Abstract
Orthopoxviruses such as variola and monkeypox viruses continue to threaten the human population. Monkeypox virus is endemic in central and western Africa and outbreaks have reached as far as the U.S. Although variola virus, the etiologic agent of smallpox, has been eradicated by a successful vaccination program, official and likely clandestine stocks of the virus exist. Moreover, studies with ectromelia virus (the etiological agent of mousepox) have revealed that IL-4 recombinant viruses are significantly more virulent than wild-type viruses even in mice treated with vaccines and/or antivirals. For these reasons, it is critical that antiviral modalities are developed to treat these viruses should outbreaks, or deliberate dissemination, occur. Currently, 2 antivirals (brincidofovir and tecovirimat) are in the U.S. stockpile allowing for emergency use of the drugs to treat smallpox. Both antivirals have advantages and disadvantages in a clinical and emergency setting. Here we report on the efficacy of a recombinant immunoglobulin (rVIG) that demonstrated efficacy against several orthopoxviruses in vitro and in vivo in both a prophylactic and therapeutic fashion. A single intraperitoneal injection of rVIG significantly protected mice when given up to 14 days before or as late as 6 days post challenge. Moreover, rVIG reduced morbidity, as measured by weight-change, as well as several previously established biomarkers of disease. In rVIG treated mice, we found that vDNA levels in blood were significantly reduced, as was ALT (a marker of liver damage) and infectious virus levels in the liver. No apparent adverse events were observed in rVIG treated mice, suggesting the immunoglobulin is well tolerated. These findings suggest that recombinant immunoglobulins could be candidates for further evaluation and possible licensure under the FDA Animal Rule.
Keywords: Immunoglobulin, Smallpox, Ectromelia, Vaccinia, Antiviral, Monkeypox
1. Introduction
Smallpox was once considered one of the biggest scourges of humanity. Characterized by high-level transmissibility and environmental stability, the disease caused significant mortality and morbidity (Fenner et al., 1988). The etiologic agent of smallpox is variola virus (VARV), an orthopoxvirus (OPV). VARV was declared eradicated in 1980 following a large-scale vaccination campaign and is stored in two official repositories in the U.S. and Russia; however, anecdotal evidence suggests that unofficial stocks of VARV exist in countries that may have a propensity towards terrorism. The re-appearance of VARV in the human population would likely be the result of bioterrorism or biowarfare and would have a massive impact on public health, infrastructure, and the economy. Monkeypox virus (MPXV) (also an OPV) remains a significant cause of morbidity and mortality in endemic areas in central and western Africa (Parker et al., 2007). Although the symptoms of human-MPX are milder than those of smallpox, the virus is almost impossible to eradicate because it can infect multiple animals of African and non-African origin (Parker and Buller 2013). Since its discovery, MPXV has emerged as a growing problem with increasingly frequent outbreaks (Beer and Rao 2019). Similar to VARV, MPXV would be an attractive vector for weaponization based on its capacity for respiratory transmission, extended incubation period, and environmental stability (Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c; Chen et al., 2011). Furthermore, a recent study using horsepox (an OPV closely related to VARV and MPXV) demonstrated that these viruses can be made de novo in most laboratories (Noyce et al. 2018).
Vaccination remains the most effective way to prevent both VARV and MPXV disease (Handley et al., 2009); however, the live replication-competent vaccines (Dryvax and ACAM2000) have sub-optimal safety profiles (Lederman et al., 2009) and are contraindicated for a large portion of the population. Some reports suggest that 25% of the U.S. population would have vaccine contraindications (Kemper et al. 2002). Safer, non-replicating vaccines such as modified vaccinia Ankara (MVA) are also available. The main disadvantage to these vaccines is the requirement for multiple administrations to achieve 100% protection, making them less useful in an emergency (Handley et al., 2009; Vollmar et al., 2006). Therefore, developing therapies for OPV disease is urgent. Two drugs have led the field in development: brincidofovir (BCV, previously CMX001) and tecovirimat (previously ST-246). BCV is a lipid conjugate of cidofovir (CDV) (Ciesla et al., 2003; Hostetler 2007), an antiviral with broad-spectrum efficacy against dsDNA viruses. Unlike CDV, BCV is orally bioavailable and has no evidence of dose-limiting nephro- or hematologic toxicity (Lanier et al., 2010). BCV has been extensively evaluated and demonstrates good efficacy against OPV challenges (Buller et al., 2004; Parker et al., 2014; Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c; Crump et al., 2017; Quenelle et al., 2007a, Quenelle et al., 2007b). Tecovirimat is the only anti-OPV drug approved for use in the U.S. (Hoy 2018; Laudisoit et al. 2018). Like BCV, tecovirimat has demonstrated a good efficacy -safety profile in testing (Berhanu et al., 2009; Duraffour et al., 2007; Grosenbach et al., 2010; Jordan et al., 2008; Quenelle et al., 2007a, Quenelle et al., 2007b). The main drawback to tecovirimat is a single point mutation can cause antiviral resistance (Lederman et al., 2012; Yang et al., 2005), whereas resistance is difficult to generate to BCV (Smee et al., 2002).
Therapeutic antibodies reactive with key antigens on OPVs use a mechanism of action different from both BCV and tecovirimat by neutralizing virus infectivity and mediating effector mechanisms including complement activation and phagocytosis of virus particles and infected cells. Passive immunotherapy constitutes an alternative approach providing immediate immunity to recently exposed individuals and long-lasting protection to those at risk of exposure. Vaccinia virus (VACV) immunoglobulin (VIG), which is purified from plasma of vaccinated donors, is licensed for treatment of complications due to smallpox vaccination (Kempe et al., 1961); however, the efficacy of VIG is low, very likely due to the small fraction of antibodies targeting the antigens of interest in the pooled immunoglobulin. In treating adverse events such as eczema-vaccinatum and progressive-vaccinia, VIG seems to have limited effect. Furthermore, vast amounts of VIG may be required to treat OPV infections. In one report, a patient with progressive-vaccinia was treated with 30 times the amount estimated to be needed for one person (Centers for Disease and Prevention 2009). It should be noted that VIG has never been subjected to any controlled clinical trials; it became approved at a time when no alternative therapeutics existed (Xiao and Isaacs 2010).
To circumvent the disadvantages of VIG, we have developed a recombinant VACV-immunoglobulin (rVIG) comprising 26 unique human IgG1 antibodies targeting different VACV proteins (Lantto et al., 2011). This recombinant product captures the diversity and specificity of the natural immune response to smallpox vaccination while having much higher specificity and activity than VIG (Lantto et al., 2011). In addition, the recombinant format allows for consistent manufacturing leading to unlimited supply and an improved safety profile.
In this study, we have further characterized the biological activity of rVIG in multiple in vitro and in vivo models to assess its potential as a therapy against OPV infections. rVIG was found to exhibit markedly higher in vitro neutralizing activity than commercially available VIG against a range of OPVs, including VACV, cowpox (CPXV), ECTV, and MPXV. rVIG also protected in vivo against multiple OPVs, both when given prophylactically and therapeutically. Furthermore, rVIG did not interfere with the development of humoral or cellular immunity against ECTV infection in mice. Altogether, these results establish rVIG as a potential prophylactic or therapeutic treatment of OPV infections.
2. Materials and methods
2.1. Cells and viruses
BSC-1 cells (ATCC CCL 26) were grown in DMEM (Lonza, Basel, Switzerland) containing 10% fetal calf serum (FCS) (Hyclone III, Logan, UT), 2 mM L-glutamine (GIBCO, Grand Island, NY), 100 U/mL penicillin (GIBCO, Grand Island, NY), and 100 μg/mL streptomycin (GIBCO, Grand Island, NY). Viruses (strains ECTV-MOS, VACV-COP, MPXV-Zaire) were purified through a sucrose cushion as described (Moss and Earl 1998). Animals were sacrificed and bled by heart-sticks to determine viral titers. Tissues were removed and ground in PBS (10% FBS w/v). Following grinding, samples were freeze-thawed thrice interrupted by 20 s sonications. Virus infectivity was calculated by titration on BSC1 monolayers (Esteban et al., 2012). Plaques were visualized by addition of 0.5 mL 0.3% crystal violet/10% formalin to each well. Arithmetic means above the limit of detection (1 × 102 plaque-forming unit (PFU)/mL) were calculated as PFU/g or PFU/mL (Wallace and Buller 1985).
2.2. Animals
The Institutional Animal Care and Use Committee at Saint Louis University and Utah State School of Veterinary Medicine approved all experimental protocols. A-strain mice were purchased from the National Cancer Institute (Frederick, MD). Cast/EiJ mice were sourced from Jackson Laboratory (Bar Harbor, ME). BALB/c mice were sourced from Charles River (Wilmington, MA).
All experimental and animal procedures using ECTV and VACV were completed at ABSL-2. MPXV procedures were completed at ABSL-3. Animals were housed in filter-top microisolator cages with corncob bedding (no more than 5 animals/cage), fed commercial mouse chow (Teklad Global 18% Protein Rodent Diet, Envigo, Huntingdon, UK) and provided water ad libitum. Animals were acclimatized for at least 1 week prior to infection. Female mice were used unless otherwise stated. For MPXV and ECTV studies, animals were 6–12 weeks old. For VACV studies, animals were 13–15 g. Experiments consisted of 4–5 animals per group unless otherwise stated. Animals were monitored daily and weighed 5 times per week. Experiments were performed at least thrice unless otherwise indicated.
For ECTV/MPXV challenges, mice were anesthetized by intraperitoneal (IP) injection of 9 mg/mL ketamine HCl and 1 mg/mL xylazine at a ratio of 0.1 mL/10 g body weight. Intranasal (IN) inoculations with 5 μL/nare of virus were used to seed the upper respiratory tract as described previously (Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c) (Esteban and Buller 2005). For VACV challenges, mice were anesthetized with ketamine (100 mg/kg) via IP injection followed by IN challenge with approximately 50 μL 1 × 105 PFU as described previously (Smee et al., 2004).
2.3. ELISAs
Direct anti-OPV ELISAs were performed using lysates from BSC1 cells infected with VACV (WR strain). Clarified cell lysate was diluted in 50 mM carbonate-bicarbonate buffer (pH 9.6) at a 1:2500 dilution, and used to coat 96-well microtiter ELISA plates at 4 °C overnight. Plates were blocked with blocking buffer (PBS + 0.05% Tween 20 + 2% normal goat serum) (Vector, Burlingame, CA) at room temperature for 30 min, and serial dilutions of mouse sera were added to wells. Following incubation at room temperature for 1 h, wells were washed with PBS-T + 0.05% Tween 20. Bound antibody was detected using biotin-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA) at 1:2500 dilution followed by streptavidin-HRP (Invitrogen, Carlsbad, CA) at 1:4000 and orthophenylenediamine (0.4 mg/mL) in 50 mM citrate buffer (pH 5.0) as a chromogen. Optical density was measured at 490 nm.
2.4. Determination of EC50 values
BSC-1 cells were plated in 24 well-plates. Monolayers were infected with ∼75 PFU of indicator virus in 0.1 mL of DMEM +5% Fetal clone III for 60 min at 37 °C. Media was aspirated and standard virus overlay media containing no drug or the test drug at concentrations ranging from 0.05 to 50 μM was added. The plates were incubated at 37 °C for 3–4 h, monolayers were stained, and plaques counted using a stereomicroscope. Each assay was performed 3-times with an N = 3 for each virus/assay. The EC50 concentration for each drug was calculated (Esteban et al., 2012).
2.5. Statistics
T-tests were used to compare means between paired groups of animals. Survival analysis was performed using Kaplan-Meier curves and log rank (Mantel Cox) test. Comparisons of weight-change over time or qPCR results between multiple groups of animals were made using one way ANOVA, Dunnett's multiple comparisons test, and Fisher's exact test. Endpoints or lowest weight points were analyzed for significance. Throughout the manuscript ‘significant’ indicates P values < 0.05.
2.6. Disease biomarker and rVIG plasma levels
Alanine amino transferase (ALT) analysis was performed on a Cobas Mira Plus Chemistry Analyzer (Roche Diagnostics, Basel, Switzerland). Samples were diluted as needed to provide adequate volume and bring the results within the linear range of the machine. For qPCR, blood was collected in EDTA microfuge tubes (BD Biosciences; San Diego, CA) and run directly in the PCR mix using Omniklentaq buffer and enzyme (DNA Polymerase Technology, St. Louis, MO) as described (Parker et al., 2012).
The levels of rVIG were measured in murine serum samples using a quantitative human IgG ELISA. Briefly, goat anti-human (Fc) IgG (Serotec, Oxford, UK) was diluted in a 50 mM sodium-carbonate buffer (pH 9.6) at a 1:500 dilution, and used to coat 96-wells microtiter ELISA plates at 4 °C overnight. Plates were blocked with blocking buffer (PBS + 0.05% Tween 20 + 2% skim milk) at room temperature for 60 min, and serial dilutions of mouse sera were added to wells. Following incubation at room temperature for 1 h on shaker, wells were washed with PBS +0.05% Tween-20. Bound antibody was detected using HRP-conjugated goat anti-human IgG (Serotec, Oxford, UK) at 1:25000 dilution. After 1 h of incubation at room temperature, TMB-Plus substrate was added followed by addition of 1M Sulfuric acid. Optical density was measured at 450 nm.
2.7. Antivirals
rVIG was provided by Symphogen, Inc. (Ballerup, Denmark). VIG was purchased from Cangene (Mississauga, Canada) for VACV studies and Southern Research Institute (Birmingham, AL) for ECTV/MPXV studies. Cidofovir (Vistide) was obtained from Gilead Sciences (Foster City, CA). Compounds were diluted in sterile saline to the desired dosage in a volume of 100 μL for IP injection. VIG (Southern Research Institute, Birmingham, AL) was IP injected according to the provider's instructions to make 2 mg/mouse.
3. Results
3.1. Recombinant rVIG neutralizes orthopoxviruses
To determine if rVIG can neutralize OPVs we performed a plaque-neutralization assay on 4 different OPVs encompassing 5 viral strains: VACV (WR and Copenhagen strains), CPXV, ECTV, and MPXV. The efficacy of rVIG (measured by EC50) was compared to VIG, the only other antibody platform approved for OPV treatment. Assays were performed 3-times with N = 3 per test. As shown in Table 1 , rVIG exhibited potent antiviral activity against all OPVs tested. In the case of VACV, CPXV and ECTV, the EC50 value was >160 fold less than for VIG. For MPXV, the EC50 value of rVIG was ∼2-fold less than for VIG. These data revealed that rVIG qualified for further evaluation.
Table 1.
rVIG neutralizes a broad range of OPVs at a lower concentration than VIG.
| Virus (Strain) | rVIG EC50a | VIG EC50a |
|---|---|---|
| VACV (Copenhagen) | 0.1 | 24.2 |
| VACV (WR) | 0.1 | 16.6 |
| CPXV (Brighton) | 0.2 | 65.9 |
| ECTV (Moscow) | 1.2 | 203 |
| MPXV (Zaire) | 14 | 31.1 |
Measured as μg/mL.
OPVs morphogenesis is complex and results in production of at least 2 different infectious particles: an enveloped intracellular mature virus (IMV) which typically remains cell-associated and are believed to be important for virus dissemination in the general environment and between different host organisms following cell lysis; and an extracellular enveloped virus (EEV) which are released from the infected cells as double-membrane enveloped virions and are thought to mediate systemic virus dissemination within an infected organism. Due to the nature of the plaque-neutralization assay using overlay media, the measurement of EEV could not be determined in this type of assay; however, removal of the overlay media results in the formation of comet-shaped plaques, which are thought to form because the EEV particles are no longer restricted in their movements. To this end, we measured EEV-specific activity of rVIG in a comet-inhibition assay using VACV (Copenhagen strain). In the comet-inhibition assay, rVIG was found to inhibit the formation of comet-tails with an EC50 of 0.23 μg/mL (data not shown). These data indicate that rVIG neutralizes both IMV and EEV particles.
3.2. rVIG protects mice against a lethal ECTV challenge
ECTV is the etiological agent of mousepox which is arguably the best respiratory challenge model of VARV (smallpox) infection in humans. Mousepox is similar to smallpox in at least 4 ways: ECTV requires a low virus inoculum in the upper respiratory tract to induce a severe and systemic lethal disease; during early disease, there appears to be no lung involvement although virus can be detected in respiratory gasses during the pre-exanthem period; disease presents with a rash similar in character to that of smallpox; and finally, mousepox is thought to fairly accurately reflect the progress of natural infection in humans (Parker et al., 2012; Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c; Parker et al., 2009). However, unlike smallpox, mousepox has a shorter disease course of 7–15 days compared to 23–28 in smallpox and the major pathology appears to involve the liver and spleen. Furthermore, rash development in mousepox is dependent on mouse strain, challenge dose, route of inoculation, and virus strain (Buller and Fenner 2007; Parker et al., 2010).
Since the ECTV/mousepox murine model is a good small-animal model of smallpox, we challenged A-strain mice with 0.6 PFU of ECTV via the IN route. Challenged mice were treated with either 160, 40 or 10 μg of rVIG via the IP route at T = −1 day post infection (T = −1 d.p.i) or T = +1 d.p.i. We also treated mice with 2 mg of VIG administered IP and used CDV as a control (2 mg administered at T = −1). As expected, mice that were challenged and treated with vehicle experienced 100% mortality by +10 d.p.i (Fig. 1 A). Mice that were mock-infected and treated with vehicle had 100% survival; however, mice treated with VIG at T = −1 and T = +1 experienced significantly reduced survival with mortality rates of 60% (p = 0.008) and 80% (p = 0.03), respectively. As expected, none of the CDV treated control mice experienced mortality (Fig. 1A). Mice treated with the highest dose of rVIG (160 μg) survived the challenge regardless of the day of treatment (Fig. 1B). The 40 μg dose of rVIG resulted in 40% mortality when given at T = −1, but this was reduced to 10% mortality when rVIG was administered at T = +1. None of the mice in the 10 μg groups survived the challenge (Fig. 1B). Morbidity was measured by weight-change; mice in the VIG T = −1 group lost ∼25% weight compared to mice in the VIG T = +1 group which lost only ∼10% weight (Fig. 1C). None of the CDV or mock-infected control mice had weight-loss. In the 160 μg group, mild weight-change (∼5%) over the first 10 days of the experiment was recorded when rVIG was administered on T = −1 d.p.i.; however, no weight-change was observed in mice administered rVIG at T = +1 d.p.i. (Fig. 1D). Mice in the 10 μg rVIG group lost weight rapidly from T = +7 with day of death not significantly extended compared to vehicle-treated controls (Fig. 1B and D). Taken together, these data reveal that mice treated with 160 μg of rVIG at T = +1 or −1 p.i. are better protected than those treated with VIG (p = 0.01 and 0.04, respectively). Moreover, at the 160 μg dose of rVIG, mice are protected against both mortality and morbidity to a similar level as those treated with CDV (no significant difference).
Fig. 1.
Mortality and morbidity of female A-strain mice following a lethal ECTV challenge and treatment with VIG, CDV or rVIG. (A and B) At T = 0 d.p.i. mice were challenged via the IN route with 0.6 PFU of ECTV or PBS (control challenge). (A) At T = −1 d.p.i., mice were treated once with either vehicle, VIG (2 mg) or CDV (2 mg). At T = +1 d.p.i., a group of mice were treated once with VIG (2 mg). (B) Groups of mice were treated once with decreasing volumes of rVIG (160, 40 or 10 μg) at T = −1 d.p.i. (prophylactic) or T = +1 d.p.i. (therapeutic). Both A and B were conducted at the same time as 1 experiment and are separated in the figure for clarity. (C and D) Mice were weighed daily Monday to Friday to measure morbidity and represent data from (A) and (B), respectively. The Figure represents data combined from three experiments performed at separate times. N = 15/group.
3.3. Determining the therapeutic window of rVIG in mousepox
Because the 160 μg treatment of rVIG protected mice comparably to CDV, and because treatment demonstrated efficacy prior to challenge (prophylactically), we decided to determine the therapeutic window of rVIG from T = −14 d.p.i. to T = +6 d.p.i. When 160 μg rVIG was given prophylactically at T = −14 d.p.i or T = −7 d.p.i, we found that the mortality rates were 20% (p < 0.0001) and 0% with negligible levels of weight-loss, respectively (Fig. 2 A and B). These results indicate that efficacious levels of rVIG remain in the mouse even when given up 2 weeks prior to challenge, and that rVIG is fully protective when given 7 days prior to challenge. When 160 μg rVIG was administered therapeutically on T = 0, +1, +2, +3, +4, +5, or +6 d.p.i. we found significant levels of protection in all groups compared to challenged mice treated with vehicle, even when rVIG was administered as far out as T = +5 and + 6 d.p.i. (p ≤ 0.0001 both days). Indeed, no mortality was observed in the T = 0, +1, +2, and +3 groups. The maximum mortality rate was 25% when rVIG administered at T = +5 or +6, with 20% mortality in the T = +4 group (Fig. 2C). Inspection of the morbidity data reveals that mice in groups with 100% survival (rVIG given at T = 0, +1, +2, or +3) did not experience significant weight-loss. As expected, groups with some mortality (rVIG given at T = +4, +5, or +6) experienced some weight-loss but this did not exceed 10% (Fig. 2D).
Fig. 2.
Therapeutic and prophylactic efficacy of rVIG following a lethal challenge with ECTV. (A and B), Female A-strain mice were challenged with 0.6 PFU of ECTV and treated once with 160 μg of rVIG at T = −14 or T = −7 d.p.i. Mortality (A) and morbidity (B) were recorded. (C and D), mice were challenged as per (A and B) and treated once with 160 μg rVIG at either T = 0, +1, +2, +3, +4, +5, or +6 d.p.i. Mortality (C) and morbidity (D) were recorded. (A–D) data were collected at the same time in a single experiment and are separated for clarity. The Figure represents data combined from three experiments performed at separate times. N = 15/group.
The data presented here demonstrates a remarkably wide therapeutic window for rVIG, with significant protection achieved following a single IP injection of rVIG from 14 days prior to challenge up to +6 d.p.i. (the last time point measured). Mice that survived challenge were bled 6 weeks later and found to be ELISA positive for anti-ECTV antibodies (data not shown). Not surprisingly, when these mice were re-challenged with a lethal ECTV dose of 0.5 PFU 63 days after the initial challenge, no mice experienced morbidity or mortality (data not shown) revealing that rVIG did not impede the generation of a memory response to challenge.
3.4. Evaluating the impact of rVIG on mousepox biomarkers
To further dissect the efficacy of rVIG, we analyzed disease biomarkers to stage the disease course and monitor the efficacy in rVIG treated animals. Several ECTV biomarkers have been previously evaluated (Parker et al., 2012); however, we focused our studies on the most robust biomarkers: viral DNA (vDNA) in whole-blood was measured by qPCR; infectious virus was measured by titers in organs; and ALT levels were determined in plasma.
Using vDNA levels as a biomarker of ECTV disease is well established in whole blood and buccal swabs; previous studies have revealed that vDNA levels increase over the course of infection and can be used effectively as a trigger to initiate antiviral drug treatment (Crump et al., 2017; Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c; Parker et al., 2012). We found that mice challenged with a lethal dose (0.5 PFU) of ECTV experienced 100% mortality by day +10 p.i (Table 2 ); however, mice receiving a single 160 μg rVIG treatment had significantly reduced mortality on all treatment days. Blood was taken from groups of mice via the sub-mandibular route on days +6, +8, +10 and + 15 d.p.i. In mice that were infected and treated with vehicle (group 2, Table 2), we found vDNA levels increased over the course of the disease up to ∼104 copies/μL before death (Fig. 3 ). However, in mice treated with a single rVIG IP treatment on day −7, 0 or +4 d.p.i. (groups 4, 5 and 6, Table 2) we found that vDNA levels were undetectable (below 13 copies/μL, the lower sensitivity level of the assay). Mice treated 14 days prior to challenge (group 3, Table 2) had detectable levels of vDNA in samples taken on day +8 and + 10 p.i.; however, the levels were significantly lower than mice infected and treated with vehicle (group 2) (p ≤ 0.001 day + 8 and p ≤ 0.0001 day + 10). Unsurprisingly, when rVIG was administered on either day +5 or +6 p.i. (groups 7 and 8), we found that vDNA levels in blood were higher compared to groups that received rVIG earlier. These higher levels corresponded with increased mortality. These data suggest that rVIG administration between day −1 through day +4 p.i., reduces vDNA levels in whole-blood to undetectable or almost undetectable levels.
Table 2.
Mortality of A-strain mice following a 0.5 PFU challenge with ECTV and treatment with 160 μg rVIG a,b.
| Group | # of mice | Challenge | Article | Time of treatment (p.i.) | % Mortality (Mean day of death) |
|---|---|---|---|---|---|
| 1 | 5 | PBS | vehicle | 0 | 0 |
| 2 | 5 | ECTV | vehicle | 0 | 100 (9.4) |
| 3 | 5 | ECTV | rVIG | −14 | 20 (13) |
| 4 | 5 | ECTV | rVIG | −7 | 0 |
| 5 | 5 | ECTV | rVIG | 0 | 0 |
| 6 | 5 | ECTV | rVIG | 4 | 0 |
| 7 | 5 | ECTV | rVIG | 5 | 40 (9) |
| 8 | 5 | ECTV | rVIG | 6 | 20 (10) |
A second group for each group shown in the table was also included. These groups were sacrificed on day 6 p.i. (not shown for clarity).
Data is representative from 1 of 2 studies. All studies had similar results.
Fig. 3.
A single treatment of rVIG suppresses blood vDNA copies following a lethal ECTV challenge. A-strain mice were challenged with a 0.5 PFU dose of ECTV via the IN route and treated with either vehicle or 160 μg of rVIG at T = −14, −7, 0, +4, +5, or +6 d.p.i. At T = +6, +8, +10, and +15 mice were bled via the sub-mandibular vein to determine vDNA copies. Data representative of 3 independent experiments with each group having an N = 5.
Although vDNA levels in whole-blood provide a good readout of disease progression, they do not quantify infectious virus. To this end, at +6 d.p.i. we sacrificed groups of mice that were treated following the same regimen as shown in Table 2 and harvested spleen, liver and lungs. In spleen and lungs, we found that viral titers fluctuated between 105 and 107 PFU/g, regardless of treatment regimen; however, liver titers were significantly reduced in all groups receiving rVIG (p ≤ 0.05) (Fig. 4 ). These findings are interesting because it is thought the cause of death in mice infected with ECTV is generalized liver failure (Fenner 1981; Esteban et al., 2012). Supporting the findings from liver titers are the results of ALT levels from plasma samples taken at days +6, +8, +10, and +15 p.i. (Fig. 5 ) from the same groups of mice sampled in Table 2. ALT is used as a readout of damaged liver cells and has been shown to increase dramatically following ECTV infection (Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c; Parker et al., 2009). Mice challenged with ECTV and treated with vehicle (group 2 in Table 2) had low but detectable levels (∼60 U/L) of ALT at day +6 p.i. and these levels increased rapidly to almost 800 U/L on day +8 p.i. (note, insufficient plasma was recovered from sick mice at day +10 p.i. for ALT analysis). Conversely, mice that received a single rVIG treatment on any administration day (groups 3–7 in Table 2) except for day +6 p.i. (group 8) had levels of ALT that did not increase over time. Mice in group 8 (rVIG treatment on day +6 p.i.) had an increase in ALT between day +6 and + 10 p.i.; however, ALT reduced dramatically by day +15 p.i. to similar levels to those of mice in groups 3–7.
Fig. 4.
A single treatment of rVIG suppresses infectious virus levels harvested from the liver following a lethal ECTV challenge. Mice were challenged and treated with rVIG as per Fig. 3. At T = +6 d.p.i. groups of mice were sacrificed and virus titers were determined from the liver, spleen and kidney. Data representative of 3 independent experiments with each group having an N = 5.
Fig. 5.
A single treatment of rVIG suppresses ALT elevation following a lethal challenge with ECTV. Mice were challenged and treated with rVIG as per Fig. 3. At T = +6, +8, +10, and +15 d.p.i. mice were bled and ALT levels were determined from the plasma. Data representative of 3 independent experiments with each group having an N = 5.
Taken together, these data suggest that rVIG not only protects mice against mortality and morbidity (as measured by weight-change), but also has a profound impact on several biomarkers of mousepox disease.
3.5. rVIG levels remain at detectable levels up to 29 days post administration
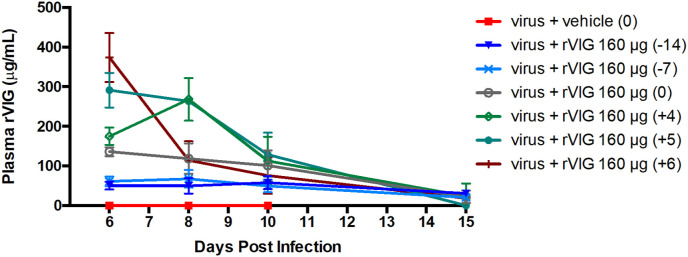
Our animal studies revealed that rVIG was protective even when administered 14 days prior to challenge. To gain insight into the pharmacokinetics of rVIG, we administered a single 160 μg IP injection at day −14, −7, 0, +4, +5, or +6 with respect to challenge (on day 0). These groups of mice were bled at day +6, +8, +10 and + 15 p.i. and plasma was analyzed using a quantitative human IgG ELISA. As shown in Fig. 6 , the highest level of rVIG was recorded at day +6 p.i. in mice that received rVIG also on day +6 p.i. (approximately 6 h earlier than being bled). Levels of rVIG dropped precipitously over time, reaching ∼20 μg/mL by day +15. Inspection of the graph reveals that levels of the recombinant human IgG in plasma were similar in the other groups, with higher levels of the drug at time points closer to administration and levels dropping precipitously to ∼20 μg/mL. Even when rVIG was administered 14 days prior to challenge, the level was still ∼20 μg/mL at +15 days p.i., or 29 days following rVIG administration. These data indicate that rVIG levels decrease rapidly in the first 4 days following administration, subsequently the curve flattens and plasma levels remain stable for several weeks and continue to provide significant protection (Fig. 6).
Fig. 6.
rVIG levels remain detectable 14 days after injection following a lethal challenge with ECTV. Mice were challenged and treated with rVIG as per Fig. 3. At T = +6, +8, +10, and +15 mice were bled via the submandibular vein to determine plasma levels of rVIG. Data representative of 3 independent experiments with each group having an N = 5.
3.6. rVIG protects against lethal VACV and MPXV challenges in mice
Our findings that rVIG is protective in a lethal mousepox model is encouraging; moreover, the data presented in Table 1 revealed that rVIG is active against several other OPVs. Therefore, we employed two other small-animal models of smallpox using VACV in BALB/c mice and MPXV in Cast/EiJ mice. It is prudent to use several other models because no animal model entirely recapitulates VARV disease in humans (Adams et al. 2007; Americo et al. 2010).
We challenged BALB/c mice with 1 × 105 PFU of VACV (IHD strain) and treated them with a rVIG IP injection 24 h following challenge. rVIG was administered at 200, 100, 30, 10, 3, 1, or 0.3 μg/mouse and 400 μg/mouse of VIG was used as a control. At T = 5 d.p.i., groups (N = 5) of mice from each treatment regimen were sacrificed to determine viral titers in the lungs. As shown in Fig. 7 A, VIG failed to protect mice against challenge; however, the 200 μg and 100 μg doses of rVIG protected 100% of mice against mortality. Doses below 100 μg/mouse were not protective. Surprisingly, unlike in the ECTV studies, we found that all mice treated with rVIG had similar levels of weight-loss (∼30%) regardless of the dose administered (Fig. 7B). When lung tissue titers were calculated, there was no significant reduction in viral titers in the lungs of mice treated with SYM002 compared to the control (data not shown). These findings are consistent with what was observed in mice challenged with ECTV and treated with rVIG (Fig. 4).
Fig. 7.
Mortality and morbidity of female BALB/c mice following a lethal VACV challenge and treatment with VIG or rVIG. At T = 0 d.p.i. mice were challenged via the IN route with 1 × 105 PFU of VACV or vehicle (control challenge). At T = +1 d.p.i., a group of mice were treated once with VIG (IP injection of 400 μg) or groups of mice were treated once with decreasing volumes of rVIG (IP injections of 200, 100, 30, 10, 3, 1, or 0.3 μg). Mortality of mice was scored daily (A) and mice were weighed (as a group) 3-times per week to measure morbidity (B). The data is representative of 1 of 4 studies. All studies had similar results. N = 10/group. Note, groups receiving the 1 and 0.3 μg doses are not shown for graph clarity.
The MPXV studies were smaller in scale but were performed to demonstrate proof-of-principle that rVIG protects against lethal OPV challenges. Wild-type mice are typically resistant to MPXV; however, 129 stat1 −/− mice were sensitive to MPXV and established the first murine small-animal model (Stabenow et al., 2010). Shortly thereafter, a second inbred mouse strain (Cast/EiJ) was demonstrated to exhibit mortality and morbidity at fairly low inocula of MPXV (Americo et al. 2010). This sensitivity is believed to be due to an augmented IFN-γ response (Earl et al. 2012). We challenged Cast/EiJ mice IN with 2.4 × 104 PFU of MPXV. At T = +1 d.p.i. mice were treated with a single 160 μg/mouse IP rVIG injection. Mice challenged with MPXV experienced 80% mortality with rapid weight loss from T = +5 d.p.i. (Fig. 8 ); however, mice treated with rVIG experienced no mortality or morbidity as measured by weight-change. These data indicate that rVIG protects mice against a lethal challenge with MPXV.
Fig. 8.
Mortality of Cast/EiJ mice following a lethal MPXV challenge and treatment with rVIG. At T = 0 d.p.i. 2 groups of mice were challenged with 2.4 × 104 PFU of MPXV via the IN route. At T = +1 d.p.i., 1 group was IP injected with vehicle and the other group was IP injected with 160 μg of rVIG. Mortality of mice was scored daily (A) and mice were weighed daily (B) to measure morbidity. The data is a combination of 2 studies with N = 10 mice per group.
4. Discussion
rVIG demonstrated excellent efficacy in vitro with EC50 values outperforming those of VIG, the most similar drug in its class. Treatment with VIG was only minimally protective against challenge. A single treatment with rVIG reduced viremia, measured as both vDNA in whole-blood and infectious virus in liver. ALT levels were also near normal, indicating strong protection from liver disease and hepatocellular damage. PK studies revealed that rVIG levels decreased rapidly after injection but were still detectable 29 days post injection. Animals treated with rVIG had no obvious toxicity or morbidity. Our findings are consistent with a similar study that evaluated a smaller rVIG cocktail in mice challenged with recombinant VACVs expressing luciferase. Challenged mice were protected from mortality and had significantly reduced viral replication in the lungs, liver and spleen (as measured by bioluminescence) (Zaitseva et al., 2011).
rVIG was highly efficacious when given as a prophylactic or therapeutic regimen. A-strain mice challenged with ≤5 PFU of ECTV typically experience 100% mortality between T = +10 to +13 d.p.i. (Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c; Parker et al., 2009), thus consistent with our findings here. We found rVIG was statistically significantly protective when given up to T = +6 d.p.i., approximately half-way through the disease course. Previously we have shown that vDNA can be detected in the blood from T = +6 d.p.i. (Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c) in A-strain mice and the results presented here are consistent. These data suggest that vDNA can be used as a biomarker to trigger intervention with rVIG within the therapeutic window of protection. Results from other mouse strains suggest that early detection of disease biomarkers can be used as a trigger to initiate treatment and are critical for providing protection (Parker et al., 2012). Other studies have evaluated the use of buccal swabs for the detection of vDNA and found vDNA appeared in blood and buccal mucosa nearly simultaneously (Parker et al., 2012; Crump et al., 2017). Although buccal swab studies (which are significantly less invasive and easier to execute than submandibular bleeds) were performed in C57BL/6 mice, they suggest that buccal swabs would have utility as a technique to detect disease in A-strain mice within the therapeutic window of rVIG.
A-strain mice are highly susceptible to ECTV compared to C57BL/6 and SKH1 strains. These strains are both sensitive to ECTV IN challenges (with significantly higher LD50 values than A-strain mice) but, unlike A-strain mice, are resistant to ECTV footpad (FP) challenges (Parker et al., 2012). These strains are thought to be better animal models of smallpox because: 1) the FP challenge models variolation, a technique where VARV was inoculated into superficially wounded skin of humans which results in a significantly milder disease course with little to no mortality (Fenner et al., 1988); and 2), the immune response in these strains appears to be mediated by a stronger Th1 response compared to A-strain mice, similar to what is thought to occur in VARV naturally infected humans (Parker et al., 2009). Evaluating rVIG in these strains, along with biomarker assays, should be considered as a next step to determine the overall efficacy of rVIG.
To date, BCV and tecovirimat are the most evaluated antivirals in the ECTV/mousepox model of smallpox. We have previously found that following a medium/low-dose IN challenge (∼50 PFU), A-strain mice experienced 100% mortality by ∼ day +9 d.p.i.; however, intervention with BCV was protective up to day+5 d.p.i. and to at least day+3 d.p.i. for tecovirimat (evaluation beyond day+3 d.p.i. was not evaluated in A-strain mice) (Quenelle et al., 2007a, Quenelle et al., 2007b; Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c). These findings suggest that rVIG will have a therapeutic window similar to BCV and tecovirimat; however, only 1 treatment with rVIG was required for protection whereas for tecovirimat, and BCV to a lesser extent, multiple administrations were required (Quenelle et al., 2007a, Quenelle et al., 2007b; Parker et al., 2008a, Parker et al., 2008b, Parker et al., 2008c; Parker et al., 2009; Yang et al., 2005). Evaluation of rVIG should be considered in comparison to BCV and tecovirimat in a controlled fashion where challenge doses are matched.
The advent of synthetic-biology and the ease with which OPVs can be genetically modified has revealed that a recombinant ECTV expressing murine IL-4 causes increased virulence and mortality even in mice vaccinated with the Dryvax vaccine – a vaccine which provides uniformly robust protection against wild-type ECTV challenges in all immunocompetent mouse strains (Jackson et al., 2001; Chen et al., 2011). These findings have been of significant concern to the bio-defense community for >20 years and played a large part in the decision to develop antivirals against OPVs, in particular VARV. Previous small-scale studies have found that the most effective strategies to protect against ECTV-IL-4 are either: 1) vaccination with Dryvax followed by a booster vaccination (28 days later) and treatment with CDV on the day of challenge; or 2) a combination treatment with BCV and ST-246 for 14 days starting on the day of challenge. Neither treatment regimen protected against significant morbidity and some mortality (insignificant) was observed (Chen et al., 2011). Consideration should be given to evaluating rVIG as a stand-alone or combination regimen against recombinant IL-4 ECTV. This is particularly important because most of the US population is not vaccinated against VARV and a significant proportion have vaccine contraindications; however, a pre-exposure (prophylactic) treatment with rVIG could be a significant treatment option.
5. Summary
rVIG provided excellent protection against multiple OPVs. In animal models it appears to offer equal, and perhaps superior, protection when compared to BCV and tecovirimat. rVIG should be considered for further evaluation in small-animal models of smallpox and consideration should be given to evaluating it against recombinant IL4 OPVs.
Funding
This work was funded by NIAID Task Order A59, contract HHSN272201000021I. The funders had no role in the study design, decision to publish, collection of data, or manuscript preparation.
Declaration of competing interest
We declare no conflict of interest.
References
- Adams M.M., Rice A.D., Moyer R.W. Rabbitpox virus and vaccinia virus infection of rabbits as a model for human smallpox. J. Virol. 2007;81:11084–11095. doi: 10.1128/JVI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Americo J.L., Moss B., Earl P.L. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J. Virol. 2010;84:8172–8180. doi: 10.1128/JVI.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhanu A., King D.S., Mosier S., Jordan R., Jones K.F., Hruby D.E., Grosenbach D.W. ST-246 inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestation. Antimicrob. Agents Chemother. 2009;53:4999–5009. doi: 10.1128/AAC.00678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R.M.L., Fenner F. Elsevier; New York: 2007. The Mouse in Biomedical Research. [Google Scholar]
- Buller R.M., Owens G., Schriewer J., Melman L., Beadle J.R., Hostetler K.Y. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318:474–481. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Centers for Disease, Control, and Prevention Progressive vaccinia in a military smallpox vaccinee - United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 2009;58:532–536. [PubMed] [Google Scholar]
- Chen N., Bellone C.J., Schriewer J., Owens G., Fredrickson T., Parker S., Buller R.M. Poxvirus interleukin-4 expression overcomes inherent resistance and vaccine-induced immunity: pathogenesis, prophylaxis, and antiviral therapy. Virology. 2011;409:328–337. doi: 10.1016/j.virol.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla S.L., Trahan J., Wan W.B., Beadle J.R., Aldern K.A., Painter G.R., Hostetler K.Y. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 2003;59:163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Crump R., Korom M., Buller R.M., Parker S. Buccal viral DNA as a trigger for brincidofovir therapy in the mousepox model of smallpox. Antivir. Res. 2017;139:112–116. doi: 10.1016/j.antiviral.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraffour S., Snoeck R., de Vos R., van Den Oord J.J., Crance J.M., Garin D., Hruby D.E., Jordan R., De Clercq E., Andrei G. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir. Ther. 2007;12:1205–1216. [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Moss B. Lethal monkeypox virus infection of CAST/EiJ mice is associated with a deficient gamma interferon response. J. Virol. 2012;86:9105–9112. doi: 10.1128/JVI.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban D.J., Buller R.M. Ectromelia virus: the causative agent of mousepox. J. Gen. Virol. 2005;86:2645–2659. doi: 10.1099/vir.0.81090-0. [DOI] [PubMed] [Google Scholar]
- Esteban D., Parker S., Schriewer J., Hartzler H., Buller R.M. Mousepox, a small animal model of smallpox. Methods Mol. Biol. 2012;890:177–198. doi: 10.1007/978-1-61779-876-4_11. [DOI] [PubMed] [Google Scholar]
- Fenner F. Mousepox (infectious ectromelia): past, present, and future. Lab. Anim. Sci. 1981;31:553–559. [PubMed] [Google Scholar]
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. World Health Organisation; Geneva: 1988. Smallpox and its Eradication. [Google Scholar]
- Grosenbach D.W., Berhanu A., King D.S., Mosier S., Jones K.F., Jordan R.A., Bolken T.C., Hruby D.E. Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:838–843. doi: 10.1073/pnas.0912134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley L., Buller R.M., Frey S.E., Bellone C., Parker S. The new ACAM2000 vaccine and other therapies to control orthopoxvirus outbreaks and bioterror attacks. Expert Rev. Vaccines. 2009;8:841–850. doi: 10.1586/erv.09.55. [DOI] [PubMed] [Google Scholar]
- Hostetler K.Y. In: De Clercq E., editor. vol. 5. 2007. Synthesis and antiviral evaluation of broad spectrum, orally active analogs of cidofovir and other acyclic nucleoside phosphonates. (Advances in Antiviral Drug Design). (Elsevir) [Google Scholar]
- Hoy S.M. Tecovirimat: first global approval. Drugs. 2018;78:1377–1382. doi: 10.1007/s40265-018-0967-6. [DOI] [PubMed] [Google Scholar]
- Jackson R.J., Ramsay A.J., Christensen C.D., Beaton S., Hall D.F., Ramshaw I.A. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virol. 2001;75:1205–1210. doi: 10.1128/JVI.75.3.1205-1210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R., Tien D., Bolken T.C., Jones K.F., Shanthakumar T.R., Strasser J., Frimm A., Corrado M.L., Strome P.G., Hruby D.E. Single-Dose Safety and Pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob. Agents Chemother. 2008;52:1721–1727. doi: 10.1128/AAC.01303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe C.H., Bowles C., Meiklejohn G., Berge T.O., St Vincent L., Babu B.V., Govindarajan S., Ratnakannan N.R., Downie A.W., Murthy V.R. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of smallpox. Bull. World Health Organ. 1961;25:41–48. [PMC free article] [PubMed] [Google Scholar]
- Kemper A.R., Davis M.M., Freed G.L. Expected adverse events in a mass smallpox vaccination campaign. Effect Clin. Pract. 2002;5:84–90. [PubMed] [Google Scholar]
- Lanier R., Trost L., Tippin T., Lampert B., Robertson A., Foster S., Rose M., Painter W., O'Mahony R., Almond M., Painter G. Development of CMX001 for the treatment of poxvirus infections. Viruses. 2010;2:2740–2762. doi: 10.3390/v2122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantto J., Haahr Hansen M., Rasmussen S.K., Steinaa L., Poulsen T.R., Duggan J., Dennis M., Naylor I., Easterbrook L., Bregenholt S., Haurum J., Jensen A. Capturing the natural diversity of the human antibody response against vaccinia virus. J. Virol. 2011;85:1820–1833. doi: 10.1128/JVI.02127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudisoit A., Tepage F., Colebunders R. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 2018;379:2084–2085. doi: 10.1056/NEJMc1811044. [DOI] [PubMed] [Google Scholar]
- Lederman E., Miramontes R., Openshaw J., Olson V.A., Karem K.L., Marcinak J., Panares R., Staggs W., Allen D., Weber S.G., Vora S., Gerber S.I., Hughes C.M., Regnery R., Collins L., Diaz P.S., Reynolds M.G., Damon I. Eczema vaccinatum resulting from the transmission of vaccinia virus from a smallpox vaccinee: an investigation of potential fomites in the home environment. Vaccine. 2009;27:375–377. doi: 10.1016/j.vaccine.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Lederman E.R., Davidson W., Groff H.L., Smith S.K., Warkentien T., Li Y., Wilkins K.A., Karem K.L., Akondy R.S., Ahmed R., Frace M., Shieh W.J., Zaki S., Hruby D.E., Painter W.P., Bergman K.L., Cohen J.I., Damon I.K. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J. Infect. Dis. 2012;206:1372–1385. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Earl P.L. Current Protocols in Molecular Biology. Wiley; 1998. Expression of proteins in mammalian cells using vaccinia virus vectors. Overview of the vaccinia virus expression system. [Google Scholar]
- Noyce R.S., Lederman S., Evans D.H. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS One. 2018;13 doi: 10.1371/journal.pone.0188453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S., Buller R.M. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S., Chen N.G., Foster S., Hartzler H., Hembrador E., Hruby D., Jordan R., Lanier R., Painter G., Painter W., Sagartz J.E., Schriewer J., Mark Buller R. Evaluation of disease and viral biomarkers as triggers for therapeutic intervention in respiratory mousepox - an animal model of smallpox. Antivir. Res. 2012;94:44–53. doi: 10.1016/j.antiviral.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S., Crump R., Foster S., Hartzler H., Hembrador E., Lanier E.R., Painter G., Schriewer J., Trost L.C., Buller R.M. Co-administration of the broad-spectrum antiviral, brincidofovir (CMX001), with smallpox vaccine does not compromise vaccine protection in mice challenged with ectromelia virus. Antivir. Res. 2014;111:42–52. doi: 10.1016/j.antiviral.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S., Nuara A., Buller R.M., Schultz D.A. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- Parker S., Schriewer J., Oberle C., Robertson A., Lanier R., Painter G., Buller R.M. Using biomarkers to stage disease progression in a lethal mousepox model treated with CMX001. Antivir. Ther. 2008;13:863–873. [PMC free article] [PubMed] [Google Scholar]
- Parker S., Schultz D.A., Meyer H., Buller R.L. In: Encyclopedia of Virology. Mahy B.W.J., Van Ragenmortel N.H.V., editors. 2008. Smallpox and monkeypox viruses. [Google Scholar]
- Parker S., Siddiqui A.M., Oberle C., Hembrador E., Lanier R., Painter G., Robertson A., Buller R.M. Mousepox in the C57BL/6 strain provides an improved model for evaluating anti-poxvirus therapies. Virology. 2009;385:11–21. doi: 10.1016/j.virol.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S., Siddiqui A.M., Painter G., Schriewer J., Buller R.M. Ectromelia virus infections of mice as a model to support the licensure of anti-orthopoxvirus therapeutics. Viruses. 2010;2:1918–1932. doi: 10.3390/v2091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S., Touchette E., Oberle C., Almond M., Robertson A., Trost L.C., Lampert B., Painter G., Buller R.M. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antivir. Res. 2008;77:39–49. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle D.C., Buller R.M., Parker S., Keith K.A., Hruby D.E., Jordan R., Kern E.R. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob.Agents Chemother. 2007;51:689–695. doi: 10.1128/AAC.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle D.C., Collins D.J., Herrod B.P., Keith K.A., Trahan J., Beadle J.R., Hostetler K.Y., Kern E.R. Effect of oral treatment with hexadecyloxypropyl-[(S)-9-(3-hydroxy-2- phosphonylmethoxypropyl)adenine] [(S)-HPMPA] or octadecyloxyethyl-(S)-HPMPA on cowpox or vaccinia virus infections in mice. Antimicrob. Agents Chemother. 2007;51:3940–3947. doi: 10.1128/AAC.00184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D.F., Sidwell R.W., Kefauver D., Bray M., Huggins J.W. 'Characterization of wild-type and cidofovir-resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob. Agents Chemother. 2002;46:1329–1335. doi: 10.1128/AAC.46.5.1329-1335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D.F., Wong M.H., Bailey K.W., Beadle J.R., Hostetler K.Y., Sidwell R.W. Effects of four antiviral substances on lethal vaccinia virus (IHD strain) respiratory infections in mice. Int. J. Antimicrob. Agents. 2004;23:430–437. doi: 10.1016/j.ijantimicag.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Stabenow J., Buller R.M., Schriewer J., West C., Sagartz J.E., Parker S. A mouse model of lethal infection for evaluating prophylactics and therapeutics against Monkeypox virus. J. Virol. 2010;84:3909–3920. doi: 10.1128/JVI.02012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar J., Arndtz N., Eckl K.M., Thomsen T., Petzold B., Mateo L., Schlereth B., Handley A., King L., Hulsemann V., Tzatzaris M., Merkl K., Wulff N., Chaplin P. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24:2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Wallace G.D., Buller R.M. Kinetics of ectromelia virus (mousepox) transmission and clinical response in C57BL/6j, BALB/cByj and AKR/J inbred mice. Lab. Anim. Sci. 1985;35:41–46. [PubMed] [Google Scholar]
- Xiao Y., Isaacs S.N. Therapeutic vaccines and antibodies for treatment of orthopoxvirus infections. Viruses. 2010;2:2381–2403. doi: 10.3390/v2102381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Buller R.L., Touchette E., Waller K., Schriewer J., Neyts J., DeClercq E., Jones K., Hruby D., Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J. Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitseva M., Kapnick S.M., Meseda C.A., Shotwell E., King L.R., Manischewitz J., Scott J., Kodihalli S., Merchlinsky M., Nielsen H., Lantto J., Weir J.P., Golding H. Passive immunotherapies protect WRvFire and IHD-J-Luc vaccinia virus-infected mice from lethality by reducing viral loads in the upper respiratory tract and internal organs. J. Virol. 2011;85:9147–9158. doi: 10.1128/JVI.00121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]