Abstract
Neurons that express a specific molecular marker are activated by ‘electroacupuncture’ stimulation. They can then mediate the treatment’s anti-inflammatory effects in a mouse model of the inflammatory condition sepsis.
Neuronal networks have evolved to control organ functions. A technique called electroacupuncture, in which specific points on the body called acupoints are stimulated electrically, has long been used to activate these networks and thereby modulate the functions of certain organs to treat various disorders. It is a key part of an emerging medical field known as bioelectronic medicine1,2. However, little is known about the neuronal networks that mediate the effects of electroacupuncture at specific acupoints1,3. On page 641, Liu et al.4 show in mice that a set of neurons expressing the protein Prokr2 are needed for electrical stimulation of a hindlimb acupoint to rein in the unbridled inflammatory responses that characterize lethal sepsis.
Acupoints have been selected on the basis of people’s responses to stimulation at these sites1. However, some acupoints are controversial, because their stimulation can produce different effects depending on the type of stimulation. The leg Zusanli (ST36) acupoint, located about 2 centimetres below the knee in humans, is the most frequently stimulated site for relieving inflammation. However, electro acupuncture stimulation (ES) of ST36 can induce opposing effects depending on the intensity. Whereas high-intensity ES of ST36 activates the sympathetic nervous system, which supports ‘fight or flight’ responses to stress, low-intensity stimulation activates the parasympathetic nervous system, which regulates physiological functions that occur during rest. Determining the neuronal networks required to activate each system will enable us to design more reliable, specific and effecti ve treatments than are currently available.
Stimulation of ST36 has the potential to suppress severe inflammation in various diseases, including infections and autoimmune disorders. A dramatic example is severe sepsis, a condition in which the body’s inflammatory reactions to an infection run out of control, eventually damaging organs and becoming more dangerous than the original infection5. Sepsis is a leading cause of death in hospitals, accounting for about 9% of all deaths in the United States6, despite the use of modern antibiotics to treat initial infections.
Previous work showed that low-intensity ES of ST36 activates a parasympathetic network in which the vagal nerve carries signals from the brain to the adrenal glands, which are located on top of the kidneys, to curb severe inflammation7. Liu et al. injected mice with a bacterial molecule to provoke an uncontrolled inflammatory reaction, which led to the production of harmful levels of inflammatory factors. The authors showed that activation of the vagal–adrenal network is mediated by Prokr2-expressing neurons at the acupoint. Selectively destroying these neurons prevented low-intensity ST36 ES from dampening the inflammation, but did not alter the ability of high-intensity ST36 ES to activate the sympathetic nervous system. Conversely, artificial activation of Prokr2-expressing neurons mimics the effects of low-intensity ST36 ES, also activating the vagal–adrenal network and controlling inflammation. These results provide, for the first time, a molecular marker of neurons that might be targeted in designing specific methods of stimulation to control discrete organ functions.
The vagal–adrenal network activated by low-intensity ST36 ES was previously found to exert its anti-inflammatory effects by inducing the production of catecholamine molecules by the adrenal glands7. Catecholamines control many processes in the healthy body, and are used to treat various disorders, including low blood pressure. The catecholamines dopamine and noradrenaline can restrain the inflammatory response by inhibiting specific immune cells and their release of inflammatory factors; they do this by activating dopaminergic type 1 receptors and β2-adrenergic receptors on these cells, respectively7–9.
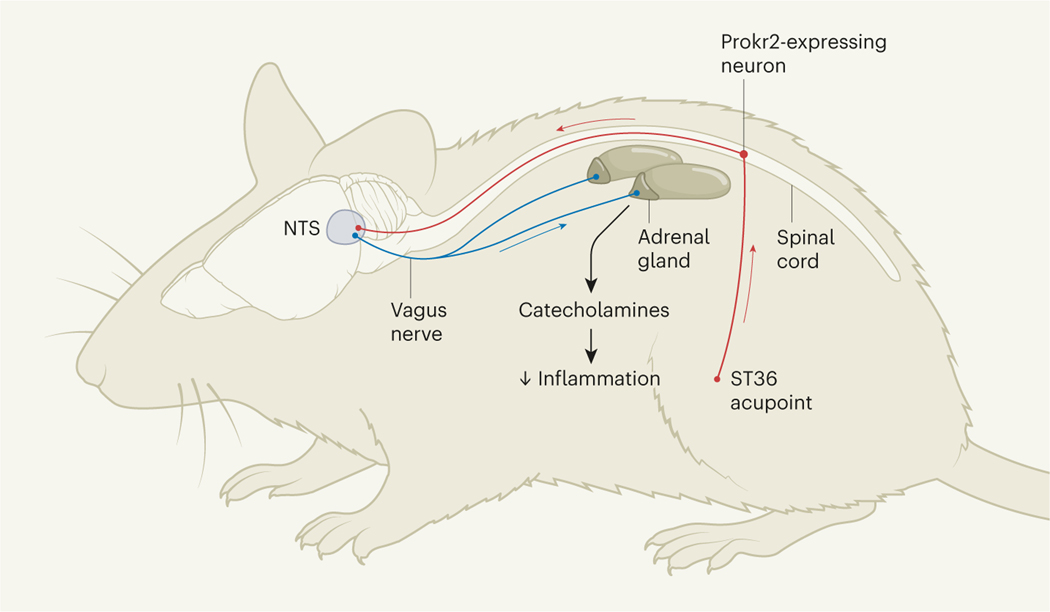
Liu and colleagues’ findings reveal the cellular route by which ST36 ES activates the vagal–adrenal network. The cell bodies of the sensory neurons that express Prokr2 are located in structures called dorsal root ganglia in the lower spinal cord, and have long processes in the sciatic (hindlimb) nerve that innervate the ST36 acupoint. These neurons carry sensory information from the hindlimb up the spinal cord towards the nucleus tractus solitarius (NTS), a structure in the brain that receives sensory information from the body’s internal organs. In the NTS, Prokr2-expressing neurons coordinate various physiological functions by activating discrete networks of neurons, such as those in the dorsal motor nuclei, brain regions that contain the cell bodies of neurons that make up the vagus nerve10.
The distribution of Prokr2-expressing neurons provides crucial information about the nerves that activate this vagal–adrenal network (Fig. 1). The ST36 acupoint is situated at the point at which the sciatic nerve splits into the sural, tibial and peroneal nerves that extend down the calf, and it was not known which of these nerves is most effective for stimulation with acupuncture7,11. The location of Prokr2-expressing neurons in the deep tissues below ST36 predicts that the anti-inflammatory effects of low-intensity ST36 ES depend on the deep innervations of the common peroneal nerve, rather than on the more-superficial skin innervations of the cutaneous sural nerves. Indeed, the authors confirmed that cutting the peroneal nerve, but not the tibial or sural nerves, blocks the anti-inflammatory effects of ST36 stimulation.
Figure 1 |. Neuronal targets of electroacupuncture.
In a treatment called electroacupuncture, certain sites on the body known as acupoints are stimulated electrically. For example, low-intensity stimulation of a leg acupoint called ST36 can reduce inflammation by activating the vagus nerve, which sends signals from the brain to the adrenal glands (located, as shown, on the kidneys), and promotes the release of anti-inflammatory catecholamine molecules such as dopamine and noradrenaline. Liu et al.4 used a mouse model of sepsis – a potentially fatal condition in which the inflammatory reaction to an infection gets out of control – to identify a population of sensory neurons that express the protein Prokr2 and that are needed for the anti-inflammatory effects of low-intensity ST36 stimulation, but not high-intensity stimulation (not shown). These neurons have cell bodies in the lower spinal cord, and have processes (red lines) that extend down the hindlimb and up to a brain structure called the nucleus tractus solitarius (NTS), which influences the activity of the vagus nerve.
Similarly, the presence of Prokr2-expressing neurons in other parts of the body might be associated with other acupoints that can activate the vagal–adrenal network. The authors did not observe Prokr2-expressing neurons in the abdominal fascia, a sheet of tissue that wraps around the abdominal organs, and confirmed that stimulation of abdominal acupoints such as the abdominal Tianshu (ST25) does not evoke the adrenal response12. By contrast, the enrichment of Prokr2-expressing neurons in the upper regions of the spinal cord that innervate the forelimbs suggested that stimulation of the elbow Shousanli (LI10) acupoint could also evoke catecholamine production, as confirmed by the authors.
These studies have considerable clinical implications because, during electroacupuncture, multiple acupoints are normally stimulated at the same time. Establishing where Prokr2-expressing neurons are distributed could help to determine which acupoints can be stimulated together to improve treatment efficacy. Further studies are needed to determine whether stimulation of Prokr2-expressing neurons at different acupoints triggers the same responses or induces varying effects on the vagus nerve and its physiological functions.
The vagus is the longest parasympathetic nerve in the body and innervates multiple organs. Its stimulation can trigger various effects, including two anti-inflammatory mechanisms. First, it can induce the release of catecholamines (mostly dopamine and noradrenaline) from the adrenal glands into the bloodstream. Second, it can induce the production of noradrenaline in the spleen to activate immune cells called lymphocytes to produce the molecule acetylcholine, which inhibits another immune cell called a splenic macrophage13–15. Future studies should determine whether different acupoints can differentially induce these two mechanisms.
The ability to activate specific neuronal networks to induce desired effects while avoiding adverse side effects would have substantial clinical advantages. Many conventional drug treatments produce nonspecific side effects as the stable drug molecules spread through the body. By contrast, catecholamines have a comparatively short half-life, of about one to four minutes, and thus act more locally — for example, to promote contraction of muscles in the heart, or relaxation of the tubes that carry air to and from of the lungs. Thus, stimulation of specific neuronal networks could drive the production of catecholamines in discrete networks and induce local effects in specific organs, avoiding nonspecific side effects.
If electroacupuncture can selectively activate specific neuronal networks, it might be feasible to design ES treatments to induce local effects, similar to pacemakers (electronic devices implanted into the chest to control the heartbeat). Thus, it might eventually be possible to use ES to evoke local anti-inflammatory mechanisms in certain parts of the body — such as an arthritic knee or specific sections of the digestive tract in individuals with chronic inflammatory bowel disorders — without suppressing the whole immune system, increasing the risk of infection or leading to side effects elsewhere in the body.
From the archive.
Musings about the value of studying wetlands, and a report of the excavation of an ancient city in Sicily.
50 years ago
Wetland ecosystems are particularly prone to human interference. Either they are drained for agriculture or they are exploited for peat, but rarely are they left untouched … The interest of these habitats lies both in their distinctive wildlife and also in that they build up a record of their own development in the peat which they form … [R]emains are often recognizable and document the vegetational succession on the site. Furthermore, the peat contains pollen which may have travelled considerable distances in the air before being deposited … and from this can be reconstructed the vegetational history of much larger areas … Such information can be valuable in the study of climatic changes in a region.
From Nature 29 October 1971
100 years ago
Motya: A Phoenician Colony in Sicily. By Joseph I. S. Whitaker — Mr. Whitaker’s investigations on the island of San Pantaleo have for the historian and the archaeologist a twofold interest … [H]e has established with reasonable probability that this island is the site of the ancient city of Motya … and he has added very considerably to our knowledge of Phoenician culture … San Pantaleo lies in a shallow lagoon at the western extremity of Sicily, not far from the ancient Lilybaeum. Motya, according to Thucydides, was one of the cities to which the Phoenicians withdrew at the advent of the Greek colonists in the middle of the seventh century b.c. It was involved in the struggle between the Greeks and Carthage, and utterly destroyed by the tyrant Dionysius in 397 b.c. … Mr. Whitaker’s excavations have been pursued in peculiarly favourable circumstances. Many of the objects brought to light by his spade, even to the very weapons of the combatants, lay where they fell during the siege. No other Phoenician site has remained undisturbed in this way.
From Nature 27 October 1921

Footnotes
The author declares no competing interests.
References
- 1.Ulloa L, Quiroz-Gonzalez S.& Torres-Rosas R.Trends Mol. Med 23, 1103–1120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavlov VA & Tracey KJ Nature Neurosci. 20, 156–166 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Sharma N.et al. Nature 577, 392–398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S.et al. Nature 598, 641–645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Poll T, van de Veerdonk FL, Scicluna BP & Netea MG Nature Rev. Immunol 17, 407–420 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Ulloa L.& Tracey KJ Trends Mol. Med 11, 56–63 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Torres-Rosas R.et al. Nature Med. 20, 291–295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassi GS et al. Neurosci. Biobehav. Rev 112, 363–373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vida G.et al. FASEB J. 25, 4476–4485 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi S.et al. Nature 587, 258–263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman N.et al. Nature Neurosci. 13, 883–888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S.et al. Neuron 108, 436–450 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulloa L.Nature Rev. Drug Discov 4, 673–684 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Wang H.et al. Nature 421, 384–388 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Huston JM et al. J. Exp. Med 203, 1623–1628 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]