14. 10.1245/s10434-014-3664-z [DOI] [PubMed] [Google Scholar]
- 12.Dhir M, Smith LM, Ullrich F, et al. A preoperative nomogram to predict the risk of perioperative mortality following gastric resections for malignancy. J Gastrointest Surg 2012;16:2026–36. 10.1007/s11605-012-2010-7 [DOI] [PubMed] [Google Scholar]
- 13.Edwards P, Blackshaw GRJC, Lewis WG, et al. Prospective comparison of D1 vs modified D2 gastrectomy for carcinoma. Br J Cancer 2004;90:1888–92. 10.1038/sj.bjc.6601790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finlayson EVA, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg 2003;138:721–5. 10.1001/archsurg.138.7.721 [DOI] [PubMed] [Google Scholar]
- 15.Smith JW, Shiu MH, Kelsey L, et al. Morbidity of radical lymphadenectomy in the curative resection of gastric carcinoma. Arch Surg 1991;126:1469–73. 10.1001/archsurg.1991.01410360039007 [DOI] [PubMed] [Google Scholar]
- 16.Harrison LE, Karpeh MS, Brennan MF. Proximal gastric cancers resected via a transabdominal-only approach. results and comparisons to distal adenocarcinoma of the stomach. Ann Surg 1997;225:678–83. 10.1097/00000658-199706000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi Y, Yoshikawa T, Tsuburaya A, et al. Is gastric carcinoma different between Japan and the United States? Cancer 2000;89:2237–46. [DOI] [PubMed] [Google Scholar]
- 18.Li H-Z, Chen J-X, Zheng Y. Laparoscopic-assisted versus open radical gastrectomy for resectable gastric cancer: systematic review, meta-analysis, and trial sequential analysis of randomized controlled trials. J Surg Oncol 2016;113:756–67. 10.1002/jso.24243 [DOI] [PubMed] [Google Scholar]
- 19.Yamada H, Kojima K, Inokuchi M, et al. Effect of obesity on technical feasibility and postoperative outcomes of laparoscopy-assisted distal gastrectomy--comparison with open distal gastrectomy. J Gastrointest Surg 2008;12:997–1004. 10.1007/s11605-007-0374-x [DOI] [PubMed] [Google Scholar]
- 20.Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-Assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224–9. 10.1016/S0140-6736(02)09290-5 [DOI] [PubMed] [Google Scholar]
- 21.van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210–8. 10.1016/S1470-2045(13)70016-0 [DOI] [PubMed] [Google Scholar]
- 22.Müller-Stich BP, Probst P, Nienhüser H, et al. Meta-Analysis of randomized controlled trials and individual patient data comparing minimally invasive with open oesophagectomy for cancer. Br J Surg 2021;108:1026–33. 10.1093/bjs/znab278 [DOI] [PubMed] [Google Scholar]
- 23.Lee H-J, Hyung WJ, Yang H-K, et al. Short-Term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann Surg 2019;270:983–91. 10.1097/SLA.0000000000003217 [DOI] [PubMed] [Google Scholar]
- 24.Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 2018;11:Cd001546. 10.1002/14651858.CD001546.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law WL, Choi HK, Lee YM, et al. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol 2007;14:2559–66. 10.1245/s10434-007-9434-4 [DOI] [PubMed] [Google Scholar]
- 26.Chok KS, Ng KK, Poon RT, et al. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg 2008;96:81–7. 10.1002/bjs.6358 [DOI] [PubMed] [Google Scholar]
- 27.Kamphues C, Bova R, Schricke D, et al. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol 2012;19:856–63. 10.1245/s10434-011-2041-4 [DOI] [PubMed] [Google Scholar]
- 28.Li Q-G, Li P, Tang D, et al. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol 2013;19:4060–5. 10.3748/wjg.v19.i25.4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DRKS trial document. Available: https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00016773 [Accessed 08 Apr 2020].
- 30.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Rev Panam Salud Publica 2015;38:506–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Slankamenac K, Nederlof N, Pessaux P, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–63. 10.1097/SLA.0000000000000948 [DOI] [PubMed] [Google Scholar]
- 32.Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7. 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 33.Nickel F, Probst P, Studier-Fischer A, et al. Minimally Invasive Versus open AbdominoThoracic Esophagectomy for esophageal carcinoma (MIVATE) - study protocol for a randomized controlled trial DRKS00016773. Trials 2021;22:41. 10.1186/s13063-020-04966-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12. 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 36.Ma G, Cao H, Wei R, et al. Comparison of the short-term clinical outcome between open and minimally invasive esophagectomy by comprehensive complication index. J Cancer Res Ther 2018;14:789–94. 10.4103/jcrt.JCRT_48_18 [DOI] [PubMed] [Google Scholar]
- 37.Huscher CGS, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232–7. 10.1097/01.sla.0000151892.35922.f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai J, Wei D, Gao CF, et al. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg 2011;28:331–7. 10.1159/000330782 [DOI] [PubMed] [Google Scholar]
- 39.Cui M, Li Z, Xing J, et al. A prospective randomized clinical trial comparing D2 dissection in laparoscopic and open gastrectomy for gastric cancer. Med Oncol 2015;32:241. 10.1007/s12032-015-0680-1 [DOI] [PubMed] [Google Scholar]
- 40.Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306–11. 10.1067/msy.2002.120115 [DOI] [PubMed] [Google Scholar]
- 41.Lee J-H, Han H-S, Lee J-H. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168–73. 10.1007/s00464-004-8808-y [DOI] [PubMed] [Google Scholar]
- 42.Jiang L, Yang K-H, Guan Q-L, et al. Laparoscopy-Assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc 2013;27:2466–80. 10.1007/s00464-012-2758-6 [DOI] [PubMed] [Google Scholar]
- 43.Kim H-H, Han S-U, Kim M-C, et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc 2013;84:123–30. 10.4174/jkss.2013.84.2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim Y-W, Yoon HM, Yun YH, et al. Long-Term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301). Surg Endosc 2013;27:4267–76. 10.1007/s00464-013-3037-x [DOI] [PubMed] [Google Scholar]
- 45.Yamashita K, Sakuramoto S, Kikuchi S, et al. Laparoscopic versus open distal gastrectomy for early gastric cancer in Japan: long-term clinical outcomes of a randomized clinical trial. Surg Today 2016;46:741–9. 10.1007/s00595-015-1221-4 [DOI] [PubMed] [Google Scholar]
- 46.Hyung WJ, Yang H-K, Han S-U, et al. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer 2019;22:214–22. 10.1007/s10120-018-0864-4 [DOI] [PubMed] [Google Scholar]
- 47.Takiguchi S, Fujiwara Y, Yamasaki M, et al. Laparoscopy-Assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg 2013;37:2379–86. 10.1007/s00268-013-2121-7 [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Xing J, Cai J, et al. Short-Term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: a multicenter randomized controlled trial. Surg Endosc 2019;33:33–45. 10.1007/s00464-018-6391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol 2016;34:1350–7. 10.1200/JCO.2015.63.7215 [DOI] [PubMed] [Google Scholar]
- 50.Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 2005;19:1172–6. 10.1007/s00464-004-8207-4 [DOI] [PubMed] [Google Scholar]
- 51.Straatman J, van der Wielen N, Cuesta MA, et al. Surgical techniques, open versus minimally invasive gastrectomy after chemotherapy (stomach trial): study protocol for a randomized controlled trial. Trials 2015;16:123. 10.1186/s13063-015-0638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haverkamp L, Brenkman HJF, Seesing MFJ, et al. Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial). BMC Cancer 2015;15:556. 10.1186/s12885-015-1551-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Veen A, Brenkman HJF, Seesing MFJ, et al. Laparoscopic versus open gastrectomy for gastric cancer (LOGICA): a multicenter randomized clinical trial. J Clin Oncol 2021;39:978–89. 10.1200/JCO.20.01540 [DOI] [PubMed] [Google Scholar]
- 54.van der Wielen N, Straatman J, Daams F, et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer 2021;24:258–71. 10.1007/s10120-020-01109-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Wielen N, Daams F, Rosati R, et al. Health related quality of life following open versus minimally invasive total gastrectomy for cancer: results from a randomized clinical trial. Eur J Surg Oncol 2022;48:553–60. 10.1016/j.ejso.2021.08.031 [DOI] [PubMed] [Google Scholar]
- 56.Wang G, Jiang Z, Zhao J, et al. Assessing the safety and efficacy of full robotic gastrectomy with intracorporeal robot-sewn anastomosis for gastric cancer: a randomized clinical trial. J Surg Oncol 2016;113:397–404. 10.1002/jso.24146 [DOI] [PubMed] [Google Scholar]
- 57.Ojima T, Nakamura M, Nakamori M, et al. Robotic versus laparoscopic gastrectomy with lymph node dissection for gastric cancer: study protocol for a randomized controlled trial. Trials 2018;19:409. 10.1186/s13063-018-2810-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Memon MA, Memon B. Laparoscopic D2 distal gastrectomy for advanced gastric cancer: a myth or a reality? Transl Gastroenterol Hepatol 2016;1:39. 10.21037/tgh.2016.05.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Medical Association . World Medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 60.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013: new guidance for content of clinical trial protocols. The Lancet 2013;381:91–2. 10.1016/S0140-6736(12)62160-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064286supp001.pdf (83.7KB, pdf)