Abstract
We previously showed that heat-killed Borrelia burgdorferi spirochetes and lipidated outer surface protein A (L-OspA) stimulated the in vitro production of interleukin-10 (IL-10) in peripheral blood mononuclear cells (PBMC) from uninfected humans and rhesus monkeys (G. Giambartolomei et al., Infect. Immun. 66:2691–2697, 1998). Here we demonstrate that uninfected human peripheral blood monocytes, but not B or T cells, are the cells that transcribe the IL-10 cytokine gene in response to heat-killed B. burgdorferi. B. burgdorferi similarly induced an upregulation of the IL-1β and IL-6 cytokine genes in monocytes and the production of IL-10 and IL-6 in culture supernatants of the human monocytic cell line THP-1. Purified L-OspA (but not unlipidated OspA [U-OspA] or U-OspC) also stimulated the production of both cytokines in THP-1 cells in a dose-dependent fashion, suggesting that acylation of the OspA protein molecule is required for the production of both anti- and pro-inflammatory cytokines in naive monocytes. A lipohexapeptide that contained the tripalmitoyl-modified cysteine motif (Pam3Cys-Hex) of B. burgdorferi lipoproteins but with an arbitrary peptide sequence had the same effect. Monoclonal antibodies (MAbs) MY4 and 60bca, both of which bind to CD14 and are known to block lipopolysaccharide (LPS)-mediated cytokine production, were able to block L-OspA-mediated IL-10 and IL-6 cytokine production. In contrast, MAb 26ic, which also binds to CD14 but does not block LPS function, failed to inhibit L-OspA-mediated cytokine production. These data suggest that activation of monocytes and production of both anti- and pro-inflammatory cytokines induced by lipoproteins proceeds via the CD14 receptor. LPS binding protein was not required for OspA-induced cytokine production. Our results demonstrate that pro- and anti-inflammatory cytokines induced by B. burgdorferi lipoproteins in PBMC are produced by monocytes and that lipoprotein and LPS signaling pathways share at least the initial signaling event that involves the CD14 receptor.
Borrelia burgdorferi, the spirochete that causes Lyme disease, can invade multiple organs and persist in them for a long time (3, 53). Spirochetal persistence in these organs has been associated with severe pathology (6, 9, 53) and may cause localized inflammation. Inflammatory foci in the joints, skin, heart, and nervous system are characteristically observed in Lyme disease patients and in some animal models of Lyme disease (29). Cytokine-stimulatory properties possessed by B. burgdorferi may explain the correlation between tissue invasion and localized inflammation (7, 13, 18, 40).
B. burgdorferi lacks lipopolysaccharide (LPS) (41), but its genome contains no fewer than 105 genes coding for putative lipoproteins (10). Since bacterial lipoproteins have potent inherent stimulatory properties (15, 16), it has been hypothesized that B. burgdorferi lipoproteins are responsible for the inflammation associated with infection (23, 33, 40, 50). Outer surface protein A (OspA) has been the lipoprotein chosen as a model to investigate the molecular basis of inflammation in Lyme borreliosis. At the most general level, OspA is capable of inducing nuclear translocation of the transcription factor NF-κB in human endothelial cells and in a human monocytoid cell line (26, 50). Other potentially inflammatory responses such as upregulation of interleukin-1β (IL-1β) and IL-6 cytokine genes in mouse macrophages (26, 33), and the production of these cytokines by human umbilical vein endothelial cells or human monocytes (14, 50), also are induced by OspA. Like LPS, OspA is capable of inducing the production not only of inflammatory cytokines but also that of anti-inflammatory cytokines such as IL-10 (11, 14). The OspA protein moiety itself does not appear to play a role in these phenomena, as heat-killed spirochetes from mutant strains of B. burgdorferi that lack the plasmid that contains the ospA gene are equally capable of stimulating the production of IL-10 in peripheral blood mononuclear cells (PBMC) (11). In addition, synthetic lipohexapeptides varying in peptide composition but all with a tripalmitoyl-Cys moiety are able to elicit inflammatory stimuli as does the OspA lipoprotein itself (33).
Since the spectrum of pro- and anti-inflammatory cytokines induced by OspA and LPS (1, 8) and the cell types involved (11, 26, 33, 50) are remarkably similar, we hypothesized that both molecules may utilize common stimulation pathways. In recent years, much insight has been gained into the mechanism(s) by which LPS acts at the cell surface to activate cells of the myeloid lineage. Several investigators (43, 47, 52) have shown that LPS interacts with an acute-phase reactant called LPS-binding protein (LBP) and that the LBP-LPS complex binds to CD14, a glycosylphosphatidylinositol-anchored protein on the cell surface.
We first elucidated which of the main cell populations within the PBMC compartment, namely, B cells, T cells, and monocytes, produce pro (IL-1β and IL-6)- and anti (IL-10)-inflammatory cytokines when stimulated with B. burgdorferi. Once the cell type involved in these phenomena was identified, we used the human monocytic cell line THP-1 as a model to investigate if LBP and CD14 are involved in the lipoprotein signaling pathway. Here, we present the results of this study.
MATERIALS AND METHODS
Reagents.
Anti-CD14 monoclonal antibodies (MAbs) 60bca immunoglobulin G1 ([IgG1]) and 26ic (IgG2b) (45) were kindly provided in ascitic fluid by Robert F. Todd (University of Michigan, Ann Arbor). Purified anti-CD14 MAb MY4 (IgG2b; 1 mg/ml) was obtained from Coulter Immunology (Hialeah, Fla.). Negative control immunoglobulins for the inhibition experiments were purified mouse IgG2b and IgG1 (Sigma Chemical Company, St. Louis, Mo.). Purified recombinant human LBP (rLBP) was kindly provided by Peter Tobias (Scripps Clinic and Research Foundation, La Jolla, Calif.). Tissue culture-tested bovine serum albumin (BSA) (endotoxin, <0.1 ng/mg; fatty acids, <0.005%), LPS from Escherichia coli O26:B6, and phytohemagglutinin (PHA) were from Sigma. The lipohexapeptide Pam3-Cys-Ser-Lys4-OH (Pam3Cys-Hex) was obtained from Boehringer Mannheim (Indianapolis, Ind.).
Bacteria and lipoproteins.
The JD1 strain of B. burgdorferi sensu stricto was used in this study (30). Heat-killed spirochetes to be used as antigen were prepared as previously described (11). Recombinant lipidated OspA (L-OspA), unlipidated OspA (U-OspA), and unlipidated OspC (U-OspC) were obtained from John Dunn, Brookhaven National Laboratories, Brookhaven, N.Y. The recombinant OspA gene was from the B31 strain of B. burgdorferi sensu stricto. U-OspA and U-OspC were equivalent to the mature Osps but lacked the cysteine in position 18 of the unprocessed proteins. The L-OspA, U-OspA, and U-OspC preparations contained less than 0.25 endotoxin units per mg of protein, as assessed by Limulus amebocyte assay (Associates of Cape Cod, Woods Hole, Mass.).
Stimulation and purification of monocytes, B cells, and T cells.
PBMC from healthy human donors were isolated as already described (11). PBMC were cultured in round-bottom polypropylene tubes (Sarstedt, Nümbrecht, Germany) in RPMI 1640 supplemented with 25 mM HEPES buffer, 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, Utah), 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphoterium B (Fungizone) per ml (RPMI), in the presence of heat-killed B. burgdorferi spirochetes (107 spirochetes/ml) or PHA (10 μg/ml) in a final volume of 1 ml. Cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and 95% air) for 24 h. At the end of the incubation, cells were centrifuged at 450 × g at 4°C. The supernatants were discarded, and the cells were washed in separation buffer (phosphate-buffered saline [PBS; 137 mM NaCl, 1.5 mM KH2PO4, 20 mM Na2HPO4, 2.7 mM KCl] containing 0.5% BSA and 5 mM EDTA). T cells, B cells, and monocytes were separated from the stimulated PBMC mixture by using immunomagnetic beads as instructed by the manufacturer (Miltenyi Biotec Inc., Auburn, Calif.). B cells were isolated by positive selection using MACS CD19-microbeads (Miltenyi) and the Miltenyi Mini MACS magnetic separation system. Monocytes were separated from T cells by positive selection using MACS CD14 microbeads (Miltenyi). Separated cells were washed, counted, and processed immediately for RNA extraction. Purity of the B-cell, T-cell, and monocyte preparations was assessed by flow cytometry analysis with a FACScalibur cell sorter (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.) and anti-CD19-phycolerythrin (B1; Becton Dickinson), anti-CD2-fluorescein isothiocyanate (T11; Becton Dickinson), and anti-CD14-fluorescein isothiocyanate MAbs. Control MAbs of the same isotype were used for each sample. T cells (99% T cells and 1% B cells), monocytes (85 to 95% monocytes and 15 to 5% T cells), and B cells (86 to 87% B cells, 12% T cells, and 1 to 2% monocytes) were used to analyze cytokine gene expression by RT-PCR.
Detection of cytokine mRNA by semiquantitative RT-PCR.
Reverse transcription (RT)-PCR was performed as previously described (11). Results were expressed as fold increase over the mRNA levels of cells cultured in the absence of antigen.
Cell culture.
Cells of the THP-1 monocyte cell line (46) were purchased from the American Type Culture Collection (catalog no. TIB 202) and cultured as described previously (20). To induce elevated expression of CD14, cells were exposed to 0.05 μM 1,25-dihydroxyvitamin D3 (Calbiochem-Nova Biochem Int., La Jolla, Calif.) for 48 h (19).
Stimulation of cytokine production.
Vitamin D3-treated THP-1 cells (106/ml) were cultured in round-bottom polypropylene tubes (Sarstedt) with RPMI, spirochetes, L-OspA, U-OspA, U-OspC, Pam3Cys-Hex, or LPS at the indicated concentration in a final volume of 0.5 ml. Cultures were incubated at 37°C in a humidified atmosphere (5% CO2 and 95% air) for 24 h. Where indicated, cultures also contained 10 μg of polymyxin B sulfate (Sigma) per ml. At the end of the culture, cells were centrifuged at 400 × g at 4°C for 10 min, and the supernatants were aliquoted and stored at −70°C until they were assayed for IL-10 or IL-6.
Inhibition of CD14 binding.
Equal volumes (125 μl) of vitamin D3-treated THP-1 cells (4 × 106/ml) and different dilutions of MAbs MY4, 60bca, and 26ic or their respective nonimmune isotype controls were mixed and incubated in round-bottom polypropylene tubes (Sarstedt) for 30 min at 4°C. This preparation was subsequently incubated with 250 μl of LPS, L-OspA, or Pam3Cys-Hex to reach a final concentration of 10 ng/ml for LPS, 100 ng/ml for L-OspA or 5 ng/ml for Pam3Cys-Hex in a final volume of 0.5 ml. Cultures were incubated for 24 h as described above, and supernatants were aliquoted and stored at −70°C until being assayed for IL-10 or IL-6.
Role of LBP in the CD14-dependent lipoprotein-induced cytokine production.
Vitamin D3-treated THP-1 cells (106/ml) were cultured in RPMI medium in round-bottom polypropylene tubes (Sarstedt) with L-OspA or LPS at the indicated concentrations in the presence of either 10% FBS, 5% BSA, or 5% BSA containing increasing amounts of rLBP in a final volume of 0.5 ml. To allow for the formation of complexes with LBP, LPS, or L-OspA were incubated with rLBP for 10 min at 37°C before addition of the cells. Cultures were incubated for 24 h as described above, and the supernatants were aliquoted and stored at −70°C until being assayed for IL-10 or IL-6.
Cytokine ELISA.
A sandwich enzyme-linked immunosorbent assay (ELISA) was used to detect IL-10 and IL-6 secretion in culture supernatants of THP-1 cells. Secreted IL-10 was quantified with MAb JDS3-9D7 (PharMingen, San Diego, Calif.) as capture antibody and biotin-conjugated MAb JDS3-12G8 (PharMingen) as detection antibody. The ELISA protocol was as described previously (11). The secretion of IL-6 was quantified with MAb MP5-20F3 (PharMingen) as capture antibody (50 ng/well) and the biotinylated MAb MP5-32C11 (PharMingen) as detection antibody (50 ng/well), using the procedure described for IL-10 (11).
RESULTS
B. burgdorferi spirochetes induce IL-10, IL-6, and IL-1β gene transcription in human peripheral blood monocytes.
We had shown that B. burgdorferi is capable of stimulating the transcription and secretion of IL-10 in uninfected human and rhesus monkey PBMC in vitro (11). To determine the cell subpopulation involved in this phenomenon, cell purification experiments were performed with uninfected human PBMC. PBMC were cultured for 24 h in the absence or presence of whole heat-killed B. burgdorferi JD1 or PHA. After culture, PBMC were separated into T-cell, B-cell, and monocyte subpopulations with appropriately tagged immunomagnetic beads, and cytokine gene expression was assessed by semiquantitative RT-PCR.
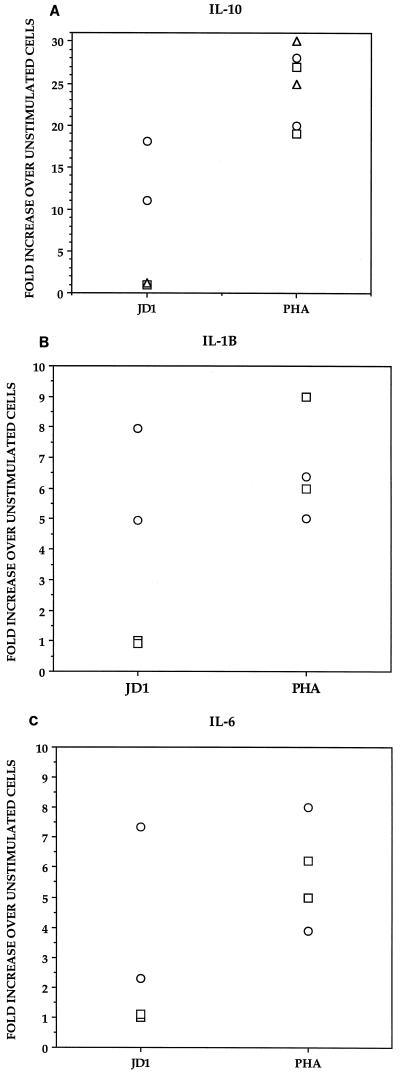
Compared to unstimulated cells, JD1 induced a marked upregulation of the expression of the IL-10 gene only in the monocyte-enriched cell population (10- to 18-fold increase over unstimulated cells). In contrast, JD1 did not ostensibly affect the expression of the IL-10 gene in normal T or B cells (onefold increase). The mitogen PHA induced the expression of the IL-10 gene in the three cell subpopulations (Fig. 1A). Heat-killed B. burgdorferi also caused an upregulation of IL-1β (five- to eightfold increase [Fig. 1B]) and IL-6 (two- to sevenfold increase [Fig. 1C]) gene expression in monocytes; a similar finding was reported for a study using OspA as the stimulant (26). JD1 did not induce an upregulation of expression of the IL-1β, or IL-6 gene in B or T cells (onefold increase) compared to unstimulated cells. PHA induced an upregulation of the expression of the IL-1β and IL-6 genes in B cells (Fig. 1A and B) but not in T cells (data not shown). Failure of purified human T cells to transcribe the IL-6 gene in response to PHA has been observed previously (48). We did not further investigate the inability of PHA to induce IL-1β gene transcription in T cells.
FIG. 1.
Cytokine mRNA expression induced by B. burgdorferi in T lymphocytes, B lymphocytes, and monocytes separated from PBMC of uninfected human donors. PBMC (3 × 106 per ml) from each of two donors were incubated for 24 h in supplemented medium (RPMI) alone or with added heat-killed B. burgdorferi JD1 spirochetes (107/ml) or PHA (10 μg/ml). After culture, PBMC were separated into T cells (▵), B cells (□), and monocytes (○) by using immunomagnetic beads tagged with specific MAbs. The induced mRNA levels of IL-10 (A), IL-1β (B), and IL-6 (C) were determined by RT-PCR. Responses are shown as fold increase over unstimulated cells. Each point represents the response of cells from an individual donor. All values were normalized with respect to GAPDH mRNA levels.
B. burgdorferi lipoproteins induce the production of pro- and anti-inflammatory cytokines in the THP-1 cell line.
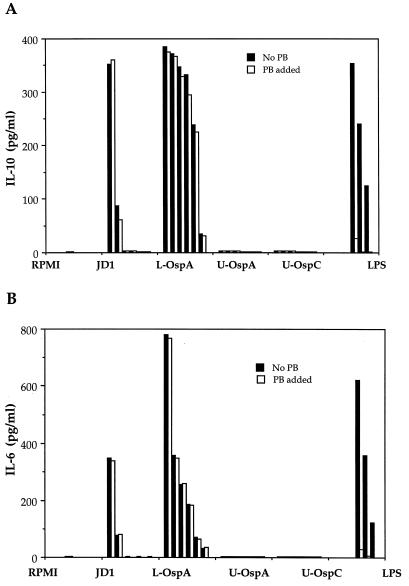
After determining that the PBMC cell type that secreted IL-10, IL-1β, and IL-6 in response to JD1 was the monocyte, we performed experiments with the well-characterized THP-1 human monocytic cell line (46). This cell line was used to further investigate the mechanisms by which B. burgdorferi lipoproteins induce the production of cytokines in uninfected human monocytes. To induce cell maturation and expression of surface CD14, THP-1 cells were pretreated with vitamin D3, a treatment previously shown to enhance the responsiveness of this cell line to LPS and B. burgdorferi lipoproteins (20, 26). Vitamin D3-treated THP-1 cells were incubated with JD1 spirochetes, L-OspA, U-OspA, U-OspC, or LPS; after 24 h of culture, the production of IL-10 and IL-6 was evaluated in the culture supernatants by ELISA. Corroborating the results obtained with purified peripheral blood monocytes, the production of both IL-10 and IL-6 was markedly higher in culture supernatants of THP-1 cells that were stimulated with JD1 than in the unstimulated cells (Fig. 2). Cytokine production was a function of the amount of spirochetes present in the culture. A marked interleukin upregulation was detected in cultures containing between 106 and 107 spirochetes/ml. As previously observed for IL-10 (11), IL-6 and IL-10 production in cultures that contained 105 spirochetes/ml or less dropped dramatically to the levels of unstimulated cells (Fig. 2). L-OspA also induced IL-10 and IL-6 in a dose-dependent fashion. Cytokine production was seen with as little as 1 ng of L-OspA per ml, and maximum production was achieved with 1,000 ng/ml (Fig. 2). IL-10 and IL-6 production by lipoprotein-stimulated THP-1 cells was dependent on the lipidation of the molecules, as U-OspA and U-OspC failed to induce cytokine production at all concentrations tested (1 to 1,000 ng/ml) (Fig. 2). Interestingly, untreated THP-1 cells also secreted IL-10 in response to LPS and L-OspA, although at concentrations of ≥100 ng/ml for both stimulants (12). Interleukin secretion was not due to LPS contamination, as the addition of polymyxin B had no effect on JD1- or L-OspA-induced cytokine production under conditions in which it completely blocked cytokine production in response to LPS concentrations ranging from 1 to 100 ng/ml (Fig. 2). These results showed that acylation of B. burgdorferi lipoproteins is crucial for the induction of both pro- and anti-inflammatory cytokines in uninfected monocytes.
FIG. 2.
B. burgdorferi and its lipoproteins induce pro- and anti-inflammatory cytokines in THP-1 monocytoid cells. Vitamin D3-treated THP-1 cells (106/ml) were incubated for 24 h with supplemented medium (RPMI), various concentrations of heat-killed strain JD1 spirochetes (from left to right, 107 to 102 spirochetes/ml, in multiples of 10), or various concentrations of L-OspA, U-OspA, U-OspC (from left to right, 1,000, 500, 250, 100, 10, and 1 ng/ml) and LPS (100, 10, and 1 ng/ml) in the presence or absence of polymyxin B (PB). IL-10 (A) and IL-6 (B) in the cell supernatants were quantified by antibody capture ELISA. Data are representative of two separate experiments. The lower limit of detection of the ELISA was 15 pg/ml.
The Pam3-modified cysteine is the molecular structure that induces the production of pro- and anti-inflammatory cytokines in THP-1 cells.
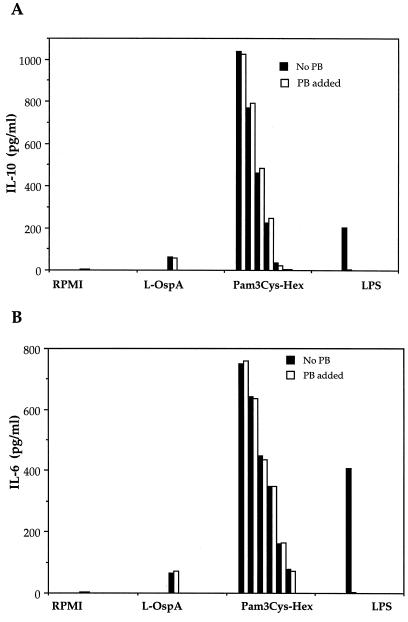
Recently, it was demonstrated that lipohexapeptides which had in common the tripalmitoyl-Cys moiety but had peptides of different amino acid compositions are qualitatively as effective as the OspA lipoprotein itself in the activation of macrophages and production of inflammatory cytokines (33). Moreover, the presence of OspA per se is not required in heat-killed spirochetes for the induction by the latter of IL-10 production in PBMC, as heat-killed spirochetes from mutant strains of B. burgdorferi that lack the plasmid that contains the ospA gene are equally effective (11). This led us to investigate the role of the Pam3-modified cysteine in the induction of both pro- and anti-inflammatory cytokines in THP-1 cells. For that purpose, vitamin D3-treated THP-1 cells were incubated with Pam3Cys-Hex, a lipohexapeptide with a peptide sequence different from B. burgdorferi lipoproteins but having the common tripalmitoyl-modified cysteine, and after 24 h of culture, the production of IL-10 and IL-6 was evaluated in culture supernatants by ELISA. Pam3Cys-Hex induced the production of IL-6 and IL-10 in a dose-dependent manner (Fig. 3). Addition of polymyxin B had no effect on the cytokine induction by Pam3Cys-Hex, which indicated that this phenomenon was not due to LPS contamination. These results demonstrate that the Pam3-modified cysteine is the molecular structure that induces the production of pro- and anti-inflammatory cytokines in THP-1 cells.
FIG. 3.
A synthetic lipohexapeptide induces pro- and anti-inflammatory cytokine production in THP-1 cells. Vitamin D3-treated THP-1 cells (106/ml) were incubated for 24 h with supplemented medium (RPMI), L-OspA (10 ng/ml), various doses of the synthetic lipohexapeptide Pam3Cys-Hex (from left to right, 50, 25, 12.5, 5, 0.5, and 0.05 ng/ml), or LPS (10 ng/ml) in the presence or the absence of polymyxin B (PB). IL-10 (A) and IL-6 (B) were quantified in the cell supernatants by antibody capture ELISA. Data are representative of two separate experiments.
B. burgdorferi lipoprotein-induced cytokine production is CD14 mediated.
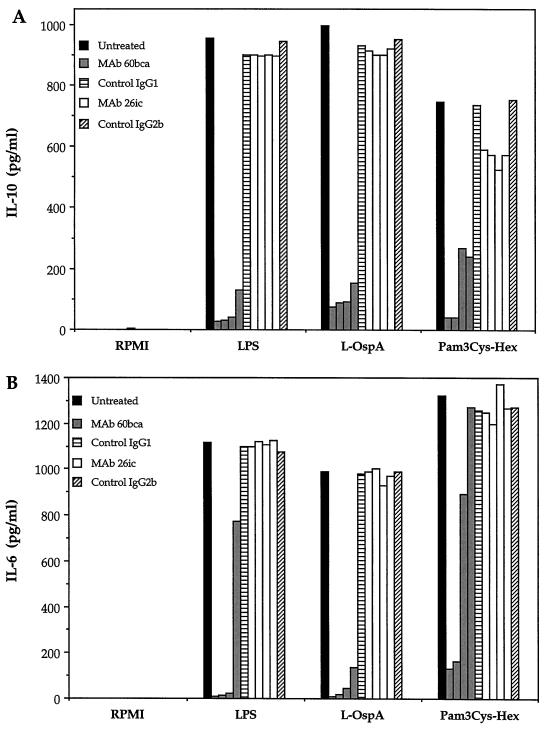
To examine the role of the CD14-dependent signaling pathway in the cytokine production induced by spirochetal lipoproteins in monocytes, vitamin D3-treated THP-1 cells were preincubated with the anti-CD14 MAbs 60bca and 26ic or their respective nonimmune controls and then cultured with LPS, L-OspA, or Pam3Cys-Hex. After 24 h of culture, the production of IL-10 and IL-6 was evaluated in culture supernatants by ELISA. As expected, MAb 60bca blocked the LPS-mediated production of both IL-10 and IL-6 in a dose-dependent manner, whereas MAb 26ic, which was previously reported to be an antibody to CD14 that does not block LPS-mediated responses (52), failed to inhibit the cytokine production induced by LPS at all dilutions tested (Fig. 4). Mimicking the effect observed on the LPS-mediated response, MAb 60bca blocked both the L-OspA- and Pam3Cys-Hex-mediated production of IL-10 and IL-6 in a dose-dependent fashion, while MAb 26ic had no effect on this response (Fig. 4). MAb MY4, which like 60bca is able to block the LPS-induced production of cytokines by monocytes, also inhibited the LPS- and L-OspA-mediated production of cytokines in a dose-dependent fashion (data not shown). Isotype control MAbs had no effect on all responses studied. These results indicate that at the concentrations of L-OspA and Pam3Cys-Hex tested, activation of monocytes and cytokine production induced by B. burgdorferi lipoproteins and LPS proceeds via the CD14 receptor. The results also suggest that the tripalmitoyl-modified cysteine is the molecular moiety of lipoproteins that binds to the CD14 molecule.
FIG. 4.
Inhibition of lipoprotein-induced cytokine production by anti-CD14 MAbs 60bca and 26ic. Vitamin D3-treated THP-1 cells (106/ml) were preincubated with RPMI (untreated) or treated with different dilutions (1/100 to 1/5,000) of ascitic fluids containing MAbs 60bca and 26ic or the respective nonimmune ascitic fluid isotype controls (1/100) IgG1 and IgG2b for 30 min at 4°C. A volume of additional RPMI was then added without (RPMI) or with LPS, L-OspA, or Pam3Cys-Hex such that the final concentrations of these three reagents were 10, 100, and 5 ng/ml, respectively, and the cells were then incubated at 37°C for 24 h. IL-10 (A) and IL-6 (B) were quantified in the cell supernatants by antibody capture ELISA. Data are representative of two separate experiments.
B. burgdorferi lipoprotein-induced cytokine production is LBP independent.
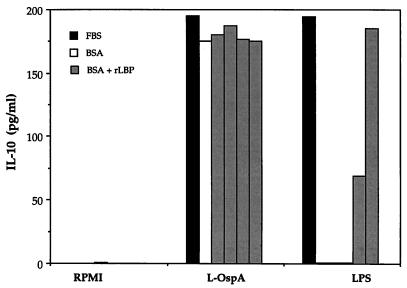
To determine the role of LBP in the CD14-mediated lipoprotein-induced cytokine production, vitamin D3-treated THP-1 cells were cultured with L-OspA or LPS in the presence of FBS or in its absence (BSA). After 24 h of culture, the production of IL-10 was evaluated in the culture supernatants by ELISA. In the absence of FBS, LPS did not induce IL-10 production in THP-1 cells, whereas the L-OspA-induced IL-10 production was unaffected (Fig. 5). Similarly, increasing concentrations of LBP restored the ability of LPS to induce IL-10 production but did not alter that of L-OspA (Fig. 5). This finding indicates that LBP is not involved in the lipoprotein-induced cytokine production by B. burgdorferi.
FIG. 5.
Effect of rLBP on IL-10 production induced by L-OspA. Vitamin D3-treated THP-1 cells (106 per ml) were incubated for 24 h in RPMI alone or with 10 ng of LPS or L-OspA per ml in the presence of 10% FBS, 5% BSA, or 5% BSA and 10-fold increasing concentrations of rLBP (0.6 to 600 ng/ml). IL-10 was quantified in the cell supernatants by antibody capture ELISA. Data are representative of two separate experiments.
DISCUSSION
Inflammation is present both in the acute and chronic phases of Lyme borreliosis and in virtually all organs affected by this disease (6). In the skin, the histopathologic hallmarks of erythema migrans are perivascular mononuclear cellular infiltrates, mostly monocytic, which appear in the superficial and deeper dermis within 1 to 2 weeks of infection both in humans and in animal models of Lyme disease (4, 28). Intermittent inflammatory arthritis is present in the early phases of disease in humans (39), and some patients may develop chronic inflammatory arthritis that resembles other forms of human inflammatory arthritis (22). In rhesus monkeys, 6 months after a B. burgdorferi infection delivered via ticks, a spectrum of inflammatory changes was evident in the joints, which included synovial cell hyperplasia, mononuclear cell infiltration of the synovium, mostly perivascular, and active chronic changes such as pannus formation (35). Joint damage was associated with presence of B. burgdorferi in these joints, as shown by immunostaining of synovial tissue and in vitro culture of synovial tissue and fluid (35).
In the nervous system, elevated levels of IL-1 and tumor necrosis factor alpha (TNF-α) have been found in neuroborreliosis patients, and IL-6 production is simulated by coincubation of C6 glioma cells (13) or cultured rat brain cells (42) with B. burgdorferi. In the rhesus monkey, peripheral neuritis involving multiple nerves was the most consistent neurologic manifestation observed 3 months postinfection when the peripheral nervous system was investigated in these animals. Both macrophages and B lymphocytes, but not T lymphocytes, were present in the cellular infiltrates. Some of the Schwann cells in lesions stained with antinitrotyrosine and anti-TNF-α antibodies. B. burgdorferi, or antigens thereof, were visualized immunohistochemically within macrophages (36).
Since bacterial lipoproteins have potent inherent stimulatory properties (15, 16), it has been hypothesized that B. burgdorferi lipoproteins are responsible for the inflammation associated with infection (23, 33, 40). Studies on the molecular pathogenesis of inflammation in Lyme borreliosis have focused on B. burgdorferi’s lipoproteins, as the general stimulatory properties of lipoproteins from other bacterial species have become better understood (15, 16). As these studies progressed (23, 24, 33, 40), it became apparent that local and systemic production of the inflammatory cytokines IL-6, IL-1β, and TNF-α, which are elicited by B. burgdorferi lipoproteins in cells such as macrophages and which have potent cell recruitment and immune response amplification capabilities (33), could explain why Lyme disease can be overtly manifest even when spirochetes are hard to find in lesions or bodily fluids. Moreover, the ability of B. burgdorferi lipoproteins to induce anti-inflammatory cytokines such as IL-10 might explain the focal and transient nature of inflammatory episodes in Lyme disease (11).
We therefore focused our studies on both IL-6 and IL-10, as we (i) determined which cells from within the PBMC compartment were stimulated by lipoproteins to produce these cytokines as well as IL-1β, (ii) demonstrated that the Pam3Cys moiety was essential for the elicitation of IL-10 and confirmed this residue’s requirement for the induction of IL-6 (33), and (iii) began to elucidate the mechanism whereby production of these cytokines is elicited by lipoproteins in monocytes.
When incubated with a mixture of cells such as PBMC for 24 h, spirochetes induced cytokine gene transcription in monocytes but not in B cells or T cells. While longer incubation periods might have led to different results, especially concerning the involvement of T cells, the results that we obtained indicate that within hours of infection, B. burgdorferi has the potential of inducing both inflammatory and anti-inflammatory responses via the same cellular source, i.e., monocytes. Indeed, mice with the severe combined immunodeficiency mutation (scid mice), which are devoid of T and B cells but have monocytes, develop an arthritis which is entirely similar to that of immunocompetent mice (2, 37); in monkeys infected with B. burgdorferi, perivascular cellular infiltrates observed in organs such as the skin, joints, and peripheral nerves are mostly monocytic in nature (28, 35, 36). This finding indicates that innate immune responses, together with spirochetal persistence in the tissues, may lead to pathology in Lyme disease.
These results were corroborated in assays using a monocytic cell line, THP-1. Whole spirochetes and L-OspA induced the secretion of IL-10 and IL-6 in a dose-dependent fashion. Neither U-OspC nor U-OspA induced cytokine secretion in THP-1 cells, demonstrating that acylation of the lipoprotein molecule is required for the production of cytokines in uninfected human monocytes. Indeed, a lipohexapeptide with a peptide sequence different from that of any B. burgdorferi lipoprotein but sharing the tripalmitoyl-modified cysteine motif had the same qualitative effect as the acylated lipoprotein. This finding indicates that the Pam3-modified cysteine hexapeptide is the molecular structure involved in the production not only of proinflammatory cytokines, as previously shown by Radolf et al. (33), but also anti-inflammatory cytokines, in cells of the monocyte/macrophage lineage.
The spectrum of cytokines, chemokines, and adhesion molecules induced by OspA and other B. burgdorferi lipoproteins (11, 17, 23, 24, 33, 34, 40, 42, 50) is similar to that induced by LPS (1, 31, 47). Furthermore, there are striking similarities between cell types that respond to LPS and OspA (11, 25, 26, 33). These facts suggest that there may be similar cell signaling pathways for these microbial molecules. The present study provides evidence that LPS and spirochetal lipoproteins activate monocytes via the same cell surface receptor, the CD14 molecule. Both L-OspA- and LPS-mediated cytokine production were blocked by increasing amounts of two different anti-CD14 MAbs, MY4 and 60bca. In contrast, cytokine production induced by L-OspA and LPS was not inhibited by MAb 26ic, which was previously reported to be a nonblocking antibody to CD14 (52). The same results were obtained when Pam3Cys-Hex was used as stimulus, strongly suggesting that the molecular structure recognized by the CD14 receptor is the tripalmitoyl-modified cysteine.
While this report was being processed for publication, it was demonstrated by others that activation of monocytic and human umbilical vein endothelial cells by spirochetal lipoproteins and lipopeptides is enhanced by CD14 (38, 51), particularly at low doses of stimulant. The dependence on CD14 was lost at higher concentrations of lipoproteins (51). Our results also indicate that, as with LPS (5), a CD14-independent stimulatory pathway becomes apparent at progressively higher concentrations of spirochetal lipoproteins (12).
Norgard and colleagues (26) had indicated that B. burgdorferi lipoproteins induce cellular activation by a mechanism that does not involve the LPS receptor. Evidence for this came from experiments performed with the CD14-negative murine pre-B-cell line 70Z/3 and the same cells transfected with human CD14. It was shown that human CD14 expression, which conferred LPS sensitivity to 70Z/3 cells, did not impart responsiveness to OspA or to other spirochetal lipopeptides. Sellati and colleagues (38) have now clarified the issue, demonstrating that CD14 is involved in cellular activation by spirochetal lipoproteins although likely by using a set of transmembrane proteins different from the one utilized by LPS to transduce the signals. THP-1 monocytic cells transfected with CD14 respond to lipoproteins, suggesting that, unlike murine pre-B70Z/3 cells, THP-1 cells constitutively express the thus far unidentified lipoprotein signal transducer. This would explain our results indicating that heat-killed spirochetes do not induce IL-10, IL-1β, or IL-6 cytokine gene transcription in purified B lymphocytes, which do express membrane-bound CD14 (21). The same line of reasoning could explain the fact that C3H/HeJ mice macrophages, which express normal levels of CD14 but do not respond to LPS, are activated by spirochetal lipoproteins. These cells would have the lipoprotein set of membrane transducers but not the LPS one. All of these results indicate that spirochetal lipoproteins utilize an alternative CD14-dependent pathway. This notion is further strengthened by our observation that monocyte stimulation by lipoproteins does not require LBP. Neither the absence of FBS, which contains LBP, nor the addition of recombinant LBP modified the cytokine production induced by L-OspA. A major function of LBP is to enable LPS to bind to either membrane or soluble CD14 (32). LBP binds to LPS via lipid A (44) and lowers the threshold stimulatory concentrations of LPS. B. burgdorferi lacks lipid A (41), suggesting, as we have confirmed, that lipoprotein-induced cytokine production via CD14 should be LBP independent. Although there appear to be differences in some of the initial events that lead to monocyte stimulation by LPS and lipoproteins, there are similarities further on in these two signaling pathways which indicate that they converge. Both LPS and lipoproteins stimulate the nuclear translocation factor NF-κB (26, 49), a transcription factor essential for the upregulated transcription of a number of genes whose products are involved in localized inflammation (1).
Our findings demonstrate that lipoproteins can induce inflammatory (IL-6 and IL-1β) and anti-inflammatory (IL-10) cytokines in the same cell type, which suggests (but does not prove) that the same signaling pathway is utilized for both types of cytokines. That these cytokines could thus act in concert within the same microenvironment of B. burgdorferi-infected tissues further underscores our hypothesis that IL-10 may play a role in the control of inflammation in Lyme borreliosis (11, 27). Indeed, IL-10 was shown to inhibit the induction of NF-κB in human monocytes (50). Finally, CD14 has been recognized as a receptor not only for LPS but also for polyuronic acid polymers, lipoarabinomannan, and other bacterial cell wall constituents (47). Our results indicate that B. burgdorferi lipoproteins also stimulate cytokine production via a CD14-mediated mechanism, thus supporting the contention that CD14 is a pattern recognition receptor by its unique ability to recognize several structurally related microbial antigens (47).
ACKNOWLEDGMENTS
This work was supported by grant U50/CCU606604 from the Centers for Disease Control and Prevention and by grant RR00164 from the National Center for Research Resources, National Institutes of Health. G.H.G. is a postdoctoral fellow of CONICET (Argentina).
We thank Christie Trew for excellent secretarial help. We also thank John Dunn (Brookhaven National Laboratory), Peter Tobias (Scripps Clinic and Research Foundation), and Robert Todd (University of Michigan) for purified recombinant OspA and OspC, purified recombinant LBP, and anti-CD14 MAbs, respectively.
REFERENCES
- 1.Baeuerle P A, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 2.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 3.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 4.Berger B W. Dermatologic manifestations of Lyme disease. Rev Infect Dis. 1989;11:S1475–S1481. doi: 10.1093/clinids/11.supplement_6.s1475. [DOI] [PubMed] [Google Scholar]
- 5.Cauwels A, Wan E, Leismann M, Tuomanen E. Coexistence of CD14-dependent and -independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect Immun. 1997;65:3255–3260. doi: 10.1128/iai.65.8.3255-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyle P K. Pathogenesis of Lyme disease. In: Manning S, editor. Lyme disease. St. Louis., Mo: Mosby Year Book; 1993. pp. 179–183. [Google Scholar]
- 7.Defosse D L, Johnson R C. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeWaal Malefyt R, Haanen J, Spits H, Roncolo M G, TeVelde A, Fidgor C, Johnson K, Kastelein R, Yssel H, DeVries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.England J D, Bohm R P, Jr, Roberts E D, Philipp M T. Mononeuropathy multiplex in rhesus monkeys with chronic Lyme disease. Ann Neurol. 1997;41:375–384. doi: 10.1002/ana.410410313. [DOI] [PubMed] [Google Scholar]
- 10.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 11.Giambartolomei G H, Dennis V A, Philipp M T. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect Immun. 1998;66:2691–2697. doi: 10.1128/iai.66.6.2691-2697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giambartolomei, G. H., V. A. Dennis, B. L. Lasater, and M. T. Philipp. Unpublished data.
- 13.Habicht G S, Katona L I, Benach J L. Cytokines and the pathogenesis of neuroborreliosis: Borrelia burgdorferi induces glioma cells to secrete interleukin-6. J Infect Dis. 1991;164:568–574. doi: 10.1093/infdis/164.3.568. [DOI] [PubMed] [Google Scholar]
- 14.Haupl T, Landgraf S, Netusil P, Biller N, Capiau C, Desmons P, Hauser P, Burmester G R. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol Med Microbiol. 1997;19:15–23. doi: 10.1111/j.1574-695X.1997.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauschildt S, Hoffmann P, Beuscher H U, Dufhues G, Heinrich P, Wiesmuller K H, Jung G, Bessler W G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990;20:63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann P, Heinle S, Schade U F, Loppnow H, Ulmer A J, Flad H D, Jung G, Bessler W G. Stimulation of human and murine adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology. 1988;177:158–170. doi: 10.1016/S0171-2985(88)80036-6. [DOI] [PubMed] [Google Scholar]
- 17.Honarvar N, Schaible U E, Galanos C, Wallich R, Simon M M. A 14,000 MW lipoprotein and a glycolipid-like structure of Borrelia burgdorferi induce proliferation and immunoglobulin production in mouse B cells at high frequencies. Immunology. 1994;82:389–396. [PMC free article] [PubMed] [Google Scholar]
- 18.Kenefick K B, Lim L C L, Alder J D, Schmitz J L, Czuprynski C J, Schell R F. Induction of interleukin-1 release by high- and low-passage isolates of Borrelia burgdorferi. J Infect Dis. 1993;167:1086–1092. doi: 10.1093/infdis/167.5.1086. [DOI] [PubMed] [Google Scholar]
- 19.Kitchens R L, Munford R S. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270:9904–9910. doi: 10.1074/jbc.270.17.9904. [DOI] [PubMed] [Google Scholar]
- 20.Kitchens R L, Ulevitch R J, Munford R S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labeta M O, Landmann R, Obrecht J P, Obrist R. Human B cells express membrane-bound and soluble forms of the CD14 myeloid antigen. Mol Immunol. 1991;28:115–122. doi: 10.1016/0161-5890(91)90094-z. [DOI] [PubMed] [Google Scholar]
- 22.Lahesmaa R, Shanafelt M C, Steinman L, Peltz G. Immunopathogenesis of human inflammatory arthritis: lessons from Lyme and reactive arthritis. J Infect Dis. 1994;170:978–985. doi: 10.1093/infdis/170.4.978. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Seiler K P, Tai K, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison T B, Weis J H, Weis J J. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J Immunol. 1997;158:4838–4845. [PubMed] [Google Scholar]
- 26.Norgard M V, Arndt L L, Akins D R, Curetty L L, Harrich D A, Radolf J D. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipoproteins proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NK-κB. Infect Immun. 1996;64:3845–3852. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philipp M T. Studies on OspA: a source of new paradigms in Lyme disease research. Trends Microbiol. 1998;6:44–47. doi: 10.1016/S0966-842X(97)01201-8. [DOI] [PubMed] [Google Scholar]
- 28.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early-disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philipp M T, Johnson B J B. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 1994;2:431–437. doi: 10.1016/0966-842x(94)90800-1. [DOI] [PubMed] [Google Scholar]
- 30.Piesman J, Mather T N, Sinsky R J, Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pober J S, Cotran R S. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Pugin J, Schurer-Maly C C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 34.Radolf J D, Norgard M V, Brandt M E, Isaacs R D, Thompson P A, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 35.Roberts E D, Bohm R, Jr, Cogswell F B, Lanners H N, Lowrie R C, Jr, Povinelli L, Piesman J, Philipp M T. Chronic Lyme disease in the rhesus monkey. Lab Investig. 1995;72:146–160. [PubMed] [Google Scholar]
- 36.Roberts E D, Bohm R P, Jr, Lowrie R C, Jr, Habicht G, Katona L, Piesman J, Philipp M T. Pathogenesis of Lyme neuroborreliosis in the rhesus monkey: the early disseminated and chronic phases of disease in the peripheral nervous system. J Infect Dis. 1998;78:722–732. doi: 10.1086/515357. [DOI] [PubMed] [Google Scholar]
- 37.Schaible U E, Gay S, Museteanu C, Kramer M D, Zimmer G, Eichmann K, Museteanu U, Simon M M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- 38.Sellati T J, Bouis D A, Kitchens R L, Darveau R P, Pugin J, Ulevitch R J, Gangloff S C, Goyert S M, Norgard M V, Radolf J D. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- 39.Steere A C. Musculoskeletal manifestations of Lyme disease. Am J Med. 1995;98:44S–48S. doi: 10.1016/s0002-9343(99)80043-6. [DOI] [PubMed] [Google Scholar]
- 40.Tai K, Ma Y, Weis J J. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–528. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayama K, Rothenberg R J, Barbour A G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987;55:2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatro J B, Romero L I, Beasley D, Steere A C, Reichlin S. Borrelia burgdorferi and Escherichia coli lipopolysaccharides induce nitric oxide and interleukin-6 production in cultured rat brain cells. J Infect Dis. 1994;169:1014–1022. doi: 10.1093/infdis/169.5.1014. [DOI] [PubMed] [Google Scholar]
- 43.Tobias P S, Soldau K, Kline L, Lee J D, Kato K, Martin T P, Ulevitch R J. Cross-linking of lipopolysaccharide (LPS) to CD14 on THP-1 cells mediated by LPS-binding protein. J Immunol. 1993;150:3011–3021. [PubMed] [Google Scholar]
- 44.Tobias P S, Soldau K, Ulevitch R J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989;264:10867–10871. [PubMed] [Google Scholar]
- 45.Todd R F, III, Van Agthoven A, Schlossman S F, Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1:329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocyte leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 47.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 48.Walz B, Stevens C, Zanker B, Melton L, Clark S C, Suthanthiran M, Storm T B. The role of interleukin-6 in mitogenic T-cell activation: detection of interleukin-2 heteronuclear RNA by polymerase chain reaction. Cell Immunol. 1991;134:511–519. doi: 10.1016/0008-8749(91)90322-3. [DOI] [PubMed] [Google Scholar]
- 49.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 50.Wooten R M, Modur V R, McIntyre T M, Weis J J. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-kappa B and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 51.Wooten R M, Morrison T B, Weis J H, Wright S D, Thieringer R, Weis J J. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- 52.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]