Abstract
Phenanthrenes are bioactive phenolic compounds found in genus Dioscorea, in which they are distributed more in peel than in flesh. Recent studies on phenanthrenes from Dioscorea sp. peels have revealed the potential for valuable biomaterials. Herein, an analytical method using high-performance liquid chromatography (HPLC) for quantitation of bioactive phenanthrenes was developed and validated. The calibration curves were obtained using the phenanthrenes (1-3) previously isolated from Dioscorea batatas concentrations in the range of 0.625-20.00 μg/ml with a satisfactory coefficient of determination (R2) of 0.999. The limit of detection (LOD) and the limit of quantification (LOQ) values of the isolated phenanthrenes ranged from 0.78-0.89 and 2.38-2.71 μg/ml, respectively. The intraday and interday precision ranged from 0.25-7.58%. The recoveries of the isolated phenanthrenes were from 95 to 100% at concentrations of 1.25, 2.5, and 5.0 μg/ml. Additionally, phenanthrenes (1-3) were found in all investigated peel extracts. Hence, the developed method was encouraging for the quantitative analysis of phenanthrenes in genus Dioscorea.
Keywords: Dioscorea, yam, HPLC, method validation, phenanthrenes, quantitative analysis
Introduction
Phenanthrenes are relatively rare, secondary metabolites derived through the formation of the oxidative coupling of the aromatic rings of stilbene precursors. More than 200 phenanthrenes have been isolated from plants belonging to the Annonaceae, Aristolochiaceae, Cannabaceae, Combretaceae, Cucurbitaceae, Dioscoreaceae, Euphorbiaceae, and Stemonaceae families [1, 2]. Phenanthrenes that have been isolated so far can be classified into three major groups by differences in their substitutents, which include monophenanthrenes, diphenanthrenes, and triphenanthrenes [3]. As of today, various biological activities of phenanthrenes have been reported, such as antimicrobial [3], antioxidant [4], anticancer [5], antiproliferative [6], anti-inflammatory [7], and antiallergic [8], and spasmolytic effects [3]. Moreover, phenanthrenes have been suggested to be a non-polar standard marker for genus Dioscorea [4].
The Dioscorea genus, widely known as yams, is the most important genus among the Dioscoreaceae family. The genus consists of over 600 species and is common in tropical and subtropical regions [9]. Yams have long been used as health food and folk medicine in some Asian countries due to their high nutritional value and various biological effects [9, 10]. The genus is regarded as an energy food source for consumers, especially in some local mountainous regions in Asia and Africa due to its high starch content, which can reach 80% on a dry weight basis [5, 11]. Reported pharmaceutical investigations showed that Dioscorea bulbifera ethanolic crude extract possesses a potent cytotoxic activity against human colorectal carcinoma (HCT116), human lung carcinoma (A549), and human breast carcinoma (MCF-7) [12]. The isolated compounds from D. bulbifera also displayed a potent effect on anti-HIV-1 integrase [13]. The anti-neuroinflammatory and neuroprotective activities of phenolic components isolated from Dioscorea nipponica were revealed in a recent study [14]. Phenanthrenes isolated from the Dioscorea genus reportedly possess anticancer [5], antioxidant [4], anticholinesterase [15], and anti-inflammatory activities [16]. Our previous studies also demonstrated anti-neuroinflammatory [17], antioxidant [4, 7], and inhibitory effects of phenanthrenes isolated from Dioscorea batatas peel on particulate matter-induced pulmonary injury [18]. Therefore, a valid quantitative analytical method for phenanthrenes is essential to evaluate the compositions of extracts from Dioscorea genus as a commercial medicinal herb or functional biomaterial source.
A recent study of ours revealed that the phenanthrenes content of D. batatas peel is markedly greater than that of its flesh [4]. Although Yoon et al. reported on the quantitative analysis method and its validation using high-performance liquid chromatography (HPLC) for phenanthrenes in roots of the Dioscorea genus [19], the method is limited in evaluating the different target analytes in the different matrix of yam peel. A dedicated analytical method was needed to simultaneously evaluate the exact amount of three bioactive phenanthrenes, which are correlated with bioactivity, in peels of the Dioscorea genus. In this investigation, we described the development and validation of a quantitative HPLC method using a photodiode array (PDA) detector to detect phenanthrenes in the peels of four Dioscorea species cultivated or collected in Korea, including D. batatas, Dioscorea polystachya, Dioscorea quinqueloba, and D. bulbifera. The results could provide a basis for quality assessment and standardization of phenanthrenes in the extracts of various Dioscorea species’ peels to discover further their full promise as functional biomaterials.
Materials and Methods
General Experimental Procedures
Acetonitrile, water, and methanol, all reagent grade, were purchased from J.T. Baker (USA). Trifluoroacetic acid (TFA) was purchased from Sigma-Aldrich (USA). Ethanol (extra-pure grade) was purchased from Duksan Pure Chemicals Co., Korea). Waters Alliance 2695 high-performance liquid chromatography (HPLC) (Waters, USA) which was performed on a Hector-M-C18 column (150 × 4.6 mm, 5 μm, RS Tech Corporation, Korea) was used for the analysis and the samples were detected by photodiode array detector (PDA, Waters 2996).
Plant Material
D. batatas and D. polystachya tubers were purchased from Oneul-Achim (Korea). D. bulbifera was collected in Jeongeup, Korea, and D. quinqueloba was collected in Damyang, Korea. Their peels were separated from flesh and washed with water, then dried with a freeze-dryer (Ilshinbiobase, Korea).
Preparation of Standard Compounds
Phenanthrenes, respectively named as 2,7-dihydroxy-4,6-dimethoxy phenanthrene (1), 6,7-dihydroxy-2,4-dimethoxy phenanthrene (2), and batatasin I (3), were isolated from the peel of D. batatas and identified following the previous description [4]. The purity of standard compounds was checked by HPLC. Their structures were confirmed by nuclear magnetic resonance (NMR) spectra and also through comparison with those reported in the literature.
Sample Preparation
The freeze-dried peels of four Dioscorea species were powdered, and 1 g of dried powder from each sample was extracted with 20 ml of 95% ethanol for 2 h. Afterward, the samples were filtered with filter paper (Hyundai Micro CO., Korea) and evaporated in vacuo. The resultant extracts were weighed and dissolved in methanol to yield a concentration of 10 mg/ml. The extracts were then filtered by 0.45 μm syringe filters (Advantec, Japan) and injected into the HPLC system for analysis.
Optimization of HPLC Analysis Conditions
The best analytical conditions were determined based on the methods suggested by Kim et al. [4] and Yoon et al.[19]. Parameters such as solvent, gradient composition, column temperature, and column type were evaluated to determine the optimal separation conditions for phenanthrenes from the Dioscorea peels. The mobile phases used were acetonitrile (A) and 0.1% TFA in water (B) at a flow rate of 1.0 ml/min. The gradient program was set as follows: 0-10 min, 20‒80% A; 10-12 min, 100% A; and 12-15 min, 20% A. The chromatograms were monitored at a wavelength of 261 nm, and the injection volume was 10 μl. The column temperature was maintained at 40°C throughout the analysis. The samples were injected 3 times each, and the averages of the peak areas on the chromatograms were obtained for quantitative analysis.
Validation of the HPLC Method
The HPLC method for the determination of phenanthrenes (1‒3) was validated in terms of linearity, detection limit (LOD), quantitation limit (LOQ), precision, and accuracy [20]. The calibration curve for each phenanthrene was prepared at the six concentrations of 0.625, 1.25, 2.5, 5, 10, and 20 μg/ml. Calibration curves were obtained by averaging the peak areas of each compound on the chromatograms acquired from 3 injections that were monitored at a wavelength of 261 nm. The LOD and LOQ values of the developed method were calculated for phenanthrenes in the peels of Dioscorea extracts. The LOD and LOQ were calculated using the standard deviation (SD) and the slope (S) of the standard curve obtained and determined by regression analysis of the injection concentration and peak area using the following formula: [LOD =3.3*SD/S, LOQ =10*SD/S]. In addition, the intraday precision was calculated by five replicates of phenanthrenes at concentrations of 1.25‒10 μg/ml on the same day. The interday precision was assessed by analyzing phenanthrenes at the same concentrations (1.25‒10 μg/ml) on five different days. The precision was verified using the RSD value, which is the relative standard deviation of each result, following the formula: [RSD (%) = (SD/mean) * 100%]. The accuracy evaluation was measured by comparing the theoretical concentration (TC) and the actual concentration (EC) by injecting the spiked extracts. The spiking process was performed by mixing 250 μl of methanol (blank) and 2.5, 5, and 10 μg/ml standard mixture with 250 μl of 95% ethanol extraction of D. batatas peel at a low concentration. The percent recovery of each phenanthrene from the D. batatas extract was calculated as follows: [Recovery (%) = EC/TC × 100 (%)].
Results and Discussion
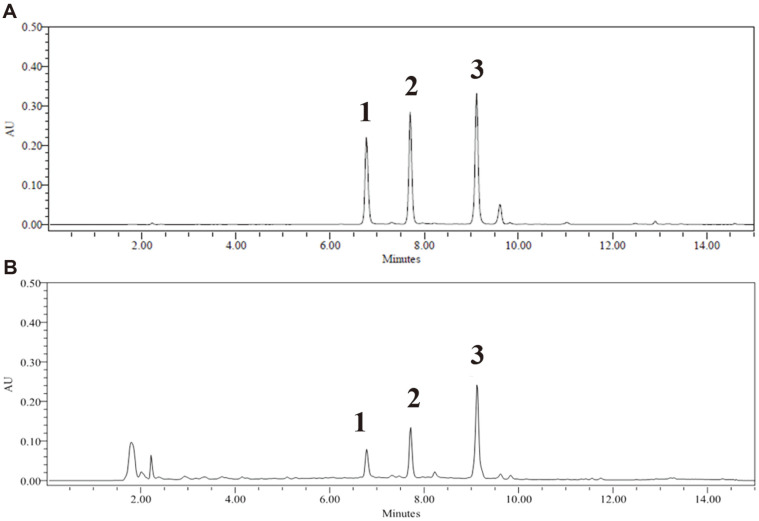
A method for quantitative analysis of three representative phenanthrenes (1‒3) (Fig. 1) in peels of four Dioscorea species using HPLC with a PDA detector was developed. The HPLC-PDA maximum absorbance wavelength of phenanthrenes was confirmed to be 261 nm. This method was modified from that of a previous report [4] by using different parameters to improve peak broadening and minimize running time and costs in routine analysis. From the chromatograms in Fig. 2, phenanthrenes (1‒3) were completely separated without overlapping with other peaks, and their retention times (r.t.) were 6.76, 7.69, and 9.10 min, respectively, shorter than those in the previous report [4]. In our study, linearity, the LOD, the LOQ, precision, and accuracy were validated [20, 21]. For the calibration curve preparation, standard solutions were prepared by serial dilution to the appropriate concentrations. As shown in Table 1, the linearity of calibration curves obtained from standard solutions was satisfactory with the determination coefficients (R2), which ranged from 0.9995 to 0.9996 in the concentration range of 0.625‒20.00 μg/ml. The LODs of phenanthrenes (1‒3) were 0.78, 0.82, and 0.89 μg/ml, respectively, and the LOQs were 2.38, 2.49, and 2.71 μg/ml, respectively (Table 1). Thus, the sensitivity of the HPLC-PDA method was determined to be appropriate for the quantitative detection of phenanthrenes in the Dioscorea genus.
Fig. 1.

Chemical structures of phenanthrenes (1‒3) isolated from D. batatas.
Fig. 2.
Chromatogram of phenanthrenes (1‒3) mixture (A) and 95% EtOH extract of the D. batatas peel monitored at 261 nm (B).
Table 1.
Calibration, LODs, and LOQs of phenanthrenes (1‒3).
| Compounds | Regression equation | Range (μg/ml) | R2(a) | LOD (μg/ml) | LOQ (μg/ml) |
|---|---|---|---|---|---|
| 1 | y = 87934x + 17518 | 0.625 – 20 | 0.9996 | 0.78 | 2.38 |
| 2 | y = 109235x + 19608 | 0.625 – 20 | 0.9995 | 0.82 | 2.49 |
| 3 | y=135505x + 28552 | 0.625 – 20 | 0.9995 | 0.89 | 2.71 |
aCoefficient of determination.
In addition, accuracy and precision are the most important validation parameters in the assessment of an analytical method [22]. Precision and accuracy were analyzed using the relative standard deviation (RSD) and recovery, respectively. As the results show in Table 2, the precisions were 0.41‒1.55% and 1.66‒5.00% for phenanthrene 1, 0.30‒1.31% and 2.39‒7.58% for phenanthrene 2, and 0.25‒0.89% and 2.89‒5.58% for phenanthrene 3, respectively. Furthermore, the recovery rates were 95.22‒100.80%, 95.07‒100.34%, and 95.87‒100.12% for phenanthrenes (1‒3), respectively (Table 3). The above results were acceptable to the method validation guideline presented by Association of Official Agricultural Chemists (AOAC) [23]. Hence, the HPLC-PDA method for phenanthrenes displayed good precision and accuracy at all concentrations in the D. batatas peel extract.
Table 2.
Intraday and interday precisions of phenanthrenes (1‒3).
| Compounds | TCa | ECb | RSDc |
|---|---|---|---|
| 1 | Intraday (n = 5) | ||
| 1.25 | 1.18 ± 0.02 | 1.55 | |
| 2.5 | 2.51 ± 0.02 | 0.81 | |
| 5 | 5.11 ± 0.02 | 0.47 | |
| 10 | 9.97 ± 0.02 | 0.41 | |
| Interday (n = 5) | |||
| 1.25 | 1.14 ± 0.06 | 5.00 | |
| 2.5 | 2.48 ± 0.05 | 1.88 | |
| 5 | 4.98 ± 0.18 | 3.52 | |
| 10 | 9.85 ± 0.16 | 1.66 | |
| 2 | Intraday (n = 5) | ||
| 1.25 | 1.18 ± 0.02 | 1.31 | |
| 2.5 | 2.52 ± 0.02 | 0.98 | |
| 5 | 5.09 ± 0.04 | 0.75 | |
| 10 | 9.97 ± 0.03 | 0.30 | |
| Interday (n = 5) | |||
| 1.25 | 1.10 ± 0.08 | 7.58 | |
| 2.5 | 2.46 ± 0.09 | 3.54 | |
| 5 | 4.92 ± 0.22 | 4.55 | |
| 10 | 9.77 ± 0.23 | 2.39 | |
| 3 | Intraday (n = 5) | ||
| 1.25 | 1.20 ± 0.01 | 0.89 | |
| 2.5 | 2.51 ± 0.02 | 0.61 | |
| 5 | 5.09 ± 0.03 | 0.57 | |
| 10 | 9.97 ± 0.02 | 0.25 | |
| Interday (n = 5) | |||
| 1.25 | 1.24 ± 0.06 | 4.73 | |
| 2.5 | 2.64 ± 0.15 | 5.58 | |
| 5 | 5.22 ± 0.15 | 2.89 | |
| 10 | 10.31 ± 0.44 | 4.23 |
aTheoretical concentration (μg/ml); bExperimental concentration (μg/ml); cRelative standard deviation.
Table 3.
Recovery of phenanthrenes (1‒3) with 95% ethanol extract of D. batatas peel.
| ASCCa | TCb | ECc | RSDd | Recovery (%) |
|---|---|---|---|---|
| Compound 1 | ||||
| Blank | 0.37 | 0.37 ± 0.00 | 0.98 | |
| 1.25 | 1.62 | 1.55 ± 0.01 | 0.90 | 95.22 ± 0.77 |
| 2.5 | 2.87 | 2.90 ± 0.03 | 0.95 | 100.80 ± 0.86 |
| 5 | 5.37 | 5.31 ± 0.06 | 1.19 | 98.77 ± 1.05 |
| Compound 2 | ||||
| Blank | 0.52 | 0.52 ± 0.01 | 2.02 | |
| 1.25 | 1.77 | 1.68 ± 0.01 | 0.46 | 95.07 ± 0.40 |
| 2.5 | 3.02 | 3.03 ± 0.04 | 1.25 | 100.34 ± 1.13 |
| 5 | 5.52 | 5.44 ± 0.06 | 1.18 | 98.60 ± 1.04 |
| Compound 3 | ||||
| Blank | 0.75 | 0.75 ± 0.02 | 2.11 | |
| 1.25 | 2.00 | 1.92 ± 0.01 | 0.44 | 95.87 ± 0.38 |
| 2.5 | 3.25 | 3.25 ± 0.04 | 1.32 | 100.12 ± 1.18 |
| 5 | 5.75 | 5.71 ± 0.08 | 1.41 | 99.31 ± 1.25 |
aAdded standard compounds concentration; bTheoretical concentration (μg/ml); cExperimental concentration (μg/ml); dRelative standard deviation.
Species of the Dioscorea genus are known for their association with low-cost food culture, traditional medicine, modern Western medicine, and the pharmaceutical industry, as they possess various nutrients and phytochemicals, which differ in chemical structure, molecular weight, polarity, and other characteristics [9]. Across different geographic regions, diverse species of Dioscorea have been adopted within different habited areas as a food source due to the high nutritional benefits and therapeutic value for the treatment and cure of certain health problems [24‒26]. Previous extensive phytochemistry investigations carried out on the genus have resulted in the obtention of various bioactive metabolites such as allantoin [27], saponins [28], dioscorin [29], flavonoids [30], phenanthrenes [15‒19], and phenolic derivatives [31]. Phenanthrene-containing plants including the Dioscorea genus have been widely used as traditional medicines for the treatment of various diseases [1, 3]. To evaluate the content of phenanthrenes in the Dioscorea genus, the subsequent quantitative analysis with HPLC-PDA was investigated on the peel extracts of four Dioscorea species, including D. batatas, D. polystachya, D. quinqueloba, and D. bulbifera. Notably, all investigated Dioscorea species were found to possess antioxidant effects in a previous report [32]. In the present study, the results showed that phenanthrene 1 was the most abundant in the peel of D. quinqueloba with content of 173.69 μg/g on the basis of weight. The content of phenanthrene 2 was 166.99 μg/g based on weight, and was highest in the peel extract of D. polystachya. On the other hand, the peel extract of D. polystachya was also rich in phenanthrene 3 with content of 419.73 μg/g on the basis of weight. Therefore, phenanthrenes were most abundant in the peel of D. polystachya and least abundant in the D. bulbifera peel extract. Based on the results in Table 4, all standard phenanthrenes (1‒3) were presented in the peels of D. batatas and D. polystachya, and phenanthrenes (2 and 3) were not detected in the peel of D. quinqueloba, whereas phenanthrene 3 was not detected in the D. bulbifera peel extract.
Table 4.
Phenanthrenes (1‒3) content in the Dioscorea genus extracts.
| Content (μg/g) | |||||
|---|---|---|---|---|---|
|
| |||||
| D. batatas | D. polystachya | D. quinqueloba | D. bulbifera | ||
| Compounds | 1 | 77.35 ± 1.48 | 107.92 ± 2.99 | 173.69 ± 3.60 | 9.79 ± 0.65 |
| 2 | 46.65 ± 1.14 | 166.99 ± 5.24 | N.D. | 4.24 ± 0.12 | |
| 3 | 97.19 ± 1.90 | 419.73 ± 12.02 | N.D. | N.D. | |
N.D.: Non-detected.
In conclusion, phenanthrenes are a promising group of biologically active natural metabolites whose potential needs to be thoroughly investigated. Lots of phenanthrene-containing plants have been used in traditional medicine, including those of the Dioscorea genus. The proposed HPLC-PDA method was validated and developed for the quantification of phenanthrenes in the peels of Dioscorea spp. The method was optimized, and sample pretreatment was validated in terms of linearity, LOD, LOQ, precision, and accuracy. The obtained results showed that the developed method was suitable for the identification and quantification of phenanthrenes in the Dioscorea genus. Moreover, phenanthrenes (1‒3) were the most abundant in the peel extracts of D. polystachya. These findings suggest that more attention should be paid to investigating the bioactive phenanthrenes in the peel of D. polystachya.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the Useful Agricultural Life Resources Industry Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant No. 121049-2), Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2021R1A6C101A416), and a project to train professional personnel in biological materials by the Ministry of Environment.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Tóth B, Hohmann J, Vasas A. Phenanthrenes: a promising group of plant secondary metabolites. J. Nat. Prod. 2018;81:661–678. doi: 10.1021/acs.jnatprod.7b00619. [DOI] [PubMed] [Google Scholar]
- 2.Cao TQ, Kim JA, Woo MH, Min BS. SARS-CoV-2 main protease inhibition by compounds isolated from Luffa cylindrica using molecular docking. Bioorg. Med. Chem. Lett. 2021;40:127972. doi: 10.1016/j.bmcl.2021.127972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovács A, Vasas A, Hohmann J. Natural phenanthrenes and their biological activity. Phytochemistry. 2008;69:1084–1110. doi: 10.1016/j.phytochem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Kim M, Gu MJ, Lee JG, Chin J, Bae JS, Hahn D. Quantitative analysis of bioactive phenanthrenes in Dioscorea batatas Decne peel, a discarded biomass from postharvest processing. Antioxidants. 2019;8:541. doi: 10.3390/antiox8110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngan NTT, Hoang NH, Hien NT, Lan NN, Lien NTK, Quang TH, et al. Cytotoxic phenanthrenes and phenolic constituents from the tubers of Dioscorea persimilis. Phytochem. Lett. 2020;40:139–143. doi: 10.1016/j.phytol.2020.10.005. [DOI] [Google Scholar]
- 6.Bús C, Kúsz N, Kincses A, Szemerédi N, Spengler G, Bakacsy L, et al. Antiproliferative phenanthrenes from Juncus tenuis: Isolation and diversity-oriented semisynthetic modification. Molecules. 2020;25:5983. doi: 10.3390/molecules25245983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim JS, Hahn D, Gu MJ, Oh J, Lee JS, Kim JS. Anti-inflammatory and antioxidant effects of 2,7-dihydroxy-4,6-dimethoxy phenanthrene isolated from Dioscorea batatas Decne. Appl. Biol. Chem. 2019;62:29. doi: 10.1186/s13765-019-0436-2. [DOI] [Google Scholar]
- 8.Matsuda H, Morikawa T, Xie H, Yoshikawa M. Antiallergic phenanthrenes and stilbenes from the tubers of Gymnadenia conopsea. Planta Med. 2004;70:847–855. doi: 10.1055/s-2004-827234. [DOI] [PubMed] [Google Scholar]
- 9.Adomėnienė A, Venskutonis PR. Dioscorea spp. Comprehensive review of antioxidant properties and their relation to phytochemicals and health benefits. Molecules. 2022;27:2530. doi: 10.3390/molecules27082530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi VV. Dictionary of medicinal plants in Vietnam. Vietnamese Publisher of Medicine. 2012;1:654–655. [Google Scholar]
- 11.Zhu F. Isolation, composition, structure, properties, modifications, and uses of yam starch. Compr. Rev. Food Sci. Food Saf. 2015;14:357–386. doi: 10.1111/1541-4337.12134. [DOI] [PubMed] [Google Scholar]
- 12.Salehi B, Sener B, Kilic M, Sharifi-Rad J, Naz R, Yousaf Z, et al. Dioscorea plants: A genus rich in vital nutra-pharmaceuticals - A review. Iran J. Pharm. Res. 2019;18:68–89. doi: 10.22037/ijpr.2019.112501.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaniad P, Wattanapiromsakul C, Pianwanit S, Tewtrakul S. Anti-HIV-1 integrase compounds from Dioscorea bulbifera and molecular docking study. Pharm. Biol. 2016;54:1077–1085. doi: 10.3109/13880209.2015.1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo KW, Kwon OW, Kim SY, Choi SZ, Son MW, Kim KH, et al. Phenolic derivatives from the rhizomes of Dioscorea nipponica and their anti-neuroinflammatory and neuroprotective activities. J. Ethnopharmacol. 2014;155:1164–1170. doi: 10.1016/j.jep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Boudjada A, Touil A, Bensouici C, Bendif H, Rhouati S. Phenanthrene and dihydrophenanthrene derivatives from Dioscorea communis with anticholinesterase, and antioxidant activities. Nat. Prod. Res. 2019;33:3278–3282. doi: 10.1080/14786419.2018.1468328. [DOI] [PubMed] [Google Scholar]
- 16.Du D, Zhang R, Xing Z, Liang Y, Li S, Jin T, et al. 9,10-Dihydrophenanthrene derivatives and one 1,4-anthraquinone firstly isolated from Dioscorea zingiberensis C.H. Wright and their biological activities. Fitoterapia. 2016;109:20–24. doi: 10.1016/j.fitote.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Lim JS, Oh J, Yun HS, Lee JS, Hahn D, Kim JS. Anti-neuroinflammatory activity of 6,7-dihydroxy-2,4-dimethoxy phenanthrene isolated from Dioscorea batatas Decne partly through suppressing the p38 MAPK/NF-κB pathway in BV2 microglial cells. J. Ethnopharmacol. 2022;282:114633. doi: 10.1016/j.jep.2021.114633. [DOI] [PubMed] [Google Scholar]
- 18.Lee W, Jeong SY, Gu MJ, Lim JS, Park EK, Baek MC, et al. Inhibitory effects of compounds isolated from Dioscorea batatas Decne peel on particulate matter-induced pulmonary injury in mice. J. Toxicol. Environ. Health A. 2019;82:727–740. doi: 10.1080/15287394.2019.1646174. [DOI] [PubMed] [Google Scholar]
- 19.Yoon KD, Yang MH, Nam SI, Park JH, Kim YC, Kim J. Phenanthrene derivatives, 3,5-dimethoxyphenanthrene-2,7-diol and batatasin-I, as non-polar standard marker compounds for Dioscorea rhizome. Nat. Prod. Sci. 2007;13:378–383. [Google Scholar]
- 20.ICH, author. International council for harmonisation of technical requirements for pharmaceuticals for human use (ICH), Validation of analytical procedures: Text and methodology Q2 (R1) 1995. Available online: https://www.ema.europa.eu/en/documents/scientificguideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf .
- 21.Choi SI, Han X, Lee SJ, Men X, Oh G, Lee DS, et al. Validation of an analytical method for the determination of thiabendazole in various food matrices. Separations. 2022;9:135. doi: 10.3390/separations9060135. [DOI] [Google Scholar]
- 22.Karnes HT, March C. Precision, accuracy, and data acceptance criteria in biopharmaceutical analysis. Pharm. Res. 1993;10:1420–1426. doi: 10.1023/A:1018958805795. [DOI] [PubMed] [Google Scholar]
- 23.AOAC, author. Appendix F: Guidelines for standard method performance requirements. AOAC Official methods of analysis. 2016. Available online: http://www.eoma.aoac.org/app_f.pdf .
- 24.Dutta B. Food and medicinal values of certain species of Dioscorea with special reference to Assam. J. Pharmacogn. Phytochem. 2015;3:15–18. [Google Scholar]
- 25.Trimanto, Hapsari L. Diversity and utilization of Dioscorea spp. tuber as alternative food source in Nganjuk Regency, East Java. Agrivita. 2015;37:97–107. doi: 10.17503/Agrivita-2015-37-2-p097-107. [DOI] [Google Scholar]
- 26.Go HK, Rahman MM, Kim GB, Na CS, Song CH, Kim JS, et al. Antidiabetic effects of yam (Dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutrients. 2015;7:8532–8544. doi: 10.3390/nu7105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu YC, Ferng LHA, Huang PY. Quantitative analysis of allantoin and allantoic acid in yam tuber, mucilage, skin, and bulbil of the Dioscorea species. Food Chem. 2006;94:541–549. doi: 10.1016/j.foodchem.2004.12.006. [DOI] [Google Scholar]
- 28.Sautour M, Mitaine-Offer AC, Lacaille-Dubois MA. The Dioscorea genus: a review of bioactive steroid saponins. J. Nat. Med. 2007;61:91–101. doi: 10.1007/s11418-006-0126-3. [DOI] [Google Scholar]
- 29.Liao YH, Tseng CY, Chen W. Structural characterization of dioscorin, the major tuber protein of yams, by near infrared Raman spectroscopy. J. Phys. Conf. Ser. 2006;28:119–122. doi: 10.1088/1742-6596/28/1/025. [DOI] [Google Scholar]
- 30.Obidiegwu JE, Lyons JB, Chilaka CA. The Dioscorea genus (Yam) - An appraisal of nutritional and therapeutic potentials. Foods. 2020;9:1034. doi: 10.3390/foods9091304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Zhao C, Jing S, Sun J, Li X, Man S, et al. Novel phenanthrene and isocoumarin from the rhizomes of Dioscorea nipponica Makino subsp. rosthornii (Prain et Burkill) C. T. Ting (Dioscoreaceae) Bioorg. Med. Chem. Lett. 2017;27:3595–3601. doi: 10.1016/j.bmcl.2017.03.095. [DOI] [PubMed] [Google Scholar]
- 32.Kim KM, Kang MK, Kim JS, Kim GC, Choi SY. Physicochemical composition and antioxidant activities of Korean Dioscorea species. J. East Asian Soc. Dietary Life. 2015;25:880–886. doi: 10.17495/easdl.2015.10.25.5.880. [DOI] [Google Scholar]