Abstract
Zoonotic orthopoxvirus outbreaks have occurred repeatedly worldwide, including monkeypox in Africa and the United States, cowpox in Europe, camelpox in the Middle East and India, buffalopox in India, vaccinia in South America, and novel emerging orthopoxvirus infections in the United States, Europe, Asia, and South America. Waning smallpox immunity may increase the potential for animal-to-human transmission followed by further community transmission person-to-person (as demonstrated by monkeypox and buffalopox outbreaks) and by contact with fomites (as demonstrated by camelpox, cowpox, and, possibly, Alaskapox). The objectives of this review are to describe the disease ecology, epidemiology, clinical manifestations, prevention, and control of human infections with animal orthopoxviruses and to discuss the association with diminished population herd immunity formerly induced by vaccinia vaccination against smallpox. Internet search engines were queried with key words, and case reports, case series, seroprevalence studies, and epidemiologic investigations were found for review.
Keywords: zoonoses, buffalopox, camelpox, cowpox, monkeypox, vaccinia, smallpox, variola
Introduction
After more than 150 y of successful vaccination against smallpox, begun by Edward Jenner in 1798, the World Health Organization declared smallpox eradicated in 1980, and smallpox vaccination with the vaccinia virus-based vaccine ceased.1, 2, 3, 4 In addition to smallpox protection, the vaccinia vaccine provided cross-protective immunity against other related orthopoxviruses, such as cowpox and monkeypox, and now can be used for postexposure prophylaxis.5 , 6 Zoonotic orthopoxvirus outbreaks have occurred repeatedly worldwide, including monkeypox in Africa and the United States, cowpox in Europe, camelpox in the Middle East and India, buffalopox in India, vaccinia in South America, and novel emerging orthopoxvirus infections in the United States, Europe, Asia, and South America. These recent outbreaks demonstrate that waning smallpox immunity likely increases the potential for animal-to-human transmission of orthopoxviruses, which can be followed by further community transmission. Moreover, an absence of immunity to smallpox virus creates a risk of greater morbidity from orthopoxvirus infections, as demonstrated by an outbreak of monkeypox in the Democratic Republic of the Congo in 2017 that resulted in 88 cases (63 confirmed and 6 deaths).5 Young persons never vaccinated for smallpox experienced the greatest morbidity and mortality during these outbreaks.6
In addition to waning smallpox immunity, other factors have played a role in the increasing prevalence of orthopoxvirus infections in humans.7 Human behavior is believed to play the greatest role for 2 reasons.7 First, the current enthusiasm for ownership of exotic animals and livestock has created an opportunity for the international movement of poxviruses, such as monkeypox, cowpox, and novel vaccinia virus, into close contact with nonimmune native animals and humans.7 Second, the transportation and abandonment of infected companion and hobby animals can release zoonotic orthopoxviruses into naïve environments and precipitate outbreaks in nonimmune animals and humans.7
Methods
The objectives of this review are to describe the disease ecology, epidemiology, clinical manifestations, prevention, and control of human infections with animal orthopoxviruses and to discuss the association with diminished population herd immunity formerly induced by vaccinia vaccination against smallpox. To meet these objectives, Internet search engines (Google, Google Scholar, PubMed, Medline, and Ovid) were queried with the keywords as search terms to examine peer-reviewed scientific articles on the most common human infections with animal orthopoxviruses worldwide. The study period was 1970 to 2020. The articles reviewed included disease surveillance studies, seroprevalence studies, review articles, case reports and series, and disease outbreak investigations. Articles excluded from review included non–English-language articles, letters to the editor, dispatches, opinion-editorial articles, clinical-pathological case conferences, and abstracts of posters and presentations at conferences and scientific meetings. The selected methodology met all recommended criteria for narrative reviews, including several keywords, use of 2 or more Internet search engines, a defined study period, and article inclusion and exclusion criteria.8
Results
Virology and Taxonomy of Orthopoxviruses
The orthopoxviruses are a group of zoonotic, phylogenetically related, double-stranded DNA viruses with large animal reservoirs in vertebrates, especially in wild and domestic mammals, food animals, and rodents (Table 1 ). Although most orthopoxviruses (family Poxviridae, subfamily Chordopoxviridae, genus Orthopoxvirus) are host-specific in animals and do not cause disease in humans, several may be transmitted to humans by respiratory droplets or by direct contact with skin lesions or contaminated fomites. There are 17 known species of orthopoxviruses, including human smallpox (variola), with new emerging species frequently reported (Table 1).9, 10, 11
Table 1.
Orthopoxviruses (family Poxviridae, subfamily Chordopoxviridae): animal reservoirs and transmission mechanisms
| Orthopoxviruses (WHO abbreviation) | Animal reservoirs | Geographic distribution | Animal-to-human transmission | Person-to-person transmission | Nosocomial transmission | Unique features in addition to a poxvirus syndromea |
|---|---|---|---|---|---|---|
| Abatino macapox virus | Monkeys | Italy, Europe (unconformed) | NR | NR | NR | NA |
| Akhmeta virus | Small mammals, cattle | Georgia (former USSR) | + | NR | NR | NA |
| Alaskapox (AK2015-poxvirus) | Small mammals | Alaska | + | NR | NR | NA |
| Buffalopox (BPXV) | Buffalo, cattle | India, Pakistan (unconfirmed) | + | + | + | Lesions usually confined to hands, axillary and inguinal lymphadenopathy lesions in oral mucosa after consumption of milk from infected animals |
| Camelpox (CMLV) | Dromedary camels | North Africa, Middle East, Afghanistan Pakistan, Southern Russia, India | + | NR | NR | Lesions usually confined to hands |
| Cowpox (CPXV) | Rarely cattle, horses, cats, rodents | Worldwide | + | + | NR | Used for early smallpox vaccination |
| Cowpox (atypical) France Amiens 2016 | Unknown, probably rodents and cats | France | NR | NR | NR | Wound eschar, spreading cellulitis with subcutaneous abscesses, regional lymphangitis and lymphadenopathy, prolonged course |
| Ectromelia (mousepox) virus (ECTV) | Mice, other rodents | Worldwide | NR | NR | NR | NA |
| Horsepox | Originally horses and then cattle, probably extinct | Mongolia (last case) | + | NR | NR | Used for early smallpox vaccination |
| Monkeypox (MPXV) | Reservoir unknown, probably rodents, especially squirrels, and small mammals hunted for bush meat | Central and West Africa, introduced into United States | + | + | + | Cervical, submandibular, and inguinal lymphadenopathy |
| Rabbitpox | Rabbits | Worldwide | NR | NR | NR | NA |
| Raccoonpox | Raccoons | Worldwide | NR | NR | NR | NA |
| Skunkpox | Skunks | Worldwide | NR | NR | NR | NA |
| Taterapox | Rodents, especially gerbils, mice, and voles | Worldwide | NR | NR | NR | NA |
| Vaccinia virus (VACV) | Cattle, rodents, humans | Worldwide, new pathogenic strains in Brazil and Colombia | + | + | + | Used for smallpox vaccination until eradication by 1980 |
| Variola virus (smallpox) | Humans | Biological weapons stockpiles worldwide | NA | + | + | NA |
| Volepox | Voles and other rodents | Worldwide | NR | NR | NR | NA |
NR, not reported; NA, not applicable; WHO, World Health Organization.
The poxvirus syndrome is characterized by an initial prodrome of fever, malaise, headache, myalgia, and, rarely, nausea and vomiting. A progressive pox stage begins after an incubation period of 10 to 14 d with successive crops of macules, papules, vesicles, pustules, ulcers, dry crusts, and depigmented scars over weeks to months. Neurologic complications include mental status changes, encephalitis, transverse myelitis, neurogenic bladder and bowel, and orbital infection with ophthalmoplegia.
Disease Ecology and Epidemiology of Human Infections with Animal Orthopoxviruses
Monkeypox in Africa and the United States
Monkeypox has emerged as the most common cause of human orthopoxvirus infections, with most cases reported from Central and West Africa.5 , 6 , 12 , 13 Monkeypox is transmitted from rodents to humans by rodent bites and close contact with infected live or dead animals or their bodily fluids, often through hunting, skinning, or butchering of bushmeat as food.5 , 6 , 12 , 13 Human-to-human transmission occurs in approximately 10% of cases by respiratory droplets and by close contact with infected lesions or bodily fluids.5 , 6 , 12 , 13 The exact host reservoir species for monkeypox is unknown, but monkeys, small mammals, rabbits, squirrels, prairie dogs, and other rodents are suspected.5 , 6 , 12 , 14 , 15
Diagnostic specimens may be obtained from skin lesion or lymph node biopsies.5 , 14 , 15 Treatment is primarily supportive. Close contacts of infected persons may receive the smallpox vaccine prophylactically to prevent transmission postexposure.5 , 15 , 16 Other recommended prevention and control strategies include avoiding physical contact with infected persons; avoiding contact with wild animals, especially those found dead; cooking all animal food products, including bushmeat, thoroughly before consumption; frequent handwashing; and early medical evaluation of all persons with signs or symptoms of monkeypox.16
Between May and July 2003, 71 cases of monkeypox were reported from 5 Midwestern US states, including Wisconsin (39), Indiana (16), Illinois (12), Kansas (1), Missouri (2), and Ohio (1).14 , 15 The outbreak was traced to imported, infected Gambian pouched rats (Cricetomys spp.) that were shipped from Texas to an exotic animal distributor in Illinois, who housed them with prairie dogs destined for retail sale.14 , 15 Of the 71 cases, 39 (55%) were in female patients; the median age of case patients was 28 y (range 1–51 y); 18 patients (26%) were hospitalized; and 2 patients, both children, developed severe, complicated illnesses.14 , 15 No deaths were reported.14 , 15 The median incubation period was 12 d (range 1–31 d).14 , 15 Of 35 laboratory-confirmed cases, 32 (91%) tested positive for monkeypox by polymerase chain reaction (PCR) assay, immunohistochemical testing, and/or electron microscopy of lesion biopsies.14 , 15 To halt transmission, 30 contacts in 6 states were vaccinated with the vaccinia smallpox vaccine.14 , 15 This outbreak was the first time monkeypox, an Old World animal orthopoxvirus, was reported in the United States in native rodents and in humans.14 , 15
Although uncommon in the United States, a diagnosis of monkeypox should be considered in any person who presents with a febrile prodrome followed by a pustular rash after travel to a country with endemic monkeypox, such as Nigeria and the Democratic Republic of Congo. In laboratory-confirmed cases, the index patient should be isolated immediately, and local and state health departments and the US Centers for Disease Control and Prevention (CDC) should be notified to initiate control investigations and conduct tracing of close contacts exposed to the index case either during travel or after arrival in the United States.
Cowpox in Europe
The cowpox virus is an orthopoxvirus with a reservoir in wild rodents and is no longer found in cows.17 , 18 Cowpox is endemic in rats and feral cats throughout Europe and Asia and is typically transmitted to humans via close contact with accidental animal hosts, especially pet rats and cats that acquire infections from mice and other wild rodents.18, 19, 20, 21, 22, 23 In 2002, Dutch investigators reported the first case of rat-to-human transmission of cowpox in a 14-y-old girl who cared for a wild Norway rat (Rattus norvegicus) for 6 d and later developed ulcerated nodules on her face.19
Although cowpox lesions may be secondarily infected and heal with scarring, the disease is typically self-limited in the immunocompetent but can be generalized and severe in the immunocompromised.24 , 25 Fatal, disseminated cowpox infection has been reported in an adolescent renal transplant recipient immunosuppressed by antirejection therapy.24 , 25 Antibiotic treatment of secondary infections should be based on wound culture and antibiotic sensitivity testing; tetanus prophylaxis should be administered if indicated.
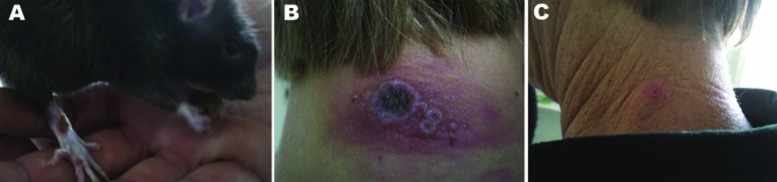
In early 2009, German investigators described a cluster outbreak of cowpox transmitted to 5 humans by infected Norway rats purchased from the same breeder and pet shop owner near Munich.22 The investigators observed that the onset and severity of the lesions were associated with the case-patients’ vaccinia virus vaccination (VVV) status, with shorter incubation periods of 3 to 5 d, multiple lesions, fever, and lymphadenopathy in patients with no history of VVV, and longer incubation periods of greater than 1 wk with single, smaller lesions and no fever or lymphadenopathy in patients with a positive history of VVV (Figure 1 ).22
Figure 1.
Cowpox lesions on a pet rat (Rattus norvegicus) and its owners during an outbreak in Germany in 2009.21 A, Pet rat with a cowpox lesion on its right forepaw. B, Cowpox lesions on the neck of a pet rat owner without previous vaccinia virus vaccination (VVV) for smallpox 13 d after direct contact with her cowpox-infected pet rat. Regional lymphadenopathy was also described. C, Milder, nearly asymptomatic lesions on the neck of the patient’s grandmother, who had a history of VVV for smallpox, 13 d after direct contact with the family’s pet rat.
In a 2013 molecular analysis of a cowpox outbreak after exposure to infected pet rats in non–smallpox-vaccinated patients in France, investigators detected the presence of cowpox DNA in lesion crusts by PCR and then extracted DNA from cell culture preparations from patient lesions to amplify viral genomes.23 The results indicated that 3 closely associated patients (2 sisters and their sick pets’ veterinarian) were infected with the same the cowpox strain.23
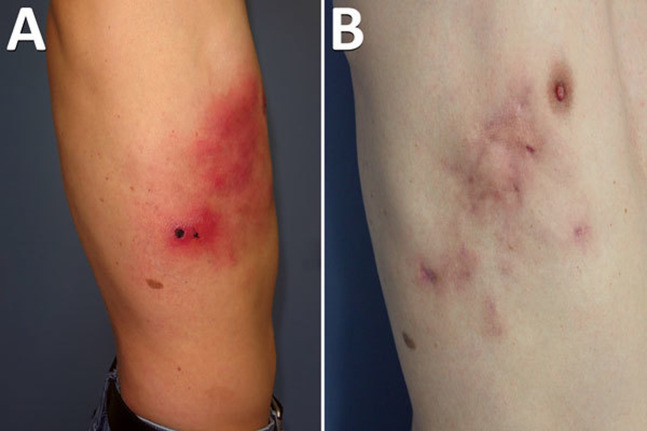
In 2019, French investigators reported an atypical case of cowpox in a 45-y-old previously vaccinia vaccinated healthy electrician who sustained a superficial puncture wound to the left lateral thorax from a metal guardrail stuck in the ground.26 The wound did not heal and developed a black eschar with painful surrounding cellulitis and subcutaneous abscesses that spread to the left anterior chest with regional lymphadenopathy over a 4-wk period (Figure 2A).26 All abscesses drained spontaneously, the cellulitis resolved by 4 mo, and the patient was discharged from follow-up care at 9 mo (Figure 2B).26 Lesion samples submitted for electron microscopy, PCR assays, genomic sequencing, and phylogenetic analysis suggested the presence of an atypical novel orthopoxvirus related to cowpox clade E3 and designated Cowpox France Amiens 2016.26 The investigators concluded that the patient’s atypical cowpox infection was most likely transmitted by the initial puncture wound made by the metal guardrail, which was contaminated by ground contact with rodent or cat urine or feces.26 Rodents and cats that eat infected rodents are well-known reservoir hosts of cowpox virus and can contract infection without clinical disease.21 , 26 This case challenged the immunologic capability of prior smallpox-vaccinated persons to maintain prolonged cross-protective immunity against other orthopoxviruses, especially atypical and novel ones.
Figure 2.
Atypical cowpox virus infection in a smallpox-vaccinated patient in France in 2016.23 A, Profile appearance of the patient’s torso 1 mo after the initial traumatic injury. B, Appearance 9 mo after the initial trauma.
Novel Vaccinia Virus in South America
Since 1999, 2 clades of vaccinia virus phylogenetically related to, but distinct from, the prototype vaccinia viruses used in smallpox vaccinations have caused repeated outbreaks of poxvirus diseases in dairy cows and in dairy and farming industry workers in Brazil and Colombia.27, 28, 29, 30 In 2015, Brazilian investigators described an outbreak of atypically severe vaccinia virus infection in 26 rural dairy farm workers, in which 12 workers who were not vaccinated against smallpox were hospitalized for severe systemic manifestations.28 All 26 patients were infected after milking dairy cows with active lesions on their udders and teats.28 All 12 hospitalized patients had high fever, prostration, regional lymphadenopathy, and painful vesicular lesions on the upper and lower extremities.28 Three hospitalized patients had nausea, vomiting, diarrhea, mental confusion, and seizures.28All 14 nonhospitalized patients had been vaccinated for smallpox in childhood and manifested fewer symptoms and fewer lesions, which were confined to the hands and arms, when compared with the 12 unvaccinated, hospitalized patients.28 All patients recovered with supportive therapy.28 The causative vaccinia virus was identified by PCR in many cases, and the authors concluded that a new vaccinia virus isolate had caused the outbreak of very severe, exanthematous vaccinia infection.28
Buffalopox in India
Buffalopox affecting domestic buffalo, cattle, and humans has occurred in sporadic and epidemic outbreaks in small villages and commercial farms throughout India.31 , 32 Despite its host specificity for buffalo and cattle, buffalopox is closer phylogenetically to vaccinia than to cowpox and causes similar clinical manifestations.31 Investigators reported an attack rate of 12% for buffalo and 7% for villagers in a 2011 descriptive epidemiologic analysis of a large outbreak of buffalopox in 351 patients from 22 villages in western India in 2009.32 The index case was a villager with a history of milking infected buffalo with lesions on the udders and teats.32 Direct contact with infected animals was the main mode of transmission, and human-to-animal spread by infected dairy workers was suspected as the main mode of spread in animals.32
Most infected patients presented with fever, malaise, and painful pox-like lesions, more often on the fingers and hands than on the face and feet, that were associated with painful axillary and inguinal lymphadenopathy.32 Children without contact with infected animals also presented with similar constitutional symptoms and painful pox-like lesions on the hands.32 In some cases, pox lesions occurred in the mouth, suggesting transmission via consumption of contaminated raw milk.32 Buffalopox was detected by electron microscopy and confirmed by virus isolation in culture and PCR identification in specimens from buffalo and humans.32
Camelpox in India
Camelpox is a host-specific orthopoxvirus infection in camels that does not infect other animal species as do monkeypox, cowpox, vaccinia, and buffalopox.33 It is transmitted to humans by direct contact with contaminated lesions on camels or fomites.33 Camelpox is closely related phylogenetically to the smallpox virus (variola).33 Since the 1990s, sporadic outbreaks of camelpox have been reported from camel-rearing areas of the world, from North Africa throughout the Middle East and including India, Pakistan, Afghanistan, and Turkmenistan.33 Clinical manifestations in camels include pox-like lesions distributed throughout hairless areas, preferentially on the head, neck, and inguinal regions.33 Similar lesions occur on the mucous membranes of the mouth and digestive and respiratory tracts.33
In a 2011 descriptive epidemiologic analysis of the first human cases associated with a prolonged outbreak of camelpox in northwestern India in 2008 to 2009, investigators reported 3 cases of human infection in camel attendants, as confirmed by PCR testing of patient scabs and isolation of camelpox virus in cultures from infected camels.33 All affected individuals had been exposed to infected camels and presented with fever and pox-like lesions confined to their hands.33 The lesions began as raised vesicles that burst within 7 to 10 d, leaving deep ulcers that crusted over with scabs that sloughed off and left scars by 15 d.33
Novel orthopoxvirus infection, Alaska, 2015
In July 2015, a non-smallpox-vaccinated female patient living near Fairbanks, Alaska, sought urgent medical care for a suspected ulcerating spider bite on her left shoulder accompanied by 5 d of fever, malaise, fatigue, and painful regional lymphadenopathy.10 A superficial ulcer 1 cm in diameter with 2 adjacent vesicles and a linear streak extending over the shoulder were confirmed by a physician, who unroofed and swabbed the vesicles for testing.10 Although the samples were negative for herpes simplex, varicella zoster, and variola viruses, a generic orthopoxvirus PCR test was positive.10 The patient’s household contacts had no serologic evidence of orthopoxvirus exposure. Swab samples from household surfaces and items and 31 small mammals collected from the house perimeter tested negative for orthopoxviruses by PCR.10 Phylogenetic analysis identified the orthopoxvirus as genetically distinct from, but related to, an Old World clade of orthopoxviruses.10 The patient’s lesions took 6 mo to resolve fully.10 The investigators concluded that the patient’s lesions represented a novel orthopoxvirus infection that resulted from exposure to unidentified, infected wild small mammals or to fomites contaminated by their excreta.10
Clinical Manifestations and Laboratory Confirmation of Animal Orthopoxvirus Infections
Although some orthopoxvirus infections are characterized by unique clinical manifestations, all share similar incubation periods and clinical manifestations. The poxvirus syndrome is characterized by an initial prodrome of fever, malaise, headache, myalgia, regional lymphadenopathy, and, rarely, nausea and vomiting. A progressive pox stage begins after an incubation period of 10 to 14 d, with successive macules, papules, vesicles, pustules, ulcers, dry crusts, and depigmented, depressed scars over 3 to 4 wk.14 , 15 In addition to viral bronchopneumonias and ocular inoculation, neurologic complications may include mental status changes, seizures, encephalitis, transverse myelitis, and neurogenic bladder and bowel.14 , 15 In addition to smallpox, the differential diagnosis of all orthopoxvirus infections should include chicken pox (varicella zoster), herpes simplex, shingles (herpes zoster), cutaneous anthrax, bubonic plague, Bartonella spp. infection, ulceroglandular tularemia, and the tickborne rickettsial diseases.19
Laboratory confirmation of orthopoxvirus infections is critical to rule out other febrile illnesses with similar cutaneous manifestations. After consultation with state health departments, specimens should be taken directly from skin lesions before smallpox vaccination and submitted to state health departments for analysis by state laboratories equipped for rapid viral identification or sent by unequipped state health departments directly to CDC labs.14 , 15 The best specimens are swabs of lesions or entire crusts from healing sores submitted for rapid viral identification by electron microscopy, immunohistochemical techniques, molecular confirmation by PCR, or viral culture in active cases.14 , 15 Serologic tests are less dependable and may be complicated by prior smallpox vaccinations or prior orthopoxvirus infections with cross-reacting antibodies.
Management, Prevention, and Control of Animal Orthopoxvirus Infections and Outbreaks
Multiple case reports and series provide evidence supporting cross-reactive immunity to animal orthopoxvirus infections in humans with less severe disease or protection from disease. During repeated monkeypox outbreaks in the Democratic Republic of Congo from 1981 to 1986, prior smallpox vaccination conferred 85% protection against monkeypox.34 Prior smallpox vaccination was associated with fewer and less severe lesions in cowpox, as noted in a household outbreak in Germany in 2009.22
In addition to vaccinia vaccination, other management strategies for human orthopoxvirus infections include intravenous vaccinia immunoglobulin (VIG), intravenous cidofovir, and oral tecovirimat. Intravenous VIG has been used to treat the complications of smallpox vaccination, such as generalized vaccinia, eczema vaccinatum, ocular vaccinia, and postvaccinial central nervous system complications.14 , 15 There were no requests for VIG in the US monkeypox outbreak in 2003.14 , 15
Although intravenous cidofovir has proven effective in the postexposure prophylaxis of monkeypox in monkeys, no data exist on the use of either VIG or cidofovir for prophylaxis or treatment of monkeypox.35 , 36 The CDC has issued guidance recommendations that cidofovir only be used for the treatment of life-threatening monkeypox and not for prophylaxis.36 In 2018, the US Food and Drug Administration (FDA) approved the first oral antiviral medication, tecovirimat, for the treatment of smallpox (FDA News, July 2018). In 2019, investigators reported the first use of oral tecovirimat in conjunction with intravenous VIG in the management of a case of laboratory-acquired vaccinia virus infection after an accidental needlestick on the finger.37 Within 48 h of initiating therapy, local pain and edema at the needlestick site decreased, and fever and axillary lymphadenopathy resolved.36
In 2021, ophthalmologists in London reported the successful use of oral tecovirimat in the management of a case of orbital cowpox in a 28-y-old female patient who presented with conjunctival necrosis, orbital swelling, and ophthalmoplegia after failed treatment with topical and intravenous antibiotics and steroids.38 Two weeks before the patient first presented with symptoms, her pet cat developed lesions on its head and paws that were scraped by a veterinarian and tested positive for orthopoxvirus by PCR.38 A PCR test on a conjunctival swab from the patient also tested positive for orthopoxvirus, and genome sequencing documented a diagnosis of cowpox in the patient and her cat.38 In addition to orbital decompression and debridement, the patient received a prolonged course of oral tecovirimat for several months.38 At 6-mo follow-up, visual acuity in the right eye was normal, but residual ptosis and restriction of extraocular movements remained.38 This case demonstrated another example of human infection with animal orthopoxvirus and confirmed the effectiveness of tecovirimat in treating cowpox virus infection. Tecovirimat is now maintained in the US Strategic National Stockpile (SNS), the nation’s largest supply of essential pharmaceuticals and medical supplies for use in public health emergencies, including a biological weapons attack with smallpox or another orthopoxvirus.36 , 37 , 39
On September 24, 2019, the FDA announced the approval of the Jynneos smallpox and monkeypox vaccine, a live, nonreplicating vaccine for the prevention of smallpox and monkeypox (FDA News, September 2019). The Jynneos vaccine does not contain live smallpox or monkeypox virus but does contains a modified nonreplicating form of the vaccinia virus, vaccinia Ankara, which does not cause human disease. The Jynneos vaccine is also maintained in the SNS.36 , 39
It is unknown how many people born before 1980 were vaccinated for smallpox and remain relatively protected against smallpox and related orthopoxviruses. Because the world population has doubled from about 4 billion in 1979 to about 8 billion in 2020, as many as 4 billion people may be unvaccinated and susceptible to smallpox and closely related orthopoxviruses. Although military personnel were routinely vaccinated for smallpox in the past, only military and civilian laboratory personnel who work directly with smallpox or related animal orthopoxviruses should be vaccinated today.36 Smallpox vaccine is no longer available to the general public in the United States.36 , 39 However, in the event of a smallpox outbreak, the SNS maintains enough smallpox vaccine to provide postexposure prophylaxis.36 , 39
Conclusions
Since the eradication of smallpox in 1980, orthopoxvirus outbreaks have occurred repeatedly worldwide. Young persons never vaccinated against smallpox experienced the greatest morbidity and mortality during these outbreaks. As population herd immunity formerly induced by vaccinia vaccination against smallpox wanes, there is increased potential for animal-to-human transmission of zoonotic orthopoxviruses, followed by fomite and person-to-person community transmission of animal orthopoxviruses. Only early recognition and management of orthopoxvirus infections in animals by isolation, quarantine, and vaccination, if available, can limit transmission of zoonotic orthopoxviruses to other animals and their human caregivers.7
Case isolation and administration of the recently approved, nonreplicating modified Ankara vaccinia vaccine and new antivirals, such as tecovirimat, may limit community contact and person-to-person transmission of orthopoxviruses after outbreaks.39 Clinicians should consider a diagnosis of orthopoxvirus infection in patients with ulcerating vesicular lesions after urban or rural domestic, wild, or exotic animal exposures, especially close contacts with rodents, feral cats, insectivores, cows, buffalo, and camels.
Acknowledgments
Financial/Material Support: All financial/material support for JHD was provided by departmental and institutional sources.
Disclosures: None.
References
- 1.Jenner E. Samuel Cooley; Springfield, MA: 1802. An inquiry into the causes and effects of the variolae vaccinae, a disease discovered in some of the western counties of England, particularly, Gloucestershire, and known by the name of the cow pox. [Google Scholar]
- 2.Schrick L., Tausch S.H., Dabrowski P.W., Damaso C.R., Esparza J., Nitsche A. An early American smallpox vaccine based on horsepox. N Engl J Med. 2017;377(15):1491–1492. doi: 10.1056/NEJMc1707600. [DOI] [PubMed] [Google Scholar]
- 3.Esparza J., Schrick L., Damaso C.R., Nitsche A. Equination (inoculation of horsepox): an early alternative to vaccination (inoculation of cowpox) and the potential role of the horsepox virus in the origin of the smallpox vaccine. Vaccine. 2017;35(2017):7222–7230. doi: 10.1016/j.vaccine.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Downie A.W. The immunological relationship of the virus of spontaneous cowpox to vaccinia virus. Br J Exp Pathol. 1939;20:158–176. [Google Scholar]
- 5.Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S., et al. Emergence of monkeypox—West and Central Africa, 1970–2017. Morb Mort Wkly Rep. 2018;67(10):306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tack D.M., Reynolds M.G. Zoonotic poxviruses associated with companion animals. Animals. 2011;1(4):377–395. doi: 10.3390/ani1040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy C.M. Writing an effective review article. J Med Toxicol. 2012;8(2):89–90. doi: 10.1007/s13181-012-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vora N.M., Li Y., Geleishvili M., Emerson G.L., Khmaladze E., Maghlakelidze G., et al. Human infection with a zoonotic orthopoxvirus in the country of Georgia. N Engl J Med. 2015;372(13):1223–1230. doi: 10.1056/NEJMoa1407647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer Y.P., Hsu C.H., Werle Z.R., Olson L.E., Cooper M.P., Castrodale L.J., et al. Novel orthopoxvirus infection in an Alaska resident. Clin Infect Dis. 2017;64(12):1737–1741. doi: 10.1093/cid/cix219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J., Gigante C., Khmaladze E., Liu P., Tang S., Wilkins K., et al. Genome sequences of Akhmeta virus, an early divergent Old World orthopoxvirus. Viruses. 2018;10(5):252. doi: 10.3390/v10050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds M.G., Carroll D.S., Karem K.L. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr Opin Virol. 2012;2:335–343. doi: 10.1016/j.coviro.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Update: Multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Morb Mort Wkly Rep. 2003;52(25):561–562. [PubMed] [Google Scholar]
- 15.Update: Multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Morb Mort Wkly Rep. 2003;52(27):616–618. [PubMed] [Google Scholar]
- 16.Eteng W.-E., Mandra A., Doty J., Yinka-Ogunleye A., Aruna S., Reynolds M.G., et al. Responding to an outbreak of monkeypox using the One Health approach—Nigeria, 2017–2018. Morb Mort Wkly Rep. 2018;67(37):1040–1041. doi: 10.15585/mmwr.mm6737a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vorou R.M., Papavassiliou V.G., Pierroutsakos I.N. Cowpox virus infection: an emerging health threat. Curr Opin Infect Dis. 2008;21:153–156. doi: 10.1097/QCO.0b013e3282f44c74. [DOI] [PubMed] [Google Scholar]
- 18.Chantry J., Meyer H., Baxby D., Begon K.J., Brown S.M., Hazel T., et al. Cowpox: reservoir hosts and geographic range. Epidemiol Infect Dis. 1999;122(3):455–460. doi: 10.1017/s0950268899002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfs T.F.W., Wagenaar J.A., Niesters H.G.M., Osterhaus A.D.M.E. Rat-to-human transmission of cowpox infection. Emerg Infect Dis. 2002;8(12):1495–1496. doi: 10.3201/eid0812.020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ninove L., Domart Y., Vervel C., Voinot C., Salez N., Raoult D., et al. Cowpox virus transmission from pet rats to humans, France. Emerg Infect Dis. 2009;15(5):781–784. doi: 10.3201/eid1505.090235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eis-Hübinger A.M., Gerritzen A., Schneweis K.E., Pfeiff B., Pullmann H., Mayr A., et al. Fatal cowpox-like virus infection transmitted by cat. Lancet. 1990;336:880. doi: 10.1016/0140-6736(90)92387-w. [DOI] [PubMed] [Google Scholar]
- 22.Campe H., Zimmerman P., Glos K., Bayer M., Bergemann H., Dreweck C., et al. Cowpox virus transmission from pet rats to humans, Germany. Emerg Infect Dis. 2009;15(5):777–780. doi: 10.3201/eid1505.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ducournau C., Ferrier-Rembert A., Ferraris O., Joffre A., Favier A.-L., Flusin O., et al. Concomitant infections with 2 cowpox virus strains in related cases, France, 2011. Emerg Infect Dis. 2013;19(12):1996–1999. doi: 10.3201/eid1912.130256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grönemeyer L.-L., Baltzer A., Broekaert S., Schrick L., Möller L., Nitsche A., et al. Generalised cowpox virus infection. Lancet. 2017;390:1769. doi: 10.1016/S0140-6736(17)31428-9. [DOI] [PubMed] [Google Scholar]
- 25.Gazzani P., Gach J.E., Colmenero I., Martin J., Morton H., Brown K., et al. Fatal disseminated cowpox virus infection in an adolescent renal transplant recipient. Pediatr Nephrol. 2017;32(3):533–536. doi: 10.1007/s00467-016-3534-y. [DOI] [PubMed] [Google Scholar]
- 26.Andreani J., Arnault J.-P., Bou Khalil J.Y., Abrahão J., Tomei E., Vial E., et al. Atypical cowpox virus infection in a smallpox-vaccinated patient. France. Emerg Infect Dis. 2019;25(2):212–219. doi: 10.3201/eid2502.171433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peres M.G., Bacchiega T.S., Appolinarion C.M., Vicente A.F., Allendorf S.D., Antunes J.M.A.P., et al. Serological study of vaccinia virus reservoirs in areas with and without reports of outbreaks in cattle and humans in Sao Paulo, Brazil. Arch Virol. 2013;158(12):2433–2441. doi: 10.1007/s00705-013-1740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahão J.S., Campos R.K., de Souza Trindade G., da Fonseca F.G., Ferreira P.C.P., Kroon E.G. Outbreak of severe vaccinia virus infection, Southeastern Brazil. Emerg Infect Dis. 2015;21(4):695–698. doi: 10.3201/eid2104.140351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroon E.G., Mota B.E.F., Abrahão J.S., da Fonseca F.G., de Souza Trindade G. Zoonotic Brazilian vaccinia virus: from field to therapy. Antiviral Research. 2011;92:150–163. doi: 10.1016/j.antiviral.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Styczynski A., Burgado J., Walteros D., Usme-Ciro J., Laiton K., Farias A.P., et al. Seroprevalence and risk factors possibly associated with emerging zoonotic vaccinia virus in a farming community, Colombia. Emerg Infect Dis. 2019;25(12):2169–2178. doi: 10.3201/eid2512.181114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh R.K., Hosamani M., Balamurugan V., Bhanuprakash V., Rasool T.J., Yadav M.P. Buffalopox: an emerging and re-emerging zoonosis. Animal Health Res Rev. 2007;8(1):105–114. doi: 10.1017/S1466252307001259. [DOI] [PubMed] [Google Scholar]
- 32.Gurav Y.K., Raut C.G., Yadav P.D., Tandale B.V., Sivaram A., Pore M.D., et al. Buffalopox outbreak in humans and animals in Western Maharashtra, India. Prev Vet Med. 2011;100(2022):242–247. doi: 10.1016/j.prevetmed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Bera B.C., Shanmugasundaram K., Barua S., Venkataseng, Virmani N., Riyesh T., et al. Zoonotic cases of camelpox infection in India. Vet Microbiol. 2011;152:29–38. doi: 10.1016/j.vetmic.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 34.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2009;48(1):e6–e8. [Google Scholar]
- 35.Andrei G., Snoeck R. Cidofovir activity against poxvirus infections. Viruses. 2010;2:2803–2830. doi: 10.3390/v2122803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Interim Centers for Disease Control and Prevention (CDC) Health Alert Network; 2003. Guidance for use of smallpox vaccine, cidofovir, and vaccinia immune globulin (VIG) for prevention and treatment in the setting of an outbreak of monkeypox infections. CDCHAN-00146-03-06-11-ALT-N. [Google Scholar]
- 37.Whitehouse E.R., Rao A.K., Yu Y.C., Yu P.A., Griffin M., Gorman S., et al. Novel treatment of a vaccinia virus infection from an occupational needlestick—San Diego, California, 2019. Morb Mort Wkly Rep. 2019;68(42):943–946. doi: 10.15585/mmwr.mm6842a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiernan M., Koutroumanos N. Orbital cowpox. N Engl J Med. 2021;384(23):2241. doi: 10.1056/NEJMicm2033620. [DOI] [PubMed] [Google Scholar]
- 39.Petersen B.W., Damon I.K., Pertowski C.A., Meaney-Delman D., Guarnizo J.T., Beigi R.H., et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. Morb Mort Wkly Rep. 2015;64(RR02):1–26. [PubMed] [Google Scholar]