Highlights
► Smallpox eradication is one of the most remarkable accomplishments of the 20th century. ► The DRC's population suffers from extreme poverty, a deteriorating health system, and civil strife. ► Despite challenges, DRC's campaign was successful, eradicating smallpox in 1971. ► The campaign in DRC provides lessons for other eradication programs including polio. ► Currently, the number of polio cases reported in DRC is 2nd highest in the world.
Keywords: Smallpox, Monkeypox, Polio, Eradication, Democratic Republic of Congo, History
Abstract
Smallpox eradication is considered to be one of the most remarkable accomplishments of the 20th century. Lessons learned from the campaign during the 1960s and 1970s in the Democratic Republic of Congo (DRC) can provide important information for the development of other eradication programs including polio.
The DRC is the third largest country in Africa; the population suffers from extreme poverty, deteriorating infrastructure and health systems, and long periods of civil strife. Despite these challenges, DRC's smallpox eradication campaign was successful, eradicating smallpox only 41 months after initiation. DRC had been polio free since 2001; however, in 2006, imported cases were identified in the country. Polio transmission has since been re-established and DRC now has the second greatest number of reported polio cases in the world. Challenges which existed during the smallpox campaign in DRC are still present today; additionally, the polio vaccine itself poses unique challenges which include requiring multiple doses to confer immunity.
In the fight against polio in DRC, it will be important to draw from the smallpox eradication experience. A number of important themes emerged during the campaign that could be beneficial to eradicating polio and future eradication programs that may follow. During the smallpox campaign, a standard vaccination program was implemented, surveillance was intensified, and there were strong collaborative programs with community involvement. These successful elements of the smallpox campaign should be adapted and applied in DRC in polio eradication programs.
1. Introduction
The global eradication of smallpox is considered one of the greatest public health achievements as the first infectious disease eradicated from the world.
Smallpox eradication in the Democratic Republic of the Congo (DRC), formerly known as Zaire (1971–1997), was particularly challenging given the vast size of the country and poor infrastructure (Fig. 1 ). The speed with which eradication was accomplished is considered to be one of the most remarkable accomplishments of the global eradication program. When the program was initiated in 1967, smallpox was widespread throughout the country; vaccination coverage was low, health services were underdeveloped, there were very few trained health care workers, and poor infrastructure made transportation and communication within the country extremely difficult. Despite these challenges, the number of smallpox cases fell steadily from an estimated peak of 3800 in 1968 until 1971, when the last case was reported [1].
Fig. 1.
Map of Democratic Republic of Congo (DRC) and adjacent countries.
It is useful to draw from the smallpox eradication experience in revamping polio eradication efforts. In1988, the World Health Assembly pledged to eradicate polio by 2000 [2]. By 2006, Wild Polio Virus (WPV) remained endemic in only four countries (Afghanistan, Pakistan, India and Nigeria) [3]. DRC had been polio free from 2001 to 2005, however in 2006, cases imported from Angola were found in the country [4], [5]. As of September 27, 2011 DRC has reported 79 cases of WPV, second only to Chad with 112 cases [3].
This paper reviews the history and unique characteristics of the smallpox eradication campaign in DRC. It further discusses key lessons learned that can be applied to the current challenges posed by the polio eradication program and future eradication programs that may follow.
2. Challenges to the implementation of an eradication program
In June 1960, DRC gained independence from Belgium. Over the next few years, the country faced protracted civil unrest due to army mutiny, tribal wars and threats of secessions from its wealthiest provinces (Katanga and East Kasai) [6]. In 1965, Sese Seiko Mobutu began a 32-year reign as president [7]. Mobutu's mismanagement of state affairs, political violence, and extreme corruption resulted in the collapse of transportation and health systems maintained during the Belgian colonial era [7].
The large size of the country combined with the steadily declining infrastructure (including roads, communication and health services throughout the country) made implementation of a reliable disease surveillance system challenging. With more than 21 million inhabitants, 80% of whom resided in rural areas, DRC had one of the most incomplete disease reporting systems in the world, making smallpox eradication seem unlikely [2], [8], [9].
In 1959, the World Health Assembly agreed to target smallpox for eradication [9]. By 1967, surrounding countries (with the exception of Sudan) were no longer reporting cases and were considered disease free [6]. However, DRC was still considered an important reservoir of smallpox virus in the African Region, home to one third of all smallpox cases in central, eastern, and southern Africa [6], [9]. Furthermore, borders between DRC and neighboring countries were porous, with frequent migration threatening to jeopardize the global eradication efforts [6].
3. The smallpox eradication campaign
In late 1965, an agreement was signed between the Congolese government and the World Health Organization (WHO), to implement a program for smallpox eradication, providing DRC with more than 8% of WHO smallpox eradication funds, the highest priority for resources [6], [9].
In 1966, the National Campaign for Smallpox Eradication (Campagne Nationale d’éradication de la variole, CNEV) was created [6], [10]. The campaign, developed for DRC, was similar to other programs throughout Africa, requiring systematic vaccination throughout the country [9]. Nevertheless, there was one key difference: concurrent administration of the Bacillus Calmette-Guérin (BCG) for tuberculosis (TB) and smallpox vaccine [6], [9].
The major goals of the CNEV were to provide simultaneous administration of freeze-dried smallpox vaccine to the entire population and BCG vaccine to children and adolescents, and to increase disease surveillance activities [6], [10].
By vehicle, boat and foot, teams strived to reach targeted populations, regardless of location. Headed by medical doctors, each of the four mass vaccination groups included one sensitization team, five vaccination teams and one assessment team (Fig. 2 ). The sensitization team played a crucial role in preparing and mobilizing the population for the arrival of the vaccination teams. They contacted village chiefs and other opinion leaders including teachers, politicians, and religious leaders to gather support [6]. Each vaccination team was composed of: a team leader, a smallpox vaccinator, a BCG vaccinator, a person to prepare vaccine, a distributor of stamped vaccination certificates, and a driver [6]. Immunization activities were conducted in a systematic manner in centralized locations, except in areas with very low population density, where the vaccination teams would make individual household visits [6]. After one week, an assessment team evaluated the activities of the vaccination team, by carrying out a rapid vaccination scar survey on a 5% sample of each village. They also vaccinated newborns and those who could not be reached during the initial visit [6].
Fig. 2.
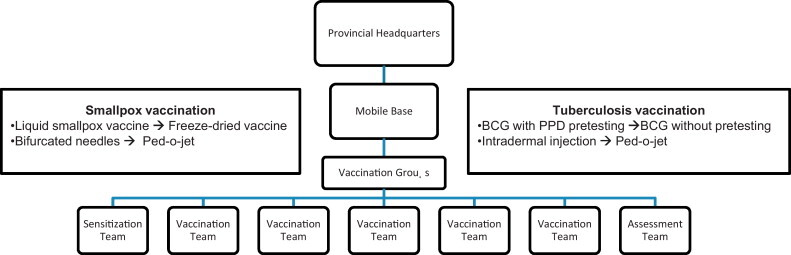
Operational strategy and improvements to the smallpox and BCG vaccination campaign in DRC.
The phases of the CNEV are outlined in Box 1 . During the pilot program, the administration of concurrent vaccinations (smallpox and BCG) posed the largest operational problem, primarily because no precedent existed [6]. While the bifurcated needles used for smallpox vaccination were efficient for individual and mass vaccination, the BCG vaccine required several cumbersome steps, making mass vaccination virtually impossible. The process involved several days because BCG vaccination required a confirmatory purified protein derivative (PPD) negative result before administration (by syringe). During the pilot project, it became evident that additional team members would be required to ensure success. At the start of the full-scale vaccination program, the pre-vaccination census in each village/territory and PPD testing were discontinued [11], [12]. In addition, the BCG syringe was replaced with the jet injection method (Ped-O-Jet), which decreased vaccine administration time [11], [12].
Box 1. National Campaign for Smallpox Eradication (CNEV) [6,10,11].
Phase 1: Preparatory (1966)
The national plan of action was prepared. This phase included recruitment and training of technical staff and completion of administrative and logistic activities.
Phase 2: Pilot Program (1967–1968)
A pilot project was launched, using two vaccination groups, to evaluate the performance of the plan and to gauge potential problems.
Phase 3: Full-Scale Vaccination Program (1968–1970)
The full vaccination program was launched, using four vaccination groups (Fig. 2)
Phase 4: Maintenance (1971–1978)
Long-term objectives included implementation of surveillance activities and long-term vaccination programs.
4. Challenges unique to DRC
The obstacles to smallpox eradication in DRC were extensive, making it one of the most challenging of all African countries. The Congolese government would frequently freeze program funds, thus halting eradication efforts [9]. The program was severely understaffed, and maintaining even three of the five vaccination teams was difficult [9]. Consequently, surveillance-containment vaccination strategy was virtually impossible. The lack of reliable transportation further complicated access to many parts of the country; of the few roads that existed, many were impassable [9]. The inaccessibility of remote villages made case reporting irregular, thus only the acute form of smallpox, variola major, was regularly reported [6].
As a result, mass vaccination campaigns were sporadic and vaccine coverage for smallpox was extremely low [6]. Poor infrastructure also resulted in poor storage conditions for the vaccine itself; hence, the quality of vaccinia vaccine was inconsistent and may have contributed to the low rate of population immunity in the country [6]. In the 1930s and 1940s, recorded vaccine uptake ranged from 9% to 64%, averaging 38% [13], [14]. Vaccine supplies were primarily imported from Belgium until transitioning to local production centers in Boma, Kisangani, Kasongo, Uvira and Lubumbashi [15]. The quality of vaccine was highest near production centers, but efficacy declined as it was transported throughout the country, due to an inadequate cold chain [15]. By 1969, the more stable, freeze-dried smallpox vaccine (produced in the USSR) had replaced the liquid vaccine, thus improving the efficacy of the vaccine [9].
In early 1971, additional staff were hired and funds to support five full time vaccination teams were secured [6], [9]. On average, each team was able to administer up to 1200 smallpox and 600 BCG vaccinations per day (dependent upon population density and accessibility) [6]. In addition, intensified surveillance activities included investigation and containment of all suspected smallpox cases leading to increased vaccine coverage [6]. Furthermore, vaccination was required and those traveling without vaccination certification were fined [16]. Between March 1968 and July 1971, 24.3 million people received smallpox vaccinations and 11.4 million children received BCG [6], [10].
In 1971, only a small number of suspected cases were reported, most of which were chickenpox [6]. The last case of smallpox was reported in June 1971 in Upper Zaire (now the Orientale province) [6]. Among DRC's neighbors, Sudan was the only country still reporting smallpox cases [6].
In August 1971, one month after DRC's last confirmed case of smallpox, 11 provincial surveillance teams were established to maintain surveillance until the country was certified smallpox free [6]. DRC was expected to be certified smallpox free two years after the last case by the Commission for Certification of Smallpox Eradication [6]. However, certification was halted after the discovery of human monkeypox (MPX) in the Equateur province [6].
Between 1970 and 1980, a total of 59 cases of human MPX were detected in central and west Africa, the majority of which originated in DRC. Monkeypox resembles variola minor. After a 1-2-week incubation period, fever and malaise follow, along with the development of vesiculopustular lesions on the skin and oropharynx, which rarely become confluent [1], [18]. Monkeypox differed from Smallpox, exhibiting prominent lymphadenopathy. It had a low secondary attack rate of less than 10% of susceptible persons, and a case fatality rate of 10% to 16%, in contrast to the 30% or greater mortality of the classic variola major [19], [20], [21]. Almost all deaths occurred in individuals unvaccinated against smallpox.
The majority of monkeypox cases were linked to exposure to infected animals and secondary transmission appeared to be rare [1]. Based on the results of monkeypox surveillance investigations, the Global Commission for the Certification for Smallpox Eradication did not consider monkeypox to be a threat to smallpox eradication, nor did it consider the presence of human monkeypox a justification for continued vaccination.
In June 1977, DRC was officially certified smallpox-free. Until the world was certified smallpox free by the World Health Organization (WHO) Advisory Committee on Orthopoxvirus Infections in 1980, surveillance teams remained active in the field and routine vaccination continued in health centers and clinics, with sporadic vaccination until 1984 [6], [17].
5. Lessons learned for polio eradication in DRC
The World Health Assembly targeted polio for eradication by 2000 [2]. From 1994 to 2006, WPV transmission was successfully interrupted throughout the majority of the world, with only four countries remaining endemic (Afghanistan, Pakistan, India and Nigeria) [2], [3].
DRC had been polio free since 2001, due in large part to National Immunization Days (NIDs) and cease-fire days in areas of conflict, however imported cases of WPV from Angola led to re-established transmission [4], [22], [23]. In most countries, polio outbreaks persisted for less than six months; however, in DRC, outbreaks continued to circulate more than a year after importation, causing outbreaks in other previously polio-free countries [3], [4]. Since the beginning of 2011, the number of WPV cases in DRC has totaled 79, making it the second most heavily affected country in the world [3].
Three decades after smallpox eradication, The Global Polio Eradication Initiative (GPEI) in DRC faces the same obstacles that plagued the smallpox eradication efforts in the 1960s (Fig. 3 ). DRC struggles to recover from a devastating multi-year conflict, encompassing mass displacement, extreme violence, and further deterioration of roads [24]. Little progress has been made in healthcare infrastructure, resulting in inadequate capacity to implement international polio outbreak guidelines, which, in turn has led to low vaccine coverage and poor surveillance [3].
Fig. 3.
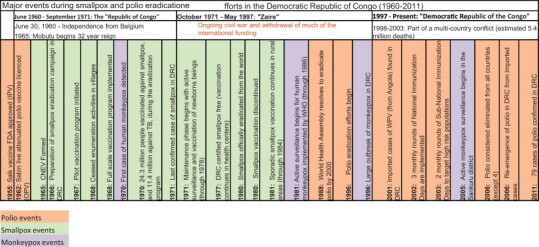
Major events during smallpox and polio eradication efforts in the Democratic Republic of Congo (1960–2011).
During the smallpox eradication campaign, eradication in DRC seemed unlikely; with few believing it was achievable. The surveillance vaccination programs implemented during the smallpox eradication efforts offer key lessons in the battle against polio as it re-emerges and threatens to spread throughout the country.
5.1. Increased vaccine coverage is necessary
Polio vaccine coverage in DRC has been suboptimal; only 73% of the population is estimated to have received at least one dose of oral polio vaccine (OPV) in 2009 [4]. However, WHO recommends four OPV doses within the first year of life, with follow-up immunizations during national and sub-national Supplementary Immunization Activities (SIAs) [25]. Nevertheless, this dosage does not guarantee seroconversion, and it appears at least five to six doses of the vaccine are needed for reliable immunity [26]. To date no serosurvey has been conducted to assess the population level immunity in DRC. Unlike OPV, the vaccinia vaccine typically induced immunity after one dose, which suggests polio vaccination may pose greater challenges for eradication. While NIDs are important to the polio eradication program, routine childhood vaccination programs cannot be overlooked.
In 2010, SIAs, including national and sub-national immunization days, vaccinated 20% to 65% of the targeted population under 5 years old [4]. Independent monitoring programs established in Bandundu and Kasai-Occidental provinces indicate that between 8% and 16% of children were excluded during the two most recent SIAs. This indicates the need for increased vaccination coverage and accurate recording for those being vaccinated, to ensure community members receive the required number of OPV doses for polio immunity [4].
Modification of the surveillance-containment strategy used in DRC may be appropriate for isolation of polio outbreaks. In areas of low population density, smallpox vaccination by household visit was common practice, accessing some of the most hard to reach populations in the country, and may again be a useful strategy for polio vaccination programs [6]. Furthermore, an assessment tool similar to the smallpox vaccination scar survey and better vaccination documentation could help ensure high rates of polio vaccine coverage [6].
5.2. Implementation of a standard vaccination program
Current polio immunization revolves around national and sub-national immunizations days [4]. The smallpox eradication campaign utilized similar activities through structured vaccination groups, comprised of a sensitization, vaccination and assessment teams (Fig. 2). Division of polio vaccination responsibilities similar to this structure may prove useful to the current polio program. External oversight may help facilitate distribution of money allocated to the local level, ensuring that adequate funds are available for transportation and storage of OPV to rural areas, which frequently left out during NIDs. Furthermore, the addition of a vaccination assessment following NIDs is appropriate.
One unique aspect of the smallpox eradication campaign in DRC was the simultaneous administration of BCG and smallpox vaccines. The addition of another vaccine (such as measles vaccine) used in conjunction with polio may be prove to be more cost-effective than administering a single vaccine, particularly in remote areas.
Furthermore, the flexibility of the smallpox eradication program in a challenging environment led to its successful eradication. A similar program should be implemented for polio with the flexibility to make needed changes as the program progresses.
5.3. Sensitive surveillance is critical
Acute flaccid paralysis (AFP) surveillance met DRC's standard detection targets in 2010, but it is unlikely that these targets represent an accurate assessment of the functioning polio surveillance system. Paralytic polio occurs in only 1% of those infected, thus most cases are subclinical, in contrast to smallpox, where subclinical cases were infrequently seen, with most people exhibiting an extensive rash [4], [27]. Recent genomic sequence analysis of WPV isolates from Katanga province indicated that AFP surveillance was insufficient to detect transmission in the eastern provinces, identifying substantial limitations associated with detection and investigation of cases [4].
Polio surveillance lacks high sensitivity for case detection, and many cases go unreported. There is a clear need to identify and develop alternative strategies which are more sensitive to increase reporting throughout the country. In the past, both cash rewards for reported smallpox cases and the surveillance-containment strategy led to highly effective surveillance [6]. The development of surveillance indicators that allow routine monitoring should be incorporated into the current surveillance structure [28]. In Mexico, the use of PCR on sewage to detect polio circulation in communities has demonstrated efficacy and maybe a useful surveillance method for DRC, reducing the burden and cost of surveying the entire population [29]. Finally, improvements in reporting guidelines including timeliness and completeness should be encouraged at the district level [28].
5.4. Establishing collaborative programs within communities and across countries
A recent review of polio in India, describes the GPEI as a vertical program with a top–down approach, falling short of the active community involvement invaluable during smallpox eradication efforts in DRC [30]. Sensitization of local leaders and public figures before vaccination teams entered villages was a critical component to community acceptance of the smallpox program. Communities were involved and prepared and willingly accepted vaccination strategies, even after campaign termination. Targeting community leaders and influencers should be incorporated into polio eradication efforts. Targeting traditional healers, birth attendants, and religious leaders can encourage community acceptance and support and assess to under-immunized populations.
The smallpox eradication campaign in DRC provides important lessons that can be applied to other disease eradication campaigns. Modifications of smallpox campaign strategies may be helpful as we move closer to polio eradication.
Conflict of interest: None.
References
- 1.WHO . World Health Organization; Geneva: 1980. The global eradication of smallpox: final report of the Global Commission for the Certification o Smallpox Eradication. [Google Scholar]
- 2.The Global Polio Eradication Initiative . Polio: global eradication initiative. 2010. History. Available from: http://www.polioeradication.org/Aboutus/History.aspx [cited 2011 June 8] [Google Scholar]
- 3.The Global Polio Eradication Initiative . Polio: Global Eradication Initiative. 2010. Infected countries: Democratic Republic of the Congo. Available from: http://www.polioeradication.org/Infectedcountries/DemocraticRepublicoftheCongo.aspx [cited 2011 June 8] [Google Scholar]
- 4.Progress toward interrupting wild poliovirus circulation in countries with reestablished transmission – Africa, 2009–2010. MMWR Morb Mortal Wkly Rep. 2011;60(10):306–311. [PubMed] [Google Scholar]
- 5.Kamadjeu R. Tracking the polio virus down the Congo River: a case study on the use of Google Earth in public health planning and mapping. Int J Health Geogr. 2009;8:4. doi: 10.1186/1476-072X-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenner F., Henderson D.A., Arita I., Jezek J., Ladnyi I.D. World Health Organization; Geneva, Switzerland: 1988. Smallpox and its eradication. [Google Scholar]
- 7.Kameba C. In: South Africa's role in conflict resolution and peace making in Africa. Southall R., editor. HSRC Press; 2006. South Africa and the DRC: is a stable developmental state possible in the Congo? pp. 155–172. [Google Scholar]
- 8.Mandjale E.A., Ngambong O.I. Environment, Organization and Research; 1993. From the Colonial Era to the present Demography of Zaire. [Google Scholar]
- 9.Henderson D.A. Prometheus Books; New York: 2009. Smallpox: the death of a disease. [Google Scholar]
- 10.Lekie R. La campagne d’éradication de la variole en République du Zaïre. Bulletin de la Société de Pathologie Exotique et de ses filiales. 1971;64:761–775. [PubMed] [Google Scholar]
- 11.ten Dam H.G., Fillastre C., Conge G., Orssaud E., Gateff C., Tanaka O., et al. The use of jet-injectors in BCG vaccination. Bull World Health Organ. 1970;43(5):707–720. [PMC free article] [PubMed] [Google Scholar]
- 12.Cerf J. La vaccination BCG sans test préalable au Congo Belge. Ann Soc Belg Med Trop. 1958;38:639–656. [Google Scholar]
- 13.Breman J.G. In: The global eradication of smallpox. Battacharya S., Messinger S., editors. Blackswan Press; New Delhi: 2010. A miracle happened there: the West and Central African smallpox eradication program and its impact; pp. 36–60. [Google Scholar]
- 14.Fasquelle R., Fasquelle A. A propos de l‘histoire de la lutte contre la variole dans les pays d‘Afrique francophone. Bull Soc Pathol Exot. 1971;64:734–756. [PubMed] [Google Scholar]
- 15.an Saceghem R. La production du vaccin antivariolique au Congo Belge. Annales de la Societe Belge de Medecine Tropicale. 1923;3:237–238. [Google Scholar]
- 16.Jezek Z., wa Mutombo M., Szczeniowski M., Dunn C. WHO; Geneva: 1983. Seroprevalence survey for specific monkeypox antibodies in Kole Zone, Kasai Oriental, Zaire, 1981–1983. p. 1–26. [Google Scholar]
- 17.The current status of human monkeypox: memorandum from a WHO meeting. Bull World Health Organ. 1984;62(5):703–713. [PMC free article] [PubMed] [Google Scholar]
- 18.Bray M., Buller M. Looking back at smallpox. Clin Infect Dis. 2004;38(6):882–889. doi: 10.1086/381976. [DOI] [PubMed] [Google Scholar]
- 19.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 20.Breman J.G. Monkeypox: an emerging infection for humans? Emerg Infect. 2000;4:45–67. [Google Scholar]
- 21.Di Giulio D.B., Eckburg P.B. Human monkeypox. Lancet Infect Dis. 2004;4(4):199. doi: 10.1016/S1473-3099(04)00967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Progress toward poliomyelitis eradication–Angola, Democratic Republic of Congo, Ethiopia, and Nigeria, January 2000–July 2001. MMWR Morb Mortal Wkly Rep. 2001;50(38):826–829. [PubMed] [Google Scholar]
- 23.Mach A. Congo polio immunisation campaign gets go ahead. BMJ. 1999;318(7186):756. doi: 10.1136/bmj.318.7186.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coghlan B., Brennan R.J., Ngoy P., Dofara D., Otto B., Clements M., et al. Mortality in the Democratic Republic of Congo: a nationwide survey. Lancet. 2006;367(9504):44–51. doi: 10.1016/S0140-6736(06)67923-3. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . World Health Organization factsheets. 2010. Poliomyelitis. Available from: http://www.who.int/mediacentre/factsheets/fs114/en/index.html [cited 2011 June 8] [Google Scholar]
- 26.Razum O. Editorial: a farewell to polio vaccination? Not anytime soon. Trop Med Int Health. 2002;7(10):811–812. doi: 10.1046/j.1365-3156.2002.00958.x. [DOI] [PubMed] [Google Scholar]
- 27.Kew O.M., Sutter R.W., de Gourville E.M., Dowdle W.R., Pallansch M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 28.Nsubuga P., McDonnell S., Perkins B., Sutter R., Quick L., White M., et al. Polio eradication initiative in Africa: influence on other infectious disease surveillance development. BMC Public Health. 2002;2:27. doi: 10.1186/1471-2458-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troy S.B., Ferreyra-Reyes L., Huang C.H., Mahmud N., Lee Y.J., Canizales-Quintero S., et al. Use of a novel real-time PCR assay to detect oral polio vaccine shedding and reversion in stool and sewage samples after a Mexican national immunization day. J Clin Microbiol. 2011;49(5):1777–1783. doi: 10.1128/JCM.02524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharya S., Dasgupta R. A tale of two global health programs. Smallpox eradication's lessons for the antipolio campaign in India. Am J Public Health. 2009;99(7):1176–1184. doi: 10.2105/AJPH.2008.135624. [DOI] [PMC free article] [PubMed] [Google Scholar]


