Abstract
The aim of this study was to determine whether the antibacterial activity of chitosan-modified Fe3O4 (CS@Fe3O4) nanomaterials against Acinetobacter baumannii (A. baumannii) is mediated through changes in biofilm formation and reactive oxygen species (ROS) production. For this purpose, the broth dilution method was used to examine the effect of CS@Fe3O4 nanoparticles on bacterial growth. The effects of CS@Fe3O4 nanoparticles on biofilm formation were measured using a semi-quantitative crystal violet staining assay. In addition, a bacterial ROS detection kit was used to detect the production of ROS in bacteria. The results showed that CS@Fe3O4 nanoparticles had a significant inhibitory effect on the colony growth and biofilm formation of drug-resistant A. baumannii (p < 0.05). The ROS stress assay revealed significantly higher ROS levels in A. baumannii subjected to CS@Fe3O4 nanoparticle treatment than the control group (p < 0.05). Thus, we demonstrated for the first time that CS@Fe3O4 nanoparticles had an inhibitory effect on A. baumannii in vitro, and that the antibacterial effect of CS@Fe3O4 nanoparticles on drug-resistant A. baumannii was more significant than on drug-sensitive bacteria. Our findings suggest that the antibacterial mechanism of CS@Fe3O4 nanoparticles is mediated through inhibition of biofilm formation in drug-resistant bacteria, as well as stimulation of A. baumannii to produce ROS. In summary, our data indicate that CS@Fe3O4 nanoparticles could be used to treat infections caused by drug-resistant A. baumannii.
Keywords: Nanozyme, antibacterial activity, Acinetobacter baumannii
Introduction
Nanozymes, nanomaterials with enzyme-like catalytic activity, are a new generation of artificial enzyme that can catalyze the substrates of biological enzymes under near physiological conditions. Their catalytic behavior, reaction kinetics and catalytic mechanisms are similar to those of natural enzymes. In 2019, Jiao et al. [1] at the Chinese Academy of Sciences first reported that Fe3O4 magnetic nanoparticles exhibited mimetic enzyme activities similar to those of natural peroxidases. Since then, more than 300 types of nanomaterials have been found to have enzymatic activity. Compared with natural enzymes and traditional mimic enzymes, nanozymes have high catalytic efficiency, multiple functions, good stability, low cost and easy large-scale preparation [2]. In the biomedical field, nanozymes can be used in clinical detection, tumor diagnosis and treatment, cell protection, anti-aging, and other important areas related to human health [3].
The Fe3O4 nanozyme is a typical metal oxide nanozyme. Fe3O4 nanoenzymes have the same catalytic characteristics as horseradish peroxidase (HRP), and can catalyze H2O2 to destroy biofilm matrices [5]. However, due to their toxic effect on cells, subsequent studies have developed various modification methods to reduce their cytotoxicity. Chitosan nanoparticles can be applied to deliver antimicrobial drugs, which further enhances the efficiency and stability of the antimicrobial agent [6]. The proposed antibacterial mechanism of chitosan under acidic conditions involves the binding of the negatively charged bacteria to the protonated amino group on the chitosan molecular chain, leading to disruption of the cell, and subsequent inhibition of bacterial growth and reproduction [4]. Tian et al. [7] demonstrated that the chitosan-modified Fe3O4 (CS@Fe3O4) nanozyme had lower cytotoxicity than the sodium oleate-modified Fe3O4 nanozyme, thereby leading to the clinical application of CS@Fe3O4 nanozymes.
Acinetobacter baumannii (A. baumannii) is a gram-negative bacilli mainly associated with community-acquired pneumonia and hospital infection. A. baumannii can secrete many virulence factors, which are related to its pathogenicity and drug resistance [8]. In addition, the extracellular matrix secreted by A. baumannii can adhere to the body, and most A. baumannii can form biofilms, which further enhance its pathogenicity [9]. A. baumannii infection leads to prolonged disease and high mortality. In addition, most A. baumannii strains have now developed resistance to a variety of drugs, including quinolones, aminoglycosides, broad-spectrum cephalosporins and other common clinical antibiotics [10]. In 2014, China's Ministry of Health issued technical guidelines for the prevention and control of hospital infections caused by multidrug-resistant bacteria, clearly defining multidrug-resistant bacteria as bacteria that are resistant to three or more types of antibiotics in clinical use at the same time. In recent years, the increasing occurrence of multidrug-resistant A. baumannii has had a serious impact on human health. Indeed, A. baumannii has become one of the most intractable pathogens in global healthcare institutions [11].
Here, CS@Fe3O4 nanozymes were used in in vitro antibacterial experiments to determine their effects on A. baumannii biofilms, as well as their impact on reactive oxygen species (ROS) levels in A. baumannii.
Materials and Methods
Bacteriostasis Assay Using the Broth Dilution Method
A 500 μg/ml working solution of CS@Fe3O4 nanoparticles (Ruixi Biotechnology, China) was prepared in double-distilled water. An A. baumannii colony was diluted in 4 ml Mueller-Hinton broth medium to prepare a bacterial suspension working solution (1 × 107 CFU/ml, OD 600 nm = 0.06) (Huankai Microbial Technology, China). The CS@Fe3O4 nanoparticles were added to the bacterial suspension (final concentration of 125 μg/ml), and the final working solution was placed in 96-well plates and cultured for 24 h. In the control group, double-distilled water replaced the CS@Fe3O4 nanoparticles.
Semi-Quantitative Crystal Violet Staining Assay
Biofilm formation was measured after 24 and 48 h, as described previously [12, 13]. An A. baumannii suspension (1 × 107 CFU/ml, OD 600 nm = 0.06) was prepared. CS@Fe3O4 nanoparticles were diluted to a concentration of 500 μg/ml. In the experimental group, the CS@Fe3O4 nanoparticle working solution (50 μl) and bacterial suspension (150 μl) were added to 96-well plates. In the control group, the CS@Fe3O4 nanoparticle working solution was replaced with double-distilled water. After incubation for 24 h, the culture medium was removed, and each well was washed three times with PBS. The biofilms were then fixed with methanol (200 μl) for 15 min, air-dried, and stained with 2% crystal violet solution (200 μl) for 5 min at room temperature. After the excess stain was removed, samples were washed three times with PBS and air-dried at room temperature. Adherent crystal violet was dissolved in 95% alcohol (200 μl) and the plates were shaken for 20 min. The optical density (OD) was measured at 570 nm using a microplate reader (Biotek, USA).
Microscopic Analysis of Crystal Violet Staining
CS@Fe3O4 nanoparticle working solution (125 μl) and bacterial suspension (375 μl) were added to 24-well plates containing a clean sterile glass cover slip. In the control group, the CS@Fe3O4 nanoparticle working solution was replaced with double-distilled water. After incubation for 24 and 48 h, the glass cover slips were gently rinsed with PBS to remove non-adherent bacteria and then stained with 2% crystal violet for 30 min. Slides were washed with water to remove excess crystal violet and then visualized using an Olympus CKX41 microscope (Japan).
ROS Stress Test
The ROS stress test was carried out as described previously [14] using a bacterial ROS fluorescence detection kit (Beibo Biotechnology, China). The experimental group was treated with a CS@Fe3O4 nanoparticle working solution (500 μg/ml) prepared using double-distilled water. The control group was treated with double-distilled water (200 μl). The fluorescence intensity (excitation light was 488 nm, emission light was 530 nm) was measured with a fluorescence enzyme reader (Biotek). After the absorbance had been measured, the bacterial solution was centrifuged at 12,000 ×g for 20 min, then placed on a glass slide and observed using an Olympus fluorescence microscope BX43.
Statistical Methods
The paired t-test was used to analyze the biofilm inhibition and ROS stimulation data. The Wilcoxon nonparametric test was used to analyze the 24 h data. All statistical analyses were performed using SPSS version 20.0 (IBM), and all figures were generated using GraphPad Prism 5.01 (GraphPad Software). p < 0.05 was considered statistically significant.
Results
CS@Fe3O4 Nanoparticles Inhibit A. baumannii
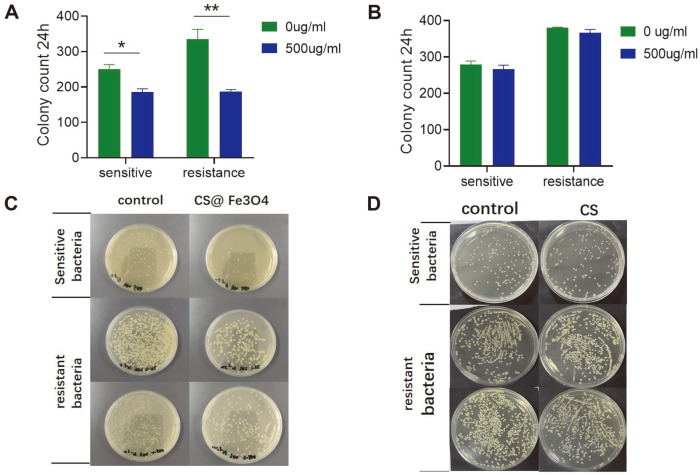
Based on our drug sensitivity test, drug-sensitive and drug-resistant bacteria were selected (Table S1). Next, we performed in vitro bacteriostatic experiments and found that CS@Fe3O4 nanoparticles had a significant inhibitory effect on A. baumannii compared with the control group in both drug-sensitive (p < 0.05) and drug-resistant (p < 0.01) bacteria (Fig. 1A). The number of colonies growing in the experimental group was significantly less than in the control group (Fig. 1C). Importantly, chitosan alone (at the same concentration) had no antibacterial effect on the number of colonies. (p > 0.05) (Fig. 1B), It also had no effect on the colony formation of A. baumannii (Fig. 1D).
Fig. 1. The inhibition rate of CS@Fe3O4 nanoparticles (A) and chitosan (B) on A. baumannii was detected by the colony formation assay. Data are shown as the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01. The broth dilution method was used to determine the inhibition rate of CS@Fe3O4 nanoparticles (C) and chitosan (D) on A. baumannii.
CS@Fe3O4 Nanoparticles Inhibit the Biofilm Formation of Drug-Resistant A. baumannii
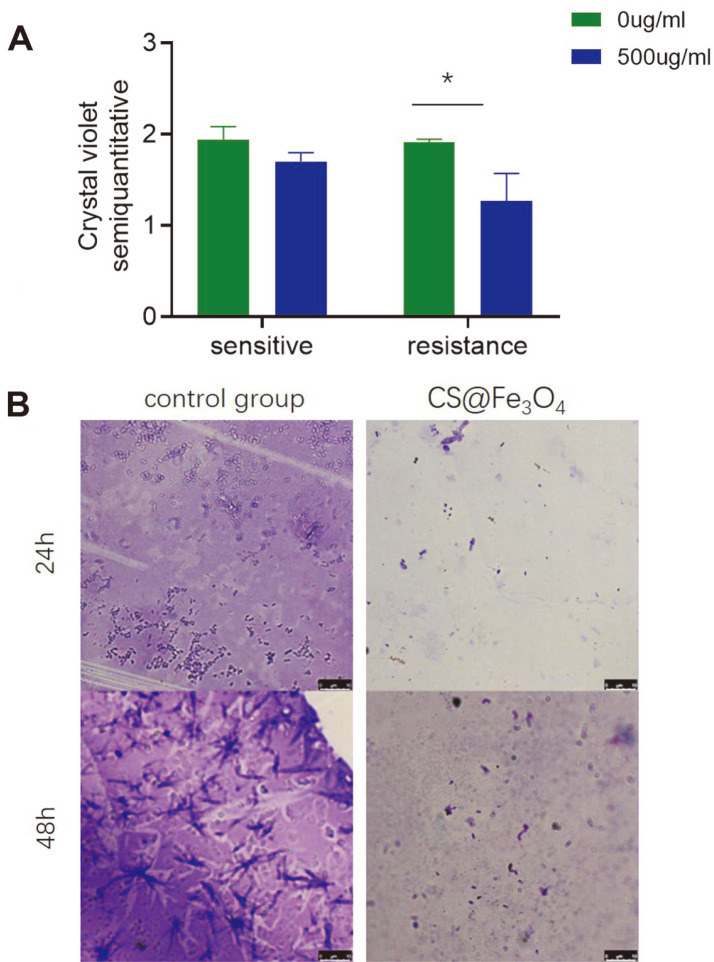
The crystal violet quantitative assay revealed that CS@Fe3O4 nanoparticles had an inhibitory effect on the biofilm formation of drug-resistant A. baumannii (p < 0.05), but not drug-sensitive bacteria (Fig. 2A). Microscopic examination of the biofilms revealed that the biofilm of the control group was large, dense and dark purple, whereas the biofilm of the experimental group was significantly smaller with light purple staining (Fig. 2B).
Fig. 2. A. The semi-quantitative crystal violet staining assay was used to determine the amount of bacterial biofilm.
OD values are given as the mean ± SD of three independent experiments. * p < 0.05. B. Microscopic examination of the effects of CS@Fe3O4 nanoparticles on biofilm formation were observed by crystal violet staining after 24 and 48 h.
CS@Fe3O4 Nanoparticles Stimulate A. baumannii to Produce ROS
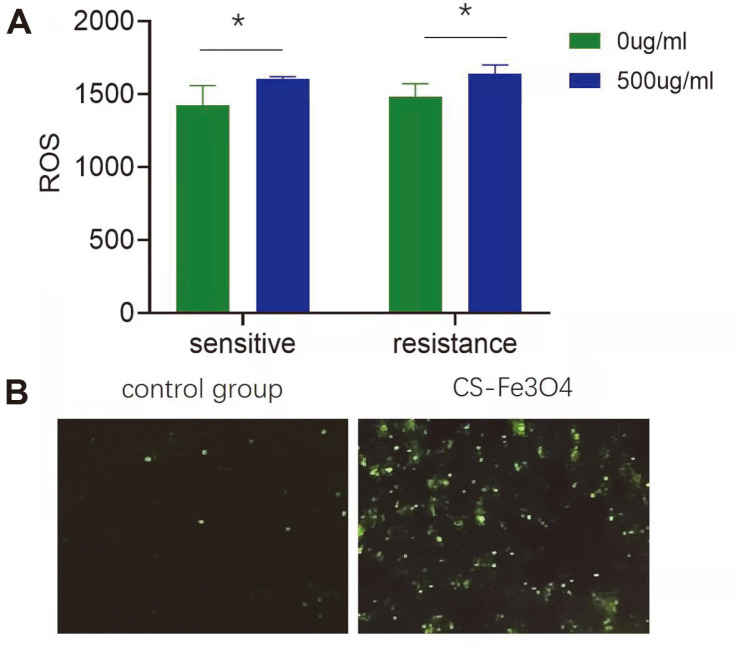
A bacterial ROS kit was used to quantify ROS production in the bacteria. ROS levels were significantly increased in the experimental group for both drug-sensitive and drug-resistant (p < 0.05) bacteria (Fig. 3A). Microscopic examination revealed an increase in both the number of ROS-positive bacteria, as well as the staining intensity in the experimental group compared to the control group (Fig. 3B).
Fig. 3. A. Quantitative analysis of the effects of CS@Fe3O4 nanoparticles on bacterial oxidative stressactivated oxygen content were determined using a commercially available bacterial ROS fluorescence detection kit. Data are shown as the mean ± SD of three independent experiments. * p < 0.05. B. Examination of the effects of CS@Fe3O4 nanoparticles on ROS levels in A. baumannii by fluorescent microscopy.
Discussion
A. baumannii has become one of the main sources of infection in intensive care unit patients, and a large number of strains are now resistant to common antibiotics [15]. Thus, the development of a new drug for multidrug-resistant A. baumannii is critical. One of the pathogenic properties of A. baumannii is the production of a large amount of biofilm. Bacterial biofilms have a protective effect on bacteria, and thus, inhibition of biofilm function or formation could reduce bacterial resistance [16]. Many chronic wound infections have been shown to be associated with biofilms [17]. A. baumannii can form biofilms that attach to the surface of ventilators and tracheal intubations [18], and can also occur in the respiratory tract and skin of patients [19]. The formation of biofilms not only enhances the resistance of bacteria to antibiotics [20], but also stimulates the host immune system to release a large number of cytokines to trigger an immune response.
Fe3O4 nanozymes exhibit triple enzyme-like activities including peroxidase, catalase, and superoxide dismutase [21], and are a typical metal oxide nanoenzyme that exhibits catalytic properties similar to HRP. Their catalytic activity is associated not only with the pH, reaction temperature and H2O2 concentration of the solution, but also with the nanoparticle size, with smaller particles leading to higher catalytic activity [22]. The Fe3O4 nanozyme was shown to catalyze H2O2 to destroy the biofilm matrix of Pseudomonas aeruginosa, and its bactericidal effect was more than 10 times higher than that of using H2O2 alone [23]. Recently, iron sulfide and Fe3O4 nanozymes were shown to not only destroy the biofilm formed by Salmonella typhimurium, but also prevent its formation [24]. In addition, nanomaterials based on cerium ions have been shown to inhibit biofilm formation [25].
ROS are an important component of the immune response, and play a crucial role in eliminating invading pathogens, as well as promoting oxidative stress and damaging cellular proteins and lipids [26]. Mammalian macrophages and neutrophils can directly internalize foreign pathogens and degrade them in lysosomes in a ROS-dependent manner. Many lysosomal enzymes catalyze the production of ROS in an acidic environment, leading to the inactivation of biological macromolecules such as nucleic acids and proteins [27]. Nanozymes have the ability to regulate ROS levels [21, 28], which may account for the antibacterial activity of nanozymes [29]. Here, we found that CS@Fe3O4 nanoparticles can stimulate ROS production in both drug-sensitive and drug-resistant A. baumannii, disrupting internal metabolism and exerting an antibacterial role.
We found that CS@Fe3O4 nanoparticles had a more significant inhibitory effect on drug-resistant bacteria than drug-sensitive bacteria. Thus, we next sought to determine the mechanisms mediating the antibacterial effect of CS@Fe3O4 nanoparticles. We found that CS@Fe3O4 nanoparticles could inhibit the formation and function of drug-resistant A. baumannii biofilms, and significantly increase the ROS content in the drug-resistant A. baumannii. Furthermore, we found that in drug-sensitive A. baumannii, CS@Fe3O4 nanoparticles stimulated the production of ROS, but had no significant inhibitory effects on the biofilm. These findings may explain the differential antibacterial effects of CS@Fe3O4 nanoparticles on drug-sensitive and drug-resistant A. baumannii, and highlight the need to develop new antibiotics for the treatment of drug-resistant bacteria [30].
In conclusion, our study is the first description of the antibacterial effects of CS@Fe3O4 nanoparticles on drug-resistant and drug-sensitive strains of A. baumannii. In addition, the antibacterial mechanism of CS@Fe3O4 nanoparticles was preliminarily explored. Future studies will examine the molecular pathways and targets within the bacteria. This study provides a solid foundation for the development of antimicrobial agents to treat drug-resistant A. baumannii.
Supplemental Materials
Supplementary data for this paper are available on-line only at http://jmb.or.kr.
Acknowledgments
This work was funded by the Science and Technology Innovation Project of Guangzhou Medical University (2019A075 & 2020A063), Zhongnanshan Medical Foundation of Guangdong Province, and the Penghua Care Fund to the Medical Pioneers against Covid-19 of Shenzhen Social Commonweal Foundation.
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Jiao J, Fan K, Hu Z, Yan X, Du P. Development trend and priority areas of nanozyme. Sci. Sin. Chim. 2019;49:1442–1453. [Google Scholar]
- 2.Wu J, Wang X, Wang Q, Lou SR, Li YY. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) Chem. Soc. Rev. 2018;48:1004–1076. doi: 10.1039/C8CS00457A. [DOI] [PubMed] [Google Scholar]
- 3.Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan XY, Cai WB. Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 2019;48:3683–3704. doi: 10.1039/C8CS00718G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahini Shams Abadi M, Mirzaei E, Bazargani A, Gholipour A, Heidari H, Hadi N. Antibacterial activity and mechanism of action of chitosan nanofibers against toxigenic Clostridioides (Clostridium) difficile isolates. Ann. Ig. 2020;32:72–80. doi: 10.7416/ai.2020.2332. [DOI] [PubMed] [Google Scholar]
- 5.Xu JB, Xing YY, Liu YT, Liu MZ, Hou XH. Facile in situ microwave synthesis of Fe3O4@MIL-100(Fe) exhibiting enhanced dual enzyme mimetic activities for colorimetric glutathione sensing. Anal. Chim. Acta. 2021;1179:338825. doi: 10.1016/j.aca.2021.338825. [DOI] [PubMed] [Google Scholar]
- 6.Rozman NAS, Tong WY, Leong CR, Tan WN, Hasanolbasori MA, Abdullah SZ. Potential antimicrobial applications of Chitosan Nanoparticles (ChNP) J. Microbiol. Biotechnol. 2019;29:1009–1013. doi: 10.4014/jmb.1904.04065. [DOI] [PubMed] [Google Scholar]
- 7.Tian MD, Zhu CM, Luo C, Gong MJ, Bi Y. Cytotoxicity of superparamagnetic iron oxide nanoparticles modified by chitosan or sodium oleate. Acad. J. Second Mil. Med. Univ. 2014;35:366–371. doi: 10.3724/SP.J.1008.2014.00366. [DOI] [Google Scholar]
- 8.Sarshar M, Behzadi P, Scribano D, Palamara AT, Ambrosi C. Acinetobacter baumannii: an ancient commensal with weapons of a pathogen. Pathogens. 2021;10:387. doi: 10.3390/pathogens10040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mea HJ, Yong PVC, Wong EH. An overview of Acinetobacter baumannii pathogenesis: motility, adherence and biofilm formation. Microbiol. Res. 2021;247:126722. doi: 10.1016/j.micres.2021.126722. [DOI] [PubMed] [Google Scholar]
- 10.Kyriakidis I, Vasileiou E, Pana ZD, Tragiannidis A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens. 2021;10:373. doi: 10.3390/pathogens10030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takoi H, Fujita K, Hyodo H. Acinetobacter baumannii can be transferred from contaminated nitrile examination gloves to polypropylene plastic surfaces. Am. J. Infect. Control. 2019;47:1171–1175. doi: 10.1016/j.ajic.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Song HS, Lee HJ, Kim SH, Suh MJ, Cho JY, et al. Comparative study of the difference in behavior of the Accessory Gene Regulator (Agr) in USA300 and USA400 community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) J. Microbiol. Biotechnol. 2021;31:1060–1068. doi: 10.4014/jmb.2104.04032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song YL, Liang X, Song X. The study on the inhibition of Shigella biofilm formation by the exopolysaccharides of Lactobacillus plantarum-12. Food Res. Devel. 2018;39:144–151. [Google Scholar]
- 14.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 15.Cha MH, Kim SH, Kim S, Lee W, Kwak HS, Chi YM, et al. Antimicrobial resistance profile of Acinetobacter spp. isolates from retail meat samples under campylobacter-selective conditions. J. Microbiol. Biotechnol. 2021;31:733–739. doi: 10.4014/jmb.2102.02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saipriya K, Swathi CH, Ratnakar KS, Sritharan V. Quorum-sensing system in Acinetobacter baumannii: a potential target for new drug development. J. Appl. Microbiol. 2020;128:15–27. doi: 10.1111/jam.14330. [DOI] [PubMed] [Google Scholar]
- 17.Evelhoch SR. Biofilm and chronic nonhealing wound infections. Surg. Clin. North Am. 2020;100:727–732. doi: 10.1016/j.suc.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Gedefie A, Demsis W, Ashagrie M, Kassa Y, Tesfaye M, Tilahun M, et al. Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: a review. Infect. Drug Resist. 2021;14:3711–3719. doi: 10.2147/IDR.S332051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pakharukova N, Tuittila M, Paavilainen S. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA. 2018;115:5558–5563. doi: 10.1073/pnas.1800961115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Yang QQ, Zhang D, Yan W. Mechanisms and control strategies of antibiotic resistance in pathological biofilms. J. Microbiol. Biotechnol. 2021;31:1–7. doi: 10.4014/jmb.2010.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan BC, Cao J, Liu J, Gu Y, Xu Z, Li D, et al. Dietary Fe3O4 nanozymes prevent the injury of neurons and blood-brain barrier integrity from cerebral ischemic stroke. ACS Biomater. Sci. Eng. 2021;7:299–310. doi: 10.1021/acsbiomaterials.0c01312. [DOI] [PubMed] [Google Scholar]
- 22.Nikolova MP, Chaval MS. Metal oxide nanoparticles as biomedical materials. Biomimetics (Basel, Switzerland) 2020;5:1–47. doi: 10.3390/biomimetics5020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Chou Y, Xu ZB, Gao LZ. Antibacterial mechanism and application of nano enzymes. Prog. Biochem. Biophys. 2018;45:118–128. [Google Scholar]
- 24.Yin YY, Wu MX, Q T, He KM, Xu N, Shi Y, et al. Effect of iron based nanoenzyme on Salmonella typhimurium biofilm. Prog. Biochem. Biophys. 2019;46:587–595. [Google Scholar]
- 25.Liu ZW, Wang FM, Ren JS, Qu XG. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials. 2019;208:21–31. doi: 10.1016/j.biomaterials.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Tao L, Lemoff A, Wang G, Zarek C, Lowe A, Yan N, et al. Reactive oxygen species oxidize STING and suppress interferon production. Elife. 2020;9:e57837. doi: 10.7554/eLife.57837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazur P, Skiba-Kurek I, Mrowiec P, Karczewska E, Drod R. Synergistic ROS-associated antimicrobial activity of silver nanoparticles and gentamicin against. Int. J. Nanomedicine. 2020;15:3551–3562. doi: 10.2147/IJN.S246484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Wan R, Gu H, Fu GQ, Tang HQ, Hu GY. Well-water-dispersed N-trimethyl chitosan/Fe3O4 hybrid nanoparticles as peroxidase mimetics for quick and effective elimination of bacteria. J. Biomater. Sci. Polym. Ed. 2020;31:969–983. doi: 10.1080/09205063.2020.1733751. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Li P, Yu D, Yan Z, Qu X. Unraveling the enzymatic activity of oxygenated carbon nanotubes and their application in the treatment of bacterial infections. Nano Lett. 2018;18:3344–3351. doi: 10.1021/acs.nanolett.7b05095. [DOI] [PubMed] [Google Scholar]
- 30.Pourhajibagher M, Hosseini N, Boluki E, Chiniforush N, Bahador A. Photoelimination potential of chitosan nanoparticlesindocyanine green complex against the biological activities of strains: a preliminary study in burn wound infections. J. Lasers Med. Sci. 2020;11:187–192. doi: 10.34172/jlms.2020.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data for this paper are available on-line only at http://jmb.or.kr.