Human monkeypox is a rare viral zoonosis endemic to central and western Africa that has recently emerged in the USA. Laboratory diagnosis is important because the virus can cause disease that is clinically indistinguishable from other pox-like illnesses, particularly smallpox and chickenpox. Although the natural animal reservoir of the monkeypox virus is unknown, rodents are the probable source of its introduction into the USA. A clear understanding of the virulence and transmissibility of human monkeypox has been limited by inconsistencies in epidemiological investigations. Monkeypox is the most important orthopoxvirus infection in human beings since the eradication of smallpox in the 1970s. There is currently no proven treatment for human monkeypox, and questions about its potential as an agent of bioterrorism persist.
Since the global eradication of smallpox in 1977, the World Health Assembly has consigned the maintenance of live variola virus to only two authorised facilities in the world.1 Recent concerns about the potential of variola virus as an agent of bioterrorism have, however, brought the virus to the forefront of the public-health and scientific-research agendas of many countries. These concerns have translated into heightened implications for any outbreak that mimics smallpox clinically, particularly if it is caused by a novel or emerging agent. In the spring of 2003, an outbreak of a pox-like illness in people occurred in the central USA. This outbreak was attributed to the monkeypox virus (MPV), a rare zoonosis that can cause illness clinically indistinguishable from smallpox. Before that outbreak, human monkeypox had never been reported in the western hemisphere. This review focuses on the clinical and epidemiological features of human monkeypox, its emergence in the USA, the similarities to smallpox and chickenpox, the potential of MPV as an agent of bioterrorism, and considerations for diagnosis, treatment, and prevention.
Causative agent
MPV is an orthopoxvirus that is genetically distinct from other members of the Poxviridae family, including the variola, vaccinia, ectromelia, camelpox, and cowpox viruses. It was first identified as the cause of a pox-like illness in captive monkeys at the State Serum Institute in Copenhagen in 1958.2 Monkeypox (figure 1 ) is regarded as the most important orthopoxvirus infection in human beings since the eradication of smallpox.3 By contrast with variola virus, however, MPV has a wide range of hosts,4 which has allowed it to maintain a reservoir in wild animals while sporadically causing human disease, and has precluded global eradication by human vaccination.
Figure 1.
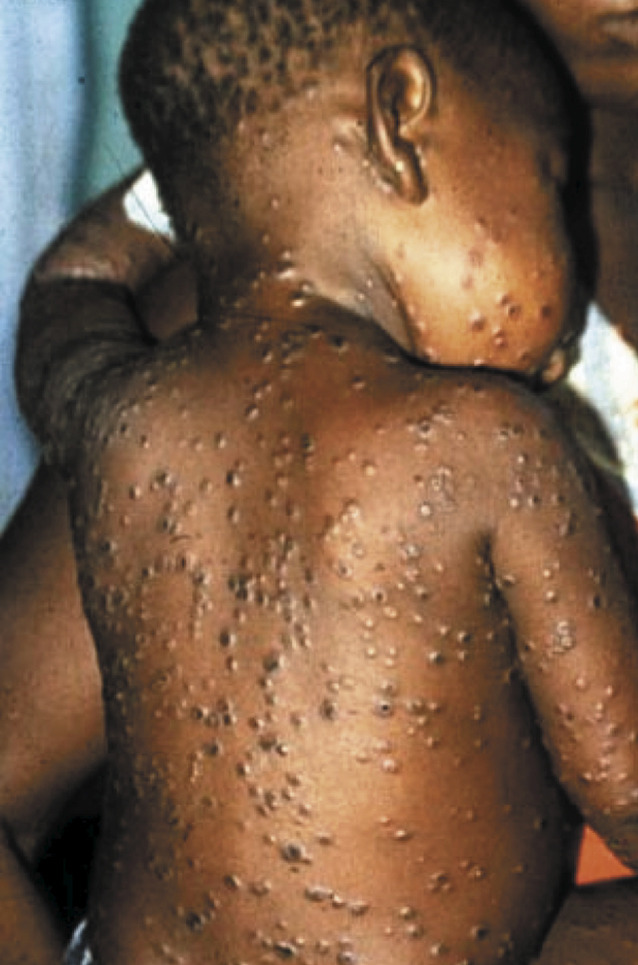
African child with disseminated monkeypox. Note postauricular adenopathy (courtesy of Leo Lanoie, Prince Albert Parkland Health Region, Saskatchewan, Canada).
Early investigations characterised the differences between MPV and variola virus on the basis of their ability to grow in cell culture and induce death of chick embryos.4 MPV was also found to induce characteristic pock morphology on chick embryo chorioallantoic membranes.5, 6 MPV and variola are closely related serologically, and they cannot be reliably differentiated by neutralisation or haemagglutination inhibition.7 Specific antisera, however, can differentiate between monkeypox and smallpox by means of specific viral antigens (mo and va, respectively).8 These monkeypox-specific antibodies were used in first defining the MPV reservoir in wild monkeys in central Africa.9 In addition, each orthopoxvirus has a specific composition of surface epitopes,10 distinctive polypeptides,11 unique DNA cleavage sites, and specific differences in the long terminal repeats of the double-stranded DNA genome.12 Despite such distinguishing characteristics, the development of rapid and reliable diagnostic tests for MPV has been difficult. Among other proposed areas of needed research, continued study of the molecular virology of MPV is essential for the further development of such tests (panel 1 ).
Panel 1. Recommended research areas for future monkeypox investigation13.
Epidemiological surveillance
Re-establish and strengthen human monkeypox surveillance systems (especially in western and central Africa), for rapid detection of suspect cases, rapid notification to national and WHO authorities, and rapid and comprehensive investigations.
Update human monkeypox case definitions.
Assess transmissibility of human monkeypox and re-explore mathematical modelling techniques.
Prospectively investigate test characteristics of orthopoxvirus and MPV-specific serological assays.
Study characteristics of monkeypox and other pox-like illnesses in HIV-infected patients in detail.
Assess the feasibility of and design ecological and natural history studies.
Design a population-based study to define the clinical, epidemiological, and ecological characteristics and laboratory diagnosis of human monkeypox in detail.
Evaluate, establish, and maintain national capability for serological and virological diagnosis.
Increase resources, training, and administrative and logistical support to ensure satisfactory surveillance and diagnostic capabilities.
Control and prevention
Expand laboratory screening for antiviral drugs against MPV and other orthopoxviruses.
Undertake preclinical and clinical trials of cidofovir for human monkeypox, especially in areas where the disease is endemic.
Clearly establish the risks of smallpox vaccination in areas where human monkeypox is endemic, especially among immunocompromised patients.
Increase training for and establish a reliable and rapid reporting system to local heath providers and regional health officials in endemic areas.
Provide local health providers and regional health officials with information on differential diagnosis, case management, notification and investigation procedures, and collection and shipment of clinical samples.
Laboratory issues
Establish a central laboratory in badly affected countries able to implement modern orthopoxvirus diagnostic assays.
WHO collaborating centres and other laboratories involved in orthopoxvirus research should continue to develop and evaluate diagnostic tests for both central laboratory and field use and continue molecular biological studies.
Increase capacity to carry out epidemiological investigations, collaborative research, and training of staff at WHO collaborating centres.
Obtain increased support for laboratory studies.
A genomic comparison of variola virus and MPV was described in 2001.12 The central region of the MPV genome encodes essential enzymes and structural proteins and is 96·3% identical to that of the variola virus. However, the end regions of the MPV genome, which encode virulence and host-range factors, differ substantially. Comparative analysis of the genomes of MPV and smallpox virus shows that MPV is a distinct species, which evolved from an orthopoxvirus ancestor independently of variola virus.14 Thus, MPV is not a direct ancestor of the variola virus (or vice versa), and variola virus cannot be readily “derived” from MPV.15 This and other evidence16 alleviated concerns that MPV could mutate into variola virus17 and reinforced confidence in the enduring success of the global smallpox vaccination programme.
Clinical features
The first human monkeypox case was reported in a child in the equatorial region of the Democratic Republic of Congo (formerly Zaire) in 1970, 9 months after the eradication of smallpox in that country.18 As the number of cases in Africa accumulated in the 1970s, human monkeypox was thought to resemble smallpox in terms of symptoms, severity, and mortality.4 By contrast with smallpox, however, it was associated with low transmissibility between human beings. As of 1980, fewer than 50 cases of human monkeypox had been recognised,” and the clinical manifestations and epidemiology remained poorly characterised.
Most clinical data on human monkeypox come from subsequent investigations of outbreaks in central and western Africa. Observational studies in the mid-1980s showed an incubation period of 10–14 days and an infectious period occurring during the first week of the rash.20 A characteristic 2-day prodrome, manifest by fever and malaise, occurs in most patients before development of the rash. In addition to the smallpox-like prodrome, severe lymphadenopathy occurs in many patients 1–2 days before the onset of the rash. Lymphadenopathy is not characteristic of smallpox, and this clinical finding is a key distinguishing feature of human monkeypox (figure 1).19, 21 About 90% of patients infected with MPV develop lymphadenopathy, which can be unilateral or bilateral and occurs in the submandibular, cervical, postauricular, axillary, or inguinal lymph nodes, or any combination of these.13, 22
The typical human monkeypox rash begins as maculopapular lesions of 2–5 mm in diameter. Reports from African outbreaks suggest that the rash becomes generalised in distribution in most cases, spreading in a centrifugal pattern (figure 1).23 A few cases have a centripetal rash, similar to that of chickenpox (table 1 ).24 The skin lesions typically progress through papular, vesicular, pustular, and crust phases over a period of 14–21 days, before sloughing and leaving dyspigmented scars.13 No haemorrhagic form of monkeypox has been described in human beings.23 In addition to smallpox (figure 2 ) and chickenpox, other syndromes to consider in the differential diagnosis of a vesiculopapular rash include drug eruptions, eczema herpeticum, dermatitis herpetiformis, rickettsialpox, and molluscum contagiosum.13
Table 1.
Comparison of clinical features between human monkeypox, smallpox, and chickenpox* (modified from Breman and Henderson24)
| Disease characteristics | Monkeypox | Smallpox† | Chickenpox |
|---|---|---|---|
| History | |||
| Recent contact with exotic animal | Yes | No | No |
| Recent exposure to patient with vesicular rash | Possible‡ | Yes | Yes |
| Previous vaccination against smallpox | 10–15% | Rare | Yes |
| Incubation period (days) | 10–14 | 10–14 | 14–16 |
| Prodromal phase (days) | 1–3 | 2–4 | 0–2 |
| Physical examination | |||
| Prodromal fever and malaise | Yes | Yes | Yes (mild) |
| Lymphadenopathy | Yes | No | No |
| Distribution of skin lesions | Centrifugal (80%) or centripetal (5%) | Centrifugal | Centripetal |
| Depth of skin lesions | Superficial | Deep | Superficial |
| Evolution of skin lesions | Monomorphic (80%) or pleiomorphic (20%) | Monomorphic | Pleiomorphic |
| Desquamation (days after onset) | 22–24 | 14–21 | 6–14 |
| Lesions on palms and soles | Common | Common | Rare |
| Extracutaneous manifestations | |||
| Secondary skin/soft-tissue infection | 19% | Possible | Possible |
| Pneumonitis | 12% | Possible | 3–16% |
| Ocular complications | 4–5% | 5–9% | No |
| Encephalitis | <1% | <1% | <1% |
| Laboratory diagnosis | |||
| DNA detection (eg, PCR) | MPV | Variola virus | VZV |
| Electron microscopy | Poxvirus particles | Poxvirus particles | Herpesvirus |
| Culture on chick chorioallantois | Characteristic pocks | Characteristic pocks | No growth |
| Serology | Orthopoxvirus and MPV antibodies | Orthopoxvirus and variola virus antibodies | Varicella antibodies |
VZV=varicella zoster virus.
Other diseases that can be confused with these infections include generalised vaccinia, disseminated infection with herpes zoster or herpes simplex virus, drug eruptions, enterovirus infections, dermatitis herpetiformis, rickettsialpox, and molluscum contagiosum.
Smallpox in previously unvaccinated patients.
Highest risk among household contacts, with secondary attack rate of about 12%.
Figure 2.

African woman with smallpox (courtesy of Department of Infectious and Parasitic Disease Pathology, Armed Forces Institute of Pathology, Washington, DC, USA)
As more patients with human monkeypox were described from the Democratic Republic of Congo in the 1980s, it became obvious that the disease characteristics differed among those with a history of smallpox vaccination.23 In patients with evidence of a smallpox vaccination scar, the monkeypox rash was milder and more likely to be pleomorphic, lymphadenopathy was evident in only 53%, and no deaths occurred. Chickenpox became the primary differential diagnostic challenge (table 1). In a follow-up study, Jezek and colleagues studied 977 patients with a rash who were not suspected of having monkeypox.25 Among 730 cases of presumed chickenpox, 3·3% had monkeypox by diagnostic testing (serology and vesicular-fluid electron microscopy or culture). Monkeypox was also discovered in 7·3% of patients with “atypical chickenpox” and 6·1% of those with rash of unknown cause. Monkeypox was probably misdiagnosed as chickenpox because of the regional pleomorphism and centripetal distribution of the skin lesions. Of note, lymphadenopathy was observed in 76% of the misdiagnosed cases of monkeypox.
The human monkeypox cases in the 2003 US outbreak (the epidemiology of which is described below) had similar clinical features to the previously described African cases, but they were generally milder in severity. Although the mode of transmission remains poorly characterised, MPV transmission in the USA was thought to occur through direct contact or respiratory droplet spread (http://www.cdc.gov/ncidod/monkeypox/infectioncontrol.htm). All 32 patients with laboratory-confirmed monkeypox in the USA reported a rash, and all but one reported at least one other clinical sign or symptom, including fever (87%), respiratory symptoms (78%), and lymphadenopathy (69%).26 Most of the patients were not seriously ill.27 Among the 78 reported US patients for whom data are available, 19 (24%) were admitted to hospital, some primarily for isolation purposes.26, 27 From published photographs, the rash appeared less severe (Figure 3 ,Figure 4 ) with fewer lesions than the generalised rash described in past African outbreaks (http://research.marshfieldclinic.org/crc/monkeypox.asp). A 9-day delay in antiviral treatment of the index case, during which time several antibacterial agents were administered, suggested that a bioterrorism algorithm was not activated and smallpox was not suspected. Two patients, both children with laboratory-confirmed MPV infection, had serious clinical illness.26, 28 The first had severe encephalitis that improved during a 14-day hospital stay, and the second had diffuse pox lesions, including oropharyngeal lesions that led to difficulty in breathing and swallowing. Mechanical ventilation was not required.27 There were no deaths among the 81 reported US cases.26
Figure 3.

Human monkeypox lesions adjacent to the site of primary inoculation in a child during the 2003 US outbreak (courtesy of Marshfield Clinic, Wisconsin, USA).
Figure 4.
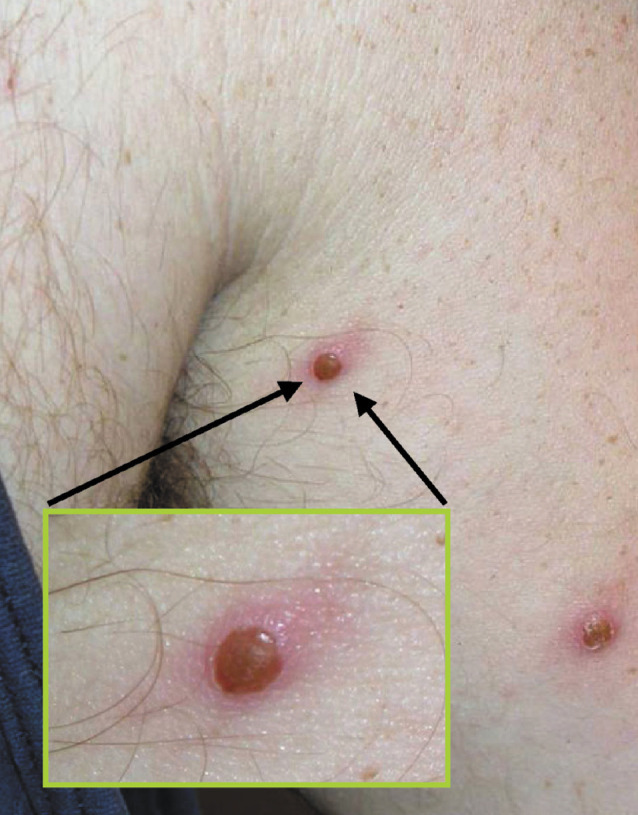
Human monkeypox lesions on the thorax of an adult male patient during the 2003 US outbreak (courtesy of Marshfield Clinic, Wisconsin, USA).
Epidemiology
The epidemiology of human monkeypox differs significantly from that of smallpox. From the first reported case of human monkeypox in 1970 until early 2003, sporadic cases were reported only from the rainforest areas of central and western Africa (including Cameroon, the Central African Republic, Gabon, Cote d'Ivoire, Liberia, Nigeria, and Sierra Leone), and large outbreaks were identified only in the Democratic Republic of Congo. Early epidemiological data came from an analysis of 47 cases of human monkeypox reported before 1980, in which the case-fatality rate was 17%, secondary transmission was the cause in 9% of cases, and the secondary attack rate of 3·3%19 was noted to be much lower than the rate observed with smallpox (37–88%; table 2 ).29 Despite the high case-fatality rate, monkeypox was not deemed a serious public-health problem at the time because, unlike smallpox, it showed no evidence of sustained transmissibility in human beings. The longest chain of documented human-to-human transmission was only five generations (four serial transmissions)30 and a stochastic model for spread of monkeypox between human beings indicated that MPV was highly unlikely to be able to maintain itself permanently in human communities.31 Accordingly, the Global Commission for the Certification of Smallpox Eradication concluded in its final report in 1979 that continued smallpox vaccination to prevent human monkeypox was not justified. In addition to the known adverse events associated with smallpox vaccination in immunocompetent patients, the emergence of AIDS in the 1980s further heightened concerns over the use of the vaccine.32 The Global Commission did, however, recommend that measures be taken to assess the public-health significance of this emerging zoonosis more accurately.3
Table 2.
Comparison of epidemiological features of human monkeypox by surveillance period and epidemiological setting
| Feature | 1970–79 | 1981–86 | 1996–97 | 2003 |
|---|---|---|---|---|
| Location | Central and western Africa | Democratic Republic of Congo | Democratic Republic of Congo | Central USA |
| Epidemiological setting | Passive surveillance | Active surveillance | Outbreak | Outbreak |
| Number of reported cases | 47 | 338 | 419* | 81 |
| % laboratory confirmed | 87 | 100 | Not known | 40 |
| Median age (years) | 4 | Not known | Not known | 27 |
| Suspected primary source(s) | Not known | “Forest animals” | Not known | Prairie dog, Gambian giant rat |
| Primary cases (%) | 91 | 72 | 22 | 100 |
| Secondary cases (%) | 9 | 28 | 78 | 0 |
| Secondary attack rate (%) | 3·3 | 3·7† | 8·0 | 0 |
| Case-fatality rate (%) | 17 | 10 | 1·5 | 0 |
| Previous vaccinia | 9% | 13% | 6% | 25%‡ |
| vaccination | (with vaccine scar) | (with vaccine scar) |
Excludes 92 cases that were identified in an earlier investigation of the same outbreak but not included in the analysis of the subsequent cases.
Among household contacts.
Proportion of the confirmed cases for which information was available.
As a result of these recommendations, an active surveillance programme for human monkeypox was established in the Democratic Republic of Congo from 1981 to 1986.33 This intensive surveillance accounted for 338 of the 404 recognised cases in Africa during this period, and it was believed to be the main reason for the eight-fold increase in the reported incidence of human monkeypox.32 Other factors that were postulated to explain the rising number of reported cases include a larger number of people unvaccinated against smallpox (ie, a growing population of susceptible individuals over time) and changes in the virus itself, although definitive data are lacking.34 Of the cases detected by active surveillance, secondary transmission accounted for 28%, a rate three times higher than for cases in the 1970s (9%; table 1). The 10% mortality rate found during the period of active surveillance was, however, similar to the 17% mortality rate reported before 1980.32 An investigation of 2510 contacts of 214 patients with monkeypox between 1980 and 1984 showed that the highest secondary attack rates (13·9%) occurred in unvaccinated household contacts aged 0-4 years.20 This same study found a rate of subclinical infection of only 3% in unvaccinated contacts, and no evidence of secondary transmission from these subclinical cases.
After the active surveillance programme ended in 1986, only 13 cases of human monkeypox were reported up to the end of 1992 (eight in Gabon, four in Cameroon, and one in the Democratic Republic of Congo), and no cases were reported between 1993 and 1995,32 probably owing to inadequate surveillance. In 1996–97, however, the largest outbreak of human monkeypox ever recorded occurred in the Kasai Oriental region of the Democratic Republic of Congo. 92 cases were identified during an initial investigation by WHO and the US Centers for Disease Control and Prevention (CDC) in February, 1997,35 and a further 419 cases were identified after a follow-up investigation in October of that year.36 Epidemiological analysis of these 419 cases gave significantly different results from previous analyses in that the proportion of cases attributed to secondary transmission was much higher (78%) and the case-fatality rate was much lower (1·5%; table 2).
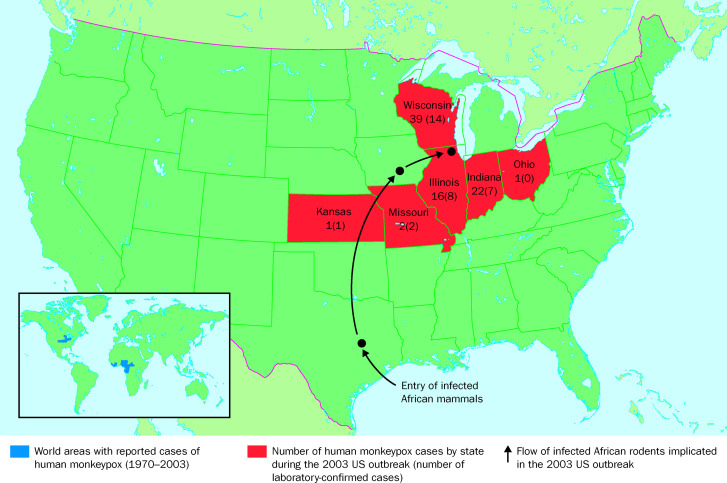
After 1997, human monkeypox attracted little attention worldwide until May, 2003, when the CDC received reports from the central USA of patients who developed fever and a rash after close contact with pet prairie dogs and other mammals.37 This outbreak, with a total of 81 identified cases (40% laboratory confirmed), was due to human monkeypox, a disease that had previously never been recorded in the western hemisphere (figure 5 ).26, 37 None of the cases were attributed to secondary transmission, and none resulted in death (table 2). Traceback investigations identified an international shipment of about 800 small mammals from Ghana to Texas as the probable source for the introduction of MPV into the USA.26 These mammals were of six genera of African rodents: rope squirrels (Funisciurus spp), tree squirrels (Heliosciurus spp), Gambian giant rats (Cricetomys spp), brushtail porcupines (Atherurus spp), dormice (Graphiurus spp), and striped mice (Hybomys spp). Laboratory testing of some of the suspect animals by virus isolation and PCR amplification at CDC revealed that at least one Gambian giant rat, two rope squirrels, and three dormice were infected with MPV. Gambian giant rats from this shipment were transported from Texas via an Iowa animal vendor to a pet distributor in the Chicago area, where they were co-housed with prairie dogs (Cynomus spp). Infected prairie dogs were subsequently transported from the distributor to a vendor in Wisconsin, where they were sold to the index patient and others (figure 5). Infected prairie dogs, which through a non-linear chain of distribution may have also been sold at “swap meets” in Illinois, Indiana, and Ohio, have been implicated as the source of primary infection for most of the US cases.26, 27, 37
Figure 5.
Geographical distribution of human monkeypox.
Hosts and reservoirs
Although much has been learned about MPV hosts and reservoirs, many questions remain. Serological surveys suggest that many animals are infected with MPV under natural conditions, including squirrels, non-human primates, and rats. The primary reservoir for human infection, however, remains unknown.21, 38 Several epidemiological studies from the Democratic Republic of Congo have implicated squirrels (especially Funisciurus anerythrus) inhabiting agricultural areas as primary candidates to sustain viral transmission among people in nearby settlements.39, 40 In one environmental survey, Funisciurus spp squirrels had a higher rate of MPV seropositivity (24%) than other animals that were tested, including Heliosciurus spp squirrels (15%) and primates (8%).40 A subsequent seroprevalence study done as part of the investigation of the outbreak in February, 1997, in the Democratic Republic of Congo showed even higher positivity rates in these squirrels (39–50% in Funisciurus spp and 50% in Heliosciurus spp squirrels).41 In addition, 16% of Gambian giant rats tested in this study had serological evidence of MPV exposure.
Whether MPV has established an enzootic reservoir in the USA remains unknown. The infection of a rabbit (family Leporidae) after exposure to a diseased prairie dog at a veterinary clinic confirmed the transmissibility of the virus between mammal species common in North America. This rabbit was implicated as the source of primary infection in one US case.37 In an attempt to halt the further spread of MPV to human beings and other species, CDC and the US Food and Drug Administration issued a joint order on June 11, 2003, banning the importation of all rodents (order Rodentia) from Africa until further notice. In addition, the order prohibits the transportation or offering for transportation in interstate commerce, or the sale, offering for sale, or offering for any other type of commercial or public distribution, including release into the environment, of prairie dogs or animals of any of the six genera of African rodents represented in the contaminated shipment (http://www.cdc.gov/ncidod/monkeypox/pdf/embargo.pdf). Despite these early measures, concerns have been raised that the virus's capability for rapid spread in rodents may have allowed it to gain a foothold in an animal reservoir in the USA,42 although there is no evidence to confirm that it has. An aggressive campaign to identify and destroy or quarantine the 800 mammals from the contaminated African shipment, along with all subsequently exposed animals, has been hampered by lack of a detailed “paper trail” in many cases.43 Information and guidance for people who have contact with animals, including animals exposed to or infected with MPV, can be found on the CDC website (http://www.cdc.gov/ncidod/monkeypox/animalbasics.htm;http://www.cdc.gov/ncidod/monkeypox/animalhandlers.htm).
If MPV has established an enzootic presence in the USA, the full implications would be difficult to predict. Although previous epidemiological analyses suggested that MPV is incapable of sustaining itself in a human population,31 the possibility of frequent reintroduction from an animal source must be considered. It has been suggested, for example, that the unusually large outbreak in 1996–97 in the Democratic Republic of Congo resulted from increased contact with infected animals by a human community displaced by civil war.32 Similarly, high and sustained rates of exposure to MPV might occur in other settings, such as the infection of wild rodent species in a metropolis such as Chicago. The coexistence of high-density populations of people and animals such as rats, mice, and squirrels must be considered in such a scenario, as well as the potential consequences of human monkeypox in immuno-suppressed people, including those with AIDS.
Little is known about coinfection with MPV and HIV. In the 1996–97 outbreak in the Democratic Republic of Congo only three of the tested case serum samples were positive for HIV, and the clinical courses of these patients were not detailed.13 Illnesses caused by certain other orthopoxviruses, including vaccinia virus,24 and molluscum contagiosum virus, are known to be more severe in AIDS patients than in non-immunosuppressed people. There are no data on the clinical course of monkeypox infection in individuals with AIDS or other immunosuppressive disorders. For these reasons and others, including waning immunity from smallpox vaccination and the unclear long-term status of the renewed smallpox immunisation initiative, the public-health implications of an enzootic animal reservoir of MPV in the USA are uncertain.
Transmissibility, lethality, and bioterrorism potential
A clear and consistent understanding of the transmissibility and mortality of MPV has been hampered by variable epidemiological data in published reports. To explain the remarkable rise in secondary cases between the 1970–86 surveillance data and the investigation of the 1997 outbreak in October of that year (9–28% vs 78%), some authors have implicated an attenuation in immunity after the widespread cessation of smallpox vaccination in the early 1980s.44, 45 If waning immunity alone accounted for such an increase in transmissibility, however, a concomitant rise in mortality due to human monkeypox would also be expected. Instead, a striking decrease was observed in the attributable mortality rate of human monkeypox in the same groups of patients over the same period (10–17% in 1970–86 vs 1·5% in 1997). The possibility of a more transmissible and less virulent strain of MPV has also been invoked to explain these epidemiological differences,44, 45 though data to support this notion directly are lacking.
Careful review of the epidemiological evidence, however, implicates an excessive number of false-positive cases in the investigation in October, 1997, as the reason for the alleged increase in transmissibility and decline in mortality of human monkeypox. The evidence suggests, furthermore, that this unusually high rate of false-positive cases resulted from the non-specific case definition used in this investigation and that a substantial proportion of these cases were actually chickenpox. For instance, the 338 cases detected by active surveillance in 1981–86 were reported only after examination by a physician and verification by laboratory testing of skin lesions, serum, or both.46 By contrast, the 419 cases detected in the investigation in October, 1997, were identified by retrospective self-reporting of clinical signs and symptoms up to 20 months after onset. Although clinical samples were collected for subsequent testing from some of the case-patients in that investigation (including serum from about 300 with historical disease, and crusted scabs or vesicular fluid from 19 with active disease), results of these tests were not used for purposes of case definition. The definition of a probable case (n=304) in the investigation in October, 1997, was the occurrence since February, 1996, of fever and a vesiculopustular rash similar to a WHO reference photo, or five or more facial pock marks in a resident of Kasai Oriental; for a possible case (n=115) the definition was the occurrence of fever and a vesicular or crusty rash.36, 44 Both of these highly sensitive case definitions lack the specificity to reliably exclude cases of varicella infection, a common disease that is characterised by a high rate of secondary transmission in susceptible people (>85%) and a low mortality in non-neonates (<0·01%).
It is evident from laboratory data that varicella cases were, in fact, misclassified as monkeypox cases in the investigation in October 1997. Analyses of the lesional material from the 19 active cases detected MPV in nine and varicella zoster virus (VZV) in four. If this rate of laboratory-proven varicella infection were extrapolated to all of the 419 cases reported as human monkeypox, 88 would be reclassified as chickenpox. To further illustrate the limitations of clinical case definitions in epidemiological investigations, of the 67 non-laboratory-confirmed US human monkeypox cases reported as of June 18, 2003, by the CDC, 19 (28%) were later excluded on the basis of an updated case definition.28 Varicella will probably continue to pose a challenge in epidemiological studies of human monkeypox. An investigation of seven suspected African monkeypox outbreaks involving 31 people in the Democratic Republic of Congo between February and August 2001 found that two outbreaks were caused by MPV, two both MPV and VZV, and two VZV alone.47 In the seventh outbreak, no evidence for either MPV or VZV was found.
Concerns about the potential use of MPV as a bioterrorism agent have fluctuated over time, but overall have been limited. The degree of concern at any given time has generally reflected the prevailing assessment of the virulence and transmissibility of MPV. By the late 1980s, there was recognition that data from active surveillance revealed no evidence that human monkeypox becomes more severe, or that the virus becomes more virulent or easily transmissible, after one or more passages through human hosts.29 Nonetheless, after initial epidemiological reports of the 1996–97 outbreak in the Democratic Republic of Congo were published, concerns re-emerged that MPV had indeed become more virulent or more transmissible.45 In addition, although there was general acceptance by the early 1990s that MPV could not evolve into variola virus, the outbreak in the Democratic Republic of Congo renewed speculation that monkeypox could fill an ecological niche vacated by smallpox.45, 48, 49 A genomic comparison of an MPV isolate from a 1996 case-patient with strains collected in Zaire in 1970–79, however, found no evidence of significant variation.35 On the basis of data available in 1998, including laboratory studies showing that a substantial portion of the 1996–97 cases were actually due to VZV infection, expert opinion was that MPV had not changed genetically or in its virulence or transmissibility.49 The conclusion was, therefore, that MPV did not represent a serious bioterrorist threat owing to its low rate of primary infection, limited transmissibility, and estimated case-fatality rate of less than 15%.49
A more guarded assessment may be warranted. Although wild-type MPV has very low potential for use as an agent of bioterrorism, how readily the virus can be genetically manipulated to exhibit greater virulence or transmissibility for such use is less clear. Evidence that genetic engineering of this type is possible comes from the inadvertent transformation of ectromelia virus (family Poxviridae), the agent of mousepox, into an unusually lethal strain.50 In studying this virus as a vector for a contraceptive vaccine, researchers developed a recombinant virus with the ability to express interleukin 4. Inoculation of a mousepox-resistant strain of mice with the control ectromelia virus led, as expected, to no deaths. Inoculation of the same strain of mice with interleukin-4-expressing virus resulted, however, in potent suppression of the cellular immune system and the death of all study animals. Furthermore, the recombinant strain was also highly lethal in mice previously immunised against ectromelia virus, resulting in 60% mortality by day 8. The same researchers reported engineering a recombinant strain of vaccinia virus with enhanced virulence that is also mediated by expression of interleukin 4.51 More recently, an independent team of investigators reported that they had replicated some of these findings, resulting in a genetically engineered mousepox virus that was uniformly lethal to vaccinated mice.52 Their results were reported at an international biodefence conference in Geneva, Switzerland, in October, 2003, but have yet to be published in a peer-reviewed journal.53 These findings suggest that certain microbes judged to have limited bioterrorism potential in the past may need to be reassessed in the era of modern molecular biology.
Diagnosis
All suspected cases of human monkeypox should be immediately reported to a local health department. Although clinical characteristics can be helpful in differentiating various poxvirus infections from other causes of vesiculopustular rashes (table 1), laboratory confirmation is required for definitive diagnosis. Case definitions used by the CDC in the US outbreak are listed in panel 2 . Suitable samples for diagnostic testing include cutaneous tissue and blood, and additional specimens may be requested by the regional public- health department. At a minimum, two scabs or material from vesicles should be collected in separate sterile containers, by use of a sterile scalpel or 26-gauge needle to unroof the lesions.54 The base of the vesicle should be vigorously swabbed with a sterile cotton or polyester swab, and the material applied to a clean microscope slide and air-dried. The swabbed material should not be stored in transport media, because dilution can affect future test results. The material should be stored on dry ice or at −20°C for transport to the CDC (or equivalent national reference laboratory) for further diagnostic testing. More detailed information on sample collection is available (http://www.cdc.gov/ncidod/monkeypox/diagspecimens.htm). Samples that potentially contain monkeypox should be handled with Biosafety Level 2 practices, containment equipment, and facilities.33
Panel 2. CDC interim case definition for human cases of monkeypox (July 2, 2003).
Human monkeypox case classification
Suspect case—meets one of the epidemiological criteria and has fever or unexplained rash and two or more other signs or symptoms with onset of first sign or symptom ⩽21 days after last exposure.
Probable case—meets one of the epidemiological criteria and has fever and vesicular-pustular rash with onset of first sign or symptom ⩽21 days after last exposure.
Confirmed case—meets one of the laboratory criteria.
Clinical criteria
Rash (macular, popular, vesicular, or pustular; generalised or localised; discrete or confluent).
Fever (subjective or measured ⩾37·4°C).
Other signs and symptoms (chills, sweats, headache, backache, lymphadenopathy, sore throat, cough, and/or shortness of breath.
Epidemiological criteria
Exposure (includes living in a household, petting or handling, or visiting a pet holding facility such as a pet store, veterinary clinic) to an exotic or wild mammalian pet (including prairie dogs, Gambian giant rats, and rope squirrels, among others to be considered on a case-by-case basis) obtained on or after April 15, 2003, with clinical signs of illness (eg conjunctivitis, respiratory symptoms, and/or rash).
Exposure (as above) to an exotic or wild mammalian pet (as above) with or without clinical signs of illness that has been in contact with a case of monkeypox in either a mammalian pet (living in a household, or originating from the same pet holding facility as another animal with monkeypox) or a human being.
Exposure (skin-to-skin or face-to-face contact) to a suspected, probable, or confirmed human case.
Laboratory criteria
Isolation of MPV in culture.
Demonstration of MPV DMA by PCR testing in a clinical sample.
Demonstration of virus morphology consistent with an orthopoxvirus by electron microscopy in the absence of exposure to another orthopoxvirus.
Demonstration of presence of orthopoxvirus in tissue by immunohistochemical testing methods in the absence of exposure to another orthopoxvirus.
Exclusion criteria
An alternative diagnosis fully explains the illness; or the case was reported on the basis of primary or secondary exposure to an exotic or wild mammalian pet or a person subsequently found not to have monkeypox, provided other possible epidemiological exposure criteria are not present; or a patient without a rash does not develop a rash within 10 days of onset of clinical symptoms consistent with monkeypox (if possible, obtain convalescent-phase serum sample from these patients); or the patient is found to be negative for non-variola generic orthopoxvirus by PCR testing of a well-sampled rash lesion by the approved Laboratory Response Network protocol.
Other clinical samples to be considered for diagnostic testing include skin biopsy tissue and blood. Biopsied skin lesions can be processed for future histopathological analysis and electron microscopy. Histopathologically, monkeypox lesions are indistinguishable from those of smallpox,55 featuring necrosis of the stratum basale, adjacent dermal papillae, and stratum spinosum. Structures similar to Guarnieri bodies can be seen in the cytoplasm of epidermal cells. Electron microscopy of monkeypox lesions has shown abundant large, brick-shaped orthopoxvirus particles in the cytoplasm of infected epidermal cells (figure 6 );55 however, this method cannot differentiate orthopoxvirus species. Although isolation of smallpox virus from blood is possible, particularly during the prodromal viraemic period, data are lacking on the use of blood cultures for MPV isolation. Blood collection for paired acute-phase and convalescent-phase serum samples can be valuable in some cases.
Figure 6.
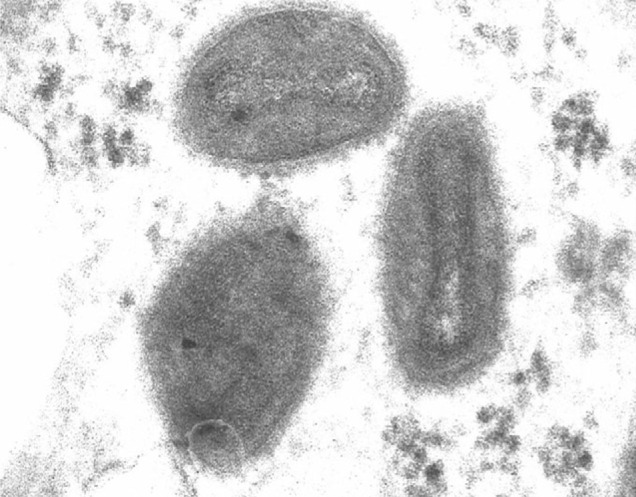
Electron micrograph depicting orthopoxvirus particles in a human skin biopsy from the 2003 US outbreak (courtesy of Marshfield Clinic, Wisconsin, USA).
Various diagnostic tests can be used to differentiate MPV infection from that of other poxviruses. Currently, the CDC is using cell culture or chick chorioallantoic membrane isolation in conjunction with DNA-based assays for the diagnosis of orthopoxvirus infection.54 DNA-based tests, such as PCR with sequencing, are the most precise methods available for orthopoxvirus identification and species assignment.54 For example, PCR amplification of a unique orthopoxvirus haemagglutinin gene can be combined with restriction-endonuclease digestion to confirm orthopoxvirus identity to the species level.56 Many PCR protocols for orthopoxvirus detection have been described.57, 58, 59 Of note, a DNA oligonucleotide microarray has lately been described as a rapid method for species-specific detection of orthopoxviruses, using the crmB gene (which encodes a receptor for tumour necrosis factor) as the target.60
Serological testing for MPV antigens is difficult because of the close antigenic relation between surface antigens among the orthopoxviruses. Various serological methods are available, including a virus-neutralising test with hyper-immune reference sera, a haemagglutination-inhibition assay with chicken erythrocytes,21 and detection of specific viral antibodies.8 The sensitivities of these tests vary (50–95%),54 however, and serological tests are not useful for the diagnosis of acute infection. Expert opinion is that no serological assay currently available can reliably diagnose orthopoxvirus infections with high sensitivity.54
Treatment and prevention
In 1968, investigators first reported that monkeys could be immunised against monkeypox by smallpox vaccination.61 In a later analysis of 215 cases of human monkeypox (209 laboratory confirmed), Fine and colleagues calculated that previous smallpox vaccination, as defined by presence of vaccination scar, conferred 85% protection against monkeypox.62 Currently, the CDC recommends pre-exposure smallpox vaccination for field investigators, veterinarians, animal-control personnel, and health-care workers who are investigating or caring for patients with suspected monkeypox and who have no contraindications to vaccination (panel 3 ).
Panel 3. People who should and should not receive smallpox vaccine for the prevention of monkeypox.
Smallpox vaccine is the best way to prevent monkeypox in someone who is exposed.
People who should receive smallpox vaccine to prevent monkeypox
People who are investigating animal or human monkeypox cases (for example, public-health and animal-control workers).
Health-care workers who are caring for monkeypox patients, may be asked to care for monkeypox patients, or have been in close contact with monkeypox patients in the past 4 days (vaccination should be considered up to 14 days after exposure).
Anyone who has had close contact with someone who is sick with monkeypox within the past 4 days (vaccination should be considered up to 14 days after exposure).
Anyone (including veterinary surgeons and technicians) who has had direct physical contact within the past 4 days with an infected animal acquired since April 15, 2003, in affected areas of the USA (vaccination should be considered up to 14 days after exposure).
Laboratory workers who handle specimens that may contain MPV (more information available in the Interim Biosafety Guidelines for Laboratory Personnel Handling Human and Animal Specimens for Monkeypox Testing).
People who should not receive smallpox vaccine even after monkeypox exposure
People with weakened immune systems should not get the smallpox vaccine, even if they have been exposed to monkeypox (cancer treatment, organ transplant, HIV infection, primary immune deficiency disorders, some severe autoimmune disorders, and medications to treat autoimmune disorders and other illnesses can weaken the immune system).
People with life-threatening allergies to latex or to the smallpox vaccine or any of its ingredients (polymyxin B, streptomycin, chlortetracycline, neomycin).
Anyone else who has been exposed to monkeypox in the past 14 days should get the smallpox vaccine, including children under 1 year of age, pregnant women, and people with skin disorders.
The role of postexposure vaccination is less clear. On the basis of findings that smallpox vaccination after exposure to smallpox is effective in preventing or ameliorating disease, the CDC currently recommends postexposure smallpox vaccination for people who are within 4 days of initial direct exposure to monkeypox, and consideration of vaccination for those who are within 2 weeks of most recent exposure. The CDC recommendations on the use of smallpox vaccination for the pre-exposure and postexposure prophylaxis of human monkeypox are summarised in panel 3.
Cidofovir is a broad-spectrum antiviral agent with in-vitro activity against virtually all DNA viruses, including MPV.63 Although this drug has been shown to have in-vivo activity against orthopoxviruses in animals and human beings,64, 65, 66 no published data are available on its effectiveness for the treatment of human monkeypox. CDC guidelines state that the use of cidofovir can be considered in severe cases of human monkeypox infection. Since it has substantial toxic effects, however, cidofovir should not be used for prophylaxis (http://www.cdc.gov/ncidod/monkeypox/treatmentguidelines.htm).
No data are available on the effectiveness of vaccinia immune globulin (VIG) in the treatment of monkeypox complications. Use of VIG can be considered in severe cases of human monkeypox, although whether it provides any benefit in this setting is unknown. VIG can be considered for prophylaxis in an exposed person with severely impaired cellular immunity for whom smallpox vaccination is contraindicated.
Closing remarks
Since smallpox was eradicated during the early 1970s, a new human orthopoxvirus infection was discovered, caused by the monkeypox virus. The term monkeypox is something of a misnomer, because evidence suggests that rodents, and not monkeys, are actually its largest natural reservoir in terms of both absolute numbers and percentages. Unlike smallpox, human monkeypox behaves like a classic zoonosis in that most cases represent primary infection from an animal source, and the causative agent appears incapable of sustained secondary transmission in human beings. When infection in human beings does occur, it can be clinically indistinguishable from smallpox, chickenpox, and other causes of a vesiculopustular rash. The most helpful distinguishing clinical feature of human monkeypox is severe lymphadenopathy, however, laboratory testing is necessary for definitive diagnosis.
The WHO's Global Commission for the Certification of Smallpox Eradication declared human monkeypox the most important orthopoxvirus infection of human beings in the post-smallpox era. As monkeypox surveillance programmes intensified during the 1980s, the incidence of this seemingly rare disease increased. Although the documented rates of transmissibility and mortality of human monkeypox appear to have changed dramatically from the 1970s to the 1990s, careful analysis suggests that these changes are an artefact caused by the use of variable case definitions in different epidemiological settings (passive vs active surveillance, outbreak investigations). Data from the 1981–86 active surveillance programme seem to be the most precise.
Once limited to the remote rainforests of central and western Africa, human monkeypox has now emerged in North America with the recent introduction of the virus through infected exotic pets. This event was predictable given US importation practices. Three of the six genera of rodents represented in the contaminated African shipment were previously documented to have high seroprevalence rates for MPV in environmental surveys. Whether MPV has established an enzootic reservoir in the USA remains to be seen. If it has, the public-health consequences will be difficult to predict from the accumulated African data owing to potential differences in population density, immunity, and rates of immunosuppression, particularly if the US reservoir includes wild rodents in an urban setting.
Vaccinia immunisation is roughly 85% effective in preventing human monkeypox, but there is no currently proven treatment for the disease. Rapid diagnosis is nonetheless important because early detection of cases is the key to limiting potential outbreaks. Although MPV has been described as having a low potential for use as an agent of biological warfare, this may no longer be the case in the era of modern molecular biology.
Of the lessons to be learned from the 2003 US outbreak of human monkeypox, perhaps two stand out. The first is that we can no longer afford to ignore uncommon, geographically restricted or seemingly conquered infectious agents. Second, governmental policies, including those pertaining to trade in wild animals, must reflect current scientific knowledge as well as the increasing global transport of people, animals, and other potential vectors of disease. If the USA is fortunate enough to have avoided the establishment of a new endemic zoonosis this time, the next outbreak caused by an emerging agent, wherever it occurs, may prove very different. The strengthening of global public-health resources may be the best way to ensure that it does not.
Search strategy and selection criteria
Data for this review were identified by searches of Medline, references from relevant articles, and internet searches of the website of the Centers for Disease Control and Prevention (http://www.cdc.gov) and ProMed database (http://www.promedmail.org). Search terms were “monkeypox virus”, “monkeypox”, “orthopoxvirus”, “bioterrorism”, and “smallpox”. Papers published in English were reviewed.
In the USA, clinical consultation on the use of VIG and cidofovir is available from staff at each state health department in the affected area. Current CDC recommendations on the use of vaccinia immunisation, cidofovir, and VIG in people exposed to or infected with MPV are available at http://www.cdc.gov/ncidod/monkeypox/smallpoxvaccine_mpox.htmandhttp://www.cdc.gov/ncidod/monkeypox/treatmentguidelines.htm.
Conflict of interest
None declared.
References
- 1.Enserink M, Stone R. Public health: dead virus walking. Science. 2002;295:2001–2005. doi: 10.1126/science.295.5562.2001. [DOI] [PubMed] [Google Scholar]
- 2.von Magnus P, Anderson EK, Petersen KB, Birch-Anderson A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 3.WHO . World Health Organization; Geneva: 1980. The global eradication of smallpox: final report of the Global Commission for the Certification of Smallpox Eradication. [Google Scholar]
- 4.Gispen R. Relevance of some poxvirus infections in monkeys to smallpox eradication. Trans R Soc Trop Med Hyg. 1975;69:299–302. doi: 10.1016/0035-9203(75)90122-4. [DOI] [PubMed] [Google Scholar]
- 5.Marennikova SS, Seluhina EM, Mal'ceva NN, Cimiskjan KL, Macevic GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 6.Marennikova SS, Shelukhina EM, Maltseva NN. Monkeypox virus and whitepox viruses. Acta Virol. 1978;22:512. [PubMed] [Google Scholar]
- 7.Rondle CJ, Sayeed KA. Studies on monkeypox virus. Bull World Health Organ. 1972;46:577–583. [PMC free article] [PubMed] [Google Scholar]
- 8.Gispen R, Brand-Saathof B. Three specific antigens produced in vaccinia, variola, and monkeypox infections. J Infect Dis. 1974;129:289–295. doi: 10.1093/infdis/129.3.289. [DOI] [PubMed] [Google Scholar]
- 9.Gispen R, Brand-Saathof BB, Hekker AC. Monkeypox-specific antibodies in human and simian sera from the Ivory Coast and Nigeria. Bull World Health Organ. 1976;53:355–360. [PMC free article] [PubMed] [Google Scholar]
- 10.Ichihashi Y, Oie M. Epitope mosaic on the surface proteins of orthopoxviruses. Virology. 1988;163:133–144. doi: 10.1016/0042-6822(88)90240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arita M, Tagaya I. Virion polypeptides of poxviruses. Arch Virol. 1980;63:209–225. doi: 10.1007/BF01315028. [DOI] [PubMed] [Google Scholar]
- 12.Shchelkunov SN, Totmenin AV, Babkin IV, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breman JG. In: Emerging infections. 4th edn. Scheld WM, Craig WA, Hughes JM, editors. ASM Press; Washington DC: 2000. Monkeypox: an emerging infection for humans? pp. 45–67. [Google Scholar]
- 14.Douglass N, Dumbell K. Independent evolution of monkeypox and variola viruses. J Virol. 1992;66:7565–7567. doi: 10.1128/jvi.66.12.7565-7567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito JJ, Nakano JH, Obijeski JF. Can variola-like viruses be derived from monkeypox virus? An investigation based on DNA mapping. Bull World Health Organ. 1985;63:695–703. [PMC free article] [PubMed] [Google Scholar]
- 16.Dumbell KR, Archard LC. Comparison of white pock (h) mutants of monkeypox virus with parental monkeypox and with variola-like viruses isolated from animals. Nature. 1980;286:29–32. doi: 10.1038/286029a0. [DOI] [PubMed] [Google Scholar]
- 17.Marennikova SS, Shelukhina EM. Whitepox virus isolated from hamsters inoculated with monkeypox virus. Nature. 1978;276:291–292. doi: 10.1038/276291a0. [DOI] [PubMed] [Google Scholar]
- 18.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 19.Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970—79. Bull WorldHealth Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 20.Jezek Z, Marennikova SS, Mutumbo M, Nakano JH, Paluku KM, Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154:551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 21.The current status of human monkeypox: memorandum from a WHO meeting. Bull World Health Organ. 1984;62:703–713. [PMC free article] [PubMed] [Google Scholar]
- 22.Jezek Z, Gromyko AI, Szczeniowski MV. Human monkeypox. J Hyg Epidemiol Microbiol Immunol. 1983;27:13–28. [PubMed] [Google Scholar]
- 23.Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 24.Breman JG, Henderson DA. Diagnosis and management of smallpox. N Engl J Med. 2002;346:1300–1308. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- 25.Jezek Z, Szczeniowski M, Paluku KM, Mutombo M, Grab B. Human monkeypox: confusion with chickenpox. Acta Trop. 1988;45:297–307. [PubMed] [Google Scholar]
- 26.Update: multistate outbreak of monkeypox-Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:616–618. [PubMed] [Google Scholar]
- 27.Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:561–564. [PubMed] [Google Scholar]
- 28.Update: multistate outbreak of monkeypox-Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:589–590. [PubMed] [Google Scholar]
- 29.Jezek Z, Grab B, Szczeniowski MV, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- 30.Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol. 1986;123:1004–1012. doi: 10.1093/oxfordjournals.aje.a114328. [DOI] [PubMed] [Google Scholar]
- 31.Jezek Z, Grab B, Dixon H. Stochastic model for interhuman spread of monkeypox. Am J Epidemiol. 1987;126:1082–1092. doi: 10.1093/oxfordjournals.aje.a114747. [DOI] [PubMed] [Google Scholar]
- 32.Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 33.Jezek Z, Fenner F. Human monkeypox. Monogr Virol. 1988;17:1–140. [Google Scholar]
- 34.Arita I, Jezek Z, Khodakevich L, Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am J Trop Med Hyg. 1985;34:781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- 35.Human monkeypox–Kasai Oriental, Zaire, 1996–1997. MMWR Morb Mortal Wkly Rep. 1997;46:304–307. [PubMed] [Google Scholar]
- 36.Human monkeypox–Kasai Oriental, Democratic Republic of Congo, February 1996—October 1997. MMWR Morb Mortal Wkly Rep. 1997;46:1168–1171. [PubMed] [Google Scholar]
- 37.Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:537–540. [PubMed] [Google Scholar]
- 38.Mutombo M, Arita I, Jezek Z. Human monkeypox transmitted by a chimpanzee in a tropical rain-forest area of Zaire. Lancet. 1983;1:735–737. doi: 10.1016/S0140-6736(83)92027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- 41.Hutin YJ, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin M. Monkeypox spreads as US public-health system plays catch-up. Lancet Infect Dis. 2003;3:461. doi: 10.1016/S1473-3099(03)00713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNeil DG. Death sought for animals in monkeypox case. New York Times. 2003 July 3 [Google Scholar]
- 44.Human monkeypox in Kasai Oriental, Democratic Republic of the Congo (former Zaire): preliminary report of October 1997 investigation Wkly Epidemiol Rec. 1997;72:369–372. [PubMed] [Google Scholar]
- 45.Cohen J. Is an old virus up to new tricks? Science. 1997;277:312–313. doi: 10.1126/science.277.5324.312. [DOI] [PubMed] [Google Scholar]
- 46.Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer H, Perrichot M, Stemmler M, et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anon Don't underestimate the enemy. Nature. 2001;409:269. doi: 10.1038/35053282. [DOI] [PubMed] [Google Scholar]
- 49.Breman JG, Henderson DA. Poxvirus dilemmas: monkeypox, smallpox, and biologic terrorism. N Engl J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 50.Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, Ramshaw IA. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol. 2001;75:1205–1210. doi: 10.1128/JVI.75.3.1205-1210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broad WJ. Bioterror researchers build a more lethal mousepox. New York Times. 2003 Nov 1 [Google Scholar]
- 53.Shane S. Building a stronger mousepox to guard nation against terror. Baltimore Sun. 2003 Nov 1 [Google Scholar]
- 54.Damon IK, Esposito JJ. In: Manual of clinical microbiology, 8th edn. Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolker MH, editors. ASM Press; Washington DC: 2003. Poxviruses that infect humans; pp. 1583–1591. [Google Scholar]
- 55.Stagles MJ, Watson AA, Boyd JF, More IA, McSeveney D. The histopathology and electron microscopy of a human monkeypox lesion. Trans R Soc Trop Med Hyg. 1985;79:192–202. doi: 10.1016/0035-9203(85)90333-5. [DOI] [PubMed] [Google Scholar]
- 56.Ropp SL, Jin Q, Knight JC, Massung RF, Esposito JJ. PCR strategy for identification and differentiation of smallpox and other orthopoxviruses. J Clin Microbiol. 1995;33:2069–2076. doi: 10.1128/jcm.33.8.2069-2076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer H, Pfeffer M, Rziha HJ. Sequence alterations within and downstream of the A-type inclusion protein genes allow differentiation of Orthopoxvirus species by polymerase chain reaction. J Gen Virol. 1994;75:1975–1981. doi: 10.1099/0022-1317-75-8-1975. [DOI] [PubMed] [Google Scholar]
- 58.Meyer H, Ropp SL, Esposito JJ. Gene for A-type inclusion body protein is useful for a polymerase chain reaction assay to differentiate orthopoxviruses. J Virol Methods. 1997;64:217–221. doi: 10.1016/S0166-0934(96)02155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim MS, Esposito JJ, Jahrling PB, Lofts RS. The potential of 5′ nuclease PCR for detecting a single-base polymorphism in Orthopoxvirus. Mol Cell Probes. 1997;11:143–147. doi: 10.1006/mcpr.1996.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lapa S, Mikheev M, Shchelkunov S, et al. Species-level identification of orthopoxviruses with an oligonucleotide microchip. J Clin Microbiol. 2002;40:753–757. doi: 10.1128/JCM.40.3.753-757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McConnell S, Hickman RL, Wooding WL, Jr, Huxsoll DL. Monkeypox: experimental infection in chimpanzee (Pan satyrus) and immunization with vaccinia virus. Am J Vet Res. 1968;29:1675–1680. [PubMed] [Google Scholar]
- 62.Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 63.De Clercq E. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 2002;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meadows KP, Tyring SK, Pavia AT, Rallis TM. Resolution of recalcitrant molluscum contagiosum virus lesions in human immunodeficiency virus-infected patients treated with cidofovir. Arch Dermatol. 1997;133:987–990. [PubMed] [Google Scholar]
- 65.Smee DF, Bailey KW, Sidwell RW. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antivir Chem Chemother. 2001;12:71–76. doi: 10.1177/095632020101200105. [DOI] [PubMed] [Google Scholar]
- 66.Smee DF, Bailey KW, Wong M, Sidwell RW. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antiviral Res. 2000;47:171–177. doi: 10.1016/s0166-3542(00)00105-4. [DOI] [PubMed] [Google Scholar]