Abstract
Purpose
Identifying cirrhotic hepatocellular carcinoma (HCC) during liver cirrhosis (LC) stage is pivotal for improving the clinical outcomes of cirrhotic HCC patients. Inflammation-driven markers play a crucial role in tumorigenesis and tumor progression. Neutrophil-to-lymphocyte ratio (NLR) is an inflammatory response marker. This study aimed to evaluate the ability of NLR to distinguish cirrhotic HCC from LC.
Methods
Data of healthy control (HC) people, LC patients, cirrhotic HCC patients, and non-cirrhotic HCC patients were retrospectively analyzed. Mann–Whitney U test and Chi-squared test were used to compare demographic and clinical parameters in different groups. Spearman correlation analysis was used to assess correlations. Receiver operating characteristic (ROC) curves were performed to determine diagnostic accuracy.
Results
A total of 419 participants were enrolled in this study, including 152 HC people, 131 LC patients, 96 cirrhotic HCC patients, and 40 non-cirrhotic HCC patients. Level of NLR was elevated significantly in LC compared with HC (P < 0.001). No significant differences were found for NLR between LC and cirrhotic HCC (P = 0.083), as well as between cirrhotic HCC and non-cirrhotic HCC (P = 0.729). NLR was positively correlated with platelet-to-lymphocyte ratio (r = 0.33, P < 0.001). The area under the ROC curve (AUC) value for NLR to distinguish LC from HC was 0.759 (P < 0.001), and AUC value to distinguish cirrhotic HCC from LC was 0.567 (P = 0.083), and AUC value to distinguish non-cirrhotic HCC from cirrhotic HCC was 0.519 (0.415–0.623) (P = 0.729).
Conclusion
NLR can distinguish LC from HC but cannot not distinguish cirrhotic HCC from LC.
Keywords: hepatocellular carcinoma, liver cirrhosis, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, total bilirubin
Introduction
The incidence of liver cancer ranks sixth among malignant tumors and is the second cause of cancer-related death in the world.1,2 A real world observation of 45 years for liver cancer survival in China showed that the overall survival of 1-, 5-, 10-, and 20-year rates were respectively 18.51%, 6.28%, 4.03%, and 2.84%.3 Hepatocellular carcinoma (HCC) is the most common primary liver cancer and its incidence and associated mortality rate continue to increase.4 Being a consequence of different causes including obesity, non-alcoholic fatty liver disease, high alcohol consumption, hepatitis B or C infection, liver cirrhosis (LC) develops after a long period of inflammation that results in replacement of the healthy liver parenchyma with fibrotic tissue and regenerative nodules.5 Growing numbers of people are being diagnosed with LC and correct treatment can help improve the quality of life.6 Patients with LC are at high risk of developing HCC and approximately 70%-90% of HCC patients have underlying LC,7,8 which means the majority of HCC patients also have LC. HCC patients with non-cirrhotic livers represent a relatively small proportion (10–30%) of HCC cases.9
The prognosis of HCC varies greatly according to tumor stage at the time of diagnosis,10 so identifying cirrhotic HCC during LC stage is pivotal for improving the clinical outcomes of cirrhotic HCC patients. Among non-invasive biomarkers, alpha-fetoprotein (AFP) is the most widely used serum marker for screening for HCC in clinical practice. However, AFP can lead to high negative and false-positive rate.11 Additionally, the expenditure to detect AFP is relatively high.
Inflammation is an important component of the tumor microenvironment and selected chronic inflammatory conditions increase the risk of developing cancer.12 Inflammation-driven markers play a crucial role in tumorigenesis and tumor progression.13 The neutrophil-to-lymphocyte ratio (NLR) represents a cost-effective and easily available but non-specific marker of inflammation.14 Compared with AFP, NLR can be determined at low cost through routine blood examinations. NLR was found to be a diagnostic factor for ovarian cancer and prostate cancer.15,16 In addition, NLR was associated with mortality and useful in identifying spontaneous bacterial peritonitis in patients with cirrhosis.17,18
To the best of our knowledge, few reports have mentioned the ability of NLR to distinguish cirrhotic HCC from LC. This study attempted to evaluate the differences of NLR among healthy control (HC) people, LC patients, cirrhotic HCC patients, non-cirrhotic HCC patients. In addition, to better investigate the abilities of inflammatory markers, platelet-to-lymphocyte ratio, another important inflammatory marker,19 was also analyzed in the present study. We attempted to find a reliable inflammatory marker used in distinguishing cirrhotic HCC from LC.
Materials and Methods
Patients
This retrospective study collected data from June 2021 to May 2022 at The Affiliated Wuxi People’s Hospital of Nanjing Medical University. According to medical records, inpatients with LC or inpatients with HCC were respectively enrolled into LC group and HCC group. The exclusion criteria of LC group included LC patients with HCC or other malignant tumors. HCC group consisted of cirrhotic HCC group and non-cirrhotic HCC group. The exclusion criteria for cirrhotic HCC group and non-cirrhotic HCC group included cholangiocarcinoma, secondary HCC and other gastrointestinal cancers. Healthy people during routine health checkup, who had no history of tumor and liver disease, were enrolled into HC group. This study was conducted following the Declaration of Helsinki and approved by the ethics committee of The Affiliated Wuxi People’s Hospital of Nanjing Medical University. Written informed consent was waived due to the retrospective nature of this study.
Any patient data that could identify individual patients were anonymized and de-identified before analysis.
Clinical Data Collection
After fasting overnight, venous blood samples were collected from each participant and placed in EDTA-K2 anticoagulation tubes, sodium citrate anticoagulation tubes and coagulation-promoting tubes. For inpatients, blood samples were collected on the first day or second day after admission. Whole blood samples of EDTA-K2 tubes were analyzed in Sysmex XE-5000 Automatic Hematology Analyzer (Sysmex Corp., Kobe, Japan) to determine routine blood test including white blood cell (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), red blood cell (RBC), hemoglobin (HGB), platelet (PLT). Blood samples in coagulation-promoting tubes were allowed to clot at room temperature for no more than 2 hours, followed by centrifugation at 3000 x g for 3 min to obtain serum. Serum samples were analyzed within 30 minutes in Beckman Coulter AU5800 Automatic Analyzer (Beckman Coulter Inc., CA, USA) to detect liver function including total bilirubin (TBIL), total protein (TP), albumin (ALB), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), alanine transaminase (ALT), aspartate transaminase (AST), creatinine (CREA). Serum samples were also analyzed in Beckman Coulter DXI800 Automatic Analyzer (Beckman Coulter Inc., CA, USA) to detect alpha-fetoprotein (AFP). Plasma samples in sodium citrate tubes were analyzed in Werfen ACL TOP 700 Coagulation Analyzer (Werfen Inc., Barcelona, Spain) or Sysmex CA2000i Coagulation Analyzer (Sysmex Corp., Kobe, Japan) to detect international normalized ratio (INR).
Neutrophil-to-lymphocyte ratio (NLR) was obtained indirectly through calculation using the following ratio: ANC/ALC. Platelet-to-lymphocyte ratio (PLR) was obtained indirectly through calculation using the following ratio: PLT/ALC. Albumin-bilirubin (ALBI) score was computed by the formula: (0.66 × log10TBIL) − (0.085 × ALB), where the unit of TBIL was μmol/L and ALB was g/L, patients were then stratified into 3 groups: ALBI grade 1 (≤ −2.60), grade 2 (> −2.60 to −1.39) and grade 3 (> −1.39).20 Model for end-stage liver disease (MELD) score was computed by the formula: 3.8[loge TBIL] + 11.2 [loge INR] + 9.6 [loge CREA] + 6.4, where the unit of TBIL was mg/dL and CREA was mg/dL.21
Tumor stages were defined according to China liver cancer (CNLC) staging system.22
Statistical Analysis
Statistical analyses were performed by SPSS 20.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 9.0 (GraphPad Inc., CA, USA). Continuous data were expressed as median (interquartile range, Q25-Q75) and compared by Mann–Whitney U test. Categorical data were shown as numbers and compared by Chi-squared test. Correlation analysis was assessed using Spearman correlation test. To assess diagnostic performance, the receiver operating characteristic (ROC) curve was constructed and the value of area under curve (AUC) with 95% confidence interval (95% CI), optimal cutoff point and corresponding sensitivity (SEN) and specificity (SPE) were determined. P < 0.05 was regarded as statistically significant.
Results
Baseline Demographic and Clinical Characteristics
A total of 419 participants were enrolled in this study, including 152 HC people, 131 LC patients, 96 cirrhotic HCC patients, 40 non-cirrhotic HCC patients. Almost all LC patients were in decompensated stages, who also had one or more complications including portal hypertension, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, variceal bleeding. High alcohol consumption, hepatitis B or C infection, autoimmune hepatitis were the most common causes of LC in this study. The results of differences between HC and LC, LC and cirrhotic HCC, cirrhotic HCC and non-cirrhotic HCC were respectively shown in Tables 1-3.
Table 1.
Differences Between HC and LC
| Parameter | HC (n = 152) | LC (n = 131) | P value |
|---|---|---|---|
| Age (y) | 58(55–65) | 59(51–71) | 0.933 |
| Male (n) | 83 | 71 | 0.945 |
| Female (n) | 69 | 60 | |
| TBIL (μmol/L) | 14.0(11.4–17.2) | 26.8(18.9–47.7) | <0.001 |
| TP (g/L) | 71.8(69.4–74.4) | 57.2(51.6–62.9) | <0.001 |
| ALB (g/L) | 43.9(42.4–5.3) | 28.3(24.9–31.6) | <0.001 |
| ALP (U/L) | 75(63–88) | 95(68–152) | <0.001 |
| ALT (U/L) | 20.2(15.7, 28.9) | 23.6(13.9, 41) | 0.072 |
| GGT (U/L) | 27(18–40) | 38(22–93) | <0.001 |
| AST (U/L) | 23(20, 28) | 39(22, 60) | <0.001 |
| WBC (109/L) | 5.8(4.9–6.88) | 3.57(2.57, 5.87) | <0.001 |
| RBC 1012/L | 4.66(4.38–5.03) | 2.95(2.57–3.42) | <0.001 |
| HGB (g/L) | 141(130–152) | 90(73–104) | <0.001 |
| NLR | 1.64(1.29–2.02) | 2.80(1.71–4.87) | <0.001 |
| PLR | 109.87(89.1–140.79) | 81.00(57.53–122.39) | <0.001 |
| ALBI score = 1 | 150 | 3 | <0.001 |
| ALBI score = 2 | 2 | 74 | |
| ALBI score = 3 | 0 | 54 |
Abbreviations: HC, healthy control; LC, liver cirrhosis; TBIL, total bilirubin; TP, total protein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; GGT, glutamyl transferase; AST, aspartate transaminase; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALBI score, albumin-bilirubin score.
Table 2.
Differences Between LC and Cirrhotic HCC
| Parameter | LC (n = 131) | Cirrhotic HCC (n = 96) | P value |
|---|---|---|---|
| Age (y) | 59(51–71) | 61(56–67) | 0.445 |
| Male (n) | 71 | 79 | <0.001 |
| Female (n) | 60 | 17 | |
| TBIL (μmol/L) | 26.8(18.9–47.7) | 22.6(16.3–44.3) | 0.204 |
| TP (g/L) | 57.2(51.6–62.9) | 59.8(54.9–65.0) | 0.02 |
| ALB (g/L) | 28.3(24.9–31.6) | 32.0(28.2–35.6) | <0.001 |
| ALP (U/L) | 95(68–152) | 114(82–172) | 0.03 |
| ALT (U/L) | 23.6(13.9, 41) | 31.4(21.6–55.6) | 0.001 |
| GGT (U/L) | 38(22–93) | 82(46–158) | <0.001 |
| AST (U/L) | 39(22, 60) | 46(31–90) | 0.002 |
| WBC (109/L) | 3.57(2.57, 5.87) | 5.14(3.83–6.65) | <0.001 |
| RBC 1012/L | 2.95(2.57–3.42) | 3.88(3.11–4.52) | <0.001 |
| HGB (g/L) | 90(73–104) | 116(99–135) | <0.001 |
| NLR | 2.80(1.71–4.87) | 3.44(1.98–6.21) | 0.083 |
| PLR | 81.00(57.53–122.39) | 99.61(65.22–150.56) | 0.064 |
| ALBI score = 1 | 3 | 8 | 0.026 |
| ALBI score = 2 | 74 | 61 | |
| ALBI score = 3 | 54 | 27 |
Abbreviations: LC, liver cirrhosis; HCC, hepatocellular carcinoma; TBIL, total bilirubin; TP, total protein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; GGT, glutamyl transferase; AST, aspartate transaminase; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALBI score, albumin-bilirubin score.
Table 3.
Differences Between Cirrhotic HCC and Non-Cirrhotic HCC
| Parameter | Total (n=136) | Cirrhotic HCC (n = 96) | Non-Cirrhotic HCC (n = 40) | P value |
|---|---|---|---|---|
| Age (y) | 63(56–71) | 61(56–67) | 70(59–77) | 0.002 |
| Male (n) | 108 | 79 | 29 | 0.198 |
| Female (n) | 28 | 17 | 11 | |
| TBIL (μmol/L) | 22.3(15.6–38.7) | 22.6(16.3–44.3) | 18.6(13.3–25.8) | 0.031 |
| TP (g/L) | 61.4(55.4–65.8) | 59.8(54.9–65.0) | 62.8(56.9–67.3) | 0.165 |
| ALB (g/L) | 32.4(28.4–36.7) | 32.0(28.2–35.6) | 34.8(30.0–38.4) | 0.023 |
| ALP (U/L) | 114(81–172) | 114(82–172) | 111(81–175) | 0.681 |
| ALT (U/L) | 31.4(21.5–62.6) | 31.4(21.6–55.6) | 31.1(19.3–82.8) | 0.767 |
| GGT (U/L) | 82(46–172) | 82(46–158) | 79(46–244) | 0.845 |
| AST (U/L) | 46(30–94) | 46(31–90) | 47(26–94) | 0.483 |
| WBC (109/L) | 5.35(3.92–7.13) | 5.14(3.83–6.65) | 6.45(4.74–7.73) | 0.053 |
| RBC 1012/L | 3.97(3.17–4.49) | 3.88(3.11–4.52) | 4.11(3.56–4.44) | 0.385 |
| HGB (g/L) | 119(101–134) | 116(99–135) | 125(110–131) | 0.428 |
| NLR | 3.52(2.03–6.01) | 3.44(1.98–6.21) | 3.52(2.18–5.41) | 0.729 |
| PLR | 109.68(73.42–174.24) | 99.61(65.22–150.56) | 143.39(108.38–192.99) | 0.002 |
| ALBI score = 1 | 15 | 8 | 7 | 0.072 |
| ALBI score = 2 | 89 | 61 | 28 | |
| ALBI score = 3 | 32 | 27 | 5 |
Abbreviations: HCC, hepatocellular carcinoma; TBIL, total bilirubin; TP, total protein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; GGT, glutamyl transferase; AST, aspartate transaminase; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALBI score, albumin-bilirubin score.
As shown in Table 1, there were no significant differences for age (P = 0.933), sex (P = 0.945) between HC and LC. NLR level was elevated significantly in LC compared with HC (P < 0.001), while PLR level was decreased in LC compared with HC (P < 0.001). LC patients showed higher levels of TBIL (P < 0.001), ALP (P < 0.001), GGT (P < 0.001), AST (P < 0.001) and lower levels of TP (P < 0.001), ALB (P < 0.001), WBC (P < 0.001), RBC (P < 0.001), HGB (P < 0.001) compared with HC people. The ALBI score was higher in LC than HC (P < 0.001).
As shown in Table 2, there were no significant differences for age between LC and cirrhotic HCC (P = 0.455). The proportion of males was significantly higher in cirrhotic HCC than LC (P < 0.001). No significant differences were found for NLR (P = 0.083) and PLR (P = 0.064) between LC and cirrhotic HCC. Cirrhotic HCC patients showed higher levels of TP (P = 0.02), ALB (P < 0.001), ALP (P = 0.03), ALT (P = 0.001), GGT (P < 0.001), AST (P = 0.002), WBC (P < 0.001), RBC (P < 0.001), HGB (P < 0.001) compared with LC patients. ALBI score was lower in cirrhotic HCC than LC (P = 0.026).
As shown in Table 3, cirrhotic HCC patients were younger than non-cirrhotic HCC patients (P = 0.002). There were no significant differences for sex between cirrhotic HCC and non-cirrhotic HCC (P = 0.198). No significant difference was found for NLR between cirrhotic HCC and non-cirrhotic HCC (P = 0.729), but PLR was lower in cirrhotic HCC than in non-cirrhotic HCC (P < 0.002). No significant differences were found for TP (P = 0.165), ALP (P = 0.681), ALT (P = 0.767), GGT (P = 0.845), AST (P = 0.483), WBC (P = 0.053), RBC (P = 0.385), HGB (P = 0.428) levels between cirrhotic HCC and non-cirrhotic. No significant difference was found for the ALBI score between cirrhotic HCC and non-cirrhotic HCC (P = 0.072).
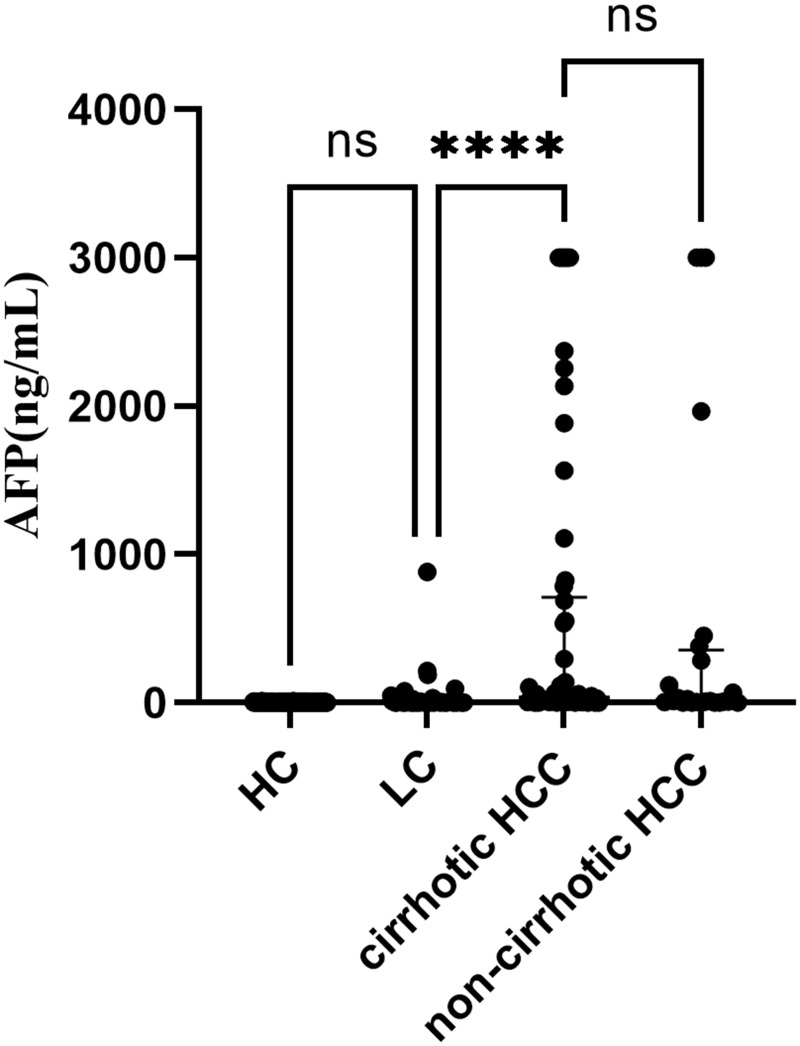
Data of AFP were not completely collected because not all LC patients and HCC patients were asked to test AFP. The differences of AFP levels among groups were shown in Figure 1. The levels of AFP were significantly higher in cirrhotic HCC than in LC (P < 0.001). However, no significant differences were found for the levels of AFP between HC and LC, between cirrhotic HCC and non-cirrhotic HCC.
Figure 1.
AFP levels in HC, LC, cirrhotic HCC and non-cirrhotic HCC. Participant numbers were respectively 152, 96, 62, 24 in HC, LC, cirrhotic HCC and non-cirrhotic HCC. NS means no significance with P >0.05, **** means P < 0.001.
Abbreviations: HC, healthy control; LC, liver cirrhosis; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein.
Data from CNLC staging could be collected in 68 HCC patients. Differences between CNLC staging groups were shown in Table 4. There were no significant differences for NLR (P = 0.772), PLR (P = 0.269), ALBI score (P = 0.092) between I/II and III/IV CNLC staging.
Table 4.
Differences Between CNLC Staging Group in HCC
| Parameter | CNLC Staging | P value | ||
|---|---|---|---|---|
| I/II (n=40) | III/IV (n=28) | |||
| Sex (n) | Male | 33 | 24 | 0.723 |
| Female | 7 | 4 | ||
| NLR | ≦3.52 | 20 | 15 | 0.772 |
| >3.52 | 20 | 13 | ||
| PLR | ≦109.68 | 24 | 13 | 0.269 |
| >109.68 | 16 | 15 | ||
| ALBI score | 1 | 6 | 0 | 0.092 |
| 2 | 27 | 21 | ||
| 3 | 7 | 7 | ||
Abbreviations: CNLC staging, China liver cancer staging; HCC, hepatocellular carcinoma; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALBI score, albumin-bilirubin score.
As shown in Supplementary Table 1 and Supplementary Table 2, MELD scores could be collected from 110 LC patients, 68 cirrhotic HCC patients, 29 non-cirrhotic HCC patients. MELD scores were higher in LC than cirrhotic HCC (P = 0.004), and were higher in cirrhotic HCC than non-cirrhotic HCC (P = 0.035).
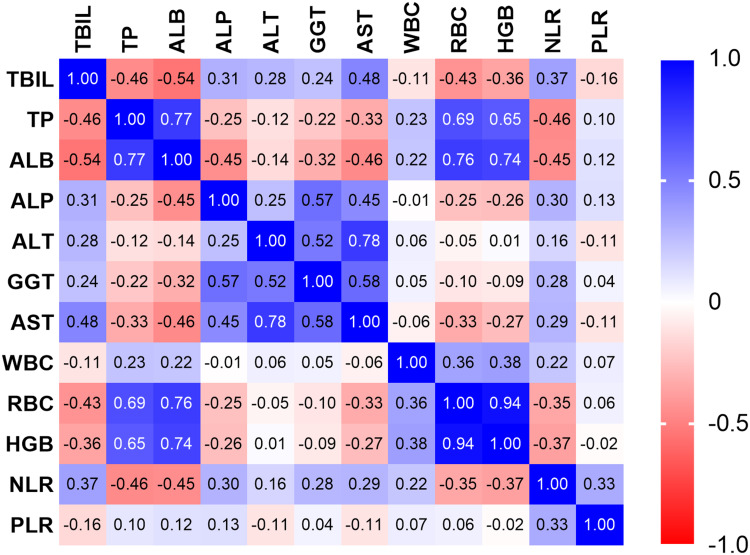
Correlation Analysis
Correlation analysis was conducted in overall participants and was summarized in Figure 2. NLR was positively correlated with TBIL (r = 0.37, P < 0.001), ALP (r = 0.30, P < 0.001), ALT (r = 0.16, P < 0.001), GGT (r = 0.28, P < 0.001), AST (r = 0.29, P < 0.001), WBC (r = 0.22, P < 0.001), PLR (r = 0.33, P < 0.001) while negatively correlated with TP (r = −0.46, P < 0.001), ALB (r = −0.45, P < 0.001), RBC (r = −0.35, P < 0.001), HGB (r = - 0.37, P < 0.001).
Figure 2.
Results of correlation matrix. The cells are colored according to the magnitude of the correlations, ranging from dark red for positive correlations to dark blue for negative correlations.
Abbreviations: TBIL, total bilirubin; TP, total protein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; GGT, glutamyl transferase; AST, aspartate transaminase; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
NLR was positively correlated with MELD score (r = 0.292, P < 0.001) in LC and HCC patients.
Diagnostic Accuracy
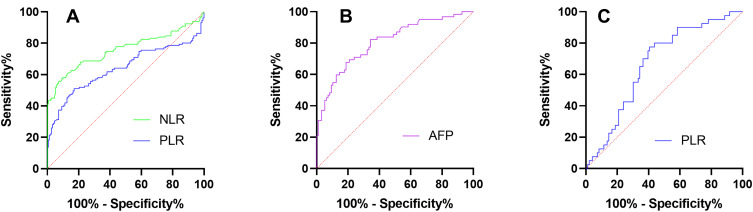
Diagnostic accuracy of NLR, PLR to distinguish LC from HC was presented in Table 5 and Figure 3A. With optimal cutoff point of 2.58, the AUC (95% CI) value for NLR was 0.759 (0.598–0.819) (P < 0.001). With optimal cutoff point of 82.44, the AUC (95% CI) value for PLR was 0.640 (0.571–0.709).
Table 5.
Diagnostic Accuracy of NLR and PLR to Distinguish Between LC and HC
| Variable | Cutoff Point | Sensitivity (%) | Specificity (%) | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| NLR | > 2.58 | 55.73 | 92.76 | 0.759 (0.598–0.819) | < 0.001 |
| PLR | < 82.44 | 51.15 | 82.89 | 0.640 (0.571–0.709) | < 0.001 |
Abbreviations: LC, liver cirrhosis; HC, healthy control; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; AUC (95% CI), area under curve (95% confidence interval).
Figure 3.
ROC curves for (A) NLR and PLR to distinguish LC from HC, (B) AFP to distinguish cirrhotic HCC from LC, (C) PLR to distinguish non-cirrhotic HCC from cirrhotic HCC.
Abbreviations: HC, healthy control; LC, liver cirrhosis; HCC, hepatocellular carcinoma; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; AFP, alpha-fetoprotein. ROC, receiver operating characteristic.
Diagnostic accuracy of NLR, PLR, AFP to distinguish cirrhotic HCC from LC was presented in Table 6. The AUC (95% CI) values for NLR and PLR were 0.567 (0.492–0.643) (P = 0.083) and 0.572 (0.497–0.647) (P = 0.064), without statistical significance existing. With optimal cutoff point of 7.95, the AUC (95% CI) value for AFP was 0.808 (0.737–0.878) (P < 0.001), as shown in Figure 3B.
Table 6.
Diagnostic Accuracy of NLR, PLR, AFP to Distinguish Between Cirrhotic HCC and LC
| Variable | Cutoff Point | Sensitivity (%) | Specificity (%) | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| NLR | 0.567 (0.492–0.643) | 0.083 | |||
| PLR | 0572 (0.497–0.647) | 0.064 | |||
| AFP | > 7.95 | 67.74 | 81.25 | 0.808 (0.737–0.878) | < 0.001 |
Abbreviations: HCC, hepatocellular carcinoma; LC, liver cirrhosis; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; AFP, alpha-fetoprotein; AUC (95% CI), area under curve (95% confidence interval).
Diagnostic accuracy of NLR, PLR to distinguish non-cirrhotic HCC from cirrhotic HCC was presented in Table 7. The AUC of NLR was 0.519 (0.415–0.623) (P = 0.729), but no statistical significance existed. With optimal cutoff point of 107.2, the AUC (95% CI) value for PLR was 0.668 (0.574–0.762) (P = 0.002) and was shown in Figure 3C.
Table 7.
Diagnostic Accuracy of NLR and PLR to Distinguish Between Non-Cirrhotic HCC and Cirrhotic HCC
| Variable | Cutoff Point | Sensitivity (%) | Specificity (%) | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| NLR | 0.519 (0.415–0.623) | 0.729 | |||
| PLR | > 107.2 | 77.5 | 59.38 | 0.668 (0.574–0.762) | 0.002 |
Abbreviations: HCC, hepatocellular carcinoma; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; AUC (95% CI), area under curve (95% confidence interval).
Discussion
In most countries, incidence rates of HCC among men are two- to four-fold higher than rates among women.23 Our study showed that incidence rates of HCC among men are three-fold higher than rates among women, in accordance with incidence rates in most countries.
Inflammation is closely related to the occurrence, development, and prognosis of tumors. Tumor-associated inflammation is a key characteristic of malignant tumors.24 In particular, inflammation can foster multiple hallmark functions of cancer.25 Inflammation contributes to carcinogenesis through several stages, particularly reactive oxygen species produced by activated inflammatory cells, which could promote genomic instability/DNA mutations.26 Considering its rapid, widely available, and relatively inexpensive assessment through routine blood count analysis, NLR in peripheral blood is being increasingly studied as a systemic inflammatory marker.27 Our study showed NLR was elevated in LC compared with HC, while no significant difference was found between LC and cirrhotic HCC. A previous study by Hu showed NLR was elevated in HCC compared to that in liver disease,11 being contrary to our study. The possible reasons for this inconsistency are as follows: firstly, in our study, HCC group consisted of cirrhotic HCC patients only while in the study by Hu HCC group consisted of both cirrhotic HCC patients and non-cirrhotic HCC patients.11 Secondly, in our study, LC group consisted of LC only, while in the study by Hu liver disease group consisted of both hepatitis and LC.11 Thirdly, the instrument used to test samples may have been different, which may affect NLR levels.
The ALBI score, a score calculated from the patient’s serum albumin and total bilirubin, was proposed for assessing liver function and subsequent long-term mortality in patients with liver disease.28 As a simple, evidence-based, objective marker of assessing liver function, ALBI score has been applied in other diseases such as heart failure, lung cancer.29,30 According to ALBI score in this study, liver functions of LC patients were worst among different populations. However, according to other markers to assess liver functions such as ALT, AST, ALP, GGT, liver functions of HCC were worst. Two reasons for these conflicting findings: firstly, hypoalbuminemia is a common complication of LC and long-term albumin administration can prolong overall survival in LC patients.31 A lot of HCC patients have relatively higher ALB levels compared with LC patients through undergoing long-term albumin administration, resulting in relatively higher ALBI scores. Secondly, ALT, AST, ALP, GGT are common biomarkers reflecting hepatocellular damage. Before progressing into HCC, hepatic cells have suffered repeated damage during cirrhotic stages, ultimately resulting in relatively higher levels of ALT, AST, ALP, GGT. Although MELD score was higher in LC patients than cirrhotic HCC patients, it should be interpreted cautiously since the INR or creatinine may be elevated due to reasons other than liver disease.21
From CNLC staging in this study, it could be concluded that no associations existed between liver function and CNLC staging, and no association existed between inflammation and CNLC staging, which should be interpreted cautiously due to the small sample.
A correlation matrix was drawn in order to comprehensively analyze correlations among markers. It can be concluded that inflammation might promote damage of hepatic cells, which is in consistent with a previous study.32
It could be concluded from this study that NLR had moderate ability to distinguish LC from HC. However, NLR could not distinguish cirrhotic HCC from LC, and also could not distinguish non-cirrhotic HCC from cirrhotic HCC. We noticed that the P value for NLR to distinguish cirrhotic HCC from LC was 0.083, suggesting that larger populations should be enrolled. Although a previous study showed AUC value of NLR to distinguish cirrhotic HCC from LC after was 0.625 (P = 0.018), all LC patients underwent splenectomy and had chronic hepatitis B virus infection,8 who only represented a portion of LC patients compared with those in our study. The abilities of PLR to distinguish LC from HC and distinguish cirrhotic HCC from LC were similar to NLR. Although PLR could distinguish non-cirrhotic HCC from cirrhotic HCC, it was unreliable due to the small sample of non-cirrhotic HCC. This study proved AFP could distinguish cirrhotic HCC from LC, which was consistent with a previous study.33
This study assessed the ability of NLR to distinguish cirrhotic HCC from LC comprehensively for the first time, which is a major strength of this study. However, this study inevitably had some limitations. Firstly, this study was only conducted based on a single center, thus the sample size was relatively small. Secondly, almost all data of LC came from decompensated LC patients and data from compensated LC patients were scarce. Thirdly, a portion of HCC patients had received hepatectomies, which could have influenced levels of AFP and other biomarkers. Fourthly, factors which affect NLR levels such as spontaneous bacterial peritonitis in LC patients could not be excluded. Finally, this study was designed as cross-sectional, thus long-term outcomes could not be analyzed.
In conclusion, NLR can distinguish LC from HC but cannot distinguish cirrhotic HCC from LC. However, these conclusions should be interpreted cautiously and more standard studies with larger population should be conducted to validate these conclusions.
Funding Statement
This research received no external funding.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Institutional Review Board Statement
This study was conducted following the Declaration of Helsinki and approved by the ethics committee of The Affiliated Wuxi People’s Hospital of Nanjing Medical University. Written informed consent was waived due to the retrospective nature of this study. Any patient data that could identify individual patients were anonymized and de-identified before analysis.
Disclosure
The authors declare no conflict of interest.
References
- 1.Chen FH, Wang JM, Wu YC, Gao Q, Zhang S. Potential biomarkers for liver cancer diagnosis based on multi-omics strategy. Front Oncol. 2022;12:822449. doi: 10.3389/fonc.2022.822449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Mattos ÂZ, Debes JD, Boonstra A, et al. Current impact of viral hepatitis on liver cancer development: the challenge remains. World J Gastroenterol. 2021;27(24):3556–3567. doi: 10.3748/wjg.v27.i24.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JG, Zhu J, Zhang YH, et al. Liver cancer survival: a real world observation of 45 years with 32,556 Cases. J Hepatocell Carcinoma. 2021;8:1023–1034. doi: 10.2147/JHC.S321346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-Gonzalez M, Beraza N. The role of the microbiome in liver cancer. Cancers. 2021;13(10):2330. doi: 10.3390/cancers13102330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398(10308):1359–1376. doi: 10.1016/S0140-6736(21) [DOI] [PubMed] [Google Scholar]
- 6.Kaplan A, Rosenblatt R. Symptom management in patients with cirrhosis: a practical guide. Curr Treat Options Gastroenterol. 2022;17:1–16. doi: 10.1007/s11938-022-00377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11) [DOI] [PubMed] [Google Scholar]
- 8.Du ZQ, Dong J, Bi JB, et al. Predictive value of the preoperative neutrophil-to-lymphocyte ratio for the development of hepatocellular carcinoma in HBV-associated cirrhotic patients after splenectomy. PLoS One. 2018;13(4):e0195336. doi: 10.1371/journal.pone.0195336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Wu H, Mei Y, et al. Clinicopathological and prognostic significance of PKM2 protein expression in cirrhotic hepatocellular carcinoma and non-cirrhotic hepatocellular carcinoma. Sci Rep. 2017;7(1):15294. doi: 10.1038/s41598-017-14813-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caviglia GP, Ciruolo M, Abate ML, et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist II and glypican-3 for the detection and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers. 2020;12(11):3218. doi: 10.3390/cancers12113218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Wang NY, Yang YF, et al. Diagnostic value of alpha-fetoprotein combined with neutrophil-to-lymphocyte ratio for hepatocellular carcinoma. BMC Gastroenterol. 2018;18(1):186. doi: 10.1186/s12876-018-0908-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281(1):57–61. doi: 10.1111/imr.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pease NA, Wise-Draper T, Privette Vinnedge L. Dissecting the potential interplay of DEK functions in inflammation and cancer. J Oncol. 2015;2015:106517. doi: 10.1155/2015/106517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soder HE, Berumen AM, Gomez KE, et al. Elevated neutrophil to lymphocyte ratio in older adults with cocaine use disorder as a marker of chronic inflammation. Clin Psychopharmacol Neurosci. 2020;18(1):32–40. doi: 10.9758/cpn.2020.18.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LF, Wang XY, Shu J, Xu S, Wu Q, Yu Y. Diagnostic value of serum D-dimer, CA125, and neutrophil-to-lymphocyte ratio in differentiating ovarian cancer and endometriosis. Int J Gynaecol Obstet. 2019;147(2):212–218. doi: 10.1002/ijgo.12949 [DOI] [PubMed] [Google Scholar]
- 16.Adhyatma KP, Prapiska FF, Siregar GP, Warli SM. Systemic inflammatory response in predicting prostate cancer: the diagnostic value of neutrophil-to-lymphocyte ratio. Open Access Maced J Med Sci. 2019;7(10):1628–1630. doi: 10.3889/oamjms.2019.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Li H, Xia J, et al. Baseline neutrophil-to-lymphocyte ratio is independently associated with 90-day transplant free mortality in patients with cirrhosis. Front Med. 2021;8:726950. doi: 10.3389/fmed.2021.726950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popoiag RE, Suceveanu AI, Suceveanu AP, et al. Predictors of spontaneous bacterial peritonitis in Romanian adults with liver cirrhosis: focus on the neutrophil-to-lymphocyte ratio. Exp Ther Med. 2021;22(3):983. doi: 10.3892/etm.2021.10415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rau CS, Wu SC, Tsai CH, et al. Association of white blood cell subtypes and derived ratios with a mortality outcome in adult patients with polytrauma. Healthcare. 2022;10(8):1384. doi: 10.3390/healthcare10081384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asrani SK, Kim WR. Clinical applications of the model for end-stage liver disease (MELD) in hepatic medicine. Clin Liver Dis. 2011;15(4):685–698. doi: 10.1016/j.cld.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CX, Wang HW, Chen RX, et al. Outcomes and recurrence patterns following curative hepatectomy for hepatocellular carcinoma patients with different China liver cancer staging. Am J Cancer Res. 2022;12(2):907–921. [PMC free article] [PubMed] [Google Scholar]
- 23.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi: 10.1002/hep.31288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Wang J, Wang HY, Zhang SQ, Wei YX, Liu SL. The interplay between inflammation and stromal components in pancreatic cancer. Front Immunol. 2022;13:850093. doi: 10.3389/fimmu.2022.850093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 26.Guerville F, Bourdel-Marchasson I, Déchanet-Merville J, et al. Does inflammation contribute to cancer incidence and mortality during aging? A Conceptual Review. Cancers. 2022;14(7):1622. doi: 10.3390/cancers14071622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paliogiannis P, Fois AG, Sotgia S, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27(147):17011322. doi: 10.1183/16000617.0113-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada S, Kaneshiro T, Yoshihisa A, et al. Albumin-bilirubin score for prediction of outcomes in heart failure patients treated with cardiac resynchronization therapy. J Clin Med. 2021;10(22):5378. doi: 10.3390/jcm10225378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S, Wang CH, Tong F, et al. Prognostic impact of albumin-bilirubin score on the prediction of in-hospital mortality in patients with heart failure: a retrospective cohort study. BMJ OPEN. 2022;12(1):e049325. doi: 10.1136/bmjopen-2021-049325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takada K, Takamori S, Shimokawa M, et al. Assessment of the albumin-bilirubin grade as a prognostic factor in patients with non-small-cell lung cancer receiving anti-PD-1-based therapy. ESMO Open. 2022;7(1):100348. doi: 10.1016/j.esmoop.2021.100348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417–2429. doi: 10.1016/S0140-6736(18) [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Lou Y, Chen Y, Yang J. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with acute-on-chronic liver failure. Int J Clin Pract. 2014;68(8):1034–1040. doi: 10.1111/ijcp.12408 [DOI] [PubMed] [Google Scholar]
- 33.Ma LN, Liu XY, Lu ZH, et al. Assessment of high-sensitivity C-reactive protein tests for the diagnosis of hepatocellular carcinoma in patients with hepatitis B-associated liver cirrhosis. Oncol Lett. 2017;13(5):3457–3464. doi: 10.3892/ol.2017.5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.