Abstract
Background
Human monkeypox, a zoonotic disease, was first reported outside of Africa during the 2003 US outbreak.
Objectives
We present two real-time PCR assays critical for laboratory diagnosis of monkeypox during the 2003 US outbreak.
Study design
A TaqMan-based assay (E9L-NVAR) targets the orthopoxvirus DNA polymerase gene and detects Eurasian orthopoxviruses other than Variola. A hybridization assay, utilizing a MGB Eclipse™ (Epoch Biosciences) probe, targets an envelope protein gene (B6R) and specifically detects monkeypox virus (MPXV). Assays were validated using coded orthopoxvirus DNA samples and used to evaluate lesion samples from five confirmed US monkeypox cases.
Results
E9L-NVAR did not detect variola (48 strains), North American orthopoxviruses (2), or DNA derived from non-poxviral rash illnesses. The assay reproducibly identified various concentrations of 13 Eurasian orthopoxvirus strains and was sensitive to 12.5 vaccinia genomes. The B6R assay recognized 15 different MPXV strains, while other orthopoxvirus (9) and bacteria (15) strains did not cross-react. Of the 13 human samples tested from confirmed cases, both assays identified 100% as containing MPXV DNA.
Conclusions
E9L-NVAR and B6R assays demonstrate 100% specificity for non-variola Eurasian orthopoxvirus and MPXV, respectively. Using two discrete viral gene targets, these assays together provide a reliable and sensitive method for quickly confirming monkeypox infections.
Abbreviations: NVAR, non-variola; RFLP, restriction fragment length polymorphism; DRC, Democratic Republic of the Congo; Ct, threshold cycle; MGB, minor groove binding
Keywords: Orthopoxvirus, Monkeypox virus (MPXV), Real-time PCR, Diagnostic
1. Introduction
Orthopoxvirus monkeypox, first isolated in 1958 from captive primate rash specimens (Magnus et al., 1959), was recognized to cause human illness in 1970 during smallpox eradication campaign intensification (Jezek and Fenner, 1988, Ladnyi et al., 1972). Between 1970 and 1986, >400 monkeypox cases were reported in Africa, 95% within Zaire (now the Democratic Republic of Congo, DRC) (Jezek and Fenner, 1988). Until the 2003 US outbreak (Reed et al., 2004), no human cases had been reported outside of Africa.
Human monkeypox, as described in the Congo Basin, typically presents with symptoms similar to discrete, ordinary smallpox. After about a 2-week, asymptomatic incubation period, infected individuals develop fever followed by disseminated rash. Both illnesses are transmissible between humans and can result in death. The case fatality rate for monkeypox (∼0–10%) (Jezek and Fenner, 1988) is much lower than for variola major (10–30%) (Fenner et al., 1998), as is the rate of human-to-human transmissibility. Control measures for these two diseases would differ, and recent events emphasize the need for monkeypox diagnostics. After discontinuation of smallpox vaccination, susceptibility to zoonotic monkeypox increased, likely contributing to increased disease reports in DRC (Hutin et al., 2001, Meyer et al., 2002, Mwanbal et al., 1997). The US monkeypox outbreak demonstrated the ability of the virus to exploit new hosts and move globally (Reed et al., 2004).
Several nucleic acid test methods have been developed for monkeypox virus (MPXV) detection and characterization (Kulesh et al., 2004, Meyer et al., 2004). Compared with other diagnostic methods, real-time PCR has the advantages of fast, high-quantity throughput and increased sensitivity. This is the first report of real-time PCR assays used to diagnose human monkeypox from clinical rash samples. The assays target different orthopoxvirus genes: DNA polymerase (E9L) and envelope protein (B6R). We report the analytic sensitivity and specificity of both assays and demonstrate their utility for US monkeypox outbreak human rash specimens. These assays represent two sensitive, rapid diagnostic tools for identification of orthopoxviral infection within clinical samples.
2. Materials and methods
2.1. Viruses, bacteria, and clinical samples homogenization
Origins, propagation, and harvesting procedures for viral and cellular isolates are documented (Esposito and Knight, 1985, Esposito et al., 1987, Frenkel et al., 1976, Gispen et al., 1967, Hanrahan et al., 2003, Likos et al., 2005, Loparev et al., 2001, Olson et al., 2004, Pulford et al., 2004, Regnery, 1971, Ropp et al., 1995, Sarmiento et al., 1979, Seki et al., 1990) or briefly described (Table 1 ). Viral samples were processed as described for clinical samples. Bacteria and Rickettsia (Table 2 ) were gifts (Holmes H and Massung R, CDC). Those bacteria with potential to contaminate clinical samples (Table 3 ) were propagated on blood agar plates, suspended in 0.85% sterile saline (0.5 McFarland turbidity), spotted (10 μL) onto slides, and processed to replicate conditions within a clinical sample.
Table 1.
Origin of viruses and cells used for DNA extraction for use in validation panels
| Organism | Sample ID | Location | Year | Patient or supplier | Material sent to CDC |
|---|---|---|---|---|---|
| Variola | V74-227 Congo 9 | 1974 | R. Gipsen | Chicken chorioallantoic membrane | |
| Variola | V77-1605 | Somalia | 1977 | Female, 9 years old | Crust |
| Variola | Somalia | Somalia | 1977 | Male, 23 years old | Swab, last naturally occurring case |
| Camelpox | V78-I-903 | Somalia | 1978 | Female camel | Crust |
| Camelpox | V78-I-2379 | Somalia | 1978 | Female camel | Crust |
| Monkeypox | V77-I-813 | Zaire | 1977 | Female, 7 years old | Crust |
| Monkeypox | V77-I-823 | Zaire | 1977 | Male, 1.5 years old | Crust |
| Monkeypox | V70-266 | Sierra Leone | 1970 | Male, 24 years old | Crust |
| Monkeypox | V81-167 | Zaire | 1981 | Male, 2 years old | Crust |
| Monkeypox | V81-I-179 Ivory Coast | Côte d’Ivoire | 1981 | Female, 3 years old | Crust |
| Monkeypox | V82-167 | Zaire | 1982 | Female, 29 years old | Swab |
| Monkeypox | V83-036 | Zaire | 1983 | Female, 3 years old | Swab |
| Monkeypox | I2003ki-DRC | DRC, formally Zaire | 1998 | ||
| Vaccinia | Lister | Great Britain | |||
| Vaccinia | Temple of Heaven | China | |||
| Vaccinia | Wyeth/Dryvax | US | |||
| Human-T-lymphoblast | SUP-T[VB] | ATCC #CRL-1942 | |||
| African Green Monkey | BS-C-40, clone of BS-C-1 | ATCC #CCL-26 | |||
| Herpesvirus | Varicella Zoster-OKa | ATCC # VR-795 | |||
| Herpesvirus | Varicella-Webster | ATCC #VR-916 |
Table 2.
E9L-NVAR assay specificity to different viral/cellular DNAa (single assay) and sensitivity to vaccinia DNA (triplicate assays)
| Organism | Sample ID | DNA | 2 ng | 200 pg | 20 pg | 2 pg | 200 fg | 20 fg | 2 fg |
|---|---|---|---|---|---|---|---|---|---|
| Eurasian orthopoxvirusa | |||||||||
| Camelpox | E2379 | Partially pure | 21.26 | 24.60 | 29.01 | 34.18 | ND | ND | ND |
| Camelpox | v78-I-903 | Crude | 19.92 | 29.67 | 36.48 | ND | |||
| Cowpox | Brighton | Partially pure | 16.05 | 24.85 | 33.11 | ND | |||
| Ectromelia | Moscow | Partially pure | 17.10 | 20.35 | 25.54 | 28.57 | 31.68 | 37.43 | ND |
| Taterapox | Gerbilpox | Partially pure | 23.56 | 24.66 | 28.60 | 31.00 | ND | 39.25 | ND |
| Monkeypox | MPXV-ZAI-1996-016 | Partially pure | 15.29 | 19.94 | 22.49 | 27.10 | 30.50 | 35.70 | ND |
| Monkeypox | V70-266 Sierra Leone | Partially pure | 16.71 | 28.23 | 33.73 | ND | |||
| Monkeypox | MPXV-ZAI-1979-005 | Crude | 19.21 | 26.66 | 32.19 | ND | |||
| Vaccinia | Lister | Partially pure | 17.48 | 26.34 | 34.55 | ND | |||
| Vaccinia | Temple of Heaven | Partially pure | 17.13 | 28.32 | 35.86 | ND | |||
| Vaccinia | IHDW | Crude | 26.19 | 30.43 | 34.21 | 36.04 | ND | ND | ND |
| Vaccinia | Wyeth/Dryvax | Crude | 26.69 | 38.19 | ND | ND | |||
| Vaccinia | WYH pGS62-9-v1-1-1 | Partially pure | 19.33 | 23.60 | 36.71 | ND | |||
| Variola | SAF65-102 | Crude | ND | ND | ND | ND | |||
| Variola | SAF65-103 | Crude | ND | ND | ND | ND | |||
| Variola | 7124 | Crude | ND | ND | ND | ND | |||
| Variola | 7125 | Crude | ND | ND | ND | ND | |||
| Variola | Variolator 4 | Crude | ND | ND | ND | ND | |||
| Variola | Garcia | Crude | ND | ND | ND | ND | |||
| Variola | BSH | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | Butler | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | ETH72-17 | Crude | ND | ND | ND | ND | |||
| Variola | Harper | Crude | ND | ND | ND | ND | |||
| Variola | Harvey | Crude | ND | ND | ND | ND | |||
| Variola | Heidelberg | Crude | ND | ND | ND | ND | |||
| Variola | Higgins | Crude | ND | ND | ND | ND | |||
| Variola | Hinden | Crude | ND | ND | ND | ND | |||
| Variola | Horn | Crude | ND | ND | ND | ND | |||
| Variola | Iran 2602 | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | Juba | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | K1629 | Crude | ND | ND | ND | ND | |||
| Variola | Kali Mathu | Crude | ND | ND | ND | ND | |||
| Variola | Hembula | Crude | ND | ND | ND | ND | |||
| Variola | Minnesota 124 | Crude | ND | ND | ND | ND | |||
| Variola | Lee | Crude | ND | ND | ND | ND | |||
| Variola | New Dehli | Crude | ND | ND | ND | ND | |||
| Variola | Nur Islam | Crude | ND | ND | ND | ND | |||
| Variola | Lahore | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | Rumbec | Crude | ND | ND | ND | ND | |||
| Variola | Shahzaman | Crude | ND | ND | ND | ND | |||
| Variola | Solaiman | Crude | ND | ND | ND | ND | |||
| Variola | Stillwell | Crude | ND | ND | ND | ND | |||
| Variola | V66-39 | Crude | ND | ND | ND | ND | |||
| Variola | V68-258 | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | V68-59 | Crude | ND | ND | ND | ND | |||
| Variola | V70-222 | Crude | ND | ND | ND | ND | |||
| Variola | V70-228 | Crude | ND | ND | ND | ND | |||
| Variola | Congo | Crude | ND | ND | ND | ND | |||
| Variola | V72-119 | Crude | ND | ND | ND | ND | |||
| Variola | V72-143 | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | Nepal 73 | Crude | ND | ND | ND | ND | |||
| Variola | V73-225 | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | v74-227 Congo 9 | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | V77-1252 | Crude | ND | ND | ND | ND | ND | ND | ND |
| Variola | v77-1605 | Crude | ND | ND | ND | ND | |||
| Variola | Yamada | Crude | ND | ND | ND | ND | |||
| Variola | Bombay | Crust | ND | ND | ND | ND | |||
| Variola | Hembula | Crust | ND | ND | ND | ND | ND | ND | ND |
| Variola | Mannan | Crust | ND | ND | ND | ND | ND | ND | ND |
| Variola | Kudano | Crust | ND | ND | ND | ND | |||
| Variola | Parvin | Crust | ND | ND | ND | ND | ND | ND | ND |
| Variola | Solaiman | Crust | ND | ND | ND | ND | ND | ND | ND |
| Variola | 7124 | Pure | ND | ND | ND | ND | ND | ND | ND |
| Variola | Variolator 4 | Pure | ND | ND | ND | ND | ND | ND | ND |
| Variola | BSH | Pure | ND | ND | ND | ND | ND | ND | ND |
| Variola | Horn | Pure | ND | ND | ND | ND | |||
| Variola | Nepal 73 | Pure | ND | ND | ND | ND | |||
| Variola | Somalia (SOM) | Pure | ND | ND | ND | ND | ND | ND | ND |
| Variola | V70-222 | Pure | ND | ND | ND | ND | ND | ND | ND |
| North American orthopoxvirusesa | |||||||||
| Raccoonpox | RCN | Partially pure | ND | ND | ND | ND | ND | ND | ND |
| Skunkpox | Skunkpox | Partially pure | ND | ND | ND | ND | |||
| Other rash illness or cellular DNAa | |||||||||
| Herpesvirus | HSV-1 HFEM | Pure | ND | ND | ND | ND | |||
| Herpesvirus | HSV-1 Justin | Pure | ND | ND | ND | ND | ND | ND | ND |
| Herpesvirus | HSV-2 patient 920 | Crust | ND | ND | ND | ND | ND | ND | ND |
| Herpesvirus | Varicella-OKA | Pure | ND | ND | ND | ND | ND | ND | ND |
| Herpesvirus | Varicella-WEB | Pure | ND | ND | ND | ND | |||
| Human | Human supT | Pure | ND | ND | ND | ND | |||
| Leporipox | Myxoma | Partially pure | ND | ND | ND | ND | |||
| Monkey | BSC-40 | Pure | ND | ND | ND | ND | |||
| Rickettsia | Rickettsia akari | Pure | ND | ND | ND | ND | ND | ND | ND |
| Rickettsia | Rickettsia conorii | Pure | ND | ND | ND | ND | |||
| Water | ND | ND | ND | ND | ND | ND | ND | ||
| Purified orthopoxvirus for determination of sensitivity | |||||||||
| Vaccinia | Wyeth/Dryvax | Pure | 16.07b | 19.47b | 23.41b | 27.53b | 31.56b | 35.69b | 39.68c |
The cycle when fluorescence crossed the threshold (Ct) for each positive sample is shown. ND, not detected.
Each sample was tested in triplicate, all three runs were positive, and the average Ct value is shown.
Each sample was tested in triplicate, one run was positive and two were negative, and the average Ct value is shown.
Table 3.
Validation of B6R assay specificity to Eurasian orthopoxvirus and bacterial DNAa
| Eurasian orthopoxvirus | Sample ID | DNA | Average Ct for samples with different amounts of viral DNA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 ng | 200 pg | 20 pg | 2 pg | 200 fg | 20 fg | 2 fg | |||
| Camelpox | v78-I2379 | Purified | ND | ND | ND | ND | ND | ND | ND |
| Cowpox | CP58 | Purified | ND | ND | ND | ND | ND | ND | ND |
| Cowpox | Brighton | Purified | ND | ND | ND | ND | ND | ND | ND |
| Monkeypox | MPXV-ZAI-1979-005 | Purified | 17.30b | 21.01b | 26.00b | 29.79b | 33.69b | 36.74b | 39.70b |
| Vaccinia | Wyeth/Dryvax | Purified | ND | ND | ND | ND | ND | ND | ND |
| Vaccinia | IHDJ | Purified | ND | ND | ND | ND | ND | ND | |
| Vaccinia | Wyeth | Purified | ND | ND | ND | ND | ND | ND | ND |
| Variola | V72-143 | Crude | ND | ||||||
| Variola | BSH | Purified | ND | ND | |||||
| Variola | Horn | Purified | ND | ND | |||||
| Bacteriac | Average Ct |
|---|---|
| Streptococcus Pyogenes ATCC 19615 | ND |
| Diphtheroid CDC#143-02 | ND |
| Peptostreptococcus anaerobius ATCC 15689 | ND |
| Propionibacterium acnes ATCC 6919 | ND |
| Staphylococcus aureus (strain 1) ATCC 12600 | ND |
| Klebsiella pneumoniae ATCC 33495 | ND |
| Staphylococcus epidermidis (strain 3) ATCC 14990 | ND |
| S. epidermidis (strain 2) ATCC 12228 | ND |
| S. epidermidis (strain 1) ATCC 49134 | ND |
| Pseudomonas aeruginosa ATCC 27853 | ND |
| Enterococcus faecalis ATCC 29212 | ND |
| Streptococcus bovis (alpha-Strep) ATCC 49147 | ND |
| S. aureus (strain 3) CDC#03-06 (TSST-1 positive) | ND |
| S. aureus (strain 2) ATCC 25925 | ND |
| Escherichia coli 25922 | ND |
Each sample was tested in triplicate; all results were negative except as indicated. ND, not detected.
All assays were positive and the average Ct value is shown.
Each sample was spotted onto a slide to mimic a clinical sample (see Section 2). The slides were processed, DNA extracted, and each DNA tested in triplicate. ND, not detected.
Clinical samples were obtained from vesicular lesions as skin biopsies (scab or vesicle roof), vesicular fluid slide (“touch prep”), or vesicular fluid swab. Recommendations for lesion sampling can be found at http://www.bt.cdc.gov/agent/smallpox/response-plan/files/guide-d.pdf. In brief, samples were processed under biosafety containment conditions to form homogenates suitable for DNA extraction:
-
1.
Skin biopsies were homogenized in sterile water or PBS (500 μL) by freezing, disruption with a disposable pestle, and vortexing. If physical disruption was insufficient, the sample underwent further freeze–thaw/grinding cycles. Finally, samples, in closed tubes, were sonicated (cup-horn sonicator, 40% maximum output).
-
2.
Nuclease-free water (100 μL) was added to each spot on the slide, scraped, and recovered into a sterile tube. Water addition/scraping was repeated twice and pooled into the same tube.
-
3.
Shafts of vesicular fluid swabs were broken near the top of the swab material. The swab was hydrated (300 μL PBS) for 5–10 min in a sterile tube and then transferred to a Swab Extraction Tube System tube (Roche Applied Science, Indianapolis, IN). The swab/tube was spun for 1 min to rinse the swab and collect the eluent.
2.2. DNA extraction
Crude virus (viral-infected cell lysates harvested 48 hpi), semi-purified virions (Esposito et al., 1981), purified virions, scabs, bacteria, and clinical samples were homogenized (as described above), and DNA extracted using the AquaPure Genomic DNA Isolation Kit (Bio-Rad, Hercules, CA), suspended in 50 μL AquaPure DNA hydration buffer, and stored at −20 °C.
2.3. E9L non-variola (NVAR) assay
The primer/probe sequences were selected from the DNA polymerase gene (E9L; GenBank L22579) with Primer Express (version 1.5; Applied Biosystems). These included E9L forward primer (5′-TCA_ACT_GAA_AAG_GCC_ATC_TAT_GA-3′), E9L reverse primer (5′-GAG_TAT_AGA_GCA_CTA_TTT_CTA_AAT_CCC_A-3′), and E9L-NVAR probe (5′TET-CCA_TGC_AAT_ATA_CGT_ACA_AGA_TAG_TAG_CCA_AC-3′). Primers and probe were synthesized in the Biotechnology Core Facility (CDC, Atlanta GA), utilizing standard phosphoramidite chemistry. The detection probe contained 5′ reporter molecule (TET) and 3′ aminomodifier (Glen Research, Sterling, VA). A 3′ quencher molecule, QSY7 (Molecular Probes, Eugene, OR), was conjugated to the 3′ amino group after synthesis.
PCR assay conditions were optimized according to standard protocols (protocol 04304449, Applied Biosystems, Foster City, CA) by adjusting primer and probe concentrations, and thermal cycling temperatures/duration. Each reaction (50 μL) contained 1× TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 0.4 μmol/L each primer, 200 nmol/L TaqMan probe, and 2 μL of template DNA. Thermal cycling conditions for the ABI7700 (Applied Biosystems, Foster City, CA): one cycle of 95 °C for 10 min; followed by 40 cycles of 95 °C for 10 s, and 60 °C for 40 s. PCR amplification is based on fluorescent emission after annealing/elongation (60 °C).
2.4. B6R MPXV-specific assay
The primer sequences were selected from the extracellular enveloped virus protein gene (B6R; GenBank L22579) using Primer Express (version 1.5; Applied Biosystems, Foster City, CA). These included B6R forward primer (5′-ATT_GGT_CAT_TAT_TTT_TGT_CAC_AGG_AAC_A-3′), and B6R reverse primer (5′-AAT_GGC_GTT_GAC_AAT_TAT_GGG_TG-3′). The MPXV-specific probe (5′-MGB/DarkQuencher-AGA_GAT_TAG_AAA_TA-FAM3′) was selected from the B6R sequence with the aid of MGB Eclipse™ By Design software (Epoch Biosciences, Bothell, WA) and has a conjugated minor groove binding (MGB) ligand and a dark quencher at the 5′-end, with the fluorophore at the 3′-end. Fluorescence of the single-stranded probe is efficiently quenched by the interaction of the terminal dye and quencher groups when not hybridized (Afonina et al., 2002a, Afonina et al., 2002b).
Each reaction (20 μL) contained 1× Eclipse Gene Expression Buffer (20 mmol/L Tris–HCl, pH 8.7, 50 mmol/L NaCl, 5 mmol/L MgCl2), 200 nmol/L MGB Eclipse™ probe, 0.25 μL 100 mmol/L deoxynucleoside triphosphate mixture, 0.4 μmol/L each primer, 0.75 μL (1.875 U) Jumpstart TaqDNA polymerase (Sigma, St. Louis, MO), and 2 μL template DNA. Thermal cycling conditions for the iCycler (Bio-Rad, Hercules, CA): one cycle of 95 °C for 30 s; followed by 45 cycles of 95 °C for 5 s, 57 °C for 15 s, and 70 °C for 20 s. PCR amplification is based on the fluorescent emission with annealed probe (57 °C).
2.5. Statistical probit analysis
Analytical sensitivity was determined using purified, photometrically quantified vaccinia DNA diluted in water (24 replicates of 5 concentrations). Probit analysis as a model of non-linear regression was accomplished with commercial software (SPSS 11.0, for Mac® OS X; SPSS, Inc., Chicago, IL). The software determines a continuous 95% confidence interval of the probability of achieving a positive result at any given input DNA concentration within the concentration range of the experiment.
3. Results and discussion
3.1. E9L-NVAR orthopoxvirus assay
Several TaqMan-based real-time PCR assays have been developed as rapid orthopoxvirus diagnostics. One diagnostic assay for orthopoxviral infections other than variola targets the viral DNA polymerase gene (E9L), amplifying a conserved gene segment within all Eurasian orthopoxviruses. The probe, however, targets 32 bases within the E9L gene containing a three nucleotide difference between variola and other orthopoxviruses (Fig. 1 ), and thus efficiently anneals to Eurasian orthopoxviruses other than variola. This orthopoxviral diagnostic can be used without raising concern that variola has been detected.
Fig. 1.
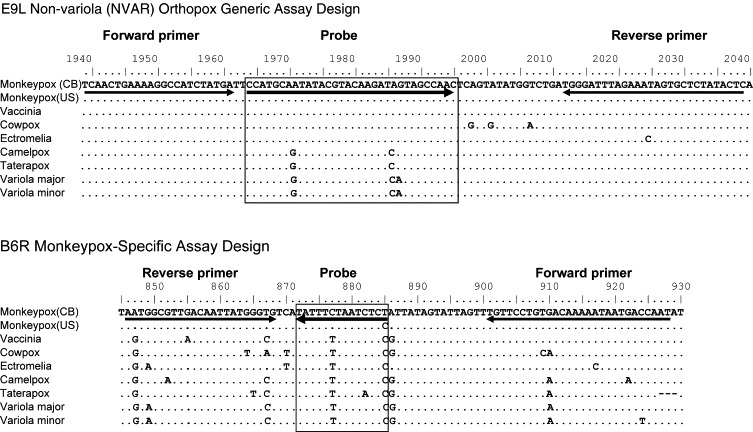
Alignment of primers and probes with orthopoxviral DNA. The primers and probe for each of the real-time PCR assays are aligned with the targeted sequence of DNA within several orthopoxviral species. The E9L-NVAR primers and probe are completely homologous with the vaccinia Copenhagen DNA sequence. The B6R primers and probe are completely homologous with the monkeypox (CB) DNA sequence. Virus strains: monkeypox (CB) MPXV-ZAI-1996-016 (Genbank AF380138); monkeypox (US) MPXV-USA-2003-039 (Genbank DQ011154); vaccinia Copenhagen (Genbank M35027); cowpox Brighton (Genbank AF482758); ectromelia Moscow (Genbank AF012825); camelpox Kazakhstan M-96 (Genbank AF438165); taterapox (Smith GL, personal communication); variola major Bangladesh (Genbank L22579); variola minor Garcia (Genbank Y16780). CB, Congo Basin; US, United States.
The specificity and sensitivity of the assay, designated E9L-NVAR, was determined utilizing a coded panel of multiple orthopoxviruses. Each sample was tested singly, and positive samples are denoted by the cycle where fluorescence crossed the threshold (Ct) (Table 2). E9L-NVAR identified all non-variola Eurasian orthopoxviruses (13 species) at concentrations between 2 pg and 20 fg of viral DNA, depending upon DNA quality. The assay detected 20 fg of partially purified MPXV DNA (∼100 genomes). Partially purified cowpox, ectromelia, and vaccinia were identified at similar efficiencies (Table 2). Partially purified camelpox and taterapox demonstrated a diminished interaction with the E9L-NVAR probe (Table 2) due to a single base difference between these viruses and variola in the probe target region (Fig. 1).
Vaccinia and MPXV sequences are identical in this region (Fig. 1), and serially diluted purified vaccinia DNA established linearity of the E9L-NVAR assay from 2 ng to 20 fg (83% reaction efficiency) (Table 2). Probit regression analysis determined assay sensitivity using the same preparation of purified DNA in 24 replicate amplification reactions. Amplification was positive in all 24 replicate reactions containing 20, 10, and 5 fg of vaccinia input DNA. Only 22 of 24 replicates containing 2.5 fg were detected, while no reactions containing 1.25 fg vaccinia DNA were positive (data not shown). Therefore, 2.54 fg viral DNA (∼12.5 genomes) is the calculated detection limit for 95% confidence.
The E9L-NVAR assay is specific for six non-variola Eurasian orthopoxviruses, not cross-reacting with variola (48 strains) or North American orthopoxviruses (2 strains). Furthermore, E9L-NVAR assay did not cross-react with any DNA derived from rash illnesses potentially confused with orthopoxviral infection, such as herpesvirus and rickettsial infections, or with human cellular DNA (Table 2), even at high concentrations (2 ng). Overall, the E9L-NVAR assay can reproducibly detect as few as 12.5 genomes of purified vaccinia or MPXV DNA without giving false positive results.
3.2. Monkeypox-specific B6R assay
Although the E9L-NVAR assay reliably detects Eurasian orthopoxviruses, other than variola, it is unable to make a species-specific identification. Due to the low G + C content (∼30%) and 90% sequence similarity to other Eurasian orthopoxviruses, it is difficult to design a monkeypox-specific TaqMan assay. To improve the reliability of a MPXV-specific assay, we utilized the MGB-based real-time PCR technology. Linking a DNA double helix MGB protein to the probe permits use of shorter probe sequences, which can detect single nucleotide polymorphisms (SNPs) (Afonina et al., 2002a, Afonina et al., 2002b, Belousov et al., 2004) such as within the MPXV envelope protein gene (B6R) (Fig. 1). The probe 5′-MGB molecule stabilizes probe-template interaction (Afonina et al., 2002a, Afonina et al., 2002b) and enhances assay specificity and sensitivity.
A coded test panel containing orthopoxviral and bacterial DNAs was assayed in triplicate using the iCycler platform (Bio-Rad, Hercules, CA) to monitor reproducibility and specificity of the B6R assay (Table 3). MPXV DNA was reproducibly detected in a linear fashion to ∼10 viral copies (2 fg). The B6R assay did not cross-react with any other orthopoxviral DNA (variola, cowpox, camelpox, and vaccinia) or with 15 bacterial species. Although certain Gram-positive bacterial DNAs were less efficiently extracted, all bacterial samples mimicked conditions expected within clinical samples. Furthermore, the presence of bacterial 16S DNA was confirmed using real-time PCR (data not shown). The B6R assay should not cross-react with these potential contaminating bacteria within clinical samples. The B6R assay detects multiple monkeypox strains; all 15 isolates of MPXV DNA were detected at 10 ng (Table 4 ), an amount often found within rash samples. Assay linearity and sensitivity were determined using purified MPXV DNA; all quantities from 2 ng to 2 fg cross-reacted with the B6R probe in a linear fashion (Table 4). The reaction efficiency was affected by the freshness of diluted DNA and B6R assay probe and primers: freshly diluted DNA produced higher reaction efficiency (97%) than DNA that had undergone multiple freeze–thaw cycles (67%). Overall, the B6R assay demonstrates a sensitive, specific, and rapid method for identifying MPXV.
Table 4.
Validation of B6R assay reactivity to different strains of monkeypox virusa and sensitivity of the assay to purified monkeypox virus DNAb
| Monkeypox straina | DNA | Geographic area | 10 ng |
|---|---|---|---|
| Ivory Coast V81-I-179 | Crude | Cote d’Ivoire (West Africa) | 16.43 |
| MPXV-LIB-1970-184 | Purified | Liberia (West Africa) | 14.30 |
| Utrecht | Crude | The Netherlands (original origin unknown) | 22.87 |
| MPXV-NIG-1978 | Crude | Nigeria (West Africa) | 17.17 |
| V70-266 Sierra Leone | Crude | Sierra Leone (West Africa) | 15.10 |
| MPXV-CAM-1990 | Crude | Cameroon (Congo Basin) | 20.23 |
| MPXV-GAB-1988-001 | Crude | Gabon (Congo Basin) | 22.53 |
| I2003ki-DRC 1998 | Crude | Zaire (Congo Basin) | 13.90 |
| MPXV-ZAI-1979-005 | Crude | Zaire (Congo Basin) | 15.00 |
| MPXV-ZAI-1996-016 | Crude | Zaire (Congo Basin) | 13.97 |
| V77-823 | Crude | Zaire (Congo Basin) | 14.20 |
| V77-813 | Crude | Zaire (Congo Basin) | 14.57 |
| V81-167 | Crude | Zaire (Congo Basin) | 14.33 |
| V82-167 | Crude | Zaire (Congo Basin) | 14.00 |
| V83-036 | Crude | Zaire (Congo Basin) | 14.03 |
| Sensitivity to purified DNAb | 2 ng | 200 pg | 20 pg | 2 pg | 200 fg | 20 fg | 2 fg |
|---|---|---|---|---|---|---|---|
| MPXV-ZAI-1996-016 (old) | 16.43 | 20.37 | 24.23 | 28.97 | 34.07 | 38.63 | ND |
| MPXV-ZAI-1996-016 (fresh) | 16.96 | 20.74 | 24.76 | 27.50 | 31.14 | 33.85 | 38.21 |
Ability of the assay to detect multiple strains of monkeypox virus. Each sample was tested in triplicate; all three assays were positive and the average Ct value is shown.
Assay limit of detection for various quantities of purified monkeypox DNA. Each sample was tested in triplicate using either freshly diluted DNA (fresh) or diluted DNA that had undergone multiple freeze–thaw cycles (old). Where all three assays were positive, the average Ct value is shown. ND, not detected.
The B6R probe did not detect any of our stocks of cowpox DNA (strain BRT (Table 3) and German isolates [GER2 and GER3] (data not shown)). However, the published cowpox GRI90 sequence is unusual in that it contains more monkeypox SNPs than cowpox signatures, including within the B6R probe region. Based on available sequence information, the B6R assay would likely cross-react with cowpox GRI90, but not other diverse cowpox isolates. At this time, we are unable to obtain cowpox GRI90 DNA to experimentally assess B6R probe cross-reactivity.
3.3. Diagnostic use of real-time PCR assays during the 2003 US monkeypox outbreak
The first US human monkeypox cases were recognized in Wisconsin (Reed et al., 2004). From five of these cases, we received a variety of rash-lesion samples at a vesicular stage of development. Samples (skin, slides, and swabs) were inoculated into tissue culture to evaluate for viral cytopathic effect and DNA extracted for various PCR-based assays. Previously established standard PCR techniques amplified the HA and ATI genes of all orthopoxviruses with subsequent Restriction Fragment Length Polymorphism RFLP analysis providing species-specific identification (Meyer et al., 1997, Ropp et al., 1995), or amplified only MPXV using monkeypox-specific primers of the E9L gene Multiplex assay (Dhar et al., 2004). Samples were assayed with both well-established assays and new real-time PCR assays (E9L-NVAR and B6R) and their performance compared.
For real-time PCR assays, a positive control (20 fg MPXV DNA) within each run established the Ct cut-off value for positivity. All monkeypox-positive samples by standard PCR or tissue culture were also detected by real-time PCR (Table 5 ). In fact, real-time PCR assays identified low levels of MPXV DNA, undetectable by standard PCR (Table 5, cases 2003-038 and 2003-040). Both real-time PCR assays detected viral DNA from various sample types: vesicle fluid (slides or swabs), and vesicle skin. As evidenced by different Ct values and days to evident cytopathic effect, viral DNA load varied between samples, possibly due to differences in sampling technique, disease stage, and/or sample type; vesicle skin having the highest viral amounts. MPXV-positive rash samples were collected anywhere from day 1 to day 22 after rash onset, indicating assay usefulness throughout multiple disease stages. Clinical assay specificity was demonstrated by samples (case 2003-072) which were orthopoxvirus negative by our PCR assays (Table 5), despite sample adequacy indicated by detection of human DNA (β-actin) (data not shown), but were confirmed to contain varicella zoster virus by PCR (National Varicella Reference Laboratory, CDC, personal communication). These real-time PCR diagnostic assays proved to be highly sensitive and specific during clinical application.
Table 5.
Analysis of clinical samples
| Case number | Sample type | Lab diagnosis | Tissue culture | Standard PCR |
||||
|---|---|---|---|---|---|---|---|---|
| HA | HA RFLP | ATI | ATI RFLP | Multiplex | ||||
| Monkeypox | ||||||||
| 2003-038 (Patient 7*) | Slide of vesicle fluid | MPX | + (1 day) | + | MPX | + | MPX | MPX |
| Skin biopsy | MPX | + (6 days) | − | NA | − | NA | Not done | |
| Swab of vesicle fluid | MPX | + (2 days) | + | Not done | + | Not done | Not done | |
| 2003-039 (Patient 4*) | Vesicle roof | MPX | + (3 days) | + | MPX | + | MPX | MPX |
| Slide of vesicle fluid | MPX | + (3 days) | + | Inconclusive | + | MPX | Not done | |
| Skin biopsy | MPX | + (3 days) | + | Inconclusive | + | MPX | Not done | |
| Swab of vesicle fluid | MPX | + (3 days) | + | Inconclusive | + | MPX | Not done | |
| Swab of vesicle fluid | MPX | + (6 days) | + | – | + | MPX | Not done | |
| 2003-040 (Patient 8*) | Vesicle skin | MPX | Inconclusive | + | Inconclusive | + | MPX | MPX |
| Slide of vesicle skin | MPX | + (6 days) | − | NA | − | NA | Not done | |
| Swab of vesicle skin | MPX | + (6 days) | + | Inconclusive | − | NA | Not done | |
| 2003-045 (Patient 11*) | Swab of vesicle skin | MPX | + (2 days) | + | Inconclusive | + | MPX | MPX |
| 2003-073 (Patient 6*) | Skin biopsy | MPX | + (1 day) | + | MPX | + | MPX | MPX |
| Varicella zoster virus | ||||||||
| 2003-072 | Vesicle skin | Negative | − (7 days) | − | NA | − | NA | − |
| Vesicle skin | Negative | − (7 days) | − | NA | − | NA | − | |
| Case number | Sample type | Real-time PCR |
Day post rash | Day post fever | |
|---|---|---|---|---|---|
| E9L-NVAR (Ct)a | B6R (Ct)a | ||||
| 2003-038 (Patient 7*) | Slide of vesicle fluid | OPX other than variola (30) | MPX (31) | 5 | 6 |
| Skin biopsy | OPX other than variola (37) | MPX (35) | 5 | 6 | |
| Swab of vesicle fluid | OPX other than variola (30) | MPX (29) | 5 | 6 | |
| 2003-039 (Patient 4*) | Vesicle roof | OPX other than variola (15) | MPX (15) | 13 | 10 |
| Slide of vesicle fluid | OPX other than variola (24) | MPX (25) | 13 | 10 | |
| Skin biopsy | OPX other than variola (24) | MPX (23) | 13 | 10 | |
| Swab of vesicle fluid | OPX other than variola (20) | MPX (21) | 13 | 10 | |
| Swab of vesicle fluid | OPX other than variola (24) | MPX (24) | 13 | 10 | |
| 2003-040 (Patient 8*) | Vesicle skin | OPX other than variola (24) | MPX (24) | 5 | 8 |
| Slide of vesicle skin | OPX other than variola (38) | MPX (37) | 5 | 8 | |
| Swab of vesicle skin | OPX other than variola (30) | MPX (29) | 5 | 8 | |
| 2003-045 (Patient 11*) | Swab of vesicle skin | OPX other than variola (28) | MPX (27) | 1 | 6 |
| 2003-073 (Patient 6*) | Skin biopsy | OPX other than variola (15) | MPX (14) | 22 | NA |
| 2003-072 | Vesicle skin | ND (40) | ND (45) | 5 | 16 |
| Vesicle skin | ND (40) | ND (45) | 5 | 16 | |
Results from tissue culture, standard PCR, and real-time PCR assays are shown for each sample. MPX, monkeypox; OPX, orthopox; NA, not applicable; ND, not detected.
Each sample was tested in triplicate and the average Ct for each positive sample is shown. Negative samples were not detected (ND).
Patient number found in NEJM (Reed et al., 2004).
4. Summary
Two rapid real-time PCR assays for the detection of orthopoxvirus and MPXV DNA have been developed. The E9L-NVAR and B6R assays target orthopoxvirus DNA polymerase and extracellular enveloped protein genes, respectively. These assays are highly sensitive (2 fg or ∼10 viral genomes) and specific. The E9L-NVAR assay detects 13 Eurasian orthopoxviruses but not variola or North American orthopoxviruses, and the B6R assay detects MPXV isolates but no other orthopoxviruses. Neither assay gave false positives with other rash illness-causing viruses or bacteria. The E9L-NVAR assay, initially developed upon the ABI7700, has provided similar results with other real-time PCR platforms such as the Lightcycler (Roche) and iCycler (data not shown). Similarly, the B6R assay, validated on the iCycler, is compatible with the ABI7700 real-time PCR technology (data not shown). Although validation was executed upon specific real-time PCR platforms, both assays are flexible and capable of using most available real-time PCR technologies and platforms, thereby allowing compatibility with laboratories regardless of which real-time PCR platform they possess.
During the 2003 US monkeypox outbreak, the E9L-NVAR and B6R assays provided reliable and sensitive identification of human monkeypox infections within a clinical context. Assay compatibility with multiple real-time PCR platforms allowed simultaneous testing of suspect samples for orthopoxvirus (E9L-NVAR) and MPXV (B6R) DNA. Interestingly, US MPXV isolates were distinct from previously characterized African Congo Basin isolates in both clinical manifestations and genomic sequences, more closely matching monkeypox isolates from West Africa (Likos et al., 2005, Reed et al., 2004). These relationships correlated well with the Ghanaian origin of the US monkeypox outbreak (Reed et al., 2004). The B6R assay was designed to detect Congo Basin MPXV; West African/US MPXV has one SNP within the B6R probe (Fig. 1). The lack of complete homology to the US monkeypox isolates did not adversely affect the detection of MPXV DNA within human samples (Table 5), confirming the B6R assay diagnostic utility for both known MPXV clades (Likos et al., 2005). Furthermore, monkeypox spread outside of Africa suggests these diagnostic assays may be relevant worldwide for identification of smallpox-like orthopox diseases.
Acknowledgments
The authors thank A. Michael Frace (CDC Biotechnology Core Facilities Branch) and Hermann Meyer (Institute of Microbiology of the Bundewehr) for the B6R sequences of cowpox GER2 and GER3, Brian Holloway (CDC Biotechnology Core Facilities Branch) for helpful discussions and critical commentary on primer and probe design, members of the Sequence Facility at the Biotechnology Core Facilities Branch for sequence information critical for assay design, and Russell Regnery (Poxvirus Program, CDC) for critical commentary on the manuscript.
References
- Afonina I.A., Reed M.W., Lusby E., Shishkina I.G., Belousov Y.S. Minor groove binder-conjugated DNA probes for quantitative DNA detection by hybridization-triggered fluorescence. Biotechniques. 2002;32:940–949. doi: 10.2144/02324pf01. [DOI] [PubMed] [Google Scholar]
- Afonina I.A., Sanders S., Walburger D.K., Belousov Y.S. Accurate SNP typing by real-time PCR: a comparison of minor groove binder-conjugated DNA probes. Pharma Genomics. 2002:48–54. [Google Scholar]
- Belousov Y.S., Welch R.A., Sanders S., Mills A., Kulchenko A., Dempcy R., et al. Single nucleotide polymorphism genotyping by two colour melting curve analysis using the MGB Eclipse Probe System in challenging sequence environment. Hum Genomics. 2004;1:209–217. doi: 10.1186/1479-7364-1-3-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A.D., Werchniak A.E., Li Y., Brennick J.B., Goldsmith C.S., Kline R., et al. Tanapox infection in a college student. N Engl J Med. 2004;350:361–366. doi: 10.1056/NEJMoa031467. [DOI] [PubMed] [Google Scholar]
- Esposito J., Brechling K., Baer G., Moss B. Vaccinia virus recombinants expressing rabiesvirus glycoprotein protect against rabies. Virus Genes. 1987;1:7–21. doi: 10.1007/BF00125682. [DOI] [PubMed] [Google Scholar]
- Esposito J.J., Cabradilla C.D., Nakano J.H., Obijeski J.F. Intragenomic sequence transposition in monkeypox virus. Virology. 1981;109:231–243. doi: 10.1016/0042-6822(81)90495-5. [DOI] [PubMed] [Google Scholar]
- Esposito J.J., Knight J.C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143:230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. Smallpox and its eradication. World Health Organization; Geneva: 1998. The epidemiology of smallpox. p. 169–208. [Google Scholar]
- Frenkel N., Locker H., Batterson W., Hayward G.S., Roizman B. Anatomy of herpes simplex virus DNA. VI. Defective DNA originates from the S component. J Virol. 1976;20:527–531. doi: 10.1128/jvi.20.2.527-531.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen R., Verlinde J.D., Zwart P. Histopathological and virological studies on monkeypox. Arch Gesamte Virusforsch. 1967;21:205–216. doi: 10.1007/BF01241445. [DOI] [PubMed] [Google Scholar]
- Hanrahan J.A., Jakubowycz M., Davis B.R. A smallpox false alarm. N Engl J Med. 2003;348:467–468. doi: 10.1056/NEJMc021898. [DOI] [PubMed] [Google Scholar]
- Hutin Y.J., Williams R.J., Malfait P., Pebody R., Loparev V.N., Ropp S.L., et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek Z., Fenner F. In: Melnick J., editor. vol. 17. Karger; Basel: 1988. Human monkeypox; pp. 1–140. (Monographs in virology). [Google Scholar]
- Kulesh D.A., Loveless B.M., Norwood D., Garrison J., Whitehouse C.A., Hartmann C., et al. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladnyi I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- Loparev V.N., Massung R.F., Esposito J.J., Meyer H. Detection and differentiation of old world orthopoxviruses: restriction fragment length polymorphism of the crmB gene region. J Clin Microbiol. 2001;39:94–100. doi: 10.1128/JCM.39.1.94-100.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P., von Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Path Microbiol Scand. 1959;46:156–176. [Google Scholar]
- Meyer H., Damon I.K., Esposito J.J. Orthopoxvirus diagnostics. Meth Mol Biol. 2004;269:119–134. doi: 10.1385/1-59259-789-0:119. [DOI] [PubMed] [Google Scholar]
- Meyer H., Perrichot M., Stemmler M., Emmerich P., Schmitz H., Varaine F., et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Ropp S.L., Esposito J.J. Gene for A-type inclusion body protein is useful for a polymerase chain reaction assay to differentiate orthopoxviruses. J Virol Meth. 1997;64:217–221. doi: 10.1016/S0166-0934(96)02155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanbal P.T., Tshioko K.F., Moudi A., Mukinda V., Mwema G.N., Messinger D., et al. Human monkeypox in Kasai Oriental, Zaire (1996–1997) Euro Surveill. 1997;2:33–35. doi: 10.2807/esm.02.05.00161-en. [DOI] [PubMed] [Google Scholar]
- Olson V.A., Laue T., Laker M.T., Babkin I.V., Drosten C., Shchelkunov S.N., et al. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–1946. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford D., Meyer H., Brightwell G., Damon I., Kline R., Ulaeto D. Amplification refractory mutation system PCR assays for the detection of variola and orthopoxvirus. J Virol Meth. 2004;117:81–90. doi: 10.1016/j.jviromet.2004.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Regnery D.C. The epidemic potential of Brazilian myxoma virus (Lausanne strain) for three species of North American cottontails. Am J Epidemiol. 1971;94:514–519. doi: 10.1093/oxfordjournals.aje.a121349. [DOI] [PubMed] [Google Scholar]
- Ropp S.L., Jin Q., Knight J.C., Massung R.F., Esposito J.J. PCR strategy for identification and differentiation of small pox and other orthopoxviruses. J Clin Microbiol. 1995;33:2069–2076. doi: 10.1128/jcm.33.8.2069-2076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P.G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979;29:1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M., Oie M., Ichihashi Y., Shida H. Hemadsorption and fusion inhibition activities of hemagglutinin analyzed by vaccinia virus mutants. Virology. 1990;175:372–384. doi: 10.1016/0042-6822(90)90422-n. [DOI] [PubMed] [Google Scholar]


