Abstract
Nitrate is an essential nutrient and signaling molecule for plant growth. Plants sense intracellular nitrate to adjust their metabolic and growth responses. Here we identify the primary nitrate sensor in plants. We found that mutation of all seven Arabidopsis NIN-Like-Protein (NLP) transcription factors abolished plant’s primary nitrate responses and developmental programs. Analyses of NIN-NLP7 chimeras and nitrate binding revealed that NLP7 is de-repressed upon nitrate perception via its N-terminus. A genetically encoded fluorescent split-biosensor, mCitrine-NLP7, enabled visualization of single-cell nitrate dynamics in planta. The nitrate-sensor domain of NLP7 resembles a bacterial nitrate sensor NreA. Substitutions of conserved residues in the ligand-binding pocket impaired nitrate-triggered NLP7 ability to control transcription, transport, metabolism, development, and biomass. We propose that NLP7 represents a nitrate sensor in land plants.
One Sentence Summary:
A dual master transcription activator and nitrate sensor central to transcriptome reprogramming and plant development.
Nitrogen, the main limiting factor for plant growth, is fundamental to agricultural productivity, animal and human nutrition, and sustainable ecosystems (1, 2). Photosynthetic plants drive the terrestrial nitrogen cycle by assimilating inorganic nitrogen into biomolecules (DNA, RNA, proteins, chlorophyll, and vitamins) that sustain plant life and the food webs that depend on plants (1–4). To compete with microbes that prefer organic nitrogen or ammonium in the soil, most plants have evolved regulatory pathways that respond to fluctuating nitrate availability (3–5). Plants that sense available nitrate will, within minutes, orchestrate transcriptome, metabolism, hormone, system-wide shoot and root growth, and reproduction responses (5–8). Here we identify the primary nitrate sensor as the NIN-Like-Protein transcription factor 7 (NLP7). We show that NLP7 acts as an intracellular nitrate sensor distinct from the plasma membrane extracellular nitrate transporter-sensor (transceptor) NRT1.1/CHL1/NPF6.3 (9–13).
NIN-like proteins 6 and 7 (NLP6/7), which were identified by homology to the regulator NODULE INCEPTION (NIN) that controls root nodule initiation and N2 fixation (14), are master transcription factors in nitrate signaling and widely conserved in land plants and major crops (9, 15–19). There are nine NLPs in Arabidopsis thaliana featuring an N-terminal nitrate response region, a conserved RWP-RK DNA-binding domain for the nitrate response cis-element (NRE), and a C-terminal PB1 protein-protein interaction domain (13, 15, 17, 20). The N-terminal nitrate-response region of NLP1–9 encompasses an evolutionarily conserved regulatory phosphorylation site critical for nitrate-induced nuclear retention and transcription activation (18, 20). Functions of putative GAF (cGMP-specific phosphodiesterase, adenylyl cyclases, and FhlA)-like domains and other conserved motifs remain unclear (17). Nitrate activates group III calcium-sensor protein kinases (CPKs), which phosphorylate NLP7 at S205 to retain NLP7 in the nucleus, where it activates primary nitrate responses that shape organ biomass and architecture (18). Nitrate-dependent transactivation activity of NLP2 requires the conserved S202 residue (20). Nitrate is required to activate the primary reporter gene despite co-expression of NLP7 and constitutively active CPK10ac in nitrate-free leaf cells (18), suggesting a missing nitrate sensor action. Here, we demonstrate that NLP7 is an intracellular nitrate sensor. The nitrate-binding domain seems evolutionarily ancient and is conserved in plant NLPs and the bacteria nitrate sensors NreA (21). Nitrate directly binds to NLP7 through residues evolutionarily conserved in other NLPs and NLP7 orthologs, triggers a conformational change, and derepresses NLP7 as a transcription activator.
Combinatorial NLPs control primary nitrate responses
All nine NLP genes are expressed in Arabidopsis shoots. Analyses of individual nlp1–9 single mutants in nitrate-mediated shoot growth revealed statistically significant defects only in nlp2 and nlp7 (9, 18, 20). To circumvent NLP redundancy and better define the overlapping or unique functions of NLP1–9, we conducted a genome-wide target gene survey. Each NLP was transiently expressed for 4.5 h in transfected leaf cells from soil-grown plants for RNA-seq analyses (15, 18, 21). Hierarchical clustering analysis of putative NLP target genes (Log2 ≥ 1 or ≤ −1; p ≤ 0.05) revealed the capability of all NLPs to activate universal primary nitrate responsive marker genes previously identified by microarray, RNA-seq, ChIP-chip, ChIP-seq, and promoter analyses (15, 16, 18, 21, 22) (Fig. 1, A and B, and table S1, S2). NLP2, NLP4, NLP7, NLP8, or NLP9 specifically activated some target genes with known functions in modulating auxin and cytokinin hormone functions, cell cycle, metabolism, peptide signaling, and shoot and root meristem activities (Fig. 1, A and B, and table S1, S2). NLP2 and NLP7 regulated broader non-redundant target genes with diverse functions, which might manifest as growth defects observed in nlp2 or nlp7 after seed germination (9, 18, 20). NLP6/7 acted predominantly as transcription activators, whereas NLP2,4,5,8,9 could activate or repress target genes. NLP1,3,6 modulated fewer target genes than other NLPs. For instance, the auxin biosynthesis gene TAR2 was only activated by NLP2,4,5,7,8,9 (23). These results are consistent with NLP variants functioning combinatorially in controlling the nitrate response network and causing retarded shoot and root development in the nlp2,4,5,6,7,8,9 septuple mutant plants grown in soil (Fig. 1C).
Fig. 1. Combinatorial NLP transcription factors are central to primary nitrate responses and developmental programs.
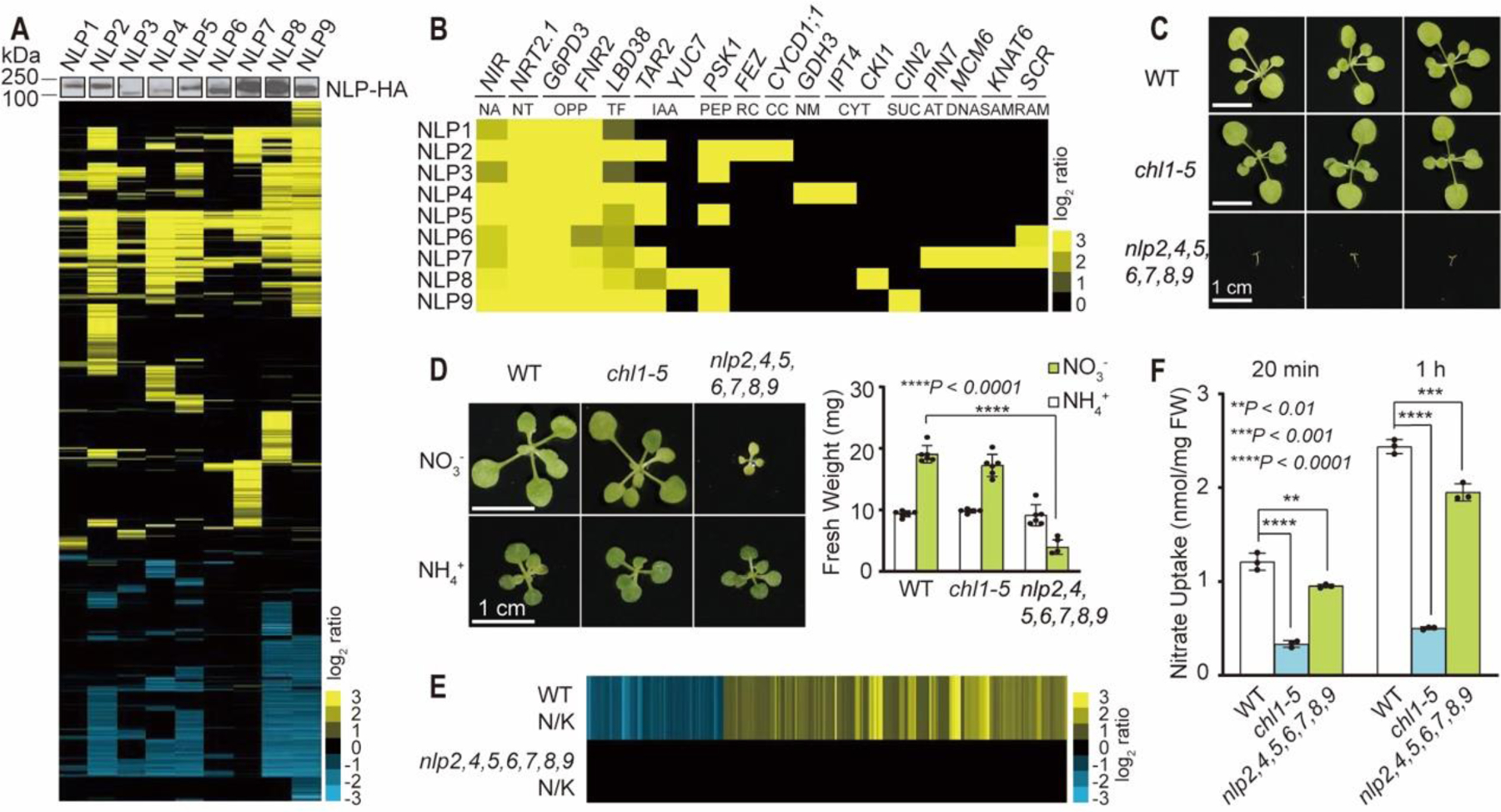
(A) NLPs regulate both common and distinct target genes. Hierarchical clustering of RNA-seq analysis defines putative target genes of NLP1–9 by transient expression in leaf cells. NLP protein expression was determined by immunoblot analyses. (B) Heatmap of nitrate-responsive core genes and unique target genes activated by NLP1–9. NA: nitrate assimilation; NT: nitrate transporter; OPP: oxidative pentose phosphate; TF: Transcription factor; IAA: IAA biosynthesis; PEP: peptide hormone; RC: root cap; CC: cell cycle; NM: nitrogen metabolism; CYT: cytokinin biosynthesis and signaling; SUC: sucrose invertase; AT: auxin transport; DNA: DNA synthesis; SAM: shoot apical meristem; RAM: root apical meristem. (C) The nlp2,4,5,6,7,8,9 mutant abolishes shoot growth in soil. (D) The nlp2,4,5,6,7,8,9 mutant displays nitrate-specific reduction in shoot and biomass. Error bars, s.d., n = 6. (E) Primary nitrate-responsive transcriptome is abolished in nlp2,4,5,6,7,8,9. (F) Nitrate uptake in nlp2,4,5,6,7,8,9. Error bars, s.d., n = 3. Scale bar, 1 cm.
NRT1.1(CHL1/NPF6.3) acts as a plasma membrane nitrate transceptor that senses external nitrate in Arabidopsis (10–12). However, the null chl1–5 mutant resembled WT when grown in the soil under light, whereas the nlp2,4,5,6,7,8,9 septuple mutant failed to develop further after germination (Fig. 1C). When grown on a sterile culture medium with 0.5% sucrose and ammonium-succinate, WT, chl1–5 and nlp2,4,5,6,7,8,9 plants were able to develop similar shoots albeit smaller than WT plants grown in nitrate medium (1, 19, 21). In contrast, shoot and root development of nlp2,4,5,6,7,8,9 remained constrained in nitrate medium (Fig. 1D, and fig. S1A–F) (20). Transcriptome reprogramming in primary nitrate responses triggered by nitrate at 20 min (Log2 ≥ 1 or ≤ −1; q ≤ 0.05) was abolished in nlp2,4,5,6,7,8,9 (Fig. 1E). The activation of primary nitrate response genes are only partially reduced in chl1–5 (fig.S2 and table S3). However, the uptake of nitrate showed a stronger reduction in chlorate-resistant chl1–5 but was only moderately reduced in nlp2,4,5,6,7,8,9 (Fig. 1F) (6, 24). Chlorate could not activate primary nitrate response genes (fig. S3). These findings further supported the essential role of NLPs in regulating primary nitrate responses (Fig. 1E, fig. S2, and table S1–3) and plant development, as well as the existence of nitrate sensors presumably distinct from NRT1.1(CHL1/NPF6.3) (9–13).
Nitrate derepresses NLP7 via the N-terminus
The N-terminus of NLPs contain the GAF-like domain and multiple conserved motifs with unknown functions (17). When fused to the LexA DNA binding domain and a nuclear localization signal, the N-terminus of NLP6(1–546) is sufficient to confer nitrate response to the 8xLexA-min:GUS reporter in nitrate-free seedlings (15). Single mutant studies showed that nlp7 but not nlp6 exhibited overt shoot growth defects (fig. S1A). NLP6 also activated fewer target genes compared with the closely related NLP7 transcription activator in transfected leaf cells (Fig. 1A and table S1, S2). Therefore, we examined the role of NLP7 and the functional domains of NLP7 for the activation of a synthetic nitrate-responsive reporter 4xNRE-min-LUC in nitrate-free Arabidopsis leaf cells (13, 15, 18). Although 4xNRE-min-LUC was activated by nitrate (10 mM, 2 h) in transfected WT and nlp6 leaf cells, its activation was diminished in nlp7 or nlp6,7, and abolished in nlp2,4,5,6,7,8,9 (fig. S1G). NRE is evolutionarily conserved in the primary nitrate responsive NIR gene promoter from Arabidopsis, spinach, bean, birch, and maize (25). These data suggested a role of NLP7 in activating NRE.
Full-length NLP7(1–959) conferred nitrate-specific 4xNRE-min-LUC activation at low 0.5 mM nitrate for 2 h in nitrate-free leaf cells. However, the N-NLP7(1–581) domain was inactive whereas the NLP7-C(582–959) domain was constitutively activated in the same reporter assay without nitrate (Fig. 2, A and B). The LjNIN transcription factor from Lotus japonicus, which controls root nodule initiation and N2 fixation and shares RWP-RK DNA-binding and PB1 domains with NLPs but not the N-terminus (15, 26), activated the NRE-based reporter in nitrate-free leaf cells. Analyses of the chimeric transcription factors N-NLP7-NIN-C and N-NIN-NLP-C suggested that N-NLP7 acted as a repressor domain in full-length NLP7 and is derepressed by nitrate (Fig. 2, A and B). As the NLP7 orthologs in diverse plant species were more ancient and arose after the green algal lineage, NIN in legume species likely evolved later, having lost autorepression and nitrate responsiveness (fig. S4) (9, 14, 17, 27).
Fig. 2. Nitrate derepresses NLP7.

(A) Reporters and effectors for transient assays in leaf cells. The nitrate-responsive reporter (4xNRE-min-LUC) contains four copies of nitrate responsive element (NRE) and the 35S minimal promoter (min) fused to firefly luciferase gene (LUC) and NOS terminator (NOS). The 35S Cauliflower mosaic virus promoter controls the Renilla luciferase gene (pCAMV-RLUC) as the internal control. The effector expression is controlled by a constitutive HBT promoter (19). Numbering are the amino acid positions. (B) Analyses of NLP-NIN chimeras reveal de-repression of NLP7 by nitrate. Transactivation assays of NRE-LUC was carried out in nlp2,4,5,6,7,8,9 leaf cells by expressing effectors for 4 h before induction (0.5 mM KNO3 for 2 h). Luciferase activity was normalized to pCAMV-RLUC activity. Error bars, s.d., n = 5 biological replicates. Effector protein expression was determined by immunoblot analyses. (C) Reporters and effector for transient assays in 293T human cells. The nitrate-responsive Human-4xNRE-min-LUC reporter contains the SV40 terminator. The herpes simplex virus (HSV) promoter driven RLUC (pHSV-RLUC) is an internal control. The MYC-NLP7-mGFP gene is controlled by a human cytomegalovirus (CMV) promoter with the β-globin gene terminator. (D) NLP7 activates Human-4xNRE-min-LUC in response to nitrate in heterologous 293T human cells. Luciferase activity was normalized to pHSV-RLUC activity (10 mM KNO3 for 2 h). Error bars, s.d., n = 3 biological replicates.
RWP-RK transcription factors are specific to vascular plants, green algae, and slime modes (17). Although human cells can transport and reduce nitrate to support an alternative plant diet-based NO pathway (28), humans have no orthologs for NRT1.1 transceptor or NLPs. We asked whether NLP7 could activate a synthetic version of the nitrate responsive reporter Human-4xNRE-min-LUC with a mammalian minimal promoter and SV40 terminator in heterologous 293T human cells (Fig. 2C). As in nitrate-free Arabidopsis leaf cells, Human-4xNRE-min-LUC was activated by NLP7 only in the presence of nitrate (Fig. 2D). Thus, NLP7 alone may function both as a nitrate sensor and a transcription activator in the heterologous human cell system.
Nitrate directly binds to NLP7
To test the hypothesis that NLP7 is both a ligand-dependent transcription activator and an intracellular nitrate sensor, we measured nitrate binding to NLP7 using microscale thermophoresis (MST) and surface plasmon resonance (SPR) (29, 30). We expressed and purified GST-NLP7–8xHIS fusion protein from E. coli (fig. S5A) and performed binding experiments by MST at different nitrate concentrations (Fig. 3A). Similar to NreA, which can bind nitrate with Kd = 22 μM, NLP7 (not HIS-GST) selectively recognized nitrate (Fig. 3A) with Kd = 52 ± 20 μM but not its structural analog chlorate or anion phosphate (Fig. 3, A and D and fig. S5B–D, F). Because the N-terminus of NLP7 is crucial for nitrate responsiveness, we also expressed and purified the N-terminus NLP7(1–581)-based GST-N-NLP7–8xHIS fusion protein and carried out the MST assay. Nitrate could bind to N-NLP7 with Kd = 86 ± 38 μM but not chlorate or phosphate (Fig. 3, B and E, fig. S5E). To confirm the interaction between NLP7 and nitrate, we performed alternative binding experiments using SPR. Purified GST-N-NLP7–8xHIS protein was immobilized on a sensor chip and different concentrations of nitrate, chlorate, or phosphate were monitored by SPR with the average Kd = 72 ± 34 μM only for nitrate (Fig. 3, C and F and fig. S5G–I).
Fig. 3. Nitrate directly binds to NLP7.

(A) NO3− binding to full-length NLP7. Microscale thermophoresis (MST). Dissociation constant, Kd = 52 ± 20 μM. Error bar, s.d., n = 3. (B) NO3− binding to N-NLP7. Kd = 86 ± 38 μM. Error bar, s.d., n = 3. (C) Analysis of NO3−-N-NLP7 interaction. Surface plasmon resonance (SPR). The result is a representative of three independent experiments. The Kd is the average of three independent experiments. (D) ClO3− does not bind to full-length NLP7. Error bars, s.d., n = 3. (E) ClO3− does not bind to N-NLP7. Error bars, s.d., n = 3. (F) SPR analysis of ClO3− -N-NLP7 interaction. The result is a representative of three independent experiments.
A genetically encoded fluorescent biosensor visualizes nitrate in plants
Ligand-sensor interaction may trigger a conformational change in the sensor protein. We generated a genetically encoded fluorescent biosensor, mCitrine-NLP7, similar to the single fluorescent protein-based glucose biosensor Green Glifon (31). We hypothesized that the nitrate-bound split mCitrine-NLP7 nitrate biosensor (sCiNiS) would reconstitute mCitrine to emit fluorescent signals (31). The predicted nuclear localization signal (630RRKKK638) of NLP7 was mutated to AAAAA to avoid competition with the endogenous NLP7, which is retained in the nucleus after nitrate induction for transcriptional activation (Fig. 4A and fig. S6, A and B) (16, 18). Quantitative confocal imaging of the cytoplasmic nitrate by sCiNiS was carried out in mesophyll cells of cotyledons and columella cells of root tips in transgenic plants (Fig. 4, B and C). The reconstituted mCitrine fluorescent signal was detected within 5 min after nitrate (10 mM), but not KCl, induction in both mesophyll cells and primary root tip cells at single-cell resolution in intact sCiNiS transgenic seedlings that developed normally (Fig. 4, B and C, and fig. S6C). Nitrate concentrations can vary from μM to mM in the soil (6). We tested different nitrate concentrations using nitrate-free transgenic seedlings and showed that the sCiNiS biosensor detected a range of nitrate concentrations from 100 μM to 10 mM in single mesophyll cells in intact plants, consistent with the sensitive and specific nitrate binding Kd of 52 μM for NLP7 in vitro (Fig. 3A and fig. S6D).
Fig. 4. The genetically encoded biosensor detects intracellular nitrate in transgenic shoots and roots.

(A) A schematic representation of domain structure and a model of the nitrate biosensor. Nitrate triggers a conformational change of split mCitrine-NLP7 nitrate biosensor (sCiNiS) and reconstitutes mCitrine to emit fluorescent signals. The predicted nuclear localization signal (630RRKKK638) of NLP7 was mutated to AAAAA to avoid competition with the nitrate induced endogenous NLP7 in the nucleus. (B) Imaging the cytoplasmic nitrate by sCiNiS in mesophyll cells of cotyledons. Dashed white line, the imaging site. Images are representative of 10 cotyledons. Blue scale bar, 1 mm. White scale bar, 30 μm. (C) Imaging the cytoplasmic nitrate by sCiNiS in in root tips. Dashed red line, the imaging site. Images are representative of 10 root tips, KCl or KNO3 (10 mM). (F-F0)/F0, the relative fluorescence intensity. Error bars, s.d., n = 10.
Evolutionary conservation of nitrate sensors in plants
Architectures of nitrate-binding sites in bacteria and cyanobacteria proteins have been solved by crystal structure analyses of cytoplasmic NreA of Staphylococci carnosus, periplasmic NarX of E. coli, and periplasmic NrtA of Synechocystis sp. These nitrate-binding proteins support anaerobic respiration and nitrate transport. Their crystal structures are available at the Protein Data Bank (PDB 4IUK, PDB 3EZH and PDB 2G29) (21). We found that seven out of eight critical residues in the nitrate-binding pocket of the NreA nitrate sensor could be aligned to the conserved motifs next to the putative GAF-like motif in NLP1–9 (17, 21) (Fig. 5, A and B, and fig. S7A). The predicted protein structure of the nitrate-binding domain in NLP7 by artificial intelligence programs, AlphaFold2 and RoseTTAfold (32, 33), also resembled that in NreA (Fig. 5C).
Fig. 5. The NLP7 sensor domain resembles NreA with conserved residues for nitrate perception and signaling.

(A) The nitrate binding domain of the bacterial nitrate sensor NreA shares homology to a region of NLP7. The homologies are in red. Numbering refers to amino acid positions. (B) Sequence alignment of the nitrate binding domain of NreA and NLP7. Black box: conserved residue. Gray box: semi-conserved residue. Three essential nitrate binding residues are outlined in red. Asterisk, the conserved residues in the nitrate-binding pocket of the NreA. (C) Comparison of the crystal structure of the NreA nitrate-binding domain and the predicted structure of NLP7. Red stick: Nitrate. Pink: NreA (I59, L61, Y95) or NLP7(H404, L406, Y436). (D) Nitrate-binding mutant screens by transient assays in leaf cells. Transactivation of 4xNRE-min-LUC by NLP7 or nitrate-binding mutants was analyzed in nlp2,4,5,6,7,8,9 leaf protoplasts (0.5 mM KNO3, 2 h). Error bars, s.d., n = 5 biological replicates. (E) The key nitrate binding residues are conserved in NLPs from major crops. Critical nitrate-binding residues are outlined in red. (F) Functional conservation of NLP7 and rice OsNLP3 for nitrate perception and transcription activation in heterologous 293T human cells. The nitrate-binding residues of NLP7(H404, L406, Y436) or OsNLP3(H392, L394, Y424) are essential for nitrate activation of human-4xNRE-min-LUC. Error bars, s.d., n = 3. (G)(H)(I) Mutant full-length NLP7 (HLY/AAA) and N-NLP7(HYL/AAA) abolish nitrate binding. Error bars, s.d., n = 3. The result is a representative of three independent experiments in SPR assays. (J) pNLP7-NLP7-GFP but not pNLP7-NLP7(HLY/AAA)-GFP complements the nlp7–1 mutant. Scale bars, 1 cm.
To functionally define the essential residues for nitrate binding in NLP7, we carried out alanine (A) scanning mutagenesis of eight putative nitrate-binding residues defined in the nitrate-NreA crystal form (21) and examined the nitrate response of mutated NLP7 in nitrate-free leaf cells. NLP7 mutations of four residues, W395A, H404A, L406A, or Y436A, significantly decreased nitrate-induced 4xNRE-min-LUC activity at low 0.5 mM nitrate for 2 h. Because H404, L406, and Y436 are conserved in NLP2,4,5,6,8,9 with similar structures in the nitrate-binding domain (fig. S7, A and B), we next generated and analyzed double (HL/AA) and triple (HLY/AAA) mutants of NLP7, which abolished nitrate-induced 4xNRE-min-LUC activity (Fig. 5D). The HLY nitrate-binding residues are also conserved among NLP7 orthologs within the structurally similar nitrate sensor domain in crop plants including rapeseed BnaNLP7, soybean GmNLP6, maize ZmNLP6, wheat TaNLP7, and rice OsNLP3 (Fig. 5E and fig. S7, C and D). We propose that NLP7 and its orthologs may serve as nitrate sensors conserved in photosynthetic plants from Charophytes to Angiosperms including eudicots and monocots but not Chlorophytes (fig. S7, C and D).
To demonstrate the function of the nitrate-binding domain in NLP7, we examined the ability of NLP7 and NLP7(HLY/AAA) to activate the nitrate responsive reporter Human-4xNRE-min-LUC in the heterologous 293T cell system. Compared to NLP7, NLP7(HLY/AAA) abrogated the LUC activity induced by nitrate-bound and activated NLP7 (Fig. 5F). Similar to NLP7, OsNLP3 as the rice NLP7 ortholog activated the nitrate-dependent Human-4xNRE-min-LUC activity, which was eliminated with OsNLP3(HLY/AAA, mutations in H392A, L394A, Y424A) (Fig. 5F). To verify that the residues, H404, L406, and Y436, are required for nitrate binding in the NLP7 sensor domain, we performed MST assays and SPR analyses using purified NLP7(HLY/AAA) or N-NLP7(HLY/AAA, 1–581) protein (Fig. 5G–I and fig. S5A). Mutation of these three amino acids impeded nitrate binding in vitro. Furthermore, NLP7-HA but not NLP7(HLY/AAA)-HA complemented shoot growth and biomass phenotypes (Fig. 5J and fig. S8A) or activation of nitrate responsive genes for nitrate transport, assimilation, and transcription in nlp7 (fig. S8B). The data support the role of these residues in NLP7 to function as a dual master transcription activator and nitrate sensor central to transcriptional reprogramming and plant development.
Discussion
Nitrate is an essential nutrient for photosynthetic plant growth in most soils and controls metabolic and developmental processes pivotal to plant vegetative and reproductive development. However, nitrogen fertilizer is energy-expensive to produce and causes pollution; its overuse in agriculture to boost crop yields has led to environmentally disastrous eutrophication worldwide. Global and regional studies indicate declining nitrogen availability on Earth (1–7). Improved plant nitrogen use efficiency could contribute to sustainable agriculture and ecosystem preservation.
Our data identified NLP 2,4,5,6,7,8,9 as transcription factors that initiate signaling for nitrate-mediated transcriptome reprogramming and coordinate transport, metabolism, hormone, signaling, transcription, and root-shoot developmental programs (5–7, 18). With genetic, genomic, cellular, imaging, biochemical, and structural analyses, we discovered that NLP7 has dual regulatory modes as a transcription activator and an intracellular nitrate sensor. Nitrate binding triggers a conformational change and transcriptional de-repression of NLP7, acting simultaneously and synergistically with CPK-dependent NLP7(S205) phosphorylation for nuclear retention (16, 18).
The sequence and structure of the nitrate-binding pocket of NLP7 is evolutionarily conserved and found in bacteria nitrate sensor NreA proteins (34), as well as in other Arabidopsis NLPs and the NLP7 orthologs of land plants and freshwater green algae in Charophytes. We speculate that NLP7 orthologs and other NLPs, but not NINs, in diverse plant species also act as sensor-transcription factors to initiate primary nitrate responses regulating overlapping and unique target genes for a range of physiological functions in the nutrient-regulatory networks (5–8, 18). The functions of NRT1.1(CHL1/NPF6.3) as an extracellular nitrate transceptor and transporter (10–12) seem distinct when plants are grown in soil or NO3− medium. The development of the genetically encoded fluorescent nitrate biosensor sCiNiS enables sensitive and specific monitoring of single-cell nitrate signals in whole seedlings, which can now support real-time live imaging and elucidate how plants dynamically translocate nitrate between tissues or cell types.
Our results reveal a regulatory mechanism that photosynthetic plants use to sense inorganic nitrogen, which then activates plant signaling networks and growth responses. Our insights may suggest avenues through which to enhance nitrogen utilization efficiency in crops, reduce fertilizer and energy inputs, and mitigate climate change from the emission of greenhouse gases in the interest of supporting more sustainable agriculture (1, 5, 6, 35).
Supplementary Material
ACKNOWLEDGMENTS
Our research was inspired by the pioneer works on NLP7 and CHL1 by A. Krapp, Y.F. Tsay and N. Crawford. We thank Jinwoo Shin and Matthew McCormack for their advice on RNA-seq data analysis. Lei Li, Fei Yu, Xiayan Liu and Andrew Diener for comments.
Funding:
Supported by National Natural Science Foundation of China grants 32170270 and 31870227 (K.-H. L.); National Institutes of Health grants GM060493 and GM129093 (J.S). Northwest A&F University for scientific research start-ups Z1090121029 (B.C.); JSPS KAKENHI 22H04977 (S.Y).
Footnotes
Competing interests: The authors declare no competing interests.
Data and materials availability: All data are available in the main text or supplementary materials. The plasmids and the transgenic Arabidopsis seeds generated in this study are available upon request. The RNA-seq raw data generated in this study have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus under accession number GSE198475.
REFERENCES AND NOTES
- 1.Bloom AJ, The increasing importance of distinguishing among plant nitrogen sources. Curr Opin Plant Biol 25, 10–16 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Mason RE et al. , Evidence, causes, and consequences of declining nitrogen availability in terrestrial ecosystems. Science 376, eabh3767 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Stitt M, Nitrate regulation of metabolism and growth. Curr Opin Plant Biol 2, 178–186 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Crawford NM, Forde BG, Molecular and developmental biology of inorganic nitrogen nutrition. The Arabidopsis book / American Society of Plant Biologists 1, e0011 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal EA et al. , Nitrate in 2020: Thirty Years from Transport to Signaling Networks. Plant Cell 32, 2094–2119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YY, Cheng YH, Chen KE, Tsay YF, Nitrate Transport, Signaling, and Use Efficiency. Annu Rev Plant Biol 69, 85–122 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Li L, Liu KH, Sheen J, Dynamic Nutrient Signaling Networks in Plants. Annu Rev Cell Dev Biol 37, 341–367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudinier A et al. , Transcriptional regulation of nitrogen-associated metabolism and growth. Nature 563, 259–264 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Castaings L et al. , The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J 57, 426–435 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Walch-Liu P, Forde BG, Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L-glutamate-induced changes in root architecture. Plant J 54, 820–828 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Ho CH, Lin SH, Hu HC, Tsay YF, CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Xing X, Wang Y, Tran A, Crawford NM, A genetic screen for nitrate regulatory mutants captures the nitrate transporter gene NRT1.1. Plant Physiol 151, 472–478 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi M, Yanagisawa S, Identification of a nitrate-responsive cis-element in the Arabidopsis NIR1 promoter defines the presence of multiple cis-regulatory elements for nitrogen response. Plant J 63, 269–282 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Schauser L, Wieloch W, Stougaard J, Evolution of NIN-like proteins in Arabidopsis, rice, and Lotus japonicus. J Mol Evol 60, 229–237 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Konishi M, Yanagisawa S, Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat Commun 4, 1617 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Marchive C et al. , Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat Commun 4, 1713 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Chardin C, Girin T, Roudier F, Meyer C, Krapp A, The plant RWP-RK transcription factors: key regulators of nitrogen responses and of gametophyte development. J Exp Bot 65, 5577–5587 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Liu KH et al. , Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 545, 311–316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez JM et al. , Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat Commun 11, 1157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konishi M, Okitsu T, Yanagisawa S, Nitrate-responsive NIN-like protein transcription factors perform unique and redundant roles in Arabidopsis. J Exp Bot 72, 5735–5750 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Niemann V et al. , The NreA protein functions as a nitrate receptor in the staphylococcal nitrate regulation system. J Mol Biol 426, 1539–1553 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Canales J, Moyano TC, Villarroel E, Gutierrez RA, Systems analysis of transcriptome data provides new hypotheses about Arabidopsis root response to nitrate treatments. Front Plant Sci 5, 22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W et al. , Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J 78, 70–79 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Liu KH, Huang CY, Tsay YF, CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11, 865–874 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi M, Yanagisawa S, Roles of the transcriptional regulation mediated by the nitrate-responsive cis-element in higher plants. Biochem Biophys Res Commun 411, 708–713 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Suzuki W, Konishi M, Yanagisawa S, The evolutionary events necessary for the emergence of symbiotic nitrogen fixation in legumes may involve a loss of nitrate responsiveness of the NIN transcription factor. Plant Signal Behav 8, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fichtner F, Dissanayake IM, Lacombe B, Barbier F, Sugar and Nitrate Sensing: A Multi-Billion-Year Story. Trends Plant Sci 26, 352–374 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Lundberg JO, Nitrate transport in salivary glands with implications for NO homeostasis. Proc Natl Acad Sci U S A 109, 13144–13145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker JL, Newstead S, Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507, 68–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutsell SQ, Kimple RJ, Siderovski DP, Willard FS, Kimple AJ, High-affinity immobilization of proteins using biotin- and GST-based coupling strategies. Methods Mol Biol 627, 75–90 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mita M et al. , Green Fluorescent Protein-Based Glucose Indicators Report Glucose Dynamics in Living Cells. Anal Chem 91, 4821–4830 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Baek M et al. , Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jumper J et al. , Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unden G, Nilkens S, Singenstreu M, Bacterial sensor kinases using Fe-S cluster binding PAS or GAF domains for O2 sensing. Dalton Trans 42, 3082–3087 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Coskun D, Britto DT, Shi W, Kronzucker HJ, Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat Plants 3, 17074 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


