Abstract
Introduction
The aim of this article was to evaluate the current perception of urologists as to what size is considered as a clinically insignificant residual fragment (CIRF).
Material and methods
A survey was globally distributed to the members of the Endourological Society via SurveyMonkey.
Results
A total of 385 participants responded to the survey on CIRF. Most participants considered 2 mm (29%) as CIRF threshold, followed by 3 mm (24%), 4 mm (22%), 0 mm (14%), 5 mm (8%) and 1 mm (3%). North American urologists considered CIRF to be smaller than urologists from Asia, Eurasia and South America, (p-values ≤0.001, 0.037 and 0.015 respectively). European urologists identified smaller CIRF in comparison to Asian urologists (p-value = 0.001). Urologists mainly using a pneumatic lithotripter accepted larger fragments as CIRF, compared to urologists mainly using ultrasonic devices or a combination of ultrasonic and pneumatic devices (p-value = 0.026 and 0.005 respectively). Similarly, urologists mainly performing X-Ray and ultrasound as post-operative imaging accepted larger fragments as CIRF in comparison to urologists mainly performing non-contrast computed tomography (p-value = 0.001).
Conclusions
What is considered as CIRF varies between urologist from different continents and seems to be associated with the lithotripter used and the post-operative imaging modality of preference to assess treatment success.
Keywords: percutaneous nephrolithotomy, survey, clinically insignificant residual fragment, imaging
INTRODUCTION
Urolithiasis is a major health problem all around the world with a steady increasing prevalence reported to be as high as 20.1% [1]. Given the improvements in technology and development of new devices, open surgery has become obsolete and minimally invasive approaches including extracorporeal shockwave lithotripsy (ESWL), ureterorenoscopy (URS) and percutaneous nephrolithotomy (PCNL) are standard treatments for urinary stone disease. Obtaining a stone-free status is one of the main parameters of surgical success and outcomes besides complications, hospital stay and renal function [2]. Although the stone-free rate is often used to compare effectiveness of different treatment modalities, there is no consensus on what constitutes a successful procedure or a stone-free status and, on the type and timing of imaging modality used to assess this status. While some clinicians would define stone-free status as having no visible or measurable fragments on follow-up imaging, others may suggest that the post-operative presence of small asymptomatic residual fragments (RF) smaller than 2 mm, 4 mm or 5 mm may still be considered a successful procedure or a stone-free patient [3, 4]. These fragments that are neither obstructing nor symptomatic are traditionally referred to as clinically insignificant residual fragments or CIRF. Although urinary stone disease is prone to recurrence, the presence of CIRF in comparison to a true stone-free status may lead to earlier stone related symptoms, complications and re-interventions [3].
In this study, we aimed to evaluate the current perception of urologists as to what constitutes a successful procedure and which size of RFs can be perceived as clinically insignificant.
MATERIAL AND METHODS
A survey, developed by EAU-YAU Endourology and Urolithiasis Working Group, was globally distributed to the members of the Endourological Society via SurveyMonkey.
The survey questions included: respondent demographics, country of practice, years of experience, type of workplace, whether endourology fellowship trained or not, annual number of PCNL cases, intraoperative lithotripsy method, post-operative imaging modality and post-operative imaging time. (Appendix 1).
Ethics committee approval is waived because survey research that does not impose risks on participants and that only enrolls competent adults.
Statistical analysis
IBM Statistical Package for Social Sciences (SPSS) version 25.0 (Armonk, New York, USA) was used for statistical analysis and p-values of <0.05 were considered significant. Shapiro Wilk was used to evaluate normal distribution of continuous variables. Non-normally distributed variables were analyzed with Mann-Whitney-U test or Kruskal-Wallis test where appropriate and results presented as medians with interquartile ranges. For multiple group comparisons, post-hoc analysis, with adjustment of the p-value with Bonferroni correction was performed.
The comparison of categorical variables was performed with Chi squared and Fisher’s exact tests and results presented as percentages.
RESULTS
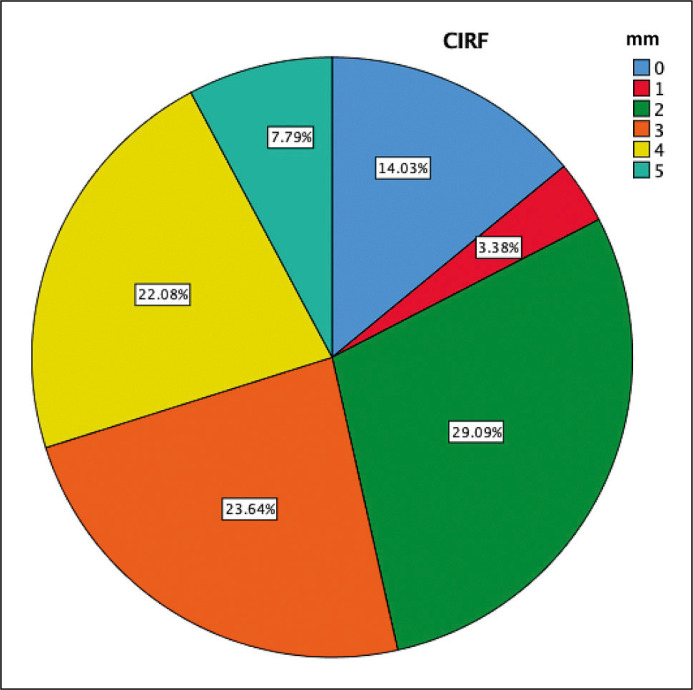
A total of 385 urologists completed the survey on CIRF. Most of the respondents considered 2 mm (29%) as CIRF threshold, followed by 3 mm (23.6%) and 4 mm (22%). A less conspicuous number of urologists considered CIRF as 0 mm (14%), 1 mm (3.4%) or 5 mm (7.8%) (Figure 1). The majority of the respondents were from North America or Europe (31.4% and 23.9%, respectively), fellowship trained (59.2%) and working at an academic center (56.9%) (Table 1). There was no significant difference between urologists working in academic versus non-academic centers (p = 0.602), between fellowship trained versus not fellowship-trained urologists (p = 0.104) and according to annual PCNL caseload in terms of CIRF size perception (p = 0.982). There was no significant association with physician’s age and the perception of what size of RF is considered clinically insignificant [p = 0.604].
Figure 1.
The distribution of perception of clinically insignificant residual fragments.
Table 1.
Demographics and characteristics of the survey respondents included the analysis
| Variables | N (%) |
|---|---|
| Age (years) 25–30 31–35 36–40 41–45 46–50 51–55 56–60 61–65 >65 |
9 (2.3) 39 (10.1) 66 (17.1) 87 (22.6) 50 (13) 56 (14.5) 33 (8.6) 25 (6.5) 20 (5.2) |
| Gender Female Male |
21 (5.5) 364 (94.5) |
| Continent Asia Africa Oceania Eurasia Europe North America South America |
72 (18.7) 12 (3.1) 8 (2.1) 24 (6.2) 92 (23.9) 121 (31.4) 56 (14.5) |
| Years in practice Junior resident Senior resident Fellow 0–5 6–10 11–20 >20 |
3 (0.8) 8 (2.1) 11 (2.9) 64 (16.6) 61 (15.8) 114 (29.6) 124 (32.2) |
| Practice setting Academic Community hospital Military hospital Private hospital VA Other |
219 (56.9) 53 (13.8) 6 (1.6) 73 (19) 3 (0.8) 31 (8.1) |
N – number; VA – veterans affairs
We did however find a significant geographical difference in perception of CIRF (P <0.0001). Urologists from North America (median/[IQR] = 2 mm/[1–3]) considered CIRF to be smaller than urologists working in Asia (median/[IQR] = 3 mm/[2–4], p <0.001), Eurasia (median/[IQR] = 3 mm/[3–4], p = 0.037) and South America (median/[IQR] = 3 mm/[2–4], p = 0.015). Similarly, European urologists considered a smaller threshold for CIRF compared to Asian urologists (median/[IQR] = 2 mm/[2–3] vs 3 mm/[2–4], p = 0.001). North American and European urologists did not have a significantly different perception on CIRF size.
Urologists mainly using a pneumatic lithotriptor accept larger fragments as CIRF (median/[IQR] = 3 mm [3–4]) in comparison to urologists mainly using ultrasonic devices only (median/[IQR] = 3 mm/[3–4], p-value = 0.026) or a combination of ultrasonic and pneumatic devices (median/[IQR] = 2 mm/[2–3] p-value = 0.005).
Urologists mainly performing non-contrast computed tomography (NCCT) as post-operative imaging tolerate smaller fragments as CIRF in comparison to urologists mainly performing X-Ray of kidneys ureters and bladder (KUB) and ultrasound (US) as post-operative imaging (median/[IQR] =2 mm/[1–3] vs 3 mm/[2–4], p-value = 0.001). The majority of urologists prefer post-operative imaging to be performed prior to discharge (43.2%), within 2 weeks (13.6%) or within 1 month (22.8%) of the procedure. The remainder would perform imaging between 6 weeks and 3 months after the procedure.
DISCUSSION
The term clinically insignificant residual fragment was first introduced in peer-reviewed literature by Kulb et al., discussing residual fragments after ESWL [5]. In this early era of ESWL, before current endoscopic and imaging technologies were widely available, the term was suggested, trying to identify a cutoff below which further interventions could be avoided [5, 6]. Given the lack of a standardized method for postoperative imaging modality, time of imaging and the threshold for CIRF size, its current utility and validity is contentious.
Despite an extensive body of literature having been dedicated to defining the cutoff size of clinical significance of RFs and their natural history, our data demonstrates that there is no globally accepted cutoff for CIRF size. Additionally, there appear to be geographical differences that may stem from differences in clinical practice such as differences in tract size or lithotripter use. Physicians preferring a NCCT for post-operative imaging seem to be more stringent, allowing significantly smaller fragments as CIRF in comparison to physicians using X-Ray and US. Although the cause and effect of this association remains elusive, it seems conceivable that if a more accurate means of detecting fragments is used, larger fragments may not be considered to be clinically insignificant.
The ideal timepoint for post-operative imaging is also a topic of debate. The end of the first month after surgery seems reasonable to avoid false positive results as a consequence of stone dust or residual fragments that pass spontaneously at early post-operative period [7].
Given the differences in perception of CIRF and what constitutes a successful procedure, several suggestions have been proposed to standardize the reporting of this most important outcome after stone surgery. In a short communication, Somani et al. proposed to report the size of the RF and the imaging modality used to assess this [8]. In search of consensus using the Delphi method, a panel of experts concluded that treatment success and stone-free status are separate outcomes [4]. The panel agreed (82.1% consensus) that stone-free status after PCNL must be defined as the complete clearance of any RFs, as assessed by means of computed tomography (CT) scan (76.2% consensus). Among 44 experts who selected a cut-off size of CIRF, 56.8% voted for 4 mm, 11.4% voted for 3 mm and 31.8% voted for 2 mm [4]. Our data confirms the lack of a consensus on CIRF size and demonstrates that we are still in dire need of a standardized way and a definition to report this most important outcome.
The results of the expert panel from Opondo et al. are somewhat reflected in published literature as a CIRF threshold of 4 mm is widely accepted in several studies [4, 9, 10]. In a study by Altunrende et al. employing a pneumatic lithotripter for stone fragmentation, KUB or CT for postoperative evaluation and a cutoff of 4 mm for CIRF, they evaluated 38 patients that had CIRF after PCNL with at least 24 months follow-up [9]. They concluded that CIRF with struvite stone composition was the main risk factor for progression of stone disease. Gokce et al. assessed the sensitivity and specificity of follow-up imaging to detect CIRF with a cutoff of 4 mm. Using NCCT as the gold standard, KUB and US demonstrated a sensitivity of 85.7% and 57.1% respectively to detect stones >4 mm [10]. In a retrospective study Osman et al. questioned the clinical insignificance of single residual stones smaller than 5 mm following PCNL [11]. They found fragments <5 mm to result in active intervention in one third of the patients on intermediate follow-up longer than 12 months. Thus, different from the aforementioned studies, they suggested that the threshold of CIRF might be ≤3 mm.
In a study comparing the stone related events after RIRS between RF smaller than 4 mm and 4 to 7 mm, authors reported that RFs larger than 4 mm were more prone to cause stone related events [12]. Chew et al. reported that RFs >4 mm after RIRS were more likely to grow with time and were more associated with complications [13]. The authors additionally found that even RF larger than 2 mm were likely to grow with time, but were not associated with complications. As such, they concluded that achieving complete stone-free status is a better strategy to prevent stone events after ureteroscopy rather than determining smaller RF thresholds. Similar to the aforementioned study, Kang et al. suggested to achieve complete stone free status following endoscopic lithotripsy methods using holmium laser. Fragments of 1 mm and <3 mm had passed spontaneously after a 2-year follow-up in only 40% and 21.4% respectively [14]. In addition, although stone growth was apparent in 18.1% and 26.6% respectively, only 4 patients (3.2%) experienced a stone related event. Rebuck et al. [15] investigated the fate of RFs ≤4 mm in 51 patients following ureteroscopy and holmium laser stone fragmentations. The authors reported that about 1 in 5 of those patients experienced a stone event over the following 1.6 years, while 21.7% became stone-free via spontaneous passage and 58.7% remained asymptomatic [15].
Evaluating the fate of patients with RFs after PCNL, Emmott et al. demonstrated that 16.9% and 28.2% of patients with RFs of <4 mm and ≥4 mm respectively required a reintervention over time. Kaplan-Meier survival analysis also identified patients with larger fragments to recur faster than patients with smaller fragments [16]. Using 2 mm as a cutoff for CIRF, Raman et al. concluded that patients with RFs larger than 2 mm after PCNL had significantly lower 3 and 5-year stone event-free survival rates compared to patients with RFs smaller than 2 mm [17].
It is clear from all these studies that leaving even small fragments appears to carry a higher risk of eventually having a recurrence or a clinical event as compared to being completely stone-free.
In fact, the term CIRF could or even should be considered a misnomer as every currently clinically insignificant residual fragment can become clinically significant over time. However, similar to its meaning after ESWL, a CIRF after PCNL can be considered a residual fragment that does not currently require or warrant an auxiliary procedure but does carry the risk of recurrence. As many CIRF do not become symptomatic over time, the active treatment of these fragments, reaching for a 100% stone-free, may be an overtreatment in a large portion of this population and the harms of such procedure should be weighed against the benefits. The term clinically acceptable residual fragment (CARF) may therefore be a more accurate description of the entity.
What the ideal cutoff is for a CIRF or CARF after endoscopic stone treatment and how these patients should be reported in published literature, remains a topic of debate and requires more high-quality research with larger populations. In the meantime, attaining a true stone-free status, as assessed by NCCT within 1 month of surgery, is the goal to be achieved with endoscopic stone treatment.
Our study is also not without limitations. The relatively low rate of response to the survey might be the most important limitation of the study. However, we think that global participation might have overcome this issue.
CONCLUSIONS
The definition of CIRF is still uncertain today. What is considered as CIRF varies between urologists from different continents and seems to be associated with the modality of post-operative imaging to assess treatment success. As CIRF is one of the most important outcome variables reported in endourological literature, this variation causes heterogeneity in data and difficulty in comparing results. There is a dire need for a consensus on a definition of what constitutes a clinically insignificant or acceptable residual fragment.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
STATEMENT OF ETHICS
Study approval statement: No formal ethical approval was necessary to conduct the study. Consent to participate statement: Consent was implied by participation.
FUNDING SOURCES
No funding was received for this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request to corresponding author.
QUESTIONS OF THE DISTRIBUTED SURVEY PERTAINING TO CIRF ASSESSMENT
-
Age:
25–30, 31–35, 36–40, 41–45, 46–50, 51–55, 56–60, >60
-
Sex:
Male/ Female
Country of clinical practice
-
Years in practice:
I am a junior resident/ I am a senior resident/ I am a fellow/ 0–5, 6–10, 11–20, >20 years
You work predominantly in: an academic hospital/ community hospital/ private hospital / Veterans Affairs hospital / military hospital
Do you have fellowship training in Endourology/stone treatment? Yes/No
-
How many PCNL procedures do you personally perform annually?
<10
10–20
21–50
51–100
>100
- Please indicate the lithotripsy modality you use
Never <25% >25%, <50% Approx. 50% >50%, <75% >75% Always Ultrasound Pneumatic Laser Electro-hydraulic Combined ultrasound/pneumatic No lithotripsy PCNL – percutaneous nephrolithotomy; CIRF – clinically insignificant residual fragment; KUB – kidneys ureters and bladder; CT – computed tomography -
At what time post-operatively do you usually perform imaging to determine stone-free status?
Prior to discharge
Two weeks
1 month
6 weeks
2 months
3 months
- Which imaging modality do you use to determine stone free status after PCNL?
Never <25% >25%, <50% Approx. 50% >50%, <75% >75% Always Nephrostogram X-Ray KUB X-Ray KUB and ultrasound Digital tomosynthesis Non-contrast CT Contrast-enhanced CT PCNL – percutaneous nephrolithotomy; CIRF – clinically insignificant residual fragment; KUB – kidneys ureters and bladder; CT – computed tomography -
What do you consider to be a clinically insignificant fragment?
0 mm: clinically insignificant fragments don’t exist
1 mm
2 mm
3 mm
4 mm
5 mm
Other: Please specify:
References
- 1.Bartoletti R, Cai T, Mondaini N, Melone F, Travaglini F, Carini M, Rizzo M. Epidemiology and risk factors in urolithiasis. Urol Int. 2007; 79 (Suppl 1): 3-7. [DOI] [PubMed] [Google Scholar]
- 2.Wang YB, Cui YX, Song JN, Yang Q, Wang G. Efficacies of various surgical regimens in the treatment of renal calculi patients: a network meta-analysis in 25 enrolled controlled clinical trials. Kidney Blood Press Res. 2018; 43: 1183-1198. [DOI] [PubMed] [Google Scholar]
- 3.Suarez-Ibarrola R, Hein S, Miernik A. Residual stone fragments: clinical implications and technological innovations. Curr Opin Urol. 2019; 29: 129-134. [DOI] [PubMed] [Google Scholar]
- 4.Opondo D, Gravas S, Joyce A, et al. Standardization of patient outcomes reporting in percutaneous nephrolithotomy. J Endourol. 2014; 28: 767-774. [DOI] [PubMed] [Google Scholar]
- 5.Kulb TB, Lingeman JE, Coury TA, et al. Extracorporeal Shock Wave Lithotripsy in Patients with a Solitary Kidney. J Urol. 1986; 136: 786-788. [DOI] [PubMed] [Google Scholar]
- 6.Hein S, Miernik A, Wilhelm K, et al. Clinical significance of residual fragments in 2015: impact, detection, and how to avoid them. World J Urol. 2016; 34: 771-778. [DOI] [PubMed] [Google Scholar]
- 7.Skolarikos A, Papatsoris AG. Diagnosis and management of postpercutaneous nephrolithotomy residual stone fragments. J Endourol. 2009; 23: 1751-1755. [DOI] [PubMed] [Google Scholar]
- 8.Somani BK, Desai M, Traxer O, Lahme S. Stone-free rate (SFR): a new proposal for defining levels of SFR. Urolithiasis. 2014; 42: 95. [DOI] [PubMed] [Google Scholar]
- 9.Altunrende F, Tefekli A, Stein RJ, et al. Clinically insignificant residual fragments after percutaneous nephrolithotomy: medium-term follow-up. J Endourol. 2011; 25: 941-945. [DOI] [PubMed] [Google Scholar]
- 10.Gokce MI, Ozden E, Suer E, Gulpinar B, Gulpınar O, Tangal S. Comparison of imaging modalities for detection of residual fragments and prediction of stone related events following percutaneous nephrolitotomy. Int Braz J Urol. 2015; 41: 86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osman Y, Harraz AM, El-Nahas AR, et al. Clinically insignificant residual fragments: an acceptable term in the computed tomography era? Urology. 2013; 81: 723-726. [DOI] [PubMed] [Google Scholar]
- 12.Atis G, Pelit ES, Culpan M, et al. The Fate of Residual Fragments After Retrograde Intrarenal Surgery in Long-Term Follow-up. Urol J. 2019; 16: 1-5. [DOI] [PubMed] [Google Scholar]
- 13.Chew BH, Brotherhood HL, Sur RL, et al. Natural History, Complications and Re-Intervention Rates of Asymptomatic Residual Stone Fragments after Ureteroscopy: a Report from the EDGE Research Consortium. J Urol. 2016; 195: 982-986. [DOI] [PubMed] [Google Scholar]
- 14.Kang M, Son H, Jeong H, Cho MC, Cho SY. Clearance rates of residual stone fragments and dusts after endoscopic lithotripsy procedures using a holmium laser: 2-year follow-up results. World J Urol. 2016; 34: 1591-1597. [DOI] [PubMed] [Google Scholar]
- 15.Rebuck DA, Macejko A, Bhalani V, Ramos P, Nadler RB. The natural history of renal stone fragments following ureteroscopy. Urology. 2011; 77: 564-568. [DOI] [PubMed] [Google Scholar]
- 16.Emmott AS, Brotherhood HL, Paterson RF, Lange D, Chew BH. Complications, Re-Intervention Rates, and Natural History of Residual Stone Fragments After Percutaneous Nephrolithotomy. J Endourol. 2018; 32: 28-32. [DOI] [PubMed] [Google Scholar]
- 17.Raman JD, Bagrodia A, Gupta A, et al. Natural history of residual fragments following percutaneous nephrostolithotomy. J Urol. 2009; 181: 1163-1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request to corresponding author.