Abstract
Orthopoxvirus monkeypox (MPXV) forms two distinct clades: the MPXV Congo Basin clade viruses are endemic in the Congo Basin, human illness typically presents with symptoms similar to discrete, ordinary smallpox and has a case fatality rate of approximately 10% in unvaccinated populations; the MPXV West African clade viruses have been isolated in West Africa and appear to cause a less severe, and less inter-human transmissible disease. Recently, monkeypox outbreaks were reported in US and Sudan caused by MPXV West African and Congo Basin strains respectively. These events demonstrated the ability and trend of the virus to exploit new hosts and emerge globally; it also emphasizes the need for the diagnosis of MPXV, especially the ability to distinguish between Congo Basin and West African monkeypox strains. In this study, three new real-time PCR assays based on TaqMan probe technology were reported: the MPXV West African specific, Congo Basin strain specific and MPXV generic assays. The new assays demonstrated good specificity and sensitivity in the validation study with multiple platforms and various PCR reagent kits, and will improve the rapid detection and differentiation of monkeypox infections from other rash illness.
Abbreviations: TNF, tumor necrosis factor; Ct, threshold cycle
Keywords: Monkeypox virus West African strain, Monkeypox virus Congo Basin strain, Real-time PCR
Monkeypox, a disease caused by the orthopoxvirus Monkeypox virus (MPXV), was discovered in 1958 from captive primate rash specimens (Magnus et al., 1959). In 1970, MPXV was first recognized to cause human illness in Africa during intensification of the smallpox eradication campaign (Ladnyi et al., 1972). The genome sequence and clinic-epidemiologic comparisons of MPXV isolates show that MPXV forms two distinct clades: human Monkeypox from MPXV strain of Congo Basin typically presents with symptoms similar to discrete, ordinary smallpox. After about a two-week, asymptomatic incubation period, infected individuals develop fever followed by disseminated rash. Congo Basin monkeypox disease has clearly been demonstrated to be transmissible between humans and can result in death. The case fatality rate is on average ∼10% in non-vaccinated individuals (Jezek and Fenner, 1988). Today, monkeypox continue to be an endemic disease in Congo Basin region and present as an important threat to public health. There is limited reported human monkeypox disease in West Africa which overall, is comparably less severe and demonstrates less human-to-human transmission than that from Congo Basin.
Recently, human monkeypox has emerged in nontraditional MPXV endemic areas. A MPXV West African strain caused the monkeypox outbreak in the U.S. in 2003; a broad range of species, including humans (Hutson et al., 2007) were infected, and the source of the outbreak was traced to the importation of MPXV infected West African rodents (Reed et al., 2004). In 2005, a monkeypox outbreak was reported in southern Sudan, where disease has never before been reported (Damon et al., 2006). DNA sequence analysis suggested that this latter outbreak was caused by a MPXV Congo Basin-like strain. The monkeypox outbreak in the US Midwest and Sudan demonstrated the ability of the virus to exploit new hosts and move globally. In recent monkeypox outbreaks, real-time PCR has become a critical tool to diagnose and monitor MPXV infections/disease due to the advantages of fast, high-quantity throughput and increased sensitivity of the assay format. Real-time PCR assay has also been used to study the virus shedding and infection course in MPXV infected animal model studies. Several MPXV generic real-time PCR assays (Li et al., 2006, Kulesh et al., 2004, Olson et al., 2004) have been developed to differentiate monkeypox virus from other orthopoxviruses either directly or using with melting curves to differentiate different orthopoxviruses after PCR amplifications. DNA sequencing after a PCR amplification is needed to differentiate MPXV West African and Congo Basin strains (Saijo et al., 2008, Meyer et al., 1997).
In this report, two new real-time PCR assays which detect specifically MPXV Congo Basin strain and MPXV West African strains, and a new MPXV generic assay which works on a broad range of real-time PCR platforms are described. The challenges to develop robust clade-specific as well as generic MPXV specific assays are to find the differentiating target sequences. MPXV Congo Basin and West African strains share 99% sequence identity (Likos et al., 2005). Other orthopoxviruses also share considerable sequence identity to MPXV (>90%) (Li et al., 2007) and further limit the sequence availability for specific assay design. The selection of the target sequences for these new assays focused on the terminal regions which demonstrate greater sequence variations among MPXV isolates than that within the central region of the genome. Previous studies showed that the TNF receptor gene, located at the terminal inverted repeat region (ITR), contains a large number of single nucleotide polymorphism (SNPs) and insertion/deletions (Indels) between two clades of MPXV and among orthopoxviruses (Likos et al., 2005). An alignment of TNF receptor gene sequences from available orthopoxviruses was used for the selection of primers and probes. Using TaqMan technology, a MPXV West African specific assay (G2R_WA) (Fig. 1A) and a new MPXV generic real-time PCR assay (G2R_G) (Fig. 1B) were developed within TNF receptor gene. Previously, a MPXV generic B6R (MPXV envelope protein gene) real-time PCR assay utilizing a beacon based probe and the minor grove binding (MGB) based real-time PCR was developed and has been used for the specific detection of MPXV DNA during the 2003 US outbreak (Li et al., 2006). The limitation for MPXV B6R assay is that its performance is related to the specific real-time PCR platform; it performs optimally only in the iCycler iQ (Bio-Rad, Hercules, CA) and SmartCycler (Cepheid, Sunnyvale, CA) platform. In comparison, these TaqMan based real-time assays performed equally well in all platforms evaluated and the manufacture of the primers and probes is easily accomplished. Multiple MPXV specific assays, which span the genome, may be useful to suggest the possibility of DNA recombination between MPXV and other orthopoxviruses. Within the TNF receptor gene, MPXV Congo Basin viruses do not present specific sequences that are long enough to reach ideal probe annealing temperatures, and therefore an alternative region was identified to design a Congo Basin strain virus specific assay. A new target gene, the complement binding protein (C3L) gene, was used for the design of MPXV Congo Basin specific assay as all known MPXV West African strains delete C3L gene. The specificity of MPXV C3L assay was determined by sequences alignment between MPXV Congo Basin strains and other orthopoxviruses (Fig. 1C). The detection probe of all three assays contains a 5′ reporter molecule (FAM) (Glen Research, Sterling, VA) and a 3′ quencher molecule (BHQ1) (Molecular Probes, Eugene, OR). MPXV West African specific (G2R_WA) assay (Fig. 1A): forward primer (5′-CACACCGTCTCTTCCACAGA), reverse primer (5′-GATACAGGTTAATTTCCACATCG), probe sequence (5′FAM-AACCCGTCGTAACCAGCAATACATTT-3′BHQ1). MPXV Congo Basin specific (C3L) assay (Fig. 1B): forward primer (5′-TGTCTACCTGGATACAGAAAGCAA), reverse primer (5′-GGCATCTCCGTTTAATACATTGAT), probe sequence (5′FAM-CCCATATATGCTAAATGTACCGGTACCGGA-3′BHQ1). MPXV generic (G2R_G) assay (Fig. 1C): forward primer (5′-GGAAAATGTAAAGACAACGAATACAG), reverse primer (5′-GCTATCACATAATCTGGAAGCGTA), probe sequence (5′FAM-AAGCCGTAATCTATGTTGTCTATCGTGTCC-3′BHQ1).
Fig. 1.
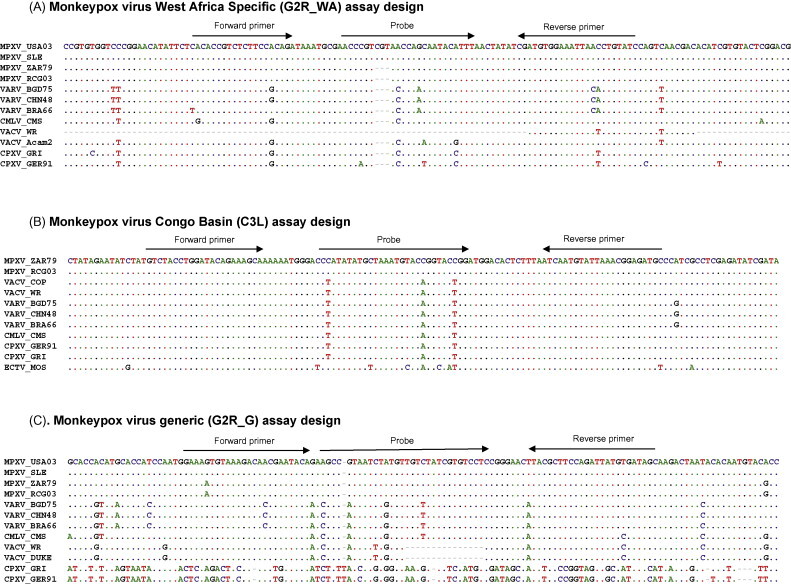
Alignment of primers and probes with orthopoxviral DNA.
The primers and probe for each of the real-time PCR assays are aligned with the targeted sequence of DNA within several orthopoxviral species. Virus strains: monkeypox West African strains MPXV_USA03, MPXV_SLE; monkeypox Congo Basin strains MPXV-ZAI79, MPXV_RCG03; variola major VARV_BGD75, VARV_CHN48; variola minor VARV_BRA66; camelpox CMLV_CMS, vaccinia VACV_WR, VACV_Acam2, VACV_DUKE; cowpox CPXV_GRI, CPXV_GER91; ectromelia Moscow ECTV_MOS. (A) The probe sequence of West African MPXV_G2R_WA assay has three bases deletion comparing to that of Congo Basin strains. (B) The MPXV West African strain lost complement binding protein C3L gene and are not in the sequences alignments. (C) The MPXV generic assay primers and probe are completely homologous within the MPXV DNA sequence except one SNPs in forward primer sequence.
A panel of MPXV and other species of orthopoxvirus DNAs isolated from infected cell culture were used for the validation of the new MPXV real-time PCR assays: including 7 MPXV Congo Basin strains and 4 MPXV West African strains, and 6 strains of other known Eurasian orthopoxviruses and 1 strain of North American orthopoxvirus (Table 1 ). Several types of samples from MPXV infected animal models, including liver and skin tissues, nasal and oral swabs, lesions and human monkeypox clinical samples were also used for the validation of the robust, specificity and sensitivity of the new assays. Origins, propagation, and harvesting procedures for viruses are documented elsewhere (Li et al., 2006, Esposito and Knight, 1985, Ropp et al., 1995). DNA extracted using the DNA was extracted from biopsy specimens with the Qiagen EZ1robot system (Qiagen, Valencia, CA), and stored at −20 °C.
Table 1.
Validation of MPXV generic and specific assaysa.
| Organism | Sample ID | MPXV real-time PCR assaysa |
||
|---|---|---|---|---|
| G2R_G | C3L | G2R_WA | ||
| Orthopoxvirus | ||||
| Monkeypox virus | ZAI79-005b | 22.9 | 25.13 | nd |
| ZAI81-167b | 25.28 | 25.91 | nd | |
| ZAI82-167b | 20.19 | 21.25 | nd | |
| ZAI83-36b | 20.98 | 20.48 | nd | |
| ZAI77-I823b | 24.42 | 24.2 | nd | |
| Cameron90b | 21.56 | 20.41 | nd | |
| Gabon88b | 28.01 | 30.23 | nd | |
| Sierra Leonec | 20.96 | nd | 20.73 | |
| Côte d’Ivoirec | 22.58 | nd | 23.15 | |
| Nigeria78c | 21.49 | nd | 21.81 | |
| USA03c | 28.19 | nd | 30.17 | |
| Variola virus | CNG70 | nd | nd | nd |
| Vaccinia virus | WR | nd | nd | nd |
| Camelpox virus | CMS | nd | nd | nd |
| Taterapox virus | DAT68 | nd | nd | nd |
| Cowpox virus | BRT | nd | nd | nd |
| Ectramelia virus | MOS | nd | nd | nd |
| Raccoonpox virus | MD61 | nd | nd | nd |
| Other rash illness or cellular DNA | ||||
| Varicella | OKa | nd | nd | nd |
| HSV-1 | HFEM | nd | nd | nd |
| Human | nd | nd | nd | |
| MPXV infected animal model study | ||||
| Swab from Congo Basin MPXV infected animals | ||||
| 25-4563 (lesion) | 24.26 | 22.76 | nd | |
| 25-4564 (liver) | 31.22 | 31.46 | nd | |
| 25-4566 (oral swab) | 17.01 | 17.16 | nd | |
| 30-4514 (skin) | 23.33 | 22.64 | nd | |
| 30-519 (lesion) | 25.66 | 25.25 | nd | |
| 30-4524 (liver) | 21.9 | 22.4 | nd | |
| 30-4539 (nasal swab) | 24.54 | 23.99 | nd | |
| 32-4535 (liver) | 19.4 | 18.66 | nd | |
| 32-4530 (lung) | 28.04 | 28.75 | nd | |
| 32-4540 (nasal swab) | 18.85 | 17.79 | nd | |
| Swab from West Africa MPXV infected animals | ||||
| 07-7116 (skin) | 37.54 | nd | 39.68 | |
| 07-0440 (nasal swab) | 38.72 | nd | 39.49 | |
| 08-0117 (skin) | 43.04 | nd | 43.14 | |
| 08-7135 (lesion) | 41.07 | nd | 42.48 | |
| 08-0451 (nasal swab) | 34.57 | nd | 34.81 | |
| 09-7118 (skin) | 28.99 | nd | 29.7 | |
| 09-7190 (oral swab) | 25.08 | nd | 25.87 | |
| 09-0442 (nasal swab) | 24.41 | nd | 24.51 | |
| 14-7123 (skin) | 32.19 | nd | 32.66 | |
| 14-0447 (nasal swab) | 28.62 | nd | 28.54 | |
| Human MPXV clinical samples | ||||
| 2003 US MPXV outbreak | ||||
| US03_086 4542 | 35.26 | nd | 35.34 | |
| US03_086 4553 | 27.35 | nd | 28.05 | |
| US03_086 4563 | 39.5 | nd | 39.3 | |
| Africa MPXV outbreak | ||||
| RCG03 358 4450 | 30.44 | 31.74 | nd | |
| RCG03 358 4451 | 17.09 | 17.41 | nd | |
| Sudan05 0016 | 24.06 | 25.41 | nd | |
| Sudan05 0017 | 26.74 | 27.4 | nd | |
The cycle when the fluorescence signal crossed the threshold (Ct) for each positive sample is shown. nd, not detected. Each reaction mixture contained 1× TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 0.4 μmol/L each primer, 200 nmol/L TaqMan probe, and 2 μL of template DNA. Thermal cycling for the ABI7900 was: one cycle 95 °C for 6 min; 45 cycles of 95 °C for 5 s, and 60 °C for 20 s for Monkeypox virus generic (G2R_G) assay and Monkeypox virus Congo Basin specific (C3L) assay; one cycle 95 °C for 6 min; 45 cycles of 95 °C for 5 s, and 62 °C for 20 s for Monkeypox virus West African specific (G2R_WA) assay.
MPXV Congo Basin strains, ZAI = Zaire.
MPXV West African strains.
All three new MPXV generic and specific assays did not cross-react with variola, cowpox, camelpox, taterapox, ectramelia, vaccinia and Raccoonpox virus DNA. The MPXV generic G2R_G assay reproducibly amplified all 11 different monkeypox viral DNAs (Table 1) and the MPXV specific G2R_WA and C3L assays reproducibly identified 4 West African monkeypox DNA and 7 Congo Basin monkeypox DNAs respectively in the initial validation study. The MPXV specific assays are especially useful in MPXV infected animal model study as infected animals may demonstrate unexpected symptoms and severity of diseases, the MPXV specific assays were used to confirm the initial viral challenge. All specimens from various animals infected with both MPXV Congo Basin and West African strains were specifically detected with respect to MPXV specific assays. Interestingly the samples from the animal models infected with MPXV Congo Basin strain consistently have a higher viral load (low Ct values) comparing to that of MPXV West African stains for the same types of samples as MPXV Congo Basin strain generally cause more severe disease in those animal models (Hutson et al., 2009). Anonymized specimen remainders from human monkeypox clinical samples were also used for the validation of the new MPXV real-time PCR assays: including West African MPXV like samples from 2003 US monkeypox outbreak; the Congo Basin MPXV like samples from Sudan MPXV outbreak in 2005, MPXV outbreak in Republic of Congo (RCG) of 2003 and Democratic Republic of Congo (DRC) MPXV outbreak in 2008 (Table 1). The MPXV G2R_G assay, MPXV G2R_WA and C3L specific assays are able to diagnose the corresponding MPXV West African and Congo Basin clinical samples specifically.
The efficiency of the new assays was measured by the triplicate assays of serially diluted DNA of purified and photometrically quantified MPXV Congo Basin isolate MPXV79_0005 and MPXV West African isolate MPXV Sierra Leone. The reaction efficiency for MPXV generic G2R_G assay, MPXV specific C3L assay, and MPXV specific G2R_WA assay are 90%, 89% and 84% respectively (data not shown). The higher efficiency of MPXV G2R assay (Table 1) manifests in the generally lower Ct values of tested DNA samples compared to that of the MPXV G2R_WA and MPXV_C3L assays. The probe sequence of MPXV G2R_WA assay contains three nucleotide Indels not present in the MPXV Congo Basin strains, and the annealing temperature of the MPXV G2R_WA assay was raised to 62 °C in the PCR cycling condition to achieve specificity (Table 1). The other two new assays used a default annealing temperature of 60 °C in their cycling conditions (Table 1). A higher annealing temperature for MPXV G2R_WA assay may have reduced the assay's efficiency. The MPXV genome contains two copies of G2R genes due to its location at the ITR terminal regions, which may, in turn, enhance the sensitivity of both the MPXV G2R_G and G2R_WA assays. The analytical sensitivity of the new assays were determined using purified, photometrically quantified MPXV Congo Basin and West African strain DNA (Li et al., 2006). DNAs were diluted serially from 5 ng to 0.05 fg. Probit regression analysis (SAS 9.2, SAS Institute, Cary, NC) determined the detection limit of each assay with 95% confidence: MPXV G2R_G assay at 0.7 fg (∼3.5 genomes); MPXV G2R_WA at 1.7 fg (∼8.2 genome), and MPXV_C3L at 9.46 fg (∼40.4 genomes). The new assays were validated in multiple platforms: ABI PRISM 7900/7500 FAST (Applied Biosystems, Foster City, CA), and Smart Cycler II system (Cepheid, Sunnyvale, CA) and with different real-time PCR Master Mix (TaqMan Universal PCR Master Mix (Applied Biosystems), qPCR SuperMix (Invitrogen, Carlsbad, CA) and SmartMix HM in bead format (Cepheid)).
In summary, a new MPXV West African strain specific, a new MPXV Congo Basin strain specific and a new MPXV generic real-time PCR assays were developed and validated. The new assays demonstrated the specificity and robustness for detection of MPXV DNA and enhanced the tools to diagnose and monitor the monkeypox outbreak and MPXV infections. The new MPXV West African specific and Congo Basin specific assays will be especially useful in the monkeypox endemic regions in Africa to confirm and differentiate monkeypox infections from other rash illness. Additionally, they can be of benefit for rapid detection and evaluation of the extent of disease, in multiple species, including humans, in the event of importations of disease causing outbreaks or epidemics.
Acknowledgments
The authors thank Christy Hutson for providing DNA sample from monkeypox virus infected animal models.
References
- Damon I.K., Roth C.E., Chowdhary V. Discovery of monkeypox in Sudan. N. Engl. J. Med. 2006;355:962–963. doi: 10.1056/NEJMc060792. [DOI] [PubMed] [Google Scholar]
- Esposito J.J., Knight J.C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143:230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Hutson C.L., Lee K.N., Abel J., Carroll D.S., Montgomery J.M., Olson V.A., Li Y., Davidson W., Hughes C., Dillon M., Spurlock P., Kazmierczak J.J., Austin C., Miser L., Sorhage F.E., Howell J., Davis J.P., Reynolds M.G., Braden Z., Karem K.L., Damon I.K., Regnery R.L. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am. J. Trop. Med. Hyg. 2007;76:757–768. [PubMed] [Google Scholar]
- Hutson C.L., Olson V.A., Carroll D.S., Abel J.A., Hughes C.M., Braden Z.H., Weiss S., Self J., Osorio J.E., Hudson P.N., Dillon M., Karem K.L., Damon I.K., Regnery R.L. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 2009;90:323–333. doi: 10.1099/vir.0.005108-0. [DOI] [PubMed] [Google Scholar]
- Jezek Z., Fenner F. In: Monographs in Virology. Melnick J., editor. Karger; Basel: 1988. Human, Monkeypox; pp. 1–140. [Google Scholar]
- Kulesh D.A., Loveless B.M., Norwood D., Garrison J., Whitehouse C.A., Hartmann C., Mucker E., Miller D., Wasieloski L.P., Jr., Huggins J., Huhn G., Miser L.L., Imig C., Martinez M., Larsen T., Rossi C.A., Ludwig G.V. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladnyi I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Org. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- Li Y., Carroll D.S., Gardner S.N., Walsh M.C., Vitalis E.A., Damon I.K. On the origin of smallpox: correlating variola phylogenics with historical smallpox records. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15787–15792. doi: 10.1073/pnas.0609268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., Davidson W., Galloway R., Khristova M.L., Reynolds M.G., Zhao H., Carroll D.S., Curns A., Formenty P., Esposito J.J., Regnery R.L., Damon I.K. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- Magnus P.V., Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Path. Microbiol. Scand. 1959;46:156–176. [Google Scholar]
- Meyer H., Ropp S.L., Esposito J.J. Gene for A-type inclusion body protein is useful for a polymerase chain reaction assay to differentiate orthopoxviruses. J. Virol. Methods. 1997;64:217–221. doi: 10.1016/S0166-0934(96)02155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson V.A., Laue T., Laker M.T., Babkin I.V., Drosten C., Shchelkunov S.N., Niedrig M., Damon I.K., Meyer H. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J. Clin. Microbiol. 2004;42:1940–1946. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., Swain G.R., Olson V.A., Sargent E.K., Kehl S.C., Frace M.A., Kline R., Foldy S.L., Davis J.P., Damon I.K. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Ropp S.L., Jin Q., Knight J.C., Massung R.F., Esposito J.J. PCR strategy for identification and differentiation of small pox and other orthopoxviruses. J. Clin. Microbiol. 1995;33:2069–2076. doi: 10.1128/jcm.33.8.2069-2076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., Ogata M., Fukushi S., Mizutani T., Iizuka I., Sakai K., Sata T., Kurata T., Kurane I., Morikawa S. Diagnosis and assessment of monkeypox virus (MPXV) infection by quantitative PCR assay: differentiation of Congo Basin and West African MPXV strains. Jpn. J. Infect. Dis. 2008;61:140–142. [PubMed] [Google Scholar]


