Abstract
Background/Aims
Clinical trials are fundamental for the development of new medicines and patient participation is based on free consent. Our study sought to identify psychological characteristics that may influence patient willingness to participate in a clinical trial.
Methods
A total of 100 participants were invited to participate with 80% positive response rate. The psychological characteristics of each patient were evaluated using the following validated psychometric scales: Self-Efficacy Scale, Curiosity, Exploration Inventory-Trait, Social Support Satisfaction, State-Trait Anxiety Inventory and Social Avoidance and Distress, and Fear of Negative Evaluation.
Results
Patients who agreed to participate in the clinical trial were significantly younger than those who refused (p=0.028). There were no differences in sex, lifestyle, employment status, monthly income or education. After adjusting for age and sex, patients who agreed to participate scored significantly higher in the following: self-efficacy total score (p<0.001), effectiveness in adversity (p<0.001), social effectiveness (p<0.001) and initiation and persistence (p<0.001); social support total score (p<0.001), family satisfaction (p=0.015), friendship satisfaction (p<0.001), social activities satisfaction (p=0.002) and intimacy (p<0.001); total curiosity score (p<0.001), absorption (p<0.001) and exploration (p<0.001). Compared with patients who agreed to participate, those who refused scored significantly higher for both state (p<0.001) and trait anxiety (p<0.001), fear of negative evaluation (p<0.001) and social avoidance and distress (p<0.001).
Conclusions
Patients who were willing to participate in clinical trials exhibited different psychological characteristics to patients who refused. Specifically, they were more curious and self-efficacious, less anxious and reported a higher level of social support than patients who declined to participate. Identifying characteristics that condition the individual’s decision to participate in a clinical trial has important implications for the development of patient-focused communication strategies and improved recruitment approaches.
Keywords: patient participation, informed consent, randomised controlled trial, clinical decision-making, community-based participatory research
WHAT IS ALREADY KNOWN ON THIS TOPIC
The heterogeneity of patient psychological profiles is an important consideration in the context of clinical trial participation: personality traits are known to significantly influence subjects’ willingness to volunteer for phase I studies.
Healthy subjects that participate in phase I clinical trials are less anxious and less socially avoidant than those who refuse to participate.
These psychological factors have an impact on study outcomes.
WHAT THIS STUDY ADDS
We have identified for the first time specific individual personality traits that influence patient participation in phase II and III clinical trials.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Individual personality traits could serve as useful predictors of clinical trial participation, and should be considered in the design of future clinical trials and recruitment strategies.
Introduction
Clinical trials are fundamental for the development of new medicines. However, poor patient recruitment is a persistent problem: patient recruitment rates are slow in the majority of clinical trial sites (around 80%) and up to 37% of investigation sites fail to achieve patient recruitment targets, with 11% not recruiting a single patient.1 2 Patient recruitment is the leading cause of trial delays, underscoring the need for more effective recruitment approaches.3 Each clinical trial patient is unique, and participation is always preceded by free and informed consent. Understanding how subject’s characteristics condition the decision to participate in a clinical trial may help improve recruitment approaches and trial design. Studies have shown that certain personality traits and interpersonal values are strong predictors of the decision to volunteer for phase I studies.4 Participants in phase I studies are recognised as more benevolent, extrovert and less anxious.4 5 Furthermore, a direct correlation has been demonstrated between anxiety traits and the incidence of adverse events reported during phase I studies.6 7 Although there may be some similarities, results obtained in healthy volunteers cannot be extrapolated to patient populations, mainly because financial compensation is provided for participation in phase I but not in phase II/III studies.8
Research on the characteristics that influence patient willingness to participate in phase II/III clinical trials is largely insufficient. Therefore, we sought to fill this knowledge gap and to better understand the role of individual self-efficacy, social support, curiosity and anxiety in the decision to participate in phase II/III clinical trials.9–15
Methods
Study design and participants
This single-centre, prospective, observational and descriptive study included 100 consecutive adult patients who had been invited to participate in a phase II or III clinical trial, and who fulfilled the eligibility criteria and consented to participate in the present study.
Patients were considered eligible for the present study if they fulfilled the following criteria: male or female patients who had been invited to enter a phase II or III clinical trial; aged 18–85 years at the moment of signing informed consent; intellectually, visually and auditorily capable; fluent in, and able to read, the language in which the study assessments are administered (ie, Portuguese). Patients were excluded if they had a clinical condition that precluded understanding informed consent or any condition that could render them unsuitable for the study.
Based on their willingness to participate in a clinical trial, patients were separated in two groups: those who accepted (group 1) and those who declined (group 2) to participate. Patients from group 1 were managed according to the requisites of the clinical trials in which they agreed to participate. Patients from group 2 were managed according to routine clinical practice (ie, received the standard of care).
Patient enrolment, ethical considerations and data collection
Patients were enrolled between August 2019 and April 2021, at a single institution (Hospital Pedro Hispano, Unidade Local de Saúde de Matosinhos, ULSM, Matosinhos, Portugal). Study participants had been invited to participate in 1 of 14 clinical trials carried out by the Departments of Cardiology, Dermatology, Endocrinology, Gynaecology, Neurology and Oncology.
The investigator ensured that participants’ confidentiality was maintained, and all data and records generated during this study were kept confidential. No risk or potential breach of privacy was identified. All participants were provided with reasonable time and conditions to properly complete all questionnaires and scales. All study material was self-administered in a designated room, and all patients filled out questionnaires on demographics, lifestyle habits and socioeconomic level. Several scales were used to assess psychological characteristics that could influence patient’s willingness to participate in the proposed clinical trial, specifically the Self-Efficacy Scale (SES, Sherer et al16), Curiosity, Exploration Inventory-Trait (CEI-T, Kashdan et al17), Social Support Satisfaction Scale (SSSS, Ribeiro,18 1999), State-Trait Anxiety Inventory (STAI, Spielberger et al,19 1970), Social Avoidance and Distress (SAD, Watson and Friend15) Scale and Fear of Negative Evaluation (FNE, Watson and Friend15). All scales had been previously validated for the Portuguese population.
Self-Efficacy Scale
Self-efficacy is a cognitive variable with a motivational function, and depending on each person’s perception of their abilities will affect their behaviour, motivation and emotional reaction. Thus, the higher the perception of self-efficacy, the more persistent and vigorous the individual’s effort will be. Conversely, those with a lower perception of self-efficacy avoid placing themselves in situations that they consider themselves incapable of managing.10
SES was developed by Sherer et al in 1982. The SES has 23 items and is divided into 2 subscales: a general self-efficacy subscale (17 items) and a social self-efficacy subscale (6 items). This scale focuses on perseverance in the face of adversity and willingness to initiate and expend effort.16 This scale was validated for the Portuguese population using 15 items with a 7-point Likert-type scale. Three dimensions are assessed in the SES: (a) initiation and persistence; (b) efficacy when facing adversities and (c) social efficacy.20
Social Support Satisfaction Scale
Social support is one of the main concepts in health psychology and is considered an important variable in the health of each individual. Social support allows stress relief in a crisis and plays a positive role in recovery from illness.11 The SSSS is a measure of knowledge of social support, taking into account that this perception is an essential dimension in the cognitive and emotional processes linked to well-being and quality of life.21 This scale consists of 15 statements, with a Likert-type scale ranging from 1 to 5 according to the grade of agreement, distributed over 4 dimensions: (a) satisfaction with friends (5 items); (b) intimacy (4 items); (c) satisfaction with family (3 items) and (d) social activities (3 items). The total score for the scale can be between 15 and 75, with a higher score corresponding to a perception of greater social support.21
Curiosity and Exploration Inventory-Trait
Curiosity is one of the personality traits in human motivation, which causes people to devote more attention to a particular activity, search for more information and better recall information. Consequently, curiosity is thought to play a key role in social relationships, happiness and meaning in life. It is also considered important in the development of psychopathology, as intolerance to uncertainty is a major risk factor for anxiety disorders.9 The CEI-T is a curiosity scale designed to measure the subject’s interest in, and recognition and seeking of, new and challenging experiences. This scale assesses two main dimensions: (a) exploration (four items), seeking challenge and novelty and (b) absorption (three items), reflects the ability to self-regulate attention to allow for immersion in these novel and challenging activities.9 This is a 7-item scale. The subject answers each item using a 7-point scale ranging from 1 (strongly disagree) to 7 (strongly agree).17 The Portuguese CEI-T version consists of an adaptation of the original scale, and shows good psychometric properties.17
State-Trait Anxiety Inventory
The distinction between state anxiety and trait anxiety is particularly important in anxiety studies. According to Spielberger et al, state anxiety refers to a transitory moment in an individual’s emotional life, whereas trait anxiety refers to a tendency to react more frequently and intensely than state anxiety.13 The STAI Scale has 40 items, consisting of 2 questionnaires of 20 items each. First, the State Anxiety Scale (S-Anxiety) (Y-1) is applied, followed by the Trait Anxiety Scale (T-Anxiety) (Y-2). The main purpose of the Y-1 scale is to assess anxiety as a state (ie, how the person feels at the moment), while the Y-2 scale assesses anxiety as a trait (ie, how the person usually feels). A 4-point scale is used, corresponding to the degree of anxiety for each item, where 1 and 4 represent the minimum and maximum levels of anxiety, respectively. The overall scale scores range from 20 to 80 points on both STAI scales. Higher scores reflect greater trait anxiety: STAI scores are classified as ‘no or low anxiety’ (20–37), ‘moderate anxiety’ (38–44) and ‘high anxiety’ (45–80). The Portuguese version, which shows good psychometric properties, was used in the present study.22
Social Avoidance and Distress Scale and Fear of Negative Evaluation Scale
Social context anxiety was defined as an experience associated with feelings of discomfort, fear and distress in social situations and deliberate avoidance of social situations. These two aspects were combined in the SAD Scale. The fear of receiving negative evaluations from others was evaluated using the FNE Scale.15 SAD measures general social anxiety, and consists of a 28-question questionnaire with a dichotomous response scale. Scores can range from a minimum of 0 to a maximum of 28, with higher SAD scores reflecting a greater tendency to avoid social interactions.23 The FNE Scale consists of 30 questions with a dichotomous response, with a total score ranging from 0 to 30. Higher scores suggest a greater likelihood of nervousness when being evaluated by others. Both scales show high internal consistency for the Portuguese population.23
Statistical analysis
Statistical analyses were performed with R software programming language (V.4.1.0; GPL, Auckland University, New Zealand).24 Quantitative variables were tested for normality using the Shapiro-Wilk test. Data are presented as the mean and SD for normally distributed variables, and as the median and IQR in all other cases. For qualitative variables, descriptive statistics included absolute and relative frequencies.
For inferential statistical analysis, the criterion for statistical significance was set at a type I error of 5% (p<0.05; two-sided tests). Independent t-tests were used for comparison of normally distributed data with homogeneity of variances. Non-normally distributed quantitative-dependent variables were compared using the Mann-Whitney rank sum test when comparing two independent groups. Analysis of covariance was used to adjust the association of clinical trial participation with age and sex.
For ordinal variables, non-parametric tests for quantitative variables were used as appropriate. For qualitative variables, Pearson’s χ2 test was used for independent samples whenever Cochran’s rules were met. In all other cases, Fisher’s exact test was used to test for independence.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Demographic and socioeconomic characterisation
A convenience sample of 100 patients was enrolled in the current study. Based on their willingness to participate in the proposed clinical trial, participants were divided into two groups: those who agreed to participate (group 1, n=80) and those who refused (group 2, n=20).
Patients were mainly invited to participate in clinical trials in neurology (60%) and endocrinology (17%). Clinical trials proposed to patients by therapeutic area are described in online supplemental table S1.
bmjoq-2022-002044supp001.pdf (109.2KB, pdf)
Sociodemographic characteristics for both groups are summarised in online supplemental table S1. Patients from group 1 were younger than those from group 2 (median (IQR): 64 (18.2) vs 68 (11.2) years; U=545.5; p=0.028). Patients were equally distributed between groups in terms of sex (group 1, 52.5% males; group 2, 50.0% males; χ2(1) =6.3×10−31; p=1.00).
No significant differences were observed between groups 1 and 2 in terms of smoking habits (21.3% vs 15.0%; χ2(1)=0.10; p=0.76), coffee intake (71.3% vs 60.0%; χ2(1)=0.50; p=0.48), physical activity (43.8% vs 25.0%; χ2(1)=1.6; p=0.20) or alcoholic beverage consumption (53.8% vs 50.0%; χ2(1)=0.003; p=0.96).
Most patients were retired or employed at the time of study inclusion (91.3% and 80.0% of patients in groups 1 and 2, respectively). There was no significant association between employment status and willingness to participate in a clinical trial (p=0.21). In both groups, the most common income range was €501–1000/month, representing 37.5% and 50.0% of patients in groups 1 and 2, respectively. There were no significant differences between groups in monthly income (U=631; p=0.72).
Regarding academic qualifications, most patients had completed only the first cycle (4 years) of basic education, corresponding to 37.5% and 55.0% in groups 1 and 2, respectively. Only 15.0% of patients had completed a bachelor’s degree or higher. No significant differences were observed between groups (U=956; p=0.16).
Patient psychological characteristics
Self-Efficacy Scale
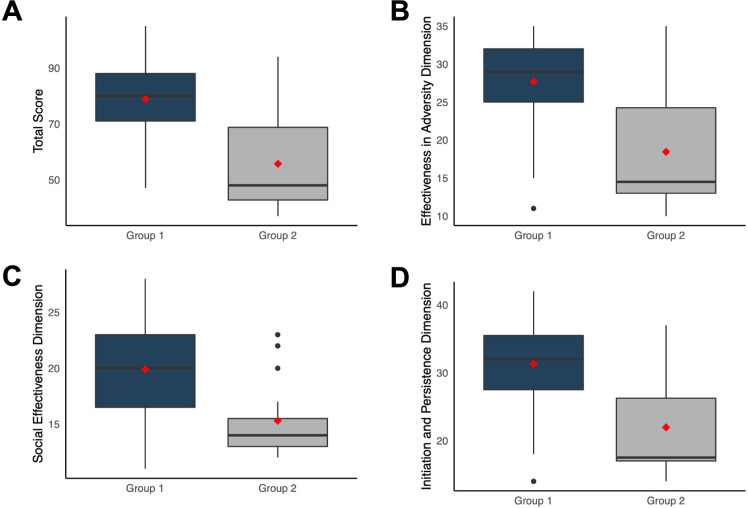
Patients who agreed to participate in a clinical trial (group 1) had a significantly higher (U=1239; p<0.001; figure 1A) total self-efficacy score (median (IQR), 80 (17)) compared with patients who refused to participate (group 2) (48 (26)). Group 1 also registered higher scores than group 2 for all three dimensions: effectiveness in adversity (median (IQR), 29 (7) vs 14.5 (11.2); U=1211; p<0.001; figure 1B); social effectiveness (20 (6.5) vs 14 (2.5); U=1208; p<0.001; figure 1C) and initiation and persistence (32 (8) vs 17.5 (9.2); U=1202; p<0.001; figure 1D). After adjusting for age and sex, all results remained statistically significant (p<0.001).
Figure 1.
Self-Efficacy Scale results according to willingness to participate in a clinical trial. Total score (A), and dimensions related to effectiveness in adversity (B), social effectiveness (C) and initiation and persistence (D) were significantly higher in patients who accepted to participate in a clinical trial (group 1) as compared with patients who refused (group 2).
Social Support Satisfaction Scale
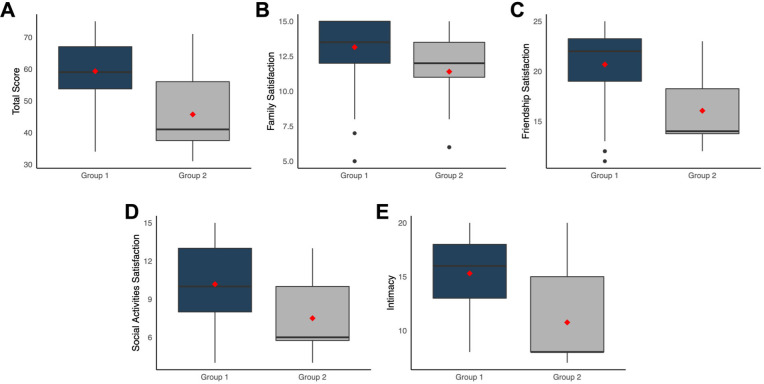
Regarding social support, total score was significantly higher in patients who agreed to participate in a clinical trial (group 1) than those who did not (group 2) (median (IQR), 59 (13.2) vs 41 (18.5); U=1253.5; p<0.001; figure 2A). This was reflected in all dimensions of the SSSS, namely family satisfaction (median (IQR), 13.5 (3) vs 12 (2.5); U=1075.5; p=0.015; figure 2B); friendship satisfaction (22 (4.2) vs 14 (4.4); U=1278.5; p<0.001; figure 2C); social activities satisfaction (10 (5) vs 6 (4.2); U=1161.5; p=0.002; figure 2D) and intimacy (16 (5) vs 8 (7); U=1270; p<0.001; figure 2E). After adjusting for age and sex, all results remained statistically significant (p<0.001).
Figure 2.
Social Support Satisfaction Scale results according to willingness to participate in a clinical trial. Patients who accepted to participate in a clinical trial (group 1) scored significantly higher than patients who refused (group 2) regarding total score (A), family satisfaction (B), friendship satisfaction (C), social activities satisfaction (D) and intimacy (E).
Curiosity and Exploration Inventory-Trait
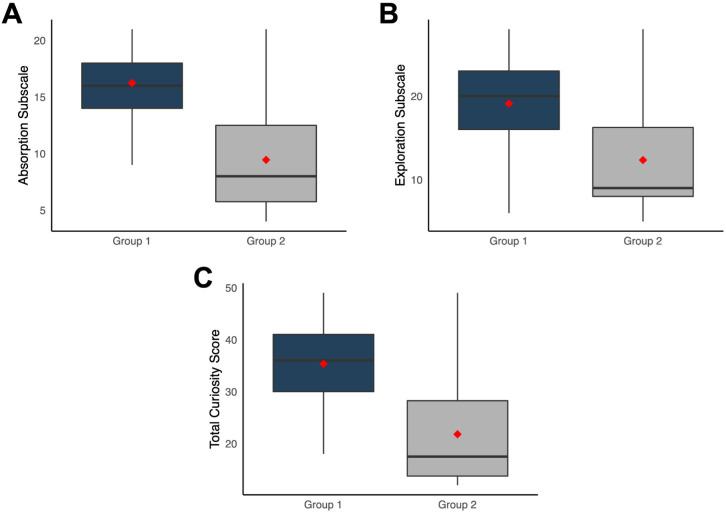
Patients who agreed to participate in a clinical trial (group 1) scored significantly higher than those who refused (group 2) in both the absorption (median (IQR), 16 (4) vs 8 (6.8); U=1386; p<0.001; figure 3A) and exploration (20 (7) vs 9 (8.2); U=1250; p<0.001; figure 3B) subscales of CEI-T. This resulted in a significantly higher total curiosity score for patients who agreed to participate than those who refused (36 (11) vs 17.5 (14.5); U=1319; p<0.001; figure 3C). After adjusting for age and sex, all results remained statistically significant (p<0.001).
Figure 3.
Curiosity and Exploration Inventory-Trait results according to willingness to participate in a clinical trial. Patients who accepted to participate in a clinical trial (group 1) scored significantly higher than patients who refused (group 2) in absorption (A), exploration (B) and total score (C).
State-Trait Anxiety Inventory
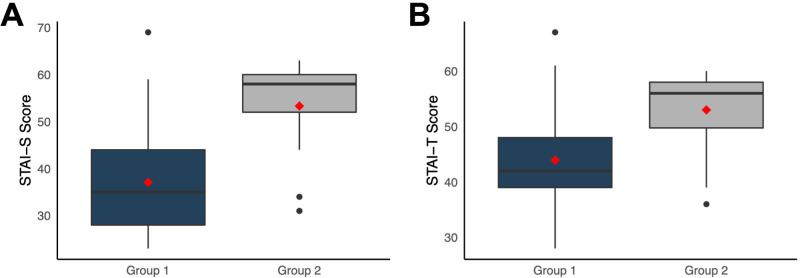
Patients who refused to participate in a clinical trial (group 2) scored significantly higher than patients who agreed (group 1) for both state (STAI-S subscale; median (IQR), 58 (8) vs 35 (16); U=209; p<0.001; figure 4A) and trait anxiety (STAI-T subscale; 56 (8.2) vs 42 (9); U=323; p<0.001; figure 4B). After adjusting for age and sex, all results remained statistically significant (p<0.001).
Figure 4.
State-Trait Anxiety Inventory results according to willingness to participate in a clinical trial. Patients who refused clinical trial participation (group 2) showed a significantly higher score than patients who accepted (group 1) for state STAI-S subscale (A) and trait anxiety STAI-T subscale (B).
Fear of Negative Evaluation and Social Avoidance and Distress Scales
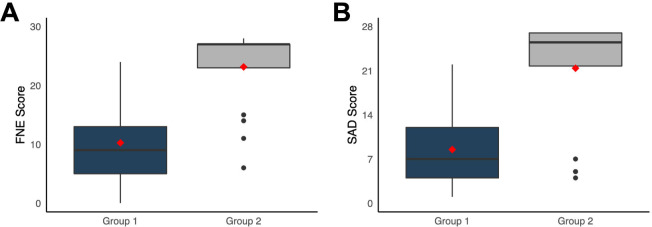
Patients who refused to participate in a clinical trial (group 2) scored significantly higher on FNE than those who agreed to participate (group 1) (median (IQR), 27 (4) vs 9 (8); U=127; p<0.001; figure 5A). Accordingly, group 2 patients also obtained higher scores on SAD (25.5 (5.2) vs 7 (8); U=182; p<0.001; figure 5B). After adjusting for age and sex, all results remained statistically significant (p<0.001).
Figure 5.
Fear of Negative Evaluation (FNE) scale (A) and Social Avoidance and Distress (SAD) scale (B) results according to willingness to participate in a clinical trial. Patients who refused clinical trial participation (group 2) scored significantly higher than patients who accepted (group 1) patients in both scales.
Discussion
To the best of our knowledge, this is the first study to explore the psychological characteristics that influence patients’ decision to participate in a therapeutic (phase II or III) clinical trial. Our findings show that patients with greater perceived self-efficacy, curiosity and social support are more willing to participate in therapeutic clinical trials. Conversely, patients with higher levels of anxiety are less likely to participate. Our findings confirm our initial hypothesis that certain personality traits significantly influence patient willingness to participate in therapeutic phase II and III clinical trials, in line with similar results reported for healthy subjects participating in phase I clinical trials.25
Patient age is an important factor to consider. According to Petty et al.,26 and Forsat et al.,27 elderly people are under-represented in clinical trials because their willingness to be included decreases with time. In line with these findings, we found that older patients were less willing to participate in a clinical trial than younger patients. We observed no sex-related differences, in agreement with the findings of Labots et al,28 who found no evidence of underrepresentation of either sex in clinical trials.
Published studies suggest that individuals of lower socioeconomic status are less likely to participate in clinical trials.29 30 However, in our study socioeconomic level did not influence patient willingness to participate. Also in line with other findings,31 our data show that level of education does not influence the likelihood of participating in a clinical study.
Willingness to participate in a clinical trial is influenced by an individual’s perceived self-efficacy beliefs.32 Using SES, we found that patients who agreed to participate in clinical trials had a higher perception of self-efficacy and were more persistent. By contrast, patients who refused to participate had a lower perception of self-efficacy, and avoided situations they perceived to be complex or considered themselves incapable of managing. When required to make a decision, patients have a propensity to choose the direction in which they feel more comfortable. Therefore, the greater the perception of self-efficacy, the greater the probability of entering a clinical trial. In terms of curiosity, patients who agreed to participate in a clinical trial scored significantly higher both in the absorption and exploration CEI-T subscales. Patients with higher scores in trait-curiosity and perceived self-efficacy were more willing to participate in a therapeutic clinical trial. Similar findings have been reported in healthy subjects: individuals more willing to participate in phase I studies showed higher curiosity/exploration scores and greater perceived initiative/persistence than those who refused.5
In our study, we observed a strong relationship between social support and willingness to participate in a clinical trial, as corroborated by findings indicating that patients participating in clinical trials report a higher level of social support.33 Family and friends play an important role because they can influence the decision of a subject to join a clinical trial or not.34 35
As previously mentioned, higher SAD scores tend to reflect a greater probability of avoiding social interactions, while higher FNE scores suggest a greater likelihood of nervousness when being evaluated by others.15 In our study, patients willing to participate in a clinical trial had significantly lower FNE and SAD scores than those who refused to participate. Furthermore, patients willing to participate in a clinical trial had lower STAI-S (state anxiety) and STAI-T (trait anxiety) scores. Overall, our data suggest that patients participating in clinical trials are less anxious and less socially avoidant than those who refuse to participate. Similar findings have been reported for healthy subjects participating in phase I clinical trials.6 23 Automated tools are increasingly used to aid clinical trial recruitment.36 The present findings could be used to tailor artificial intelligence algorithms in order to ensure more effective identification of recruitable patients.36 37
The main limitations of this work are the relatively small number of subjects (n=100), corresponding to a homogenous patient population from a single hospital in a Western country. Studies in larger populations with cultural and linguistic differences will be required to assess the generalisability of the results to other settings and to determine whether the findings are valid across different therapeutic indications.
Studying patients’ personality traits is very important as they may affect study outcomes (eg, the likelihood of reporting adverse events during a clinical trial).6 38 It is crucial to conduct further studies in patients to understand whether personality traits may condition clinical trial outcomes, induce placebo or nocebo effects39 or even affect unblinding rates. Several studies of efficacy outcomes have investigated the influence of specific personal characteristics on placebo responses.39 40 Regarding the association between increased self-awareness of adverse events and the risk of unblinding, a recent trial of patients with Alzheimer’s disease found that the perception of headaches in the treatment arm may have conditioned patients’ expectations as to the drugs’ effect, as well as their responses to quality of life questionnaires.41 42
Conclusion
To our knowledge, this is the first study to examine individual psychological characteristics that influence patient willingness to participate in phase II and III clinical trials. Clinical trials are conducted in a self-selected population of consenting participants. Our findings suggest that patients who are willing to participate in clinical trials exhibit personality traits distinct from those of patients who refuse to participate. In general, patients who agree to participate show more positive traits and fewer negative traits. This means that participants in a clinical trial may not be truly representative of the target population of patients who will be treated with the drug under development. The impact of this self-selection bias on study outcomes is unknown and warrants further research. On the other hand, while respecting free informed consent, it is important to develop communication strategies that do not exclude patients with fewer positive traits and more negative personality traits in order to increase the likelihood of their participation in clinical trials and, ultimately, to reduce self-selection bias.
Acknowledgments
The authors thank Joana Antão for support with statistical analysis. Thanks also to the staff at the Departments of Cardiology, Dermatology, Endocrinology, Gynecology, Neurology and Oncology at Pedro Hispano Hospital and to all patients for their essential contributions to this work.
Footnotes
Contributors: Conceptualisation: RG, VTC and LA; methodology: RG and LA; formal analysis: RG and LA; analysis and interpretation of data: RG, VTC and LA; writing—original draft preparation: RG; writing—review and editing: RG, VTC and LA; writing—final approval of the version to be published: RG, VTC and LA; supervision: VTC and LA; project administration: RG and LA; guarantor: RG, VTC and LA. All authors have read and approved the final version of the manuscript.
Funding: This work was supported by BlueClinical, Ltd.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
All clinical trials had been previously approved by the National Ethics Committee for Clinical Research (CEIC; Lisbon, Portugal) and the National Authority of Medicines and Health Products (INFARMED; Lisbon, Portugal). The current study was approved by the local ethics committee and the board of the Hospital Pedro Hispano, and was conducted in accordance with the requirements established in Portuguese legislation on biomedical research, personal data protection and bioethics. Prior to inclusion in the study, written informed consent was obtained from all participants.
References
- 1.Brøgger-Mikkelsen M, Ali Z, Zibert JR, et al. Online patient recruitment in clinical trials: systematic review and meta-analysis. J Med Internet Res 2020;22:e22179. 10.2196/22179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhari N, Ravi R, Gogtay NJ, et al. Recruitment and retention of the participants in clinical trials: challenges and solutions. Perspect Clin Res 2020;11:64–9. 10.4103/picr.PICR_206_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006;6:34. 10.1186/1471-2288-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida L, Vaz-da-Silva M, Coelho R, et al. Interpersonal values of healthy subjects who volunteer for phase I clinical trials. Pharmaceut Med 2009;23:299–303. 10.1007/BF03256785 [DOI] [Google Scholar]
- 5.Almeida L, Kashdan TB, Coelho R, et al. Healthy subjects volunteering for phase I studies: influence of curiosity, exploratory tendencies and perceived self-efficacy. Int J Clin Pharmacol Ther 2008;46:109–18. 10.5414/cpp46109 [DOI] [PubMed] [Google Scholar]
- 6.Almeida L, Kashdan TB, Nunes T, et al. Who volunteers for phase I clinical trials? influences of anxiety, social anxiety and depressive symptoms on self-selection and the reporting of adverse events. Eur J Clin Pharmacol 2008;64:575–82. 10.1007/s00228-008-0468-8 [DOI] [PubMed] [Google Scholar]
- 7.Almeida L, Coelho R, Albino-Teixeira A, et al. Adverse non-drug-related complaints by healthy volunteers in phase I studies compared to the healthy general population. Int J Clin Pharmacol Ther 2008;46:574–83. 10.5414/cpp46574 [DOI] [PubMed] [Google Scholar]
- 8.Fisher JA, McManus L, Kalbaugh JM, et al. Phase I trial compensation: how much do healthy volunteers actually earn from clinical trial enrollment? Clin Trials 2021;18:477–87. 10.1177/17407745211011069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashdan TB, Gallagher MW, Silvia PJ, et al. The curiosity and exploration Inventory-II: development, factor structure, and Psychometrics. J Res Pers 2009;43:987–98. 10.1016/j.jrp.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandura A. Self-Efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215. 10.1037//0033-295x.84.2.191 [DOI] [PubMed] [Google Scholar]
- 11.Rodin J, Salovey P. Health psychology. Annu Rev Psychol 1989;40:533–79. 10.1146/annurev.ps.40.020189.002533 [DOI] [PubMed] [Google Scholar]
- 12.Revista Portuguesa de Psicologia - Santos, S. C. e Silva, D. R . Adaptação do State-Trait anxiety inventory (STAI) – form Y para a população portuguesa: Primeiros dados. Revista Portuguesa de Psicologia, 32, 85-98, 1997. Available: https://sites.google.com/site/revistaportuguesadepsicologia/numeros-publicados/vol-32-1997/resumo-32-85 [Accessed 23 March 2022].
- 13.Spielberger CD, Sydeman SJ. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In: The use of psychological testing for treatment planning and outcome assessment. Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc, 1994: 292–321. [Google Scholar]
- 14.Farmer AS, Kashdan TB, Affective KTB. Affective and self-esteem instability in the daily lives of people with generalized social anxiety disorder. Clin Psychol Sci 2014;2:187–201. 10.1177/2167702613495200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson D, Friend R. Measurement of social-evaluative anxiety. J Consult Clin Psychol 1969;33:448–57. 10.1037/h0027806 [DOI] [PubMed] [Google Scholar]
- 16.Sherer M, Maddux JE, Mercandante B, et al. The self-efficacy scale: construction and validation. Psychol Rep 1982;51:663–71. 10.2466/pr0.1982.51.2.663 [DOI] [Google Scholar]
- 17.Kashdan TB, Rose P, Fincham FD. Curiosity and exploration: facilitating positive subjective experiences and personal growth opportunities. J Pers Assess 2004;82:291–305. 10.1207/s15327752jpa8203_05 [DOI] [PubMed] [Google Scholar]
- 18.Pais-Ribeiro J. Escala de Satisfação com o Suporte Social (ESSS) [Satisfaction with Social Support Scale]. Análise Psicológica 1999;3:547–58. [Google Scholar]
- 19.Spielberger CD, Gorsuch RL, R.E L. Manual for the State-Trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press, 1970. [Google Scholar]
- 20.Pais-Ribeiro J. Adaptação de uma escala de avaliação da auto-eficácia geral. Epub ahead of 366 print 5 Jun 1995, 1995. 10.13140/2.1.3971.1682 [DOI] [Google Scholar]
- 21.Ribeiro JLP. Escala de Satisfação com O Suporte social. Lisboa: Placebo Editora, LDA, 2011. [Google Scholar]
- 22.Silva D, Campos R. Alguns dados normativos do Inventário de Estado-Traço de Ansiedade – forma Y (STAI – Y) – de Spielberger, para a população portuguesa. Rev Port Psicol;33. [Google Scholar]
- 23.Gouveia P, et al. Ansiedade social: Utilização DOS questionários de auto-resposta sad, FNE E SISST NuMA população Portuguesa. Psiquiatria Clínica 1986;7:43–8. [Google Scholar]
- 24.R: the R project for statistical computing. Available: https://www.r-project.org/ [Accessed 18 June 2021].
- 25.Almeida L, Falcão A, Coelho R. The role of socioeconomic conditions and psychological factors in the willingness to volunteer for phase I studies. Pharm Med 2008;22:367–74. [Google Scholar]
- 26.Petty DR, Zermansky AG, Raynor DK, et al. "No thank you": why elderly patients declined to participate in a research study. Pharm World Sci 2001;23:22–7. 10.1023/a:1011276924820 [DOI] [PubMed] [Google Scholar]
- 27.Forsat ND, Palmowski A, Palmowski Y, et al. Recruitment and retention of older people in clinical research: a systematic literature review. J Am Geriatr Soc 2020;68:2955-2963. 10.1111/jgs.16875 [DOI] [PubMed] [Google Scholar]
- 28.Labots G, Jones A, de Visser SJ, et al. Gender differences in clinical registration trials: is there a real problem? Br J Clin Pharmacol 2018;84:700–7. 10.1111/bcp.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol 2019;44:148–72. 10.1016/j.cpcardiol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 30.Baquet CR, Commiskey P, Daniel Mullins C, et al. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev 2006;30:24–33. 10.1016/j.cdp.2005.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gitanjali B, Raveendran R, Pandian DG, et al. Recruitment of subjects for clinical trials after informed consent: does gender and educational status make a difference? J Postgrad Med 2003;49:109–13. [PubMed] [Google Scholar]
- 32.Bandura A. Guide for constructing self-efficacy scales. In: Self efficacay beliefs of adolescents. USA: Information Age Publishing, 2005: 307–37. [Google Scholar]
- 33.Reblin M, Uchino BN. Social and emotional support and its implication for health. Curr Opin Psychiatry 2008;21:201–5. 10.1097/YCO.0b013e3282f3ad89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book 2019;39:105–14. 10.1200/EDBK_243729 [DOI] [PubMed] [Google Scholar]
- 35.Hajjaj FM, Salek MS, Basra MKA, et al. Non-Clinical influences on clinical decision-making: a major challenge to evidence-based practice. J R Soc Med 2010;103:178–87. 10.1258/jrsm.2010.100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weissler EH, Naumann T, Andersson T, et al. The role of machine learning in clinical research: transforming the future of evidence generation. Trials 2021;22:537. 10.1186/s13063-021-05489-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calaprice-Whitty D, Galil K, Salloum W, et al. Improving clinical trial participant Prescreening with artificial intelligence (AI): a comparison of the results of AI-Assisted vs standard methods in 3 oncology trials. Ther Innov Regul Sci 2020;54:69–74. 10.1007/s43441-019-00030-4 [DOI] [PubMed] [Google Scholar]
- 38.Almeida L, Falcão A, Vaz-da-Silva M, et al. Personality characteristics of volunteers in phase 1 studies and likelihood of reporting adverse events. Int J Clin Pharmacol Ther 2008;46:340–8. 10.5414/cpp46340 [DOI] [PubMed] [Google Scholar]
- 39.Kern A, Kramm C, Witt CM, et al. The influence of personality traits on the placebo/nocebo response: a systematic review. J Psychosom Res 2020;128:109866. 10.1016/j.jpsychores.2019.109866 [DOI] [PubMed] [Google Scholar]
- 40.Jakšić N, Aukst-Margetić B, Jakovljević M. Does personality play a relevant role in the placebo effect? Psychiatr Danub 2013;25:17–23. [PubMed] [Google Scholar]
- 41.Tampi RR, Forester BP, Agronin M. Aducanumab: evidence from clinical trial data and controversies. Drugs Context 2021;10:1–9. 10.7573/dic.2021-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu KY, Howard R. Can we learn lessons from the FDA's approval of aducanumab? Nat Rev Neurol 2021;17:715–22. 10.1038/s41582-021-00557-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2022-002044supp001.pdf (109.2KB, pdf)
Data Availability Statement
Data are available on reasonable request.