Abstract
Previously, we demonstrated that an experimental smallpox DNA vaccine comprised of four vaccinia virus genes (4pox) administered by gene gun elicited protective immunity in mice challenged with vaccinia virus, and in nonhuman primates challenged with monkeypox virus (Hooper JW, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol 2004;78:4433–43). Here, we report that this 4pox DNA vaccine can be efficiently delivered by a novel method involving skin electroporation using plasmid DNA-coated microneedle arrays. Mice vaccinated with the 4pox DNA vaccine mounted robust antibody responses against the four immunogens-of-interest, including neutralizing antibody titers that were greater than those elicited by the traditional live virus vaccine administered by scarification. Moreover, vaccinated mice were completely protected against a lethal (>10LD50) intranasal challenge with vaccinia virus strain IHD-J. To our knowledge, this is the first demonstration of a protective immune response being elicited by microneedle-mediated skin electroporation.
Keywords: Poxviruses, DNA vaccine, Electroporation
1. Introduction
Our research is aimed at developing a molecular smallpox vaccine. Smallpox as a naturally occurring disease was eradicated after a world-wide vaccination campaign; however, the threat that smallpox or a related poxvirus could be used as a biological weapon remains. One way to counter this threat is to vaccinate. The vaccine currently licensed by the Food and Drug Administration is composed of live vaccinia virus (VACV) administered by skin prick with a bifurcated needle. This technology was developed more than two centuries ago by Edward Jenner. Although undoubtedly effective, there are several drawbacks to this vaccine including (1) nonserious and serious adverse events that make the vaccine contraindicated in large segments of the population (e.g., persons who are immunodeficient, immunosuppressed, pregnant, breastfeeding, or have history of cardiac disease), and (2) because this vaccine results in a localized skin infection containing infectious virus (i.e., pock), the infection can spread to other sites on the body (e.g., ocular autoinoculation) or to persons who come in close contact with the vaccinee [2]. Vaccine candidates comprised of attenuated versions of VACV have been produced and tested in humans. These vaccines appear to be safer than the classic smallpox vaccine because the virus used in the vaccine is incapable of dissemination and transmission. However, recent studies caution that these attenuated viruses fail to induce protective immunity in immunocompromised rhesus macaques, possibly due to defects in antibody class switching [3]. Further studies to evaluate the efficacy of alternative live poxvirus vaccines are in progress. Nevertheless, it is prudent to consider that these vaccines involve infection with live, albeit attenuated, poxviruses that encode approximately 200 genes, many with immunomodulatory properties and some with unknown function. Identification of the genes associated with protective immunity and, conversely, the genes associated with adverse events unrelated to dissemination or transmission will be important for characterizing the next-generation smallpox vaccines and for engineering future smallpox vaccines.
There are two infectious forms of orthopoxviruses: the intracellular mature virion (IMV) and the extracellular enveloped virion (EEV). IMV are released from lysed cells and are likely the form of the virus that would be used in a biological attack due to their stability in the environment. EEV consist of IMV that have been wrapped with additional cell-derived membranes during morphogenesis and have budded from infected cells. EEV are not stable outside the host; however, they are suspected to be the primary form of the virus involved in long-range spread within the host [4]. The 4pox DNA vaccine is comprised of four plasmids two of which encode proteins found on the membrane of the IMV and two encode proteins found on the membrane of the EEV. pWRG/L1R and pWRG/A27L are plasmids that express the VACV L1R or A27L open reading frame gene products. These plasmids have been shown to elicit IMV neutralizing antibodies in mice and nonhuman primates vaccinated by gene gun [5], [6]. pWRG/A33R and pWRG/B5R are plasmids that express the VACV A33R and B5R open reading frame gene products, and these plasmids can elicit protective anti-EEV antibodies in mice and nonhuman primates vaccinated by gene gun [5], [6]. Similar plasmids expressing the VACV A33R or B5R genes were shown to protect mice when injected intramuscularly [7].
Several DNA vaccines have been evaluated for safety and efficacy in clinical trials [8]. The location and method of delivery plays a significant role in the efficacy of DNA-based vaccines. For example, DNA vaccines administered by needle-injection intramuscularly have elicited only weak antibody responses (except when followed by a protein boost), whereas DNA vaccines administered to the skin by particle-mediated epidermal delivery using a gene gun have elicited impressive immune responses in humans, and importantly, protective immunity [9], [10], [11], [12]. The enhanced immunogenicity of DNA vaccines delivered by gene gun likely involves the direct introduction of plasmid DNA to cells in the skin, including specialized antigen-presenting cells (e.g., Langerhan's cells). While the gene gun has yielded among the most promising immune responses for a DNA vaccine thus far, as with any technology, there is the possibly that all of the criteria required for successful product development will not be satisfied (e.g., safety, acceptance, efficacy, practicality). Hence, it is important to continue to evaluate alternative technologies that might have attributes that facilitate the development of licensed human vaccines.
Alternative means of delivering DNA vaccines under investigation include the use of electric field technologies. Electroporation is a process where cells are transiently permeabilized by high-intensity electric field pulses [9], [13], [14]. Here, we tested a novel device capable of targeting electroportion to the dermis using a microneedle array (Fig. 1 ). The plasmid DNA is dried onto the tips of the microneedles. These microneedles (≤1 mm long) are inserted into the skin where the DNA dissolves in interstial fluid and is then transfected into the surrounding cells by electroporation.
Fig. 1.
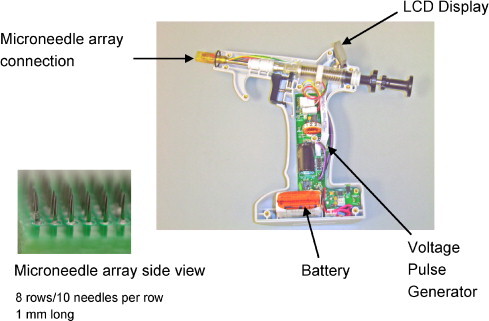
Easy Vax™ vaccine delivery system. The Easy Vax™ vaccine delivery system has two parts: an electrically conductive microneedle array coated with dried vaccine DNA and an Easy Vax™ device designed to insert the array into skin and to provide the electrical pulses to deliver DNA into cells. The Easy Vax™ device contains a battery powered pulse generator capable of delivering pulses up to 120 volts. The microneedle arrays used in this study have 80 needles arranged in an 8 × 10 matrix with a 0.6 mm distance tip to tip within and between rows of needles (see inset). Depth of insertion was set by adjusting a collar surrounding the needle array and by adjusting the force and speed of array insertion with a spring adjustment. The average insertion depth of needles in this study was 0.45 mm.
2. Methods
2.1. Viruses and cells
VACV Connaught vaccine strain (derived from the New York City Board of Health strain), VACV strain WR (ATCC VR-1354), and VACV strain IHD-J (obtained from Dr. Alan Schmaljohn) were all maintained in VERO cell (ATCC CRL-1587) monolayers grown in Eagle minimal essential medium, containing 5% heat-inactivated fetal bovine serum (FBS), 1% antibiotics (100 U/ml penicillin, 100 μg/ml of streptomycin, and 50 μg/ml of gentamicin), 10 mM HEPEs (cEMEM). COS cells (ATCC CRL-1651) were used for transient expression experiments. BS-C-1 cells (ATCC CCL-26) were used for plaque reduction neutralization tests (PRNT).
2.2. Plasmids and immune serum
The VACV gene-containing plasmids used in this study, pWRG/L1R(x), pWRG/A33R(x), pWRG/B5R, and pWRG/A27L have all been described previously [6]. Negative control plasmids contained irrelevant genes encoding Hantaan virus glycoproteins or hepatitis B antigen in the same or different backbone, pWRG/HTN-M(x) [15] and pRc/CMV-HBs(S) (Aldevron, Fargo, ND), respectively.
For L1, initial experiments used pWRG/L1R(x), however low levels of expression prompted us to construct a new plasmid. To construct the new plasmid, pWRG/TPA-L1R, the L1R open reading frame was subcloned into the NheI and BglII sites of vector pWRG/TPA. pWRG/TPA contains a CMV promoter identical to pWRG but also contains a tissue plasminogen secretion signal sequence (TPA). L1R was amplified by PCR using the forward primer
GGGGGGCTAGCATGGGTGCCGCAGCAAGC and the reverse primer GGGTCTAGATCAGTTTTGCATATCCG. These primers contain a NheI and BglII site, respectively. The resultant PCR product was cut with NheI and BglII, gel purified and ligated into pWRG/TPA vector, in frame with the TPA signal sequence. Sequence analysis confirmed that the L1R insert was in frame with the TPA signal sequence.
2.3. Vaccine delivery
Adult (16–23 g) female BALB/c mice were vaccinated in the skin of the thigh. An Easy Vax™ DNA vaccine delivery system was used to deliver the vaccine plasmids. The Easy Vax™ DNA vaccine delivery system consisted of a microneedle array and a self-contained device used to insert the array into skin and apply the electrical pulses. The microneedle array used in this study was an 80-needle array arranged in eight rows of 10 needles. DNA was applied as a liquid to individual needles in the array and dried onto the needles before use. The arrays were stored under desiccation at room temperature. The pulse protocol consisted of six pulses of 100 Volts, 100 μS pulse duration and 125 mS pulse interval. Mice vaccinated with the 4pox DNA vaccine were administered four arrays, each coated with 30 μg of a separate plasmid (four total). Each array was administered to a separate site (inner and outer right and left thigh). Mice vaccinated with the negative controls plasmids were administered using one array to an inner thigh.
2.3.1. Vaccination by tail scarification
Anesthetized mice were scarified by placing 10 μl of PBS containing live VACV (∼8 × 106 plaque forming units of VACV, Connaught strain) on the tail, ∼1 cm from the tail base. A 26 gauge 5/8″ needle was used to scratch the tail (10×, ∼2 mm scratches) to facilitate infection/vaccination. A lesion (pock) at the site of scarification on day 10 indicated successful vaccination.
2.4. VACV infected-cell-lysate ELISA
ELISAs were performed essentially as described elsewhere [5]. Here, VACV strain WR infected-cell-lysate was used as antigen. Endpoint titers were determined as the highest dilution with an absorbance value greater than the mean absorbance value from negative control plasmid-vaccinated animals plus three standard deviations. The secondary antibody used to detect mouse IgG was peroxidase-conjugated goat anti-mouse Ig diluted 1:1000 (Sigma). When geometeric mean titers (GMT) were calculated for a group of sera, the GMT were equal to the anti-log of the arithmetic mean of the log10-transformed titer values for each serum sample.
In the antibody isotyping experiment, ELISA endpoint titers were not determined, but rather, the specific O.D. from a dilution series was graphically represented as a stacked graph for each sample. The secondary antibody used to detect mouse IgG1 or IgG2a was peroxidase-conjugated goat anti-mouse IgG1 and peroxidase-conjugated goat anti-mouse IgG2a diluted 1:1000 (Bethyl Laboratories). For isotype controls, two monoclonal antibodies that bind VACV D8L protein were used. MAb-5B8 is an IgG2a antibody and MAb-1D3 is an IgG1 antibody (J.W. Hooper and A. Schmaljohn, unpublished data).
2.5. VACV plaque reduction neutralization test (PRNT)
The PRNT was performed essentially as described previously [5]; however, BS-C-1 cells and a semisolid overlay was used. Briefly, VACV strain IHD-J was diluted in cEMEM to give ∼250 pfu/ml. Aliquots of this viral suspension (100 μl) were incubated with an equal volume of serum diluted in cEMEM (serum samples were heat activated, 56 °C for 30 min, before dilution) for 1 h at 37 °C and then 180 μl of sample was adsorbed to confluent BS-C-1 cell monolayers in 6-well plates for 1 h in a 37 °C 5% CO2 incubator. A 2 ml semisolid overlay (Earle's basal minimal essential medium, 1.5% methyl cellulose, 5% heat-inactivated FBS, antibiotics (100 U/ml penicillin, 100 μg/ml of streptomycin, and 50 μg/ml of gentamicin) was added to each well. After 4 days in 37 °C 5% CO2 incubator, cell monolayers were stained with 1 ml of crystal violet staining solution. Plaques were counted and the percent neutralization was calculated relative to the number of plaques in the absence of antibody. Titers represent the reciprocal of the highest dilution resulting in a 50% reduction in the number of plaques. GMT were equal to the anti-log of the arithmetic mean of the log10-transformed titer values for each serum sample.
2.6. Indirect immunofluorescence antibody test (IFAT)
COS cells grown on 15-mm glass cover slips in 12-well cell culture plates were transfected with 0.5 μg of DNA using Fugene6 (Roche) as described by the manufacturer. Two days after transfection, cover slips were rinsed once with PBS (pH 7.4) and fixed with acetone:methanol (equal volumes) for 10 min at room temperature. Slides were rinsed three times in PBS and blocked 10 min in PBS containing 5% goat serum. Mouse sera were diluted 1:100 in blocking buffer and then incubated on transfected cells for 1 h at room temperature. Cover slips were rinsed three times with PBS and incubated 30 min at 37 °C with Alexafluor-488 goat anti-mouse IgG heavy and light (Molecular Probes, Eugene, OR). Cover slips were rinsed three times with PBS and then placed on a drop of Prolong Gold anti-fade reagent with DAPI (Molecular Probes) on glass slides. Slides were cured overnight at room temperature and then visualized by a Nikon E600 fluorescence microscope. Images were taken using a SPOT camera (Diagnostic Instruments, INC). For some experiments, cells were stained with the L1-specific monoclonal antibody, MAb-10F5 diluted 1:100 [5].
2.7. Fluorescently activated cell sorting (FACS)
COS-7 cells were plated on T25 flasks and transfected with 5 μg DNA encoding pWRG/L1R, pWRG/TPA-L1R or pWRG/TPA using Fugene6 as described by the manufacturer. Two days post-transfection, cells were washed with PBS and trypsinized. Cells were then resuspended in 5 ml medium containing 5% FBS and placed in 15 ml plastic tubes. Tubes were spun at low speed for 5 min at RT and the medium was removed. Cells were resuspended in 5 ml PBS and 1 ml (∼5 × 105 cells) was transferred to 1.5 ml tubes. Cells were subsequently incubated with MAb-10F5 diluted 1:500 in PBS containing 5% FBS for 1 h at RT with constant agitation. After antibody incubation, cells were washed twice with PBS and low speed centrifugation. Cells were then incubated with Alexafluor-488-goat anti-mouse IgG heavy and light diluted 1:500 for 30 min at RT with constant agitation. Cells were washed three times with PBS, after the final spin cells were resuspended in 1 ml PBS and added to tubes containing 1 ml of FACS buffer. Stained cells were analyzed on a FACScalibur FACS machine (BD Biosciences, San Jose, CA). For each experiment, a total of 10,000 cells were counted.
2.8. Challenge
Mice were anesthetized and weighed before intranasal injection of 50 μl (25 μl per nare) of PBS containing 2 × 106 pfu of VACV strain IHD-J using a plastic pipette tip. After challenge, mice were observed and weighed daily for 3 weeks. Moribund mice (>30% body weight) were euthanized.
3. Results
3.1. Microneedle-mediated skin electroporation elicits neutralizing antibodies against poxviruses
We performed an experiment to test the capacity of a microneedle-mediated skin electroporation device to deliver the 4pox DNA vaccine. A group of eight mice was vaccinated with the 4pox DNA vaccine administered using the Easy Vax™ skin electroporation device on weeks 0, 3, and 8. A negative control group of 16 mice was vaccinated with negative control plasmids using the same device and same schedule. A positive control group of eight mice was vaccinated with a live vaccine strain of VACV (VACV strain Connaught, which a New York Board of Health strain) by tail scarification on week 0. Serum samples were collected immediately before the first vaccination and 2 weeks after the last vaccination, and at the time of challenge (5 weeks after the last vaccination). To determine if the 4pox DNA vaccine elicited an antibody response after administration by skin electroporation, the post-vaccination serum was evaluated by a VACV infected-cell-lysate ELISA. Serum from all of the mice vaccinated with the 4pox DNA vaccine or scarified with VACV had antibodies that bound to the VACV ELISA antigens; whereas pre-vaccination serum or serum from mice vaccinated with the negative control plasmid did not (Fig. 2a). To determine if the sera contained neutralizing antibodies, PRNT were performed. Serum from all of the mice vaccinated with the 4pox DNA vaccine or scarified with VACV were positive for VACV IMV neutralizing antibodies (Fig. 2b). ELISA and PRNT titers for individual mice are shown in Table 1 .
Fig. 2.
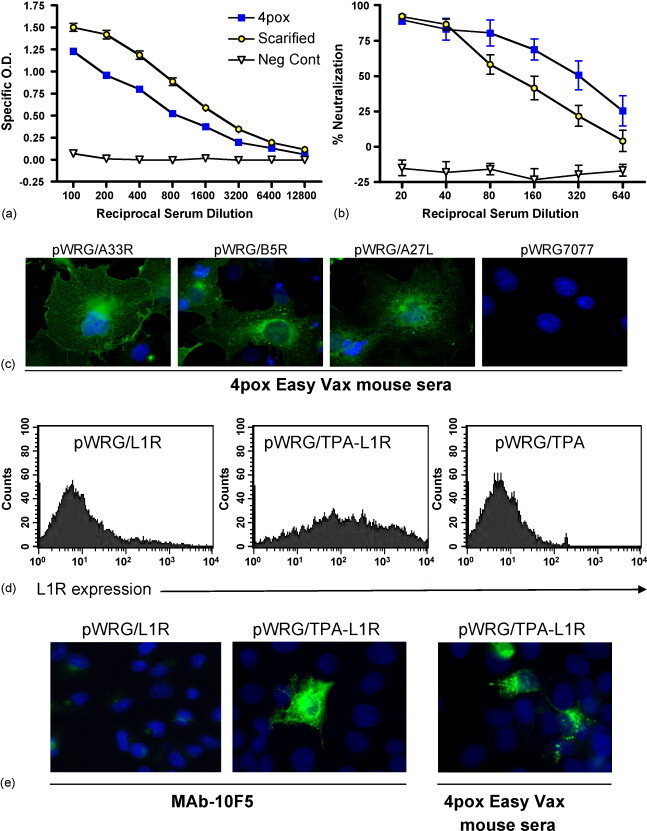
Antibody responses after microneedle-mediated skin electroporation. Groups of mice were vaccinated with the 4pox DNA vaccine negative control DNA vaccine, or tail-scarified with live VACV as a positive control. (a) ELISA using VACV infected-cell-lysate as antigen or (b) PRNT were performed on serial dilutions of heat-inactivated serum from individual mice. Symbols represent mean values ± standard deviations for all of the mice in each group. (c) COS-7 cells were plated on coverslips in 12-well plates and transfected with the indicated plasmid. 72 h post-transfection, cells were fixed and incubated with serum (1:100) from a mouse vaccinated with the 4pox vaccine using Easy Vax™. Bound antibody was detected using Alexafluor488-anti-mouse IgG secondary antibody and fluorescent microscopy (magnification 1000×). (d) COS-7 cells were transfected in T25 plates with pWRG/L1R, pWRG/TPA-L1R or pWRG/TPA. 48 h post-tranfection, cells were trypsinized and placed in 1.5 ml tubes as indicated in the materials and methods. Cells were incubated for 1 h with Mab-10F5 (1:100). Subsequently, cells were incubated in secondary Alexfluor488-anti-Mouse (1:500) for 1 h and stained cells were then analyzed by FACS. For each graph, 10,000 cells were counted. (e) COS-7 cells were transfected with the indicated plasmid and after 48 h, fixed cells were incubated with either Mab-10F5 (1:100) or serum from a mouse vaccinated with the 4pox vaccine using Easy Vax™. Bound antibody was detected using Alexafluor488-anti-mouse IgG secondary antibody and fluorescent microscopy (magnification × 1000).
Table 1.
Immunogenicity and survival data for individual mice
| Mouse ID | Antibody responsesa |
Challengeb (day of death) | ||||||
|---|---|---|---|---|---|---|---|---|
| ELISA | PRNT80 | PRNT50 | IFAT A27 | IFAT B5 | IFAT A33 | FACS L1 | ||
| 4pox-Easy Vax DNA vaccine | ||||||||
| 268 | 1600 | 160 | 320 | + | + | + | + | Survived |
| 269 | 1600 | 80 | 640 | + | + | + | − | Survived |
| 270 | 6400 | 80 | 160 | nd | nd | nd | nd | Survived |
| 271 | 1600 | 80 | 160 | + | + | + | − | Survived |
| 272 | 1600 | 160 | 320 | + | + | + | + | Survived |
| 273 | 3200 | 160 | 320 | + | + | + | + | Survived |
| 274 | 3200 | 160 | 640 | nd | + | + | + | Survived |
| 275 | 1600 | <20 | 20 | hb | hb | hb | + | Survived |
| Negative control-Easy Vax DNA vaccine | ||||||||
| 276 | <100 | nd | nd | − | − | − | − | 6 |
| 278 | <100 | nd | nd | 6 | ||||
| 279 | <100 | nd | nd | 6 | ||||
| 280 | <100 | nd | nd | 6 | ||||
| 281 | <100 | nd | nd | 6 | ||||
| 282 | <100 | nd | nd | 7 | ||||
| 283 | <100 | nd | nd | 7 | ||||
| 284 | nd | <20 | <20 | 8 | ||||
| 285 | nd | <20 | <20 | 8 | ||||
| 286 | nd | <20 | <20 | 8 | ||||
| 287 | nd | <20 | <20 | 8 | ||||
| 288 | nd | <20 | <20 | 7 | ||||
| 289 | nd | <20 | <20 | 7 | ||||
| 290 | nd | <20 | <20 | 8 | ||||
| Scarified VACV vaccine | ||||||||
| 291 | 6400 | 40 | 80 | Survived | ||||
| 292 | 3200 | 80 | 320 | Survived | ||||
| 293 | 6400 | 20 | 40 | DUE | ||||
| 294 | 3200 | 40 | 160 | Survived | ||||
| 295 | 6400 | 20 | 40 | Survived | ||||
| 296 | 3200 | 40 | 80 | Survived | ||||
| 297 | 6400 | 40 | 80 | Survived | ||||
| 298 | 3200 | 40 | 40 | Survived | ||||
PRNT, plaque reduction neutralization test where PRNT80 and PRNT50 is defined as the highest dilution reducing plaque number by 80% or 50%, respectively, relative to the number of plaques in the absense of test serum. IFAT, indirect immunofluorescence antibody test where cells were transfected with plasmid expressing A27, B5, or A33. FACS, fluorescently activated cell sorting where cells were transfected with pWRG/TPA-L1. ‘+’ Indicates positive signal relative to cells transfected with empty vector, − indicates negative signal. DUE, death unrelated to experiment. nd, not done; hb, high background.
ELISA titer defined as lowest dilution aivina a specific O.D. areater than the mean specific O.D. of negative control serum (1:100) plus three standard deviations.
VACV strain IHD-J administered by intranasal route (2 × 106 pfu).
3.2. The 4pox vaccine delivered using Easy Vax elicits antibody responses against four poxvirus immunogens
To determine if antibody responses to the individual immunogens contained in the 4pox DNA vaccine could be detected in vaccinated mice, IFAT were performed on COS cells transfected with pWRG/A27L, pWRG/B5R, pWRG/A33R, or pWRG/L1R. All of the serum that were tested were positive for anti-A27, anti-B5, and anti-A33 antibodies (Table 1). IFAT results for a representative serum sample are shown in Fig. 2c. Antibodies to L1 were not detected using cells transfected with pWRG/L1R (data not shown). We suspected that the low sensitivity of the L1 IFAT was due to inefficient expression of correctly folded L1. To overcome this, we constructed a plasmid containing the L1R open reading frame (including the transmembrane region) fused to an up stream tissue plasminogen activator secretion signal sequence (TPA). This sequence has been used previously to target proteins through the endoplasmic reticulum and to the secretory pathway [16], [17], [18]. Because TPA-L1 retained its transmembrane domain, it was expected to localize to the plasma membrane of transfected cells. When COS cells were transfected with pWRG/TPA-L1R, high levels of surface expressed L1 protein were detected using the L1-specific monoclonal antibody MAb-10F5 by FACS (Fig. 2d). Using this plasmid, L1-specific antibodies were readily detected by IFAT in the serum from mice vaccinated with 4pox vaccine using Easy Vax™ (Fig. 2e). We tested the serum from mice vaccinated with the 4pox DNA vaccine for reactivity in the L1 FACS assay. Five of the seven sera were positive for anti-L1 antibodies (Table 1). We did not detect poxvirus protein expression using pre-immune serum in the IFAT or FACs (data not shown). These findings therefore show that antibody responses to all four proteins could be elicited in mice vaccinated with the combination vaccine.
3.3. The 4pox DNA protects mice against a lethal challenge with VACV administered via mucosal route
To evaluate the protective efficacy of the 4pox-Easy Vax vaccine, a challenge was performed 5 weeks after the last vaccination. Mice were challenged intranasally with 2 × 106 pfu of VACV strain IHD-J. An additional group of eight unvaccinated mice received a 1/10 dose (2 × 105 pfu) to ensure the challenge dose was at least 10 LD50. Mice were weighed every day for the next 3 weeks. Moribund animals were euthanized. Survival curves and weight loss of survivors are shown in Fig. 3 . Our findings indicated that not only did all of the mice vaccinated with the 4pox DNA vaccine using the Easy Vax™ device survive, but also weight loss was minimal and indistinguishable from that observed in the mice scarified with live VACV. In contrast, all of the negative control mice lost >10% of weight starting by day 4 and succumbed between days 6 and 9. Moreover, over half of the mice challenged with 1/10 the challenge dose succumbed and all survivors appeared unhealthy 3 weeks after challenge (i.e., had not regained weight and fur remained ruffed). These data clearly show that mice vaccinated with the 4pox DNA vaccine using the Easy Vax™ device were completely protected from intranasal (mucosal) challenge with >10 LD50 of VACV, strain IHD-J.
Fig. 3.
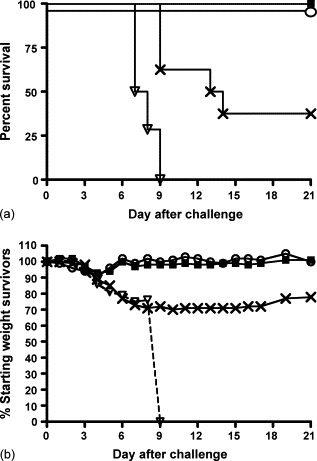
Protection in intranasal challenge model. Groups of mice were vaccinated with (solid square) the 4pox DNA vaccine, (open triangle) a negative control DNA vaccine, or (open circle) tail-scarified with live VACV as a positive control and then challenged intranasally with 2 × 106 pfu of VACV strain IHD-J (day 0). A group of mice (X symbol) was challenged with 1/10 dose (2 × 105 pfu of VACV strain IHD-J). (a) Symbols represent the percentage of surviving mice on the indicated day after challenge. (b) On each day after challenge, the average weight of the survivors was determined and the percentage of average of starting weight (on day 0) was calculated. Mice were monitored for 21 days.
3.4. Analysis of the IgG subclasses induced by microneedle-mediated skin electroporation
Intradermal DNA-vaccination by gene gun elicits a Th2 response characterized by production of IgG1 subclass antibodies [19]. To provide insight as to the whether intradermal electroporation using microneedles elicits a Th1 or Th2 response, we determined the type of IgG subclass elicited upon Easy Vax™ vaccination. To this end, an ELISA on VACV infected-cell-lysate was performed (Fig. 4 ). As predicted, mice vaccinated with the gene gun produced high levels of IgG1 indicating skewing towards a Th2 response. In contrast, serum from mice vaccinated with Easy Vax™ or scarified with live VACV contained similar levels of IgG1 and IgG2a. These findings indicate that delivery of the 4pox plasmids to the skin using microneedles and electroporation results in an antibody response that is isotypically similar to the response elicited by live virus, but different to the response elicited by particle bombardment using a gene gun. These findings are consistent with reports that the method of DNA vaccination, not necessarily the route, can affect the type of immune response generated by the vaccine [19]. Whether a Th1, Th2, or balanced response is optimal to confer protective immunity against poxvirues is poorly understood. Existing mouse data indicate that CD8+ are important for protection of unvaccinated animals against poxvirus infection [20]; however, CD8+ cells are not necessary for protective immunity in vaccinated animals and antibody is necessary and sufficient for protection. Cell depletion studies in vaccinated nonhuman primates indicated that B cells but not CD8+ cells are necessary and sufficient to protect against a lethal monkeypox challenge [3]. Moreover, passive transfer of vaccinia immune globulin was sufficient to protect nonhuman primates against lethal monkeypox emphasizing the importance of antibody in immunity in the lethal monkeypox model [3]. Additional studies will be required to determine if the more balanced IgG1/IgG2a response, and presumably more balanced lymphocyte response, produced in mice vaccinated with 4pox using Easy Vax™ versus gene gun is beneficial or detrimental to the overall efficacy of the smallpox vaccine.
Fig. 4.
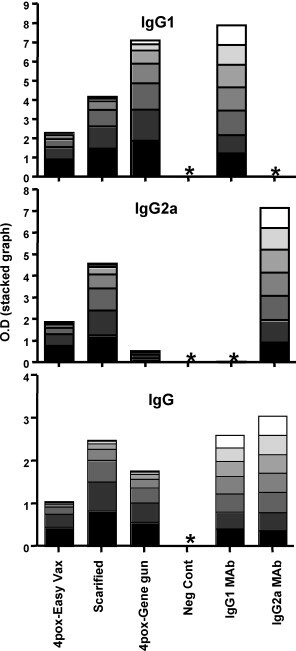
IgG1/IgG2a isotyping antibody responses in vaccinated mice. Serum pools from mice vaccinated with the 4pox DNA vaccine using Easy Vax™ (Easy Vax), scarified with live VACV (Scarified), vaccinated with the 4pox DNA vaccine using a gene gun (gene gun), or normal mouse serum (Neg Cont) were serially evaluated by VACV infected-cell-lysate ELISA. Serial dilutions of the pooled serum or antibody control mouse monoclonal antibodies (IgG1 MAb and IgG2a MAb) were tested for levels of IgG1, IgG2a, and total IgG. Specific O.D. for each dilution are represented as a stacked graph. *Below detection. Sera from mice vaccinated with the gene gun were obtained from a previously published study [6].
4. Discussion
This is the first vaccine study in which microneedle-mediated electroporation has been used to immunize animals. Previous skin electroporation studies have involved topical administration of DNA followed by electroporation [21], [22], [23] or subcutaneous injection of DNA followed by electroporation [24], [25], [26]. Others have demonstrated improved delivery of DNA vaccines to the skin by micromechanical disruption [27]; however this procedure did not involve electroporation. In this report, we demonstrated that immune responses against multiple poxvirus immunogens, including neutralizing antibodies, could be generated by microneedle-mediated skin electroporation. And finally, we demonstrated that protective immunity could be elicited by this vaccine delivery technology.
The antibody titers in the serum of mice vaccinated with the 4pox DNA vaccine were lower than those vaccinated with live virus by scarification when measured by VACV infected-cell-lysate ELISA. This is not surprising because the ELISA measures the additive antibody responses to dozens of VACV immunogens that are produced during live VACV infection (the scarified group) [27a]. Since the mice vaccinated with the 4pox DNA vaccine were immunized with only 4 immunogens, it is expected that the ELISA titers would be lower. Interestingly, and more critical to protection, the neutralizing antibody responses were actually higher in the 4pox DNA vaccine immunized mice than in the scarified group. This indicates that the level of functional antibodies (e.g., IMV neutralizing antibodies) elicited by the 4pox DNA vaccine was similar to the level raised by live virus vaccination despite a lower overall level of anti-VACV antibody.
Recent reports indicate that antibody responses play a critical role in protective immunity produced by poxvirus vaccines [3]. To assess the antibody responses induced by different smallpox vaccination strategies, we compared the geometric mean ELISA and PRNT titers elicited in mice vaccinated with the 4pox vaccine delivered by Easy Vax™ to those elicited by the 4pox DNA vaccine delivered by gene gun or live virus administered by scarification (Table 2 ). The gene gun resulted in the most consistent and robust antibody responses as measured by these assays. This is impressive because the amount of DNA delivered by the gene gun was <10 micrograms per vaccination; whereas the amount of DNA delivered using the Easy Vax device was ∼120 micrograms per vaccination. Despite lower ELISA titers, Easy Vax™ skin electroporation resulted in PRNT titers that were within ∼two-fold of those elicited by gene gun and were higher than those elicited by scarification in two of three experiments.
Table 2.
Comparison of antibody responses elicted by three different vaccines
| Vaccine | Antibody responses (GMT) |
|
|---|---|---|
| ELISAa | PRNT50b | |
| 4pox-Easy Vax™ DNA vaccine (n = 8) | 2,263 | 226 |
| 4pox-gene gun DNA vaccine (n = 10) | 11,143 | 485c |
| 4pox-gene gun DNA vaccine (n = 10) | 23,886 | 211c |
| 4pox-gene gun DNA vaccine (n = 7) | 42,001 | 476c |
| Scarified VACV vaccine (n = 8) | 4,525 | 80 |
| Scarified VACV vaccine (n = 7) | 5,797c | 49c |
| Scarified VACV vaccine (n = 9) | 16,127 | 243c |
GMT, geometric mean titer (GMT calculated from data in Table 1 shown in bold). n, number of mice.
VACV infected-cell-lysate ELISA. Titers for individual mice defined as highest serum dilution giving a specific O.D. greater than the mean specific O.D. of negative control sera plus three standard deviations.
PRNT, plaque reduction neutralization test where PRNT50 is defined as the highest dilution reducing plaque number by 50% relative to the number of plaques in the absense of test serum.
Previously reported, see Reference [6].
This study demonstrates that immune responses in animals can be elicited when microgram quantities of dried DNA are delivered to the skin by a device that combines microneedles and electroporation. Attributes of this vaccine that are favorable in terms of safety and practicality include: (1) the quantity of DNA required per vaccine is orders of magnitude lower than conventional injected DNA vaccines, (2) the DNA component of the vaccine is dry and therefore potentially very stable over time even when stored at room temperature, (3) the microneedle arrays do not penetrate to a depth rich in nerves, so pain is reduced, (4) the device does not deliver inorganic particles (e.g., gold) that could potentially remain visible at the site of injection. Finally, the fact that we could elicit antibody responses against four different immunogens demonstrates that this technique is broadly applicable. The stainless steel arrays used in this study were hand made, reusable, and consisted of 80 microneedles. In the future, the manufacturing process will be automated and the devices consisting of 300 microneedles pre-loaded with DNA will be disposable.
In terms of a future-generation smallpox vaccine, several lines of evidence now support the concept that a molecular subunit vaccine might play a role in a strategy to protect against a smallpox attack. Molecular vaccines comprised of plasmids encoding individual poxvirus immunogens [5], [6], [7], [28], or individual purified proteins or peptides plus adjuvant [29], [30], [31], [32], [33], have been shown to protect or partially protect against in several different lethal disease models involving VACV challenge in mice. Greater protection in mice has been achieved when the molecular vaccines were comprised of combinations of EEV and IMV immunogens [5], [6], [30]. A four-gene combination DNA vaccine protected nonhuman primates against monkeypox virus [1], and a single-gene protein vaccine protected mice against a highly lethal natural poxvirus disease caused by ectromelia virus [34].
If the 4pox-Easy Vax vaccine proves efficacious in both the mouse mucosal challenge model (as demonstrated here) and in the nonhuman primate severe skin-lesion monkeypox model or alternative poxvirus infection model that mimics human disease, then it would be a strong candidate for clinical trials. To date, there has been only one published report of the testing of a molecular subunit smallpox vaccine in nonhuman primates [1]. The plasmids used in that study were identical to those used in our present study, and the target tissue was the same (i.e., skin), so the prospects of successful immunization of nonhuman primate with the microneedle-mediated skin electroporation technology are high. The predicted safety of this candidate vaccine in humans is also high because it does not involve infection with a live virus, but rather it involves the introduction into the skin of very small quantities of four highly defined plasmids known to contribute to protective immunity. Advances in molecular vaccine immunogen delivery, like that reported here, will facilitate the transition of proof-of-concept laboratory vaccines to products that are precisely defined, efficacious, safe, practical, and acceptable to the intended recipients.
Acknowledgements
The authors thank Dr. Victoria Wahl-Jensen for assistance with IFAT. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army. The research described herein was sponsored by the Military Biological Defense Research Program, U.S. Army Medical Research and Material Command, Project No. E2_X001_04_RD_B.
References
- 1.Hooper J.W., et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78:4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane J., Goldstein J. Evaluation of 21st-century risks of smallpox vaccination and policy options. Ann Intern Med. 2005;138:488–493. doi: 10.7326/0003-4819-138-6-200303180-00014. [DOI] [PubMed] [Google Scholar]
- 3.Edghill-Smith Y., Golding H., Manischewitz J., King L., Scott D., Bray M., et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. Nat Med. 2005;11(7):740–747. [Google Scholar]
- 4.Smith G.L., Vanderplasschen A., Law M. The formation and function of extracellular enveloped vaccinia virus. Gen Virol. 2002;83(12):2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 5.Hooper J.W., Custer D.M., Schmaljohn C.S., Schmaljohn A.L. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 6.Hooper J.W., Custer D.M., Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306:181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galmiche M.C., Goenaga J., Wittek R., Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254:71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J.J., Wahren B., Liu M.A. DNA vaccines: progress and challenges. J Immunol. 2005;175:633–639. doi: 10.4049/jimmunol.175.2.633. [DOI] [PubMed] [Google Scholar]
- 9.Roy M.J., Wu M.S., Barr L.J., Fuller J.T., Tussey L.G., Speller S., et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19(7–8):764–768. doi: 10.1016/s0264-410x(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 10.Rottinghaus S.T., Poland G.A., Jacobson R.M., Barr L.J., Roy M.J. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine. 2003;21(31):4604–4608. doi: 10.1016/s0264-410x(03)00447-x. [DOI] [PubMed] [Google Scholar]
- 11.Roberts L.K., Barr L.J., Fuller D.H., McMahon C.W., Leese P.T., Jones S. Clinical safety and efficacy of a powdered Hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine. 2005;23(40):4867–4878. doi: 10.1016/j.vaccine.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Drape R.J., Macklin M.D., Barr L.J., Jones S., Haynes J.R., Dean H.J. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006;24(21):4475–4481. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Widera G., et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 14.Babiuk S., et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- 15.Hooper J.W., Custer D., Thompson E., Schmaljohn C.S. DNA vaccination with the Hantaan virus M gene protects hamsters against three of four HFRS hantaviruses and elicits a high-titer neutralizing antibody response in rhesus monkeys. J Virol. 2001;75:8469–8477. doi: 10.1128/JVI.75.18.8469-8477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delogu G., Li A., Repique C., Collins F., Morris S. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect Immun. 2002;70(1):292–302. doi: 10.1128/IAI.70.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashok M., Rangarajan P. Protective efficacy of a plasmid DNA encoding Japanese encephalitis virus envelope protein fused to tissue plasminogenactivator signal sequences: studies in a murine intracerebral virus challenge model. Vaccine. 2002;20(11–12):1563–1570. doi: 10.1016/s0264-410x(01)00492-3. [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Howard A., Delogu G., Collins F., Morris S. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect Immun. 1999;67(9):4780–4786. doi: 10.1128/iai.67.9.4780-4786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feltquate D., Heaney S., Webster R., Robinson H. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA Immunization. J Immunol. 1997;158(5):2278–2284. [PubMed] [Google Scholar]
- 20.Karupiah G., Buller M., Van Rooijen N., Duarte C., Chen J. Different roles for CD4+ and DC8+ lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70(12):8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titomirov A.V., Sukharev S., Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991;1088:131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- 22.Dujardin N., Van Der Smissen P., Preat V. Topical gene transfer into rat skin using electroporation. Pharm Res. 2001;18:61–66. doi: 10.1023/a:1011026726938. [DOI] [PubMed] [Google Scholar]
- 23.Babiuk S., et al. Needle-free topical electroporation improves gene expression from plasmids administered in porcine skin. Mol Ther. 2003;8:992–998. doi: 10.1016/j.ymthe.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Drabick J.J., Glasspool-Malone J., King A., Malone R.W. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther. 2001;3:249–255. doi: 10.1006/mthe.2000.0257. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Nolan E., Kreitschitz S., Rabussay D.P. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta. 2002;1572:1–9. doi: 10.1016/s0304-4165(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Widera G., Rabussay D. Enhancement of the effectiveness of electroporation-augmented cutaneous DNA vaccination by a particulate adjuvant. Bioelectrochemistry. 2004;63:369–373. doi: 10.1016/j.bioelechem.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Mikszta J.A., et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]; (a) Jones-Trower A., Garcia A., Meseda C.A., He Y., Weiss C., Kumar A., et al. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005;343:128–140. doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Pulford D.J., Gates A., Bridge S.H., Robinson J.H., Ulaeto D. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine. 2004;22:3358–3366. doi: 10.1016/j.vaccine.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Demkowicz W.E., Maa J.S., Esteban M. Identification and characterization of vaccinia virus genes encoding proteins that are highly antigenic in animals and are immunodominant in vaccinated humans. J Virol. 1992;66:386–398. doi: 10.1128/jvi.66.1.386-398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogg C., et al. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78:10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies D.H., McCausland M.M., Valdez C., Huynh D., Hernandez J.E., Mu Y., et al. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79(18):11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder J.T., Belyakov I.M., Dzutsev A., Lemonnier F., Berzofsky J.A. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J Virol. 2004;78(13):7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai C.F., Gong S.C., Esteban M. The purified 14 kilodalton envelope protein of vaccinia virus produced in Escherichia coli induces virus immunity in animals. J Virol. 1991;65:5631–5635. doi: 10.1128/jvi.65.10.5631-5635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang M., Cheng H., Dai Z., Bu Z., Sigal L.J. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology. 2006;345:231–243. doi: 10.1016/j.virol.2005.09.056. [DOI] [PubMed] [Google Scholar]


