Dear Editor
We read with great interest the paper by Orviz et al.1 about human Monkeypox (MPX), a neglected zoonosis caused by an Orthopoxvirus identified as responsible of human disease in West and Central Africa where viral transmission occurs through contact with animals or - in the case of human-to-human transmission - by close and prolonged direct contacts or exposure to respiratory droplets and fomites2. Since 2003, a limited number of outbreaks of Monkeypox virus (MPXV) in non-endemic countries were reported3, 4. In early 2022, many reports outlined an unprecedented global outbreak of human MPX featuring unusual characteristics such as the absence of a epidemiological link with endemic regions, the predominant involvement of men-who-have-sex-with-men (MSM) and unusual clinical presentation5, 6. We aimed to characterize the clinical course of human MPX in subjects attending a sexual health clinic with a retrospective observational study enrolling confirmed cases between May-July 2022.
Demographic/epidemiological and clinical characteristics were collected as well as a plasma sample and swabs taken from the oropharynx and skin lesions. Initial screening real-time PCR targeting variola virus and non-variola Orthopoxvirus species was used, followed by a homemade real-Time PCR for MPXV confirmatory test7.
Characteristics of the 32 enrolled patients are shown in Table 1 . MPXV was identified in 51 (56%) skin lesions’ swabs, from 29 (32%) oropharynx swabs and from 11 (12%) plasma samples.
Table 1.
Epidemiological characteristics of the study population.
| Characteristics | N = 32 |
|---|---|
| Age, median [IQR] | 38 [34–42] |
| Male biological sex, n (%) | 32 (100) |
| Self-identified as MSM, n (%) | 32 (100) |
| Comorbidities other than HIV, n (%) | 3 (9.4) |
| PLWH (n = 17), n (%) | 17 (53.1) |
| HIV-RNA 50 cp/mL, n (%) | 16 (94.1) |
| CD4, median [IQR] | 678 [526, 933] |
| On PrEP (n = 15), n (%) | 8 (25) |
| Previous STIs, n (%) | 27 (84.4) |
| Concomitant STIs, n (%) | 7 (21.9) |
| Smallpox vaccination, n (%) | 2 (6.2) |
| Travel outside Italy in the 30 days before symptoms onset, n (%) | 6 (18.7) |
| Presumed nation of MPXV acquisition, n (%) | |
| Italy | 26 (81.2) |
| France | 2 (6.2) |
| Germany | 2 (6.2) |
| Spain | 1 (3.1) |
| Switzerland | 1 (3.1) |
| Transmission rank†, n (%) | |
| Isolated | 21 (65.6) |
| Index | 4 (12.5) |
| Secondary | 7 (21.9) |
| Self-reported sexual activity in the last month, n (%) | |
| Unprotected sexual intercourse | 25 (78.1) |
| Protected sexual intercourse only | 4 (12.5) |
| Protected sexual intercourse but unprotected oral sex | 3 (9.4) |
List of abbreviations: n, number; IQR, inter quartile range; PLWH, people living with HIV; PrEP, pre-exposure prophylaxis; STIs, sexual transmitted infections; MPXV, monkeypox virus.
According to the classification applied by Adler et al. [14].
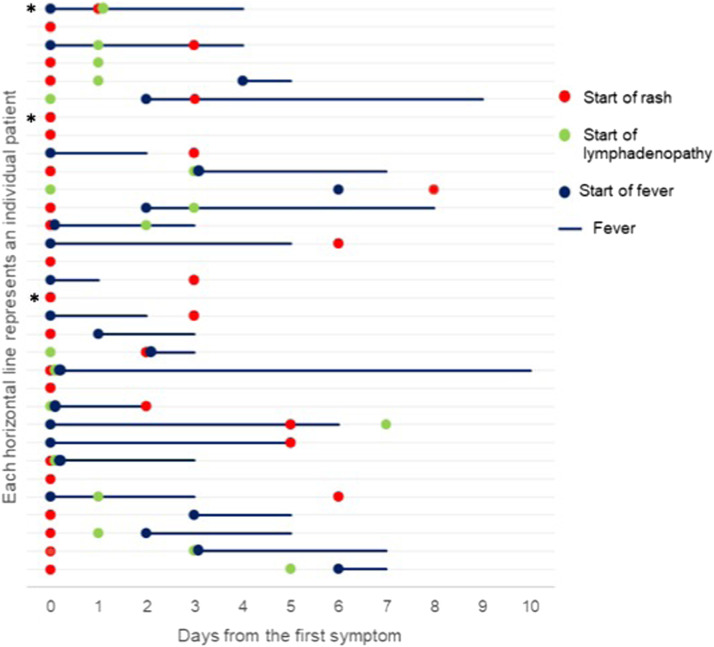
A presumed date of MPXV acquisition was available for 16 patients with an estimated median incubation period was 11 days (IQR 11–16). A graphical representation of illness is presented in Fig. 1 . Rash was the first symptoms in half of individuals (50%), followed by fever (28.1%) and lymphadenopathy (9.4%). Other symptoms were common (71.9%), represented by fatigue, asthenia and malaise (62.5%), headache (21.9%), sore throat (18.7%) and back pain (15.6%).
Fig. 1.
Graphical representation of illness progression among individuals with monkeypox virus infection starting from the first symptoms onset among fever, rash and lymphadenopathy*. Each line represents one of the 32 subjects included in the study. * In three subjects other systemic symptoms comprehensive of fatigue, asthenia, malaise, back pain, abdominal symptoms, sore throat and headache were reported 1–4 days preceding fever and/or rash and/or lymphadenopathy onset. In all other cases when other systemic symptoms were presented were contextual to one among fever, rash and lymphadenopathy.
In 15 cases (46.9%) the rash started from the perianal region, in 13 cases (40.6%) started from the genital area and in three cases (9.4%) from face and oral cavity subsequently involving the perianal (56.2%) and genitals (46.9%) areas. All skin lesions were asynchronous classified as benign according to the rash extension (≤25 umbilicated vesicles evolving into pustules).
Fever was present in 75% of cases with a median duration of 3 days (IQR 2–4). Lymphadenopathy was present in 18 patients (56.2%), especially in inguinal area (n = 10, 55.6%).
When compared to those living without HIV, PLWH showed similar epidemiological characteristics (Supplementary Table 1), clinical manifestation and disease course (Supplementary Table 2).
The median time of skin lesions healing was 17 days (IQR 15–24 days) with a complete restitution ad integrum. Since symptoms onset, the median time of resolution was 20 days (IQR 16–25). Seven patients (21.9%) showed a complication by ulcerations and pharyngitis. Four (12.5%) subjects required hospital admission because of such complications.
The demographic and epidemiological characteristics of our population are similar to those noted in recent with speculation about the possible link to a transmission chain related to sexual intercourse and/or close interpersonal contact. This hypothesis recalls the cases of Vaccinia virus transmission via sexual intercourse with a smallpox vaccinee occurred in USA9 and puts a new light to the high rate of genital lesions (68%) observed in Nigeria4. The frequent involvement of the genital and perianal region that seems to be the hallmark of the present multistate outbreak5, 6 was confirmed in our study with the majority of subjects experiencing the onset of the rash from the perianal region (46.9%) and genital area (40.6%). Interestingly, half of our cases manifested the rash as the first clinical sign again in contrast with the human MPX where rash was preceded by a prodromal febrile10. A similar observation has been previously made by Reynolds et al. during the 2003 US; in particular, in cases with noninvasive exposure (i.e., close contact), the clinical course was characterized by a short prodrome phase (2–3 days) with fever, chills, lymphadenopathy, headache, sore throat, myalgia, and gastrointestinal symptoms, followed by the onset of the rash which seems to resemble the classical human MPX description. On the contrary, for patients with a complex exposure (i.e., animal's bite or scratch) the rash usually preceded the acme of febrile illness3. The evolution of the rash observed in our study is also different from the classical human MPX usually presenting with monomorphic and synchronous vesicular umbilicated lesions. Our speculation is that sexual intercourse could be considered as a “complex exposure” leading to an atypical MPXV inoculation.
In our study we observed a benign course of the disease with complications occurring in seven cases (21.9%) mainly related to the ulceration and superinfection of skin or genital and perianal lesions in line with what has been observed during the present outbreak5, 6, 7, 8, with only one patient requiring antiviral therapy with cidofovir due to the extensive location of an ulcerative lesion in the nose. One potential explanation of the limited number of serious is the prevalent involvement of young individuals which showed in our study only living with HIV as major comorbidity (53.1%), of whom all on effective antiretroviral treatment showing a good immunological situation; in fact, we did not observe any difference when compared to those living without HIV. The higher risk of disseminated vaccinia and severe MPX10 seems to be relegated in times when effective antiretroviral treatment was not widely implemented. In our study we observed a median of 17 days from symptoms onset to the complete healing of the skin lesions.
In our study, MPX usually features genital and perianal rash as first symptoms of presentation. The course of the disease is generally benign and self-limiting. The characteristics of the rash onset, location and disease natural history observed in our study suggests a potential “complex exposure” to MPXV occurred during close contacts or sexual intercourses.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
D. Moschese, G.P., A.G., M.V.C., M.B..: writing-original draft preparation; D. Moschese, A.G., S.A., G.R.: writing—review and editing; D. Moschese, G.P., M.B.: were directly involved in the patient care; D. Mileto, A.R., M.R.G., performed the microbiological assays; D. Moschese, A.G., S.A., G.R.: reviewed and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Ethical considerations
The study was approved by the Comitato Etico Milano Area 1 (protocol number n. 2022/ST/124). All patient signed written informed consent including consent for pictures and reports.
Declaration of Competing Interest
All authors: No reported conflicts of interest related to the present manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.08.019.
Appendix. Supplementary materials
References
- 1.Orviz E., Negredo A., Ayerdi O., Vázquez A., Muñoz-Gomez A., Monzón S., Clavo P., Zaballos A., Vera M., Sánchez P., Cabello N., Jiménez P., Pérez-García J.A., Varona S., Del Romero J., Cuesta I., Delgado-Iribarren A., Torres M., Sagastagoitia I., Palacios G., Estrada V., Sánchez-Seco M.P. Grupo Viruela del Simio Madrid CNM/ISCIII/HCSC/Sandoval. Monkeypox outbreak in Madrid (Spain): clinical and virological aspects. J Infect. 2022 doi: 10.1016/j.jinf.2022.07.005. Jul 10S0163-4453(22)00415-7Epub ahead of print. PMID: 35830908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marennikova S.S., Seluhina E.M., Mal'ceva N.N., Cimiskjan K.L., Macevic G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972;46(5):599–611. [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds M.G., Yorita K.L., Kuehnert M.J., et al. Clinical manifestations of human Monkeypox influenced by route of infection. J Infect Dis. 2006;194(6) doi: 10.1086/505880. 773-80PMID: 16941343. [DOI] [PubMed] [Google Scholar]
- 4.Ogoina D., Iroezindu M., James H.I., et al. Clinical course and outcome of human Monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–e214. doi: 10.1093/cid/ciaa143. PMID: 32052029. [DOI] [PubMed] [Google Scholar]
- 5.Mileto D., Riva A., Cutrera M., et al. New challenges in human monkeypox outside Africa: a review and case report from Italy. Travel Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102386. Jun 20Epub ahead of print. PMID: 35738529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S., Apea V., Boesecke C., Vandekerckhove L., Yakubovsky M., Sendagorta E., Blanco J.L., Florence E., Moschese D., Maltez F.M., Goorhuis A., Pourcher V., Migaud P., Noe S., Pintado C., Maggi F., Hansen A.E., Hoffmann C., Lezama J.I., Mussini C., Cattelan A., Makofane K., Tan D., Nozza S., Nemeth J., Klein M.B. Orkin CM; SHARE-net clinical group. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022 doi: 10.1056/NEJMoa2207323. Jul 21Epub ahead of print. PMID: 35866746. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC Duration of isolation procedures for human Monkeypox. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html, 2022. Accessed 15 July 2022.
- 9.Centers for Disease Control and Prevention (CDC) Vulvar vaccinia infection after sexual contact with a military smallpox vaccinee–Alaska, 2006. MMWR Morb Mortal Wkly Rep. 2007;56(17):417–419. 4PMID: 17476203. [PubMed] [Google Scholar]
- 10.Di Giulio D.B., Eckburg P.B. Human Monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.