Abstract
Background:
Haemophilus influenzae serotype a (Hia) can cause invasive disease similar to serotype b; no Hia vaccine is available. We describe the epidemiology of invasive Hia disease in the United States overall and specifically in Alaska during 2008–2017.
Methods:
Active population- and laboratory-based surveillance for invasive Hia disease was conducted through Active Bacterial Core surveillance sites and from Alaska statewide invasive bacterial disease surveillance. Sterile-site isolates were serotyped via slide agglutination or real-time polymerase chain reaction. Incidences in cases per 100,000 were calculated.
Results:
From 2008–2017, an estimated average of 306 invasive Hia disease cases occurred annually in the United States (estimated annual incidence: 0.10); incidence increased by an average of 11.1% annually. Overall, 42.7% of cases were in children aged <5 years (incidence: 0.64), with highest incidence among children aged <1 year (1.60). Case fatality was 7.8% overall and was highest among adults aged ≥65 years (15.1%). Among children aged <5 years, incidence was 17 times higher among American Indians and Alaska Native (AI/AN) children (8.29) than among children of all other races combined (0.49). In Alaska, incidences among all ages (0.68) and among children aged <1 year (24.73) were nearly 6 and 14 times higher, respectively, than corresponding U.S. incidences. Case fatality in Alaska was 10.2%, and the vast majority (93.9%) of cases occurred among AI/AN.
Conclusions:
Incidence of invasive Hia disease has increased since 2008, with the highest burden among AI/AN children. These data can inform prevention strategies, including Hia vaccine development.
Keywords: Haemophilus influenzae, serotype a, invasive disease, surveillance, epidemiology, bacterial infections, American Indian and Alaska Native
Background
Invasive Haemophilus influenzae disease is an important cause of morbidity and mortality in young children and older adults, and in those with certain underlying medical conditions [1–3]. H. influenzae bacteria may be either encapsulated (typeable) or nonencapsulated (nontypeable), with six encapsulated serotypes designated a through f according to their distinct capsular polysaccharides. H. influenzae can cause asymptomatic nasopharyngeal carriage or clinical disease, including meningitis, bacteremia, and pneumonia. The greatest burden of disease occurs at both ends of the life spectrum—in infants and in older adults [1, 2, 4].
After the introduction of H. influenzae serotype b (Hib) vaccines in the 1980s–1990s, the incidence of invasive H. influenzae disease among children aged <5 years decreased by >99% in the United States [5–8]. Following this dramatic reduction, the serotype distribution of invasive H. influenzae cases shifted [9]. Though nontypeable H. influenzae and H. influenzae serotype f now cause the majority of invasive disease in the United States, H. influenzae serotype a (Hia) is of particular concern because incidence increased by an average of 13% annually from 0.02 per 100,000 in 2002 to 0.14 per 100,000 in 2015 [9], and elevated incidence has been reported among children and indigenous populations in the United States and Canada [10–17]. In Alaska, the epidemiology of Hia is distinct from that in the rest of the United States, and multiple invasive Hia disease outbreaks have occurred [10, 17, 18]. Hia can cause disease of similar clinical presentation and severity as Hib [10, 19], and Hib vaccines offer no cross-protection against Hia. We analyzed data from active, population- and laboratory-based surveillance during 2008–2017 to describe the current epidemiology of invasive Hia disease in the United States overall and in Alaska specifically.
Methods
Active Bacterial Core Surveillance and Laboratory Methods
Active, population- and laboratory-based surveillance for invasive H. influenzae disease was conducted as part of Active Bacterial Core surveillance (ABCs) [9, 20]. ABCs is supported by the Centers for Disease Control and Prevention (CDC) as part of the Emerging Infections Program Network [21]. ABCs surveillance areas included California (3 San Francisco Bay area counties, 2008–2017), Colorado (5 Denver area counties, 2008–2017), Connecticut (statewide, 2008–2017), Georgia (20 Atlanta area counties, 2008–2009; statewide, 2010–2017), Maryland (statewide, 2008–2017), Minnesota (statewide, 2008–2017), New Mexico (statewide, 2008–2017), New York (15 Rochester and Albany area counties, 2008–2017), Oregon (statewide, 2008–2017), and Tennessee (11 counties, 2008–2009; 20 counties, 2010–2017). The population under surveillance represented 11.9% and 13.7% of the U.S. population in 2008 and 2017, respectively [22].
A case of invasive Hia disease was defined as isolation of Hia from a normally sterile site (e.g., blood or cerebrospinal fluid [CSF]) in an ABCs surveillance area resident. Epidemiologic and clinical information was abstracted from medical records. Outcome (alive/dead) was based on patient status at discharge. Infants with a gestational age ≤22 weeks were excluded from ABCs.
State public health laboratories serotyped and sent H. influenzae isolates to CDC, where species and serotyping were confirmed for all isolates and whole genome sequencing was performed on select isolates [9, 23–25].
Alaska Surveillance and Laboratory Methods
Statewide laboratory-based surveillance for invasive H. influenzae disease in Alaska was conducted by CDC’s Arctic Investigations Program (AIP) in Anchorage, Alaska [10, 17]. Invasive H. influenzae cases are reportable by clinicians and laboratories to the Alaska Section of Epidemiology (AKSOE). AIP and ASKOE participate in reciprocal notification of cases. Case information is routinely abstracted from medical records using a standardized form. Clinical laboratories send H. influenzae isolates recovered from a normally sterile site in Alaska residents to AIP for laboratory confirmation and characterization. Prior to 2017, isolates received by AIP were confirmed by culture (first by growth on chocolate agar, and then by X,V-factor requirement testing and ALA-Porphyrin testing); serotype was confirmed by slide agglutination. Since 2017, species confirmation and serotyping has been performed by rt-PCR [24, 25].
Statistical Analysis
Data from January 1, 2008 through December 31, 2017 were included in this analysis. Cases of invasive Hia disease were categorized as meningitis if a clinical diagnosis of meningitis was recorded in the medical record and Hia was isolated from CSF or other sterile sites, as bacteremic pneumonia if pneumonia was recorded in the patient’s medical record and Hia was isolated from a blood or pleural fluid, and as isolated bacteremia if Hia was isolated from blood and the patient did not have another clinical syndrome. All other clinical syndromes were classified based upon source of isolate or information noted in the medical record. Clinical syndromes were not considered mutually exclusive in this analysis. Race was categorized as white, black, AI/AN, or Asian/Pacific Islander; however, within the AI/AN category, the distribution of persons who identify as AI vs. AN likely varied between the ABCs and Alaska data. Case-fatality ratios were calculated using the proportion of cases with known outcomes as the denominator. Wilcoxon rank-sum tests were used to compare continuous variables and Pearson’s χ2 test was used for categorical variables.
For the ABCs sites, incidence rates were reported as cases per 100,000 population and calculated using National Center for Health Statistics’ bridged-race postcensal population estimates [22]; nationwide estimates were calculated by directly standardizing to the age and race distribution of the U.S. population. For race-stratified nationwide incidence estimates, missing race was multiply imputed using sequential regression multiple imputation [26] via IVEware software (Institute for Social Research, University of Michigan, Ann Arbor). Variance estimates were calculated using standard combining rules for multiply imputed data. The 95% confidence intervals (CI) around the directly standardized rates were calculated using a method derived from the relationship between the Poisson distribution and the gamma distribution, while estimated age-, race-, and serotype-specific 95% CIs were calculated using exact CI for a Poisson random variable [27]. Theoretically, the projected nationwide estimates include Alaska; however, the epidemiology of Hia in Alaska is distinct from that in the rest of the United States and is therefore presented separately. Incidence in Alaska was calculated using State of Alaska population estimates [28]. Incidence trends over time were assessed using Cochrane-Armitage tests for trend. A negative binomial model with 95% CIs was used to estimate annual percent changes in incidence from 2008–2017. To identify potential secondary cases of Hia, all instances where 2 Hia cases were reported to ABCs from the same county within <60 days were reviewed for epidemiologic links.
This project was reviewed in accordance with CDC human research protection procedures and was determined to be non-research public health surveillance. At each ABCs site, it was deemed either a public health assessment or human subjects research, for which approval was granted by local institutional review boards.
Results
United States Hia Epidemiology
During 2008–2017, 386 cases of invasive Hia disease were reported from ABCs sites; 30 (7.8%) were fatal (Table 1). The median annual incidence among ABCs sites was 0.10 cases per 100,000, with site-specific incidences ranging from 0.01 (Connecticut) to 0.47 (New Mexico). Nationwide, the estimated annual number of cases ranged from 160 in 2008 to 459 in 2017 (estimated annual average: 306), with an estimated average annual national incidence of 0.10 cases per 100,000 (Table 1). Hia incidence increased by an average of 11.1% annually in the United States, from 0.05 in 2008 to 0.14 in 2017 (Figure 1).
Table 1.
Average annual incidence, average annual percent change in incidence, and case-fatality ratios for invasive H. influenzae serotype a disease, by age group—United States and Alaska, 2008–2017
| Age (years) | United States |
Alaska |
||||
|---|---|---|---|---|---|---|
| Estimated incidence (95% CI) | Average annual percent change in incidence (95% CI) | CFR, % | Incidence (95% CI) | Average annual percent change in incidence (95% CI) | CFR, % | |
| <1 | 1.60 (1.46-1.75) | 14.6 (11.4-17.8) | 6.2 | 24.73 (16.30-36.00) | 1.2 (−11.2-15.3) | 7.4 |
| 1-4 | 0.41 (0.36-0.45) | 5.6 (2.8-8.5) | 1.1 | 4.14 (2.45-6.54) | 17.7 (−0.8-39.5) | 11.1 |
| 5-17 | 0.03 (0.03-0.03) | 7.8 (2.0-14.0) | 5.0 | 0.07 (0.00-0.41) | −1.0 (−29.8-39.5)a | 0.0 |
| 18-34 | 0.02 (0.01-0.02) | 16.4 (8.3-25.1) | 14.3 | 0.00 (0.00-0.00) | 0.0 | |
| 35-49 | 0.05 (0.04-0.05) | 4.0 (−0.1-8.3) | 11.1 | 0.07 (0.00-0.41) | 0.0 | |
| 50-64 | 0.10 (0.10-0.11) | 23.4 (19.5-27.3) | 7.9 | 0.07 (0.00-0.41) | 0.0 | |
| ≥65 | 0.14 (0.14-0.15) | 7.9 (5.0-10.9) | 15.1 | 0.15 (0.00-0.85) | 100.0 | |
| Total | 0.10 (0.10-0.10) | 11.1 (9.7-12.6) | 7.8 | 0.68 (0.50-0.89) | 4.9 (−5.0-15.8) | 10.2 |
Abbreviations: CFR, case-fatality ratio; CI, confidence interval.
Note: Incidence presented in cases per 100,000 persons per year.
Due to small numbers, age groups were combined. This average annual percent change in incidence is for persons aged ≥5 years in Alaska.
Figure 1.
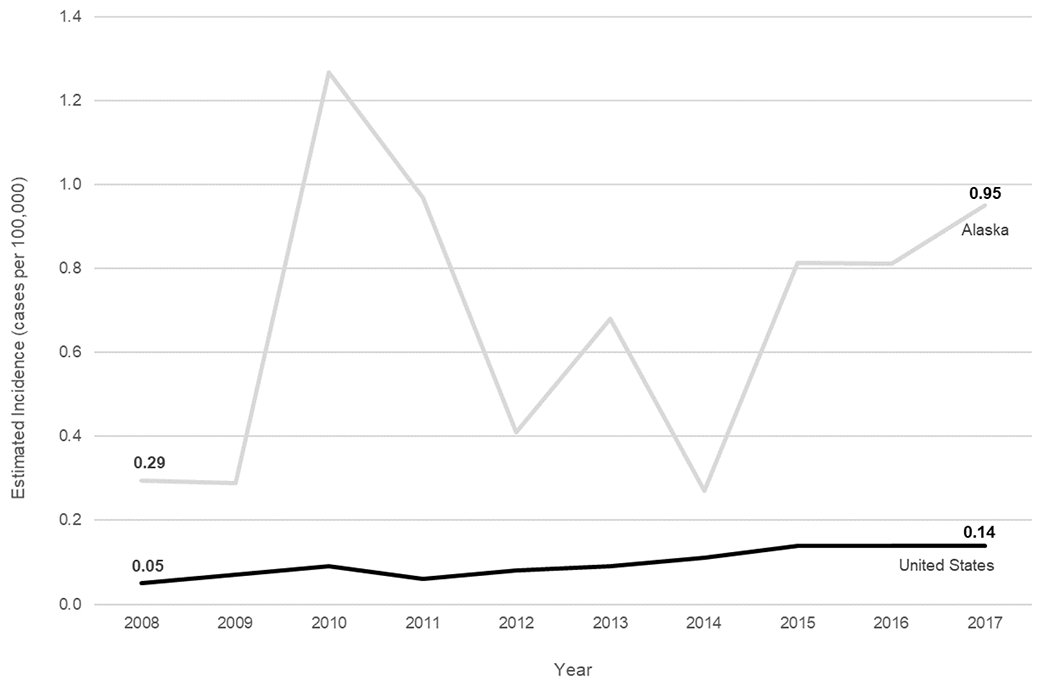
Trends in incidence of invasive H. influenzae serotype a disease in the United States and Alaska, 2008–2017.
Estimated national invasive Hia disease incidence was highest among children aged <1 year (1.60) and lowest among adults aged 18–34 years (0.02) (Table 1). Case-fatality ratio was highest among adults aged ≥65 years (15.1%), with 80.0% of deaths occurring in adults. Overall, exactly half of patients were male; 59.3% were White, 14.8% Black, 14.8% AI/AN, 1.3% Asian/Pacific Islander, and 9.8% of unknown race (Table 2). Incidence was highest in AI/AN populations (1.01), though steady incidence increases were also seen in White and Black populations (Supplemental Figure). Median patient age was 30 years (range: 0–92 years). Information on clinical syndrome was available for 99.7% of cases: 41.3% had bacteremic pneumonia, 32.0% had bacteremia, 23.9% had meningitis, 7.8% had septic arthritis, 4.4% had cellulitis, and 3.9% had epiglottitis (syndromes were not mutually exclusive). The median age of patients with bacteremic pneumonia was 56 years (interquartile range [IQR]: 36–66 years), whereas the median age was 30 years (IQR: 1–62 years) among patients with bacteremia and 0 years (IQR: 0–1 years) among those with meningitis (P<0.0001); all of the patients with epiglottitis were aged >50 years.
Table 2.
Epidemiologic and clinical characteristics of patients with invasive H. influenzae serotype a disease—United States and Alaska, 2008–2017
| Characteristic | United Statesa (N=386) | Alaska (N=49) |
|---|---|---|
| Age, median (range) | 30 years (0–92) | 1 year (0–77) |
| Sex: | ||
| Male | 50.0% | 61.2% |
| Race: | ||
| White | 59.3% | 4.1% |
| Black | 14.8% | 0.0% |
| AI/AN | 14.8% | 93.9% |
| Asian/PI | 1.3% | 2.0% |
| Unknown | 9.8% | 0.0% |
| Ethnicity: | ||
| Hispanic/Latino | 15.0% | N/A |
| Non-Hispanic/Latino | 68.1% | N/A |
| Unknown | 16.8% | N/A |
| Clinical syndromeb | ||
| Bacteremic pneumonia | 41.3% | 18.4% |
| Bacteremia | 32.0% | 12.2% |
| Meningitis | 23.9% | 38.8% |
| Septic arthritis | 7.8% | 16.3% |
| Cellulitis | 4.4% | 12.2% |
| Epiglottitis | 3.9% | 0.0% |
| Other invasive disease | 0.5% | 2.1% |
| Hospitalized | 94.8% | 87.8% |
| Duration of hospitalization, median (range) | 7 days (0–126) | 8 days (0–68) |
| Diedc | 7.8% | 10.2% |
Abbreviations: AI/AN, American Indian and Alaska Native; N/A, not available; PI, Pacific Islander.
Note: data shown in this column are from Active Bacterial Core surveillance sites and do not include all cases in the United States.
Information on clinical syndrome was available for 385/386 (99.7%) cases in the United States and 49/49 (100%) cases in Alaska. Clinical syndrome was not mutually exclusive.
Information on outcome was available for 384/386 (99.5%) cases in the United States and 49/49 (100%) cases in Alaska.
The majority (94.8%) of patients were hospitalized; median duration of hospitalization was 7 days (range: 0–126 days). The duration of hospitalization also varied by syndrome, with a median of 6 days (IQR: 3–10 days) for bacteremic pneumonia, 5 days (IQR: 3–8 days) for bacteremia, and 14 days (IQR: 9–18 days) for meningitis (P<0.0001). Case-fatality ratio did not significantly vary by syndrome.
During 2008–2017, 165 (42.7%) cases of invasive Hia disease in children aged <5 years were reported from ABCs sites, corresponding to an estimated national incidence of 0.64 cases per 100,000: 1.60 among infants aged <1 year, 0.86 among children aged 1 year, 0.25 among children aged 2–4 years (Table 3). Of the 81 (21.0%) infants aged <1 year with invasive Hia disease, 7 (8.6%) were aged <2 months, 5 (6.2%) were 2–<4 months, 22 (27.2%) were 4–<6 months, and 47 (58.0%) were 6–<12 months. Among invasive Hia disease cases in children aged <5 years reported to ABCs, 3.6% were fatal (of which 83.3% were in infants aged 3–11 months and 16.7% were in children aged 1–4 years); clinical syndromes included meningitis (47.9%), bacteremia (32.7%), septic arthritis (13.9%), bacteremic pneumonia (12.1%), and cellulitis (3.0%).
Table 3.
Average annual incidence (95% CI) of H. influenzae serotype a disease, by age and race, among children aged <5 years—United States and Alaska, 2008–2017
|
United States
|
Alaska
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | White | Black | AI/AN | Asian/PI | Total | White | Black | AI/AN | Asian/PI | Total |
| <1 | 1.45 (1.28-1.61) | 1.22 (1.01-1.45) | 17.78 (11.95-23.56) | 0.00 (.00-.00) | 1.60 (1.46-1.75) | 0.00 (.00-.00) | 0.00 (.00-.00) | 82.39 (53.82-120.72) | 8.64 (.22-48.15) | 24.73 (16.3-35.99) |
| 1 | 0.52 (.39-.64) | 1.05 (.76-1.33) | 16.07 (9.18-22.89) | 0.00 (.00-.00) | 0.86 (.72-1.00) | 0.00 (.00-.00) | 0.00 (.00-.00) | 41.96 (22.34-71.75) | 0.00 (.00-.00) | 12.02 (6.40-20.55) |
| 2–4 | 0.18 (.15-.21) | 0.29 (.21-.37) | 3.20 (1.68-4.71) | 0.10 (.03-.16) | 0.25 (.22-.29) | 0.44 (.01-2.46) | 0.00 (.00-.00) | 4.31 (1.18-11.05) | 0.00 (.00-.00) | 1.53 (.50-3.57) |
|
| ||||||||||
| <5 | 0.50 (.45-.54) | 0.64 (.55-.71) | 8.29 (6.76-10.73) | 0.06 (.04-.08) | 0.64 (.60-.69) | 0.27 (.01-1.49) | 0.00 (.00-.00) | 27.74 (20.07-37.40) | 1.70 (0.04-9.45) | 8.27 (6.03-11.07) |
Abbreviations: AI/AN, American Indian and Alaska Native; CI, confidence interval; PI, Pacific Islander.
Note: Incidence presented in cases per 100,000 persons per year. Incidence in the United States is an estimated national incidence.
Alaska Hia Epidemiology
During 2008–2017, 49 cases of invasive Hia disease were reported in Alaska; 5 (10.2%) were fatal, with 4/5 fatalities occurring among children aged <5 years (Tables 1 and 2). In comparison to the United States overall, the incidence of invasive Hia disease in Alaska was nearly 6 times higher among all ages (0.68 cases per 100,000), and 14 times higher among children aged <1 year (24.73) (Figures 1 & 2, Table 1). Incidence increased by an average of 4.9% annually. The vast majority (93.9%) of invasive Hia disease cases in Alaska occurred in AI/AN people. Median patient age in Alaska was much younger than that in the United States overall (1 vs. 30 years, respectively). The most common clinical syndrome observed in Alaska was meningitis (38.8%), followed by bacteremic pneumonia (18.4%), and septic arthritis (16.3%). Similar to the United States overall, most (87.8%) patients were hospitalized; median duration of hospitalization was 8 days (range: 0–68 days).
Figure 2.
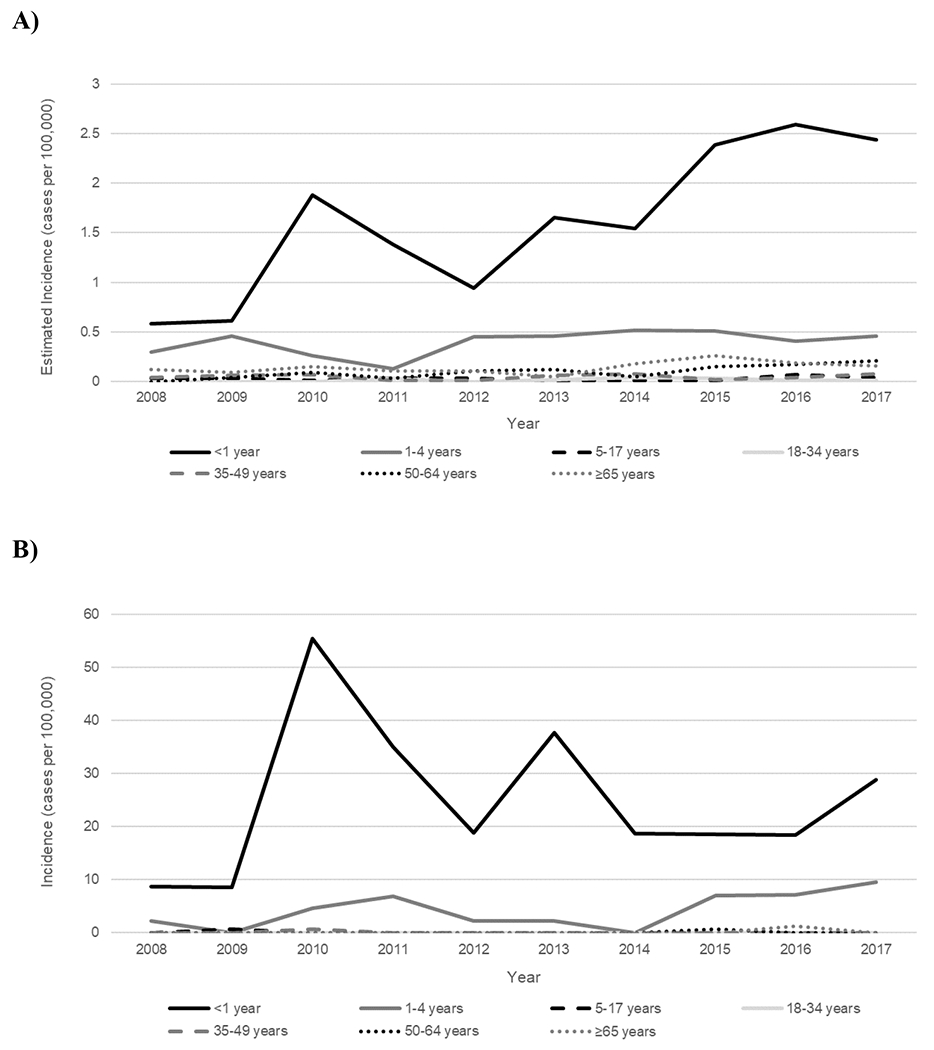
Trends in incidence of invasive H. influenzae serotype a disease, by age group—United States (A) and Alaska (B), 2008–2017.
In Alaska, 45 cases of invasive Hia disease in children aged <5 years were reported during 2008–2017, with an incidence of 8.27: 24.73 among infants aged <1 year, 12.02 among children aged 1 year, and 1.53 among children aged 2–4 years (Table 3). Of the 4 fatal cases in children aged <5 years, 2 were in infants and 2 were in children aged 1–4 years. The age distribution of the 27 Alaskan infants aged <1 year with invasive Hia disease was slightly older than that in the United States overall: 1 (3.7%) was aged <2 months, 3 (11.1%) were 2–<4 months, 4 (14.8%) were 4–<6 months, and 19 (70.4%) were 6–<12 months (Figure 3). All but 1 of the cases in infants aged <1 year were in the AI/AN population.
Figure 3.
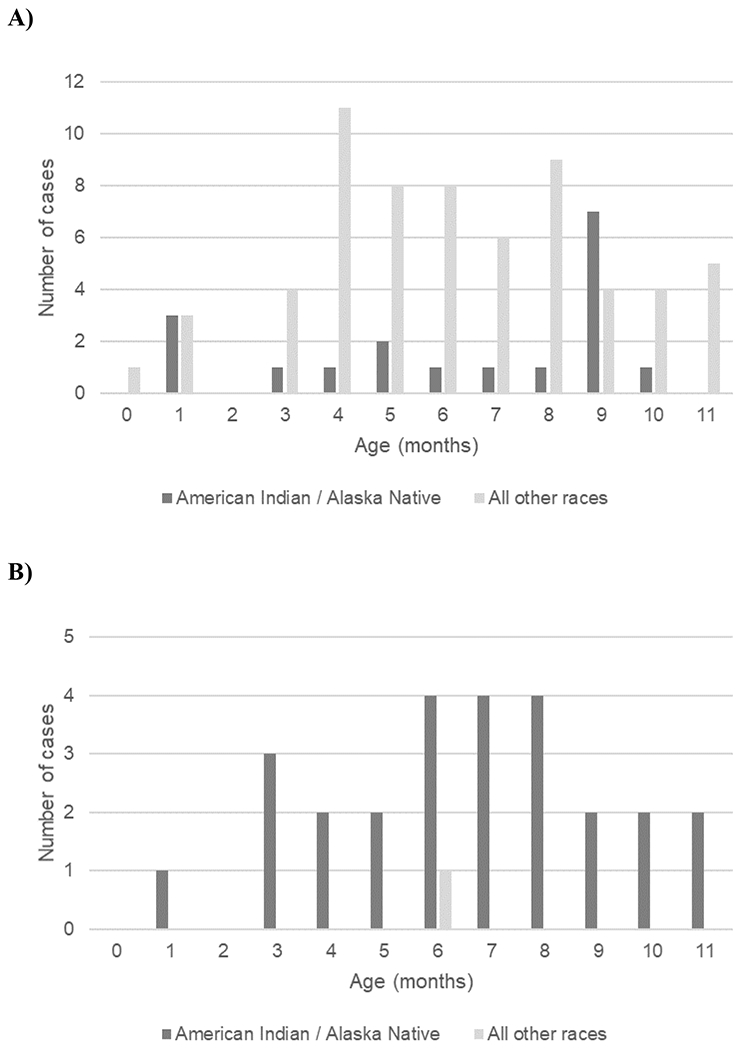
Infants aged <1 year with invasive H. influenzae serotype a disease, by age in months and race—Active Bacterial Core surveillance sites (A) and Alaska (B), 2008–2017.
Hia Epidemiology in American Indian and Alaska Native Children
In the United States overall, the burden of invasive Hia disease among AI/AN children is much greater than that in the general U.S. population, with an estimated incidence of 8.29 per 100,000 among AI/AN children aged <5 years (16.9 times the incidence among all other races combined) and 17.78 among AI/AN infants aged <1 year (13.9 times the incidence among all other races combined) (Table 4). These incidence rate ratios are even more pronounced in Alaska, with an incidence of 27.74 among AI/AN children aged <5 years (54.4 times the incidence among all other races combined) and 82.39 among AI/AN infants aged <1 year (63.9 times the incidence among all other races combined). Stratifying by both age (<5 vs. ≥5 years) and race (AI/AN vs. all other races), incidence of invasive Hia disease was markedly higher among AI/AN children aged <5 years than among all other age-race subgroups, both the United States overall and in Alaska (Figure 4).
Table 4.
Average annual incidencea and incidence rate ratios for H. influenzae serotype a disease among American Indian and Alaska Native persons compared to persons of all other races—United States and Alaska, 2008–2017
|
United States
|
Alaska
|
|||||
|---|---|---|---|---|---|---|
| Estimated Cases | Estimated Incidence | Incidence Rate Ratio | Cases | Incidence | Incidence Rate Ratio | |
| All ages | ||||||
| AI/AN | 433 | 1.01 | 11.2 | 46 | 3.22 | 4.7 |
| All other races | 2,700 | 0.09 | Ref | 3 | 0.68 | Ref |
|
| ||||||
| Children aged <5 years | ||||||
| AI/AN | 313 | 8.29 | 16.9 | 43 | 27.74 | 54.4 |
| All other races | 968 | 0.49 | Ref | 2 | 0.51 | Ref |
|
| ||||||
| Infants aged <1 year | ||||||
| AI/AN | 136 | 17.78 | 13.9 | 26 | 82.39 | 63.9 |
| All other races | 504 | 1.28 | Ref | 1 | 1.29 | Ref |
Abbreviations: AI/AN, American Indian and Alaska Native; CI, confidence interval.
Note: Incidence presented in cases per 100,000 population.
Figure 4.
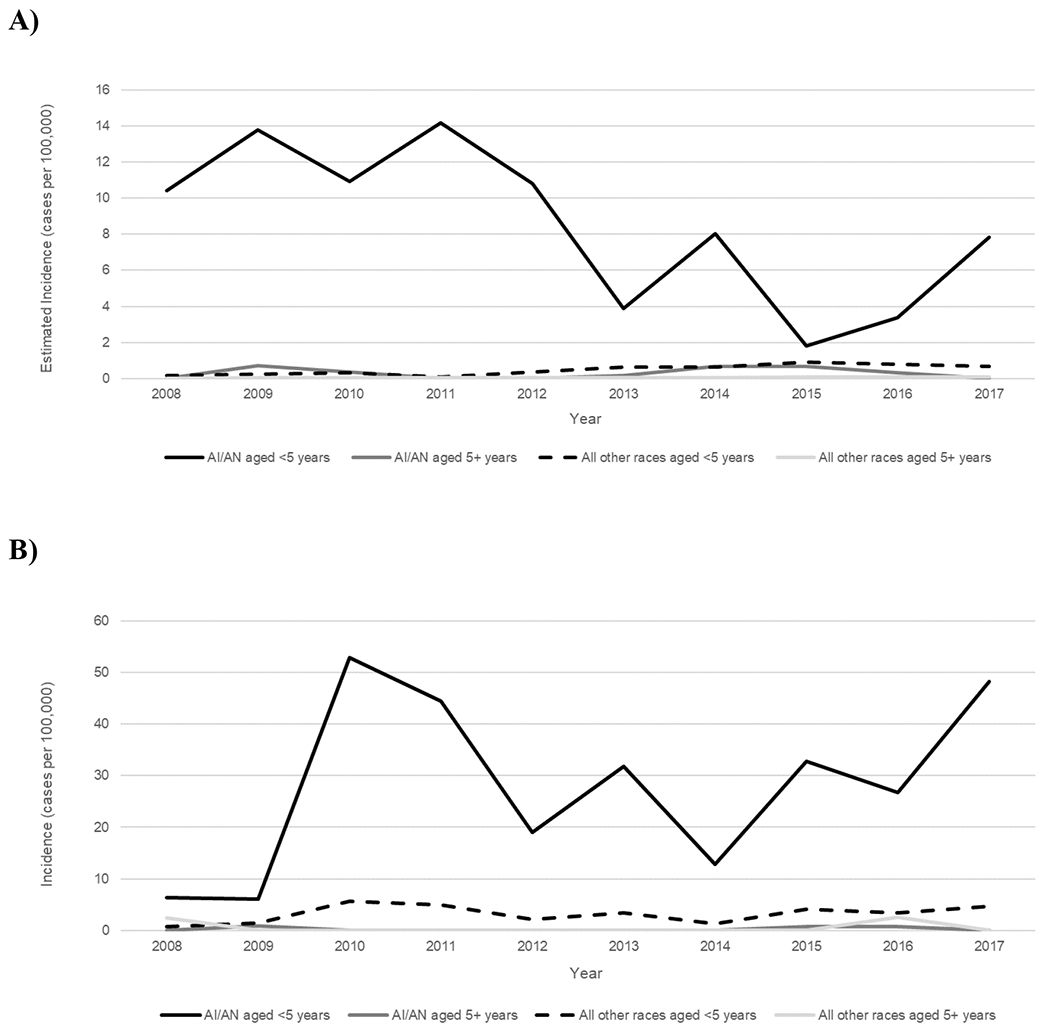
Trends in incidence of invasive H. influenzae serotype a disease, by age group and race—United States (A) and Alaska (B), 2008–2017.
Potential Secondary Case of Hia
Only one potential secondary case was identified in ABCs or Alaska data. In Georgia, a 15-year-old male and his 56-year-old mother, neither of whom had underlying conditions, became ill several days apart and were diagnosed with Hia bacteremic pneumonia. Isolates from both patients were found to be sequence type 56, with only 1 single nucleotide polymorphism difference between them.
Discussion
Although the incidence of invasive Hia disease was 5-fold higher in Alaska than in the United States overall, in both settings, incidence increased from 2008–2017 and was highest among AI/AN children aged <5 years. In total, an estimated 11% of reported invasive Hia disease cases occurred in AI/AN children aged <5 years, who experienced a disease incidence 17 and 54 times that of children aged <5 years of all other races in the United States and Alaska, respectively.
Other recent reports from the United States have highlighted Hia as a pathogen of increasing importance [11, 29]. In 2018, an Hia outbreak involving 4 children aged <2 years occurred in a remote Alaskan village. In response, chemoprophylaxis was administered to village residents aged <10 years and a community-wide carriage study was conducted. A similar Alaskan village outbreak was reported in 2003 [18]. Moreover, Hia hospitalization rates have increased in Texas and Utah in recent years, with Hia as the predominant cause of H. influenzae meningitis [11, 16].
Most reports of Hia have been from North America and Brazil [9, 30, 31]; surveillance elsewhere in the world has rarely detected invasive Hia disease [32–34]. However, reporting of H. influenzae in many locations is still limited to Hib vs. non-b, with no serotyping capacity for Hia. Therefore, the true geographic range and disease burden of Hia globally is unknown.
Numerous reports have shown that Hia can cause disease with clinical severity similar to Hib [10, 11, 19], perhaps influenced by the close similarity between the Hia and Hib capsular polysaccharides [32, 35]. A chart review of a subset of ABCs Hia cases from 2011–2015 found evidence of severe disease, and that children aged <1 year had the highest proportion of cases with both short- and long-term adverse clinical outcomes, including hearing loss, developmental delay, and speech delay [19]. In the present analysis, the case-fatality ratio was higher in Alaska than in ABCs, possibly because a higher proportion of cases presented with meningitis and occurred among young children living in remote locations. Additionally, we showed a notable proportion of Hia cases presenting with septic arthritis (7.7% in ABCs, 16.3% in Alaska), mainly among children aged <5 years. Hia septic arthritis has been previously reported [11, 36, 37], but is not a clinical presentation historically associated with invasive H. influenzae disease.
To prevent secondary cases of invasive Hib disease, the Advisory Committee on Immunization Practices (ACIP) recommends antibiotic chemoprophylaxis for household and daycare contacts of cases with specific circumstances [38]. However, the ACIP recommendations also state that “chemoprophylaxis is not recommended for contacts of persons with invasive disease caused by nontype b H. influenzae because cases of secondary transmission of disease have not been documented” [38]. The mother-son pair of Hia cases reported here, as well as two genetically-indistinguishable Hia cases reported within the same daycare center in Texas [11] represent an important documentation of potential secondary transmission of a non-b serotype and may indicate a need to revisit chemoprophylaxis recommendations. In fact, the 2018 Red Book updated language around chemoprophylaxis to state that “clinicians may consider prophylaxis of contacts of index cases of Hia invasive disease, using the same criteria as that recommended … for Hib disease” [39]. Following an Hia village outbreak in Alaska in 2018, Alaska issued a bulletin indicating that “chemoprophylaxis should be considered” for certain contacts of patients with invasive Hia [40]. To note, as serotyping is often performed at a state or reference public health laboratory, results are often not available in time to inform serotype-specific chemoprophylaxis interventions.
Given the recent increases in Hia incidence, clear burden of disease among AI/AN children, clinical disease severity similar to Hib [19], and lack of cross-protection of Hib vaccines against Hia, improved Hia disease prevention measures are needed. The National Research Council of Canada and the Public Health Agency of Canada recently developed a vaccine with the Hia capsular polysaccharide conjugated to a CRM protein [41, 42]. Proof of concept has been established, as all tested Hia strains were killed by conjugated vaccine-derived sera. There are ongoing efforts to move this vaccine candidate towards phase 1 clinical trials, with the hopes that the vaccine could become available to high-risk populations at a reasonable cost. Because 44% of AI/AN infants with invasive Hia disease were aged 2–6 months, any Hia vaccine would need careful consideration of which carrier protein would be needed to provide early protection (i.e., after 1 dose). Though there are acknowledged challenges to license and introduce a Hia vaccine for a relatively small sub-population, increasing incidence in non-AI/AN populations suggest that a Hia vaccine could potentially have broader appeal by the time it completes the licensure pathway.
Incidence of invasive Hia disease is increasing in the United States overall and Alaska in particular, with the highest burden among AI/AN infants and children. In the context of increasing Hia incidence and clinical severity similar to Hib, new prevention strategies, including development of an Hia vaccine, could prevent morbidity and mortality among these vulnerable populations. Additional questions remain regarding the frequency with which secondary Hia transmission occurs, the potential need for updated chemoprophylaxis recommendations, and, appropriate target populations for a potential Hia vaccine.
Supplementary Material
Key points:
Invasive H. influenzae serotype a disease has increased, with the highest incidence among American Indian and Alaska Native children.
Acknowledgments
The authors are grateful to the following individuals for their contributions to the establishment and maintenance of the ABCs system. California Emerging Infections Program: Susan Brooks and Hallie Randel. Colorado Emerging Infections Program: Benjamin White, Deborah Aragon, Meghan Barnes, and Jennifer Sadlowski. Connecticut Department of Public Health: Matt Cartter, Carmen Marquez, and Michelle Wilson. Georgia Emerging Infections Program: Stephanie Thomas, Amy Tunali, Wendy Baughman, Ashley Moore, Lauren Lorentzson, and Melissa Tobin-D’Angelo. Maryland Emerging Infections Program: Joanne Benton, Terresa Carter, Rosemary Hollick, Kim Holmes, and Andrea Riner. Minnesota Emerging Infections Program: Kathryn Como-Sabetti, Lori Triden, Corinne Holtzman, Richard Danila, and Kerry MacInnes. New Mexico Emerging Infections Program: Kathy Angeles, Joseph Bareta, Lisa Butler, Sarah Khanlian, Robert Mansmann, and Megin Nichols. New York Emerging Infections Program: Kari Burlaff, Suzanne McGuire, Glenda Smith, Nancy Spina, and Rachel Wester. Oregon Emerging Infections Program: Mark Schmidt, Jamie Thompson, and Tasha Poissant. Tennessee Emerging Infections Program: Brenda Barnes, Karen Leib, Katie Dyer, Tiffanie Markus, and Lura McKnight. Arctic Investigations Program, CDC: Debby Hurlburt, Danielle Lecy, Gail Thompson, Sara Seeman, Alisa Reasonover, and Carolynn Debyle. Division of Bacterial Diseases, CDC: Melissa Arvay, Olivia Almendares, Huong Pham, the Bacterial Meningitis Laboratory.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding
This work was supported by a cooperative agreement with the Emerging Infections Program of the Centers for Disease Control and Prevention (CDC-RFA-CK17-1701).
Footnotes
Potential conflicts of interest
Lee Harrison has served as a consultant to GSK, Merck, Pfizer, and Sanofi Pasteur. William Schaffner has served as a consultant to Roche Diagnostics and a member of data safety monitoring boards for Merck and Pfizer. Ruth Lynfield served as a co-editor on a book on Infectious Disease Surveillance, Public Health and Preventive Medicine and American Academy of Pediatrics Committee on Infectious Disease, with all proceeds donated to the Minnesota Department of Health. All other authors: No reported conflicts.
References
- 1.MacNeil JR, Cohn AC, Farley M, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease--United States, 1989–2008. Clin Infect Dis 2011; 53(12): 1230–6. [DOI] [PubMed] [Google Scholar]
- 2.Blain A, MacNeil J, Wang X, et al. Invasive Haemophilus influenzae Disease in Adults ≥65 Years, United States, 2011. Open Forum Infect Dis 2014; 1(2): ofu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briere EC, Jackson M, Shah SG, et al. Haemophilus influenzae type b disease and vaccine booster dose deferral, United States, 1998–2009. Pediatrics 2012; 130(3): 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livorsi DJ, Macneil JR, Cohn AC, et al. Invasive Haemophilus influenzae in the United States, 1999–2008: epidemiology and outcomes. J Infect 2012; 65(6): 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams WG, Deaver KA, Cochi SL, et al. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA 1993; 269(2): 221–6. [PubMed] [Google Scholar]
- 6.Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis 1998; 4(2): 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease C, Prevention. Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children--United States, 1998–2000. MMWR Morb mortal wkly rep 2002; 51(11): 234–7. [PubMed] [Google Scholar]
- 8.Centers for Disease C, Prevention. Progress toward elimination of Haemophilus influenzae type b disease among infants and children--United States, 1987–1995. MMWR Morb mortal wkly rep 1996; 45(42): 901–6. [PubMed] [Google Scholar]
- 9.Soeters HM, Blain A, Pondo T, et al. Current Epidemiology and Trends in Invasive Haemophilus influenzae Disease-United States, 2009–2015. Clin Infect Dis 2018; 67(6): 881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plumb ID, Lecy KD, Singleton R, et al. Invasive Haemophilus influenzae Serotype a Infection in Children: Clinical Description of an Emerging Pathogen-Alaska, 2002–2014. Ped Infect Dis J 2018; 37(4): 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallejo JG, McNeil JC, Hulten KG, Sommer LM, Dunn JJ, Kaplan SL. Invasive Haemophilus influenzae Disease at Texas Children’s Hospital, 2011 to 2018. The Ped Infect Dis J 2019; 38(9): 900–5. [DOI] [PubMed] [Google Scholar]
- 12.Tsang RS, Proulx JF, Hayden K, et al. Characteristics of invasive Haemophilus influenzae serotype a (Hia) from Nunavik, Canada and comparison with Hia strains in other North American Arctic regions. Int J Infect Dis 2017; 57: 104–7. [DOI] [PubMed] [Google Scholar]
- 13.Tsang RS, Li YA, Mullen A, et al. Laboratory characterization of invasive Haemophilus influenzae isolates from Nunavut, Canada, 2000–2012. Int J Circumpolar Health 2016; 75: 29798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruce MG, Deeks SL, Zulz T, et al. Epidemiology of Haemophilus influenzae serotype a, North American Arctic, 2000–2005. Emerg Infect Dis 2008; 14(1): 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millar EV, O’Brien KL, Watt JP, et al. Epidemiology of invasive Haemophilus influenzae type A disease among Navajo and White Mountain Apache children, 1988–2003. Clin Infect Dis 2005; 40(6): 823–30. [DOI] [PubMed] [Google Scholar]
- 16.Bender JM, Cox CM, Mottice S, et al. Invasive Haemophilus influenzae disease in Utah children: an 11-year population-based study in the era of conjugate vaccine. Clin Infect Dis 2010; 50(7): e41–6. [DOI] [PubMed] [Google Scholar]
- 17.Bruce MG, Zulz T, DeByle C, et al. Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983–2011. Emerg Infect Dis 2013; 19(6): 932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammitt LL, Block S, Hennessy TW, et al. Outbreak of Invasive Haemophilus influenzae Serotype a Disease. Ped Infect Dis J 2005; 24(5): 453–6. [DOI] [PubMed] [Google Scholar]
- 19.Bozio CH. Clinical severity and sequelae of invasive Haemophilus influenzae serotype a cases — United States, 2011–2015. 2019. [submitted] [DOI] [PubMed] [Google Scholar]
- 20.Langley G, Schaffner W, Farley MM, et al. Twenty Years of Active Bacterial Core Surveillance. Emerg Infect Dis 2015; 21(9): 1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynfield R, Schaffner W. Emerging Infections Program−-20 Years of Achievements and Future Prospects. Emerg Infect Dis 2015; 21(9): 1497–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. Bridged-Race Population Estimates, United States. Available at: https://wonder.cdc.gov/bridged-race-population.html. Accessed July 16, 2019.
- 23.Potts CC, Topaz N, Rodriguez-Rivera LD, et al. Genomic characterization of Haemophilus influenzae: a focus on the capsule locus. BMC Genomics 2019; 20(1): 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Mair R, Hatcher C, et al. Detection of bacterial pathogens in Mongolia meningitis surveillance with a new real-time PCR assay to detect Haemophilus influenzae. Int J Med Microbiol 2011; 301(4): 303–9. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae, 2nd edn. Geneva: World Health Organization, 2011. [Google Scholar]
- 26.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology 2001; 27(1): 85–95. [Google Scholar]
- 27.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997; 16(7): 791–801. [DOI] [PubMed] [Google Scholar]
- 28.State of Alaska Department of Labor and Workforce Development. Alaska population estimates. Available at: http://live.laborstats.alaska.gov/pop/index.cfm. Accessed December 13.
- 29.Konduri A, Wolf A, Boppana SB. Invasive Disease Caused by Haemophilus influenzae Type A. Clin Ped 2019; 58(4): 470–3. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro GS, Lima JB, Reis JN, et al. Haemophilus influenzae meningitis 5 years after introduction of the Haemophilus influenzae type b conjugate vaccine in Brazil. Vaccine 2007; 25(22): 4420–8. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro GS, Reis JN, Cordeiro SM, et al. Prevention of Haemophilus influenzae type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of Hib immunization in Brazil. J Infect Dis 2003; 187(1): 109–16. [DOI] [PubMed] [Google Scholar]
- 32.Ulanova M, Tsang RSW. Haemophilus influenzae serotype a as a cause of serious invasive infections. Lancet Infect Dis 2014; 14(1): 70–82. [DOI] [PubMed] [Google Scholar]
- 33.Whittaker R, Economopoulou A, Dias JG, et al. Epidemiology of Invasive Haemophilus influenzae Disease, Europe, 2007–2014. Emerg Infect Dis 2017; 23(3): 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suga S, Ishiwada N, Sasaki Y, et al. A nationwide population-based surveillance of invasive Haemophilus influenzae diseases in children after the introduction of the Haemophilus influenzae type b vaccine in Japan. Vaccine 2018; 36(38): 5678–84. [DOI] [PubMed] [Google Scholar]
- 35.Kapogiannis BG, Satola S, Keyserling HL, Farley MM. Invasive infections with Haemophilus influenzae serotype a containing an IS1016-bexA partial deletion: possible association with virulence. Clin Infect Dis 2005; 41(11): e97–103. [DOI] [PubMed] [Google Scholar]
- 36.Pavlik DF, Johnston JJ, Eldredge JD, Dehority W. Non-Type b Haemophilus influenzae Septic Arthritis in Children. J Pediatric Infect Dis Soc 2017; 6(3): e134–e9. [DOI] [PubMed] [Google Scholar]
- 37.Albrecht T, Poss K, Issaranggoon Na Ayuthaya S, Triden L, Schleiss KL, Schleiss MR. Case report of congenital asplenia presenting with Haemophilus influenzae type a (Hia) sepsis: an emerging pediatric infection in Minnesota. BMC Infect Dis 2019; 19(1): 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briere EC, Rubin L, Moro PL, et al. Prevention and control of Haemophilus influenzae type b disease: recommendations of the advisory committee on immunization practices (ACIP). MMWR Morb mortal wkly rep 2014; 63(RR-01): 1–14. [PubMed] [Google Scholar]
- 39.Committee on Infectious Diseases, American Academy of Pediatrics, Kimberlin DW, Brady MT, Jackson MA, Long SS. Haemophilus influenzae Infections. Red Book 2018: American Academy of Pediatrics, 2018:367–75. [Google Scholar]
- 40.Zulz T, Bruce M, Singleton R, Massay S, Tiffany A. Update on Haemophilus influenzae Type a Invasive Disease — Alaska, 2014–2018. State of Alaska Epidemiology Bulletin 2018; 9. [Google Scholar]
- 41.Cox AD, Barreto L, Ulanova M, Bruce MG, Tsang RSW. Developing a vaccine for Haemophilus influenzae serotype a: Proceedings of a workshop. Can Commun Dis Rep 2017; 43(5): 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barreto L, Cox AD, Ulanova M, Bruce MG, Tsang RSW. The emerging Haemophilus influenzae serotype a infection and a potential vaccine: Implementation science in action. Can Commun Dis Rep 2017; 43(3): 85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


