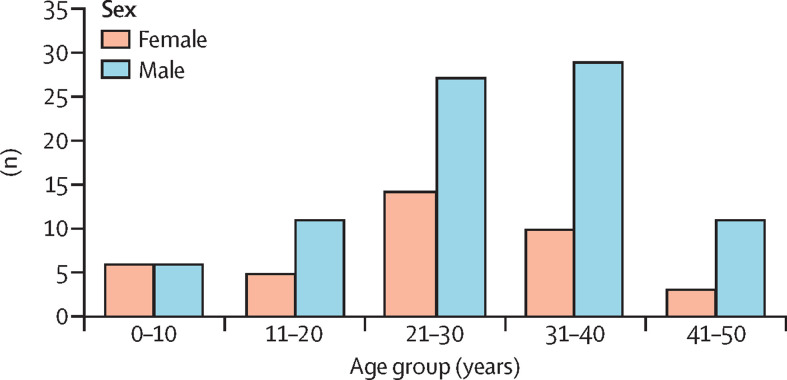Abstract
Background
In September, 2017, human monkeypox re-emerged in Nigeria, 39 years after the last reported case. We aimed to describe the clinical and epidemiological features of the 2017–18 human monkeypox outbreak in Nigeria.
Methods
We reviewed the epidemiological and clinical characteristics of cases of human monkeypox that occurred between Sept 22, 2017, and Sept 16, 2018. Data were collected with a standardised case investigation form, with a case definition of human monkeypox that was based on previously established guidelines. Diagnosis was confirmed by viral identification with real-time PCR and by detection of positive anti-orthopoxvirus IgM antibodies. Whole-genome sequencing was done for seven cases. Haplotype analysis results, genetic distance data, and epidemiological data were used to infer a likely series of events for potential human-to-human transmission of the west African clade of monkeypox virus.
Findings
122 confirmed or probable cases of human monkeypox were recorded in 17 states, including seven deaths (case fatality rate 6%). People infected with monkeypox virus were aged between 2 days and 50 years (median 29 years [IQR 14]), and 84 (69%) were male. All 122 patients had vesiculopustular rash, and fever, pruritus, headache, and lymphadenopathy were also common. The rash affected all parts of the body, with the face being most affected. The distribution of cases and contacts suggested both primary zoonotic and secondary human-to-human transmission. Two cases of health-care-associated infection were recorded. Genomic analysis suggested multiple introductions of the virus and a single introduction along with human-to-human transmission in a prison facility.
Interpretation
This study describes the largest documented human outbreak of the west African clade of the monkeypox virus. Our results suggest endemicity of monkeypox virus in Nigeria, with some evidence of human-to-human transmission. Further studies are necessary to explore animal reservoirs and risk factors for transmission of the virus in Nigeria.
Funding
None.
Introduction
Human monkeypox is a zoonotic smallpox-like illness caused by the monkeypox virus, which belongs to the Orthopoxvirus genus. This genus also comprises variola virus (the causative agent of smallpox), vaccinia virus, and cowpox virus.1, 2, 3 The first recognised human case was in a boy aged 9 months in DR Congo in 1970.3 Between 1970 and 2017, several outbreaks and sporadic cases have been reported in several areas of central and west Africa, including Cameroon, Central African Republic, Congo Brazzaville, Côte d'Ivoire, DR Congo, Gabon, Liberia, Nigeria, Sierra Leone, and South Sudan.4, 5, 6 The first outbreak outside Africa occurred in the USA in 2003, where 47 human cases were attributed to close contact with prairie dogs infected by rodents imported from Ghana.1, 7 After the eradication of smallpox in 1980, monkeypox has gradually emerged as the most important orthopoxvirus from a public health perspective, because it is the most prevalent in human beings.1, 2, 6, 7, 8
Two distinct clades of monkeypox virus were responsible for the outbreaks in central and west Africa: the Congo Basin clade and the west African clade, respectively.1, 8 Initially, human-to-human transmission had been documented only for the Congo Basin clade, which is also associated with more severe disease and higher case fatality than the west African clade.1, 9, 10 However, in Nigeria in 1971, two cases of the west African clade were reported in a 4-year-old girl and her mother, who both lived in Ihie Umduru (present-day Abia state). The presumption was that the child transmitted the disease to her mother.5, 11 Although information about monkeypox in Nigeria is scarce, a third case was documented in a man aged 35 years in Omifunfun (Oyo state) in 1978.5, 11
Research in context.
Evidence before this study
We searched Google Scholar, MEDLINE, Embase, African Journals Online, Web of Science, the Cochrane database, and infectious diseases and public health journals with the medical subject heading terms “monkeypox”, “human monkeypox”, “monkeypox outbreaks”, “West African clade monkeypox”, “clinical and epidemiological characteristics”, “transmission”, “risk factors”, and “treatment and outcomes” for articles published in English up to March 8, 2019. According to previous studies, the west African clade of monkeypox is rare and associated with milder disease than the Congo Basin clade, which is predominantly found in central Africa. No direct evidence of human-to-human transmission of the west African clade had been reported. The number of cases of monkeypox reported in Africa has increased in the past decade.
Added value of this study
Our study provides detailed clinical and epidemiological data for the 2017–18 outbreak of monkeypox virus infection in Nigeria, which is the largest known outbreak of the west African clade of monkeypox virus to date. Genomic analysis of seven samples from Rivers state, combined with contact information, suggested that there have been multiple introductions into the human population, with some evidence of human-to-human transmission. Seven people with monkeypox virus infection died, four of whom had HIV infection. By contrast with previous outbreaks, which mostly affected children and rural dwellers, cases were mostly young adults and people living in urban or peri-urban areas.
Implications of all the available evidence
Human monkeypox is no longer a rare disease and is a public health concern. Our findings have implications for clinical and public health responses to human monkeypox outbreaks, with respect to contact follow-up, infection prevention and control measures, and assessment of comorbidities. A sensitive human and animal disease surveillance system is required.
On Sept 22, 2017, the Nigeria Centre for Disease Control was notified of a suspected case of monkeypox in an 11-year-old boy, who presented with an 11-day history of fever, malaise, and progressive appearance of a vesiculopustular rash on his skin and oral and nasal mucosae, with associated generalised lymphadenopathy. The Nigeria Centre for Disease Control thereafter activated a national outbreak response, including enhanced monkeypox surveillance.12 Subsequent cases were reported in multiple states—the first cases in nearly four decades—in what would become the largest documented outbreak of the west African clade, despite monkeypox not being a priority disease in Nigeria's Integrated Disease Surveillance and Response framework.13 Sporadic cases continue to be recorded in the country, often without known epidemiological linkages.14 In this Article, we describe the clinical and epidemiological features of the 2017–18 human monkeypox outbreak in Nigeria.
Methods
Data collection
In this epidemiological report, we used data from Sept 22, 2017, to Sept 16, 2018, to generate evidence about the burden of west African clade monkeypox in Nigeria, to describe the clinical and epidemiological features of the disease, and to explore potential human-to-human transmission. After detection of the first suspected case, the Nigeria Centre for Disease Control developed standard case definitions (panel ) and interim guidelines based on previous outbreak reports from DR Congo and the USA.1, 15 Case-based surveillance was instituted, and an electronic Surveillance Outbreak Response and Analysis System introduced to improve digitalisation and timeliness of the surveillance system.16
Panel. Case definintions used during the 2017–18 Nigeria human monkeypox outbreak.
-
•
Suspected case: any person presenting with a history of sudden onset of fever, followed by a vesiculopustular rash occurring mostly on the face, palms, and soles of feet
-
•
Confirmed case: any suspected case with laboratory confirmation (ie, viral identification by real-time PCR, antibody detection, or viral isolation)
-
•
Probable case: any suspected case in whom laboratory testing could not be done but who could be epidemiologically linked with a confirmed case
-
•
Case: any probable or confirmed case
-
•
Contact person: any person who has no symptoms but had been in physical contact with a suspected case or with body fluids (ie, skin secretions, oral secretions, urine, faeces, vomitus, or blood) of a case in the past 3 weeks
-
•
Monkeypox death: any death in a confirmed monkeypox case that occurred during the course of monkeypox rash illness and that has no other suggestive cause of death
A detailed and standardised case investigation form (appendix pp 1–5) was developed on the basis of available literature and guidelines for human monkeypox.5, 6, 12 The form, which was used to collect clinical and epidemiological data, was completed for all suspected cases of human monkeypox by clinicians, who clinically examined patients, and surveillance officers (public health officers employed by local and state government to work with the community and health facilities to detect and report priority diseases) and epidemiologists, who reviewed patients' clinical records. A paper-based form was used, which was later complemented with the electronic surveillance system. Data collected included sociodemographic characteristics, clinical symptoms and signs, smallpox vaccination history, detailed contact history, and other surveillance-related information. In many cases, clinical photographs of rashes and other lesions were also documented (after obtaining patients' verbal consent). All data were collected for the purpose of diagnosis and outbreak control as part of constitutional disease control procedures in Nigeria. Thus, specific ethics approval was not required.
Laboratory investigations
At least one type of specimen (either blood, lesion swab, or crust [ie, dried rash debris—serum, pus, or blood—on the skin surface) was collected from each patient for investigation. Real-time PCR, serology, and culture were done on the samples. The appendix (p 6) contains information about specimen processing. In the first 6 weeks of the outbreak, PCR testing was done at Institut Pasteur de Dakar (Dakar, Senegal), Redeemer's University (Ede, Nigeria), and the US Centers for Disease Control and Prevention (Atlanta, GA, USA). Thereafter it was done at the National Reference Laboratory of the Nigeria Centre for Disease Control (Abuja, Nigeria). Serology and culturing (which was optional for confirmation) were done at the US Centers for Disease Control and Prevention. Specimens were also tested for varicella zoster virus at the National Reference Laboratory. A specimen was considered positive for monkeypox virus if there was viral identification by PCR, antibody detection (IgM), or viral isolation. The date of collection of the specimen relative to disease onset was taken into consideration when interpreting results. IgM has been detected in samples collected between 5 and 56 days after onset of disease.17
Whole-genome sequencing was attempted on some samples that tested positive on real-time PCR at the US Centers for Disease Control and Prevention. We used a targeted hybridisation protocol to enrich monkeypox virus DNA from clinical samples. The captured and amplified DNA was sequenced with the Illumina MiSeq (San Diego, CA, USA) benchtop platform. We used Sanger sequencing to fill gaps. Whole-genome sequences were assembled using methods outlined previously.18 All generated sequences were submitted to GenBank (accession numbers MK783027–MK783033).
Statistical analysis
We calculated means with SDs for continuous data, and percentages for nominal data. A quantum geographical information system was used to create case distribution maps. Single-nucleotide polymorphism data matrices with gaps and ambiguous bases removed were analysed with PopART and a minimum spanning network algorithm.19, 20 Published data for disease progression and mutations between transmission events were used to interpret molecular data in light of epidemiological evidence.21, 22, 23 Haplotype analysis results, genetic distance data, and epidemiological data were used to infer a likely series of events for potential human-to-human transmission of the west African clade of monkeypox virus, and to investigate the hypothesis of multiple introductions from wildlife during the outbreak. We set an a-priori threshold of ten mutations (one log higher than the number of mutations accumulated in the transmission event identified by Likos and colleagues23). Below this threshold, human-to-human transmission could not be ruled out. Above this threshold, if there was no epidemiological link between cases, we assumed that separate introductions from wildlife had occurred. We used Epi Info (version 7) and Microsoft Excel 2016 for all analyses.
Role of the funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
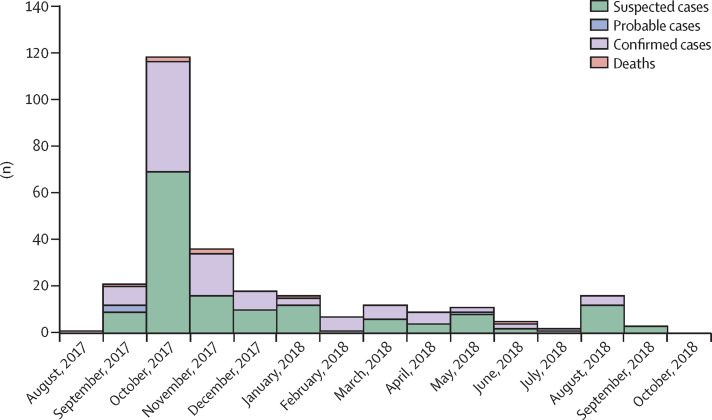
Between Sept 11, 2017, and Sept 16, 2018, we identified 276 suspected cases of human monkeypox, including 118 (43%) confirmed cases, four (1%) probable cases, and seven (3%) deaths. In the 253 patients for whom laboratory testing of samples was done, 104 (41%) had positive PCR results and a further 14 (6%) had positive IgM results only. Among the remaining 135 (53%) patients negative for monkeypox virus, 15 (11%) were positive for orthopoxvirus IgG. 90 (74%) of the 122 confirmed or probable cases were reported in the last quarter of 2017, and 32 (26%) were reported in 2018 (the last before our data cutoff was reported on Sept 16, 2018). 47 cases were recorded in October, 2017—the highest number of cases in 1 month, and the time when the public was first notified of the disease. The number of confirmed notifications fell progressively in subsequent months (figure 1 ).
Figure 1.
Temporal distribution of suspected, confirmed, and probable human monkeypox cases in Nigeria, 2017–18 (n=276)
The median age of the 122 confirmed or probable cases was 29 years (IQR 14; range 2 days to 50 years), and 84 (69%) were male (figure 2 ). Complete information about occupation was available for 91 (75%) confirmed or probable cases: 24 (26%) were traders, 18 (20%) were students, 14 (15%) were artisans (eg, carpenters, masons, welders, mechanics), five (5%) were farmers, five (5%) were prison inmates, four (4%) were teachers, four (4%) were housewives, four (4%) were health workers (including two doctors directly involved in the management of confirmed cases), two (2%) were religious leaders, two (2%) were factory workers, one (1%) was a naval officer, one (1%) was a security guard, and seven (8%) were children.
Figure 2.
Confirmed human monkeypox cases in Nigeria by age and sex, 2017–18 (n=122)
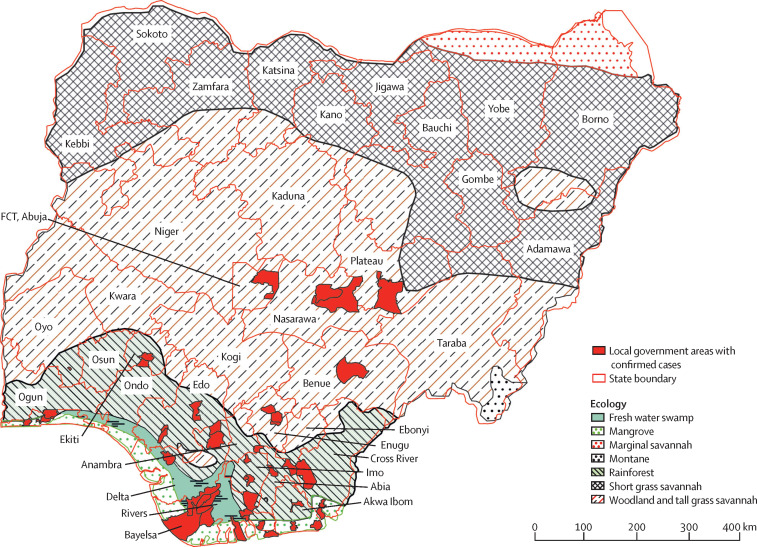
At least one suspected case was reported in 26 (70%) of the 37 Nigerian states and federal territories, and at least one confirmed case was reported in 17 (46%; figure 3 ). 66 (54%) of the 122 confirmed or probable cases were reported in three states whose terrain is characterised by fresh water swamps and mangrove vegetation areas, 47 (39%) were reported in 11 states characterised by rainforest terrain, and eight (7%) were reported in states with primarily savannah vegetation (figure 3).
Figure 3.
Geographical distribution of confirmed human monkepox cases in Nigeria, 2017–18 (n=122)
Discrepancies between state boundaries and the Nigerian border are because ecological data come from a different source and were further georeferenced and digitised. The white area in Borno state is Lake Chad, which was not included as an ecological zone. Discrepancies in the south are caused by the presence of creeks and the shoreline, and the different versions of boundary layers that were available. FCT=Federal Capital Territory.
In the 17 states with at least one confirmed case, cases were recorded in 48 (6%) of 774 local government areas (mean number of confirmed cases per local government area was three [SD 4]). Most cases were reported in the urban local government areas of affected states (figure 3). 41 (34%) of all confirmed or probable cases were reported in two local government areas in Rivers and Bayelsa states. Additionally, the Nigeria Centre for Disease Control received reports in September, 2018, of two people with monkeypox virus infection in the UK, and, in October, 2018, of one person with monkeypox virus infection in Israel, all of whom had returned from travel in Nigeria. These three cases were not included in the subsequent analysis.
Of the 122 confirmed or probable cases, 36 (30%) had an epidemiological link with people with similar lesions before the onset of monkeypox. Of these 36 people, 12 (33%) were epidemiologically linked with a confirmed case. Seven (58%) of these 12 people shared a household or had intimate contact with a confirmed case, four (33%) were inmates in the same prison as a confimed case, and one was a health worker who treated a confirmed case. Dates of first contact and dates of disease onset were available for 12 cases. The time from first contact to disease onset ranged from 3 days to 34 days (mean 13 [SD 9]; median 9·5 days [IQR 11]). Clustering of cases was noted in households and in a prison facility, but we did not identify an epidemiological link between these clusters. The largest household cluster was in a household of six members, three of whom had confirmed monkeypox and three of whom had probable disease, which developed in a 27-day period. Among all confirmed cases, ten patients reported contact with animals (two with monkeys, two with rodents, two with unspecified wild animal [consumed as meat—ie, bush meat], and four with domestic animals). No one reportedcontact with sick or dead animals.
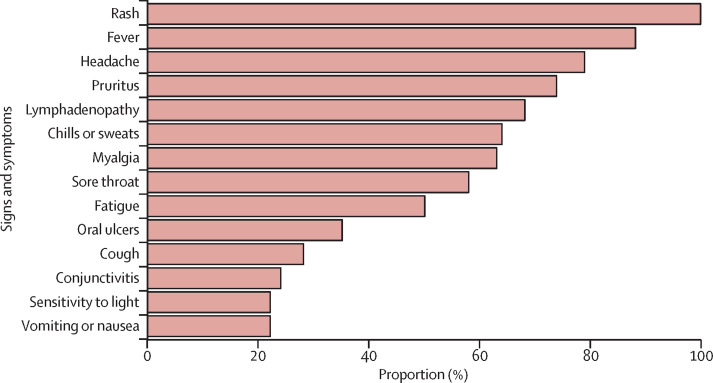
All 122 confirmed or probable cases had vesiculopustular rash. 81 (88%) of 92 cases with available data had fever, 61 (79%) of 77 cases had headache, 57 (73%) of 78 cases had pruritus, 45 (69%) of 65 cases had lymphadenopathy, 42 (63%) of 67 cases had myalgia, and 45 (58%) of 77 cases had sore throat (figure 4 ). Fever preceded rash in only 46 (57%) of the 81 patients with fever. Rashes were reported all over the body, but the most affected parts were the face (in 68 [96%] of 71 cases with available data), legs (63 [91%] of 69), trunk (56 [80%] of 70), arms (55 [79%] of 70), palms (48 [69%] of 70), genitalia (44 [68%] of 65) and soles of feet (42 [64%] of 66). The case investigation form did not explicitly include assessment of the scalp, yet aneccdotal reports from clinicians and available clinical photographs suggest that the scalp was also frequently affected. Spontaneous abortion at 26 weeks' gestation was recorded in one woman who was pregnant during her illness.
Figure 4.
Frequency of signs and symptoms in people with confirmed monkeypox virus infection in Nigeria, 2017–18
Percentages were calculated individually for each symptom on the basis of the number of patients with available data.
Seven people with monkeypox virus infection (five male, two female; mean age 27 years [SD 14]) among the 118 confirmed cases died while ill—a case fatality rate of 6%. According to information reported by the attending clinician, four of the people who died had HIV with features of AIDS. Of these four people, three had stopped antiretroviral therapy more than 3 months before monkeypox virus infection and one had never been on antiretroviral therapy. The four people with HIV rapidly deteriorated and died, but information about the exact cause of death or the presence of concomitant opportunistic infections in addition to monkeypox was not available. Two of the other deaths occurred after secondary bacterial infection of the monkeypox skin lesions, with associated features of sepsis as diagnosed by the managing clinicians. The seventh death was recorded in a baby aged 1 month, whose mother had died from a similar illness (suspected monkeypox) 2 weeks previously.
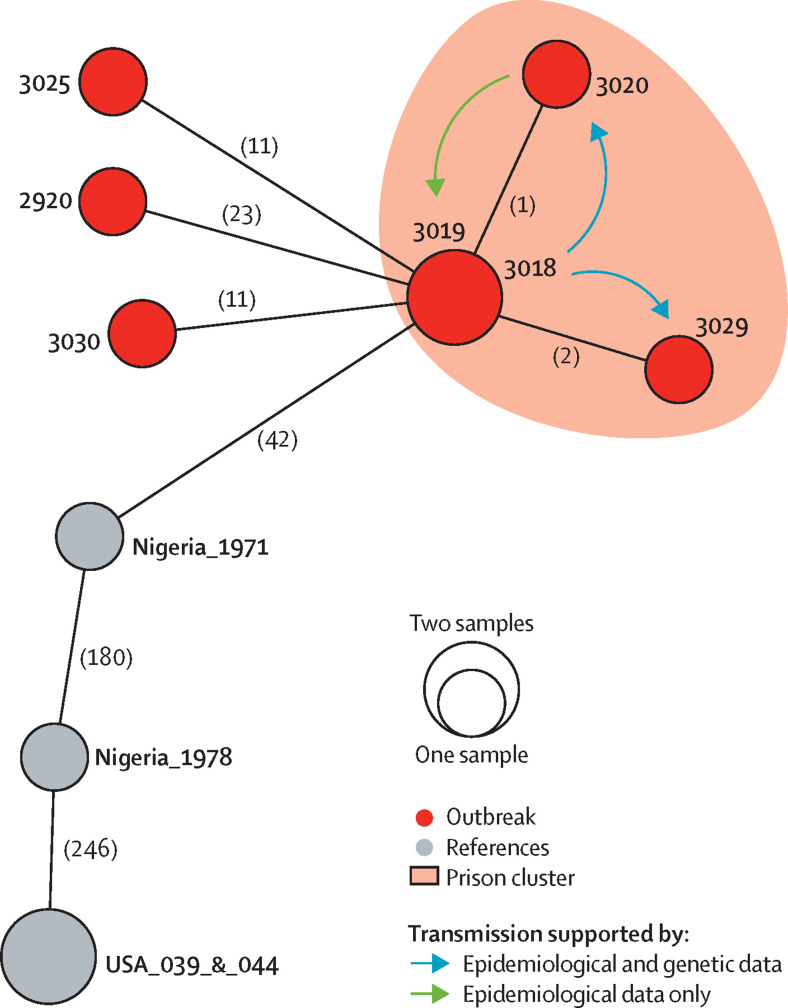
Whole-genome sequencing was attempted on nine samples (six swabs and three serum samples), and sequences were successfully generated for seven samples (six swabs and one serum sample). The seven samples were from Rivers state, and all had more than 42 mutations' difference from a sample taken during the 1971 human monkeypox outbreak in Nigeria (figure 5 ). These seven cases included four cases from a prison facility. Sequences from case numbers 3018 and 3019 were identical, and those from case numbers 3020 and 3029 were one and two mutations apart, respectively (figure 5). Case 3018 was the first prison inmate to report symptoms and was a cellmate of case 3029. Case 3029 in turn had unspecified contact with case 3020 before either developed symptoms. Although epidemiological data suggest human-to-human transmission from case 3020 to case 3018, the reversal of the single mutation is unlikely. Therefore, we postulate that case 3019 became ill because of unreported exposure to case 3018 or to the source from which case 3018 became ill. The other three cases (2920, 3025, and 3030) were each at least 11 mutations different from the prison cases (figure 5). No epidemiological links were discovered between these three cases, or between these cases and the prison cases.
Figure 5.
Haplotype network of seven monkeypox samples from Rivers state, Nigeria, in 2017
Circles indicate unique haplotypes (genomic sequences), with smaller circles representing one sample and larger circles representing two samples with identical examined sequences. Numbers in parentheses along the branches show mutations between samples. The examined single-nucleotide polymorphism sequences 3018 and 3019 were identical, as were two samples (one human, one from a prairie dog) from the US outbreak in 2003, which were used as a reference. US samples have a single mutational difference, which was not identified in this analysis because of missing data at that nucleotide position for other samples.
Discussion
In this epidemiological study, we identified 122 confirmed or probable cases of human monkeypox during the 2017–18 Nigerian outbreak, which represents the largest ever documented outbreak of the west African clade of the virus. The heightened public awareness after the detection and confirmation of the index case and the initiation of monkeypox surveillance for the first time in Nigeria could largely account for the progressive detection of cases across the country. The wide geographical distribution of unrelated cases across various regions of the country and the genomic similarity between the viral strains of the 2017–18 and the 1971 human monkeypox outbreaks could suggest that the monkeypox virus has remained enzoonotic in Nigeria for many decades in an animal reservoir that has yet to be identified.24 Waning or loss of orthopoxvirus-related herd immunity from previous smallpox vaccination and possible increased human contact with monkeypox-related animal reservoirs facilitated by trade, deforestation, animal husbandry, and climate change, among others, are potential contributors to the re-emergence of the disease in Nigeria and other west African countries.8, 25, 26
Human monkeypox is known to be endemic in Africa, mainly in rainforest areas, and most outbreaks in endemic countries are reported in rural communities.5, 10 In our study, most cases occurred in the known ecological niche of the monkeypox virus (or its suspected reservoirs)—in the freshwater swamps and mangrove areas of the South South geopolitical zone—but some cases occurred in the central savannah vegetation areas of the Federal Capital Territory and Nasarawa state. The ecological niche of monkeypox virus in west Africa should be investigated further because additional data could alter or refine niche modelling results. The predominance of cases in urban areas could suggest the presence of an unrecognised risk factor for monkeypox that should be explored further.6, 27, 28
Genomic analysis of a subset of samples from Rivers state and epidemiologically assessed contact information suggested that there have been multiple introductions from animal reservoirs into the human population and that human-to-human transmission has occurred. Based on epidemiological data and mutational differences, the prison cluster that we identified is probably indicative of multiple human-to-human transmission events. Mutational differences captured in the haplotype analysis are within the a-priori threshold previously established for human-to-human transmission. Although human-to-human transmission of the west African clade of monkeypox virus was not reported in the 2003 US outbreak, our results seem to support concomitant primary zoonotic and human-to-human transmission due to clustering of cases within households and a prison facility. The occurrence of monkeypox in two health-care workers who developed the disease after treating confirmed cases provides further evidence of human-to-human transmission. Because animal surveys for viral reservoirs were not done, and risk factors related to animal exposure were not established, we can only speculate on a possible animal source of infection.
The temporal and geographical patterns of the 2017–18 outbreak suggest that monkeypox cases could have been sporadically occurring throughout the country since the 1970s, mainly driven by sporadic introduction from animal reservoirs with scarce and self-limiting human-to-human transmission chains. The likelihood of monkeypox infection being confused with varicella zoster virus infection, and the fact that neither infection is a notifiable disease in Nigeria, could have resulted in monkeypox virus remaining undetected for many decades. A population-based serological survey could improve understanding of the extent to which monkeypox occurred sporadically—or even endemically—in different age groups and geographical regions between the 1971 and 2017–18 outbreaks. Another possibility is that monkeypox could have been latent because of immunity afforded by the smallpox vaccination, with a resurgence now because of low immunity or other risk factors that have yet to be identified.
The preponderance of adults infected in the 2017–18 outbreak contrasts with outbreaks caused by the Congo Basin clade, which predominantly (>90%) infects children.5, 10 Although the 2003 US outbreak of the west African Clade was more prevalent in adults than in children, it was directly linked to close contact with infected pets, whereas our findings suggest infection from multiple sources.8, 29 We did not identify the reason for the predominance of adult cases in our study, and future studies should aim to identify risk factors that could have promoted increased acquisition of infection among adults.
The clinical characteristics of the 2017–18 Nigerian monkeypox outbreak are largely similar to those of previous outbreaks in the USA (west African clade) and DR Congo (Congo Basin clade). However, unlike the west African clade outbreak in the USA, in which no deaths were reported, the 2017–18 Nigerian outbreak was associated with a case fatality rate of 6%, and four of the seven patients who died had concomitant HIV/AIDS. Although previous studies have shown no relation between HIV and monkeypox infection, our results suggest a need to further explore the potential connection between the two diseases, especially in west Africa, where both are endemic.30, 31 12% of confirmed cases in the 2017–18 outbreak did not present with fever, and thus the case definition for suspected monkeypox that was developed and used during the outbreak should be reviewed.
Nosocomial transmission of monkeypox was previously reported in the Central African Republic.32, 33 Post-outbreak assessment of health workers in the USA in 2003 also suggested possible acquisition of asymptomatic infection in hospital settings.32 Nosocomial infection in a health-care worker who cared for a patient with confirmed monkeypox was also reported in the UK.34 The infection of health-care workers suggests a possible breach in infection prevention and control measures while caring for patients with monkeypox. The potential for infection of health-care workers during monkeypox outbreaks calls for concerted efforts by hospitals to strengthen and routinely follow recommended infection prevention and control practices during patient care.
Our study had several limitations. First, genomic analyses were done for only seven samples, and the threshold of ten or more mutations used to differentiate human-to-human transmission from indepedent introduction from wildlife was based on evidence from different settings.23 Second, we could not collect and examine samples from potential animal reservoirs. In the future, we intend to do genomic analysis of additional cases and compare the findings with contact tracing and risk factor data to further test our hypotheses that human-to-human trasmission and independent introduction from animal reservoirs have characterised this outbreak. In view of the study design, we could not explore all possible risk factors for disease transmission. Incomplete data collection during the outbreak was also a limitation.
In summary, inclusion of monkeypox in the list of priority diseases in Nigeria and in the WHO African region's integrated disease surveillance and response guidelines should be explored to improve its surveillance and engender a better understanding of its epidemiology. Improved animal surveillance is required to establish the animal reservoir and mode of transmission of monkeypox virus in Nigeria.
Acknowledgments
Acknowledgments
We acknowledge the support of the Federal and State Ministries of Health in Nigeria; the Pox Virus Team at the US Centers for Disease Control and Prevention; WHO; the Nigeria Centre for Disease Control Monkeypox Technical Working Group members; the epidemiology teams of Nigerian state ministries of health; the German Center for Infection Research, which provided emergency flexible funds to allow ad-hoc roll out of the Surveillance Outbreak Reponse Management and Analysis System for suporting the response to this outbreak (fund number TTU 01.920); and the patients and their families involved in this research, whose cooperation was core to the success of this work.
Contributors
AY-O prepared the first draft of the Article, which was revised by OAr, MD, DO, GK, CI, and the CDC Monkeypox Outbreak Team. AY-O, OAr, DO, AK, OO, GK, and CI contributed to the best practice and innovations implemented. CI supervised and approved all best practices. AY-O OAr, MD, MS, OI, YD, and IM developed the data collection tool. AY-O, YD, IN, NA, PU, DJ, and PW implemented field investigations and data collection, and documented best practices. AAk, AAh, JB, AAded, NM, and the CDC Monkeypox Outbreak Team did the laboratory testing, and the Outbreak Team also did the whole-genome sequencing (and interpretation thereof). AN, EN, LM, AMo, OI, AAdey, and JM extracted and analysed the data. AAdey worked on the Quantum Geographical Information System. DT-A, BS, OAd, and GK developed the digital Surveillance Outbreak Response Management and Analysis System, trained field investigators in how to use the system, and contributed to data processing and cleaning. All authors reviewed the Article, contributed to interpretation, and approved the final version.
CDC Monkeypox Outbreak Team
Anna Mandra, Whitni Davidson, Mary G Reynolds, Victoria Olson, Andrea M McCollum, Jeffrey Doty, Kimberly Wilkins, Yu Li, Kay Radford, Matthew R Mauldin, Hui Zhao, Michael Townsend, Jillybeth Burgado, Panayampalli Subbian Satheshkumar.
Declaration of interests
We declare no competing interests.
Contributor Information
CDC Monkeypox Outbreak Team:
Anna Mandra, Whitni Davidson, Victoria Olson, Yu Li, Kay Radford, Hui Zhao, Michael Townsend, Jillybeth Burgado, and Panayampalli S. Satheshkumar
Supplementary Material
References
- 1.Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutson CL, Nakazawa YJ, Self J, et al. Laboratory investigations of African pouched rats (Cricetomys gambianus) as a potential reservoir host species for monkeypox virus. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Monkeypox. http://www.who.int/news-room/fact-sheets/detail/monkeypox
- 5.Breman JG, Kalisa-Ruti, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 6.Foster SO, Brink EW, Hutchins DL, et al. Human monkeypox. Bull World Health Organ. 1972;46:569–576. [PMC free article] [PubMed] [Google Scholar]
- 7.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eke RA. Monkeypox in a four year old: a case report. West Afr Med J. 1972;21:21–22. [PubMed] [Google Scholar]
- 9.Reynolds MG, Davidson WB, Curns AT, et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007;13:1332–1339. doi: 10.3201/eid1309.070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutin YJ, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolen LD, Osadebe L, Katomba J, et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis. 2016;22:1014–1021. doi: 10.3201/eid2206.150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yinka-Ogunleye A, Aruna O, Ogoina D, et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federal Ministry of Health Nigeria Technical guidelines for integrated disease surveillance and response in Nigeria. https://www.ncdc.gov.ng/themes/common/docs/protocols/4_1476085948.pdf14
- 14.Nigeria Centre for Disease Control Nigeria monkeypox outbreak report. https://www.ncdc.gov.ng/themes/common/files/sitreps/24004f4681760b8cc1575df9f2fb1f01.pdf
- 15.Nigeria Centre for Disease Control Monkeypox outbreak response interim national guidelines. https://ncdc.gov.ng/themes/common/docs/protocols/50_1508912430.pdf
- 16.Adeoye O, Tom-Aba D, Ameh C, et al. Implementing Surveillance and Outbreak Response Management and Analysis System (SORMAS) for public health in west Africa—lessons learnt and future direction. Int J Trop Dis Health. 2017;22:1–17. [Google Scholar]
- 17.Karem KL, Reynolds M, Braden Z, et al. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Gigante C, Khmaladze E, et al. Genome sequences of Akhmeta virus, an early divergent old world orthopoxvirus. Viruses. 2018;10:252. doi: 10.3390/v10050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316–323. doi: 10.1093/infdis/jiq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhao H, Wilkins K, Hughes C, Damon IK. Real-time PCR assays for the specific detection of monkeypox virus west African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 22.Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010;140:229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 24.Faye O, Pratt CB, Faye M, et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect Dis. 2018;18:246. doi: 10.1016/S1473-3099(18)30043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107:16 262–16 267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberia issues alert on monkeypox outbreak. http://www.xinhuanet.com/english/2018-04/11/c_137101492.htm
- 27.Arita I, Henderson DA. Monkeypox and whitepox viruses in west and central Africa. Bull World Health Organ. 1976;53:347–353. [PMC free article] [PubMed] [Google Scholar]
- 28.Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of monkeypox—west and central Africa, 1970–2017. MMWR Morb Mortal Wkly Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sejvar JJ, Chowdary Y, Schomogyi M, et al. Human monkeypox infection: a family cluster in the midwestern United States. J Infect Dis. 2004;190:1833–1840. doi: 10.1086/425039. [DOI] [PubMed] [Google Scholar]
- 30.WHO Technical Advisory Group on Human Monkeypox. Report of a WHO meeting. https://apps.who.int/iris/handle/10665/65998
- 31.Moss WJ. 9th edn. Elsevier; London: 2013. Hunter's tropical medicine and emerging infectious disease. [Google Scholar]
- 32.Fleischauer AT, Kile JC, Davidson M, et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40:689–694. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- 33.Nakoune E, Lampaert E, Ndjapou SG, et al. A nosocomial outbreak of human monkeypox in the Central African Republic. Open Forum Infect Dis. 2017;4 doi: 10.1093/ofid/ofx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.