Abstract
The Brighton strain of cowpox virus causes lethal bronchopneumonia when delivered as a small-particle (1 μm) aerosol to weanling BALB/c mice. We showed previously that this disease can be prevented or cured with one subcutaneous injection of cidofovir (HPMPC, Vistide®). To determine whether even better results could be obtained by delivering the drug directly to the respiratory tract, we administered cidofovir by small-particle aqueous aerosol before or after aerosolized cowpox infection. In a series of five experiments, aerosol doses of 0.5–5 mg/kg were always more effective than 25 mg/kg and sometimes more effective than 100 mg/kg injected subcutaneously, as measured by changes in body and lung weight, lung viral titers, pulmonary pathology and survival. A cyclic analog ((1-[(S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl] cytosine) (cHPMPC) was less protective. The results suggest that aerosolized cidofovir would be effective for prophylaxis or early post-exposure therapy of human smallpox or monkeypox virus infection.
Keywords: Cowpox virus, Orthopoxvirus, Smallpox, Cidofovir, HPMPC, Cyclic HPMPC, Aerosol, Antiviral therapy
1. Introduction
Virulent orthopoxviruses continue to pose a threat to human health. The discontinuation of vaccination against smallpox, as a result of global eradication in 1977, has rendered most of the world's population vulnerable to severe or fatal infection, in the event that any illicitly preserved stocks of variola virus, the agent of smallpox, were to be employed as a biological weapon (Fenner et al., 1988; Henderson, 1998; Orent, 1998; O'Toole, 1999). In addition, monkeypox virus continues to circulate in animal reservoirs in rain forest regions of Africa, causing sporadic outbreaks of human disease (Fenner et al., 1986; Hutin et al., 2001). Although monkeypox appears to be both less virulent and less contagious than variola, nothing is known about its potential for epidemic spread in populous areas. The disease could potentially become more prevalent in Africa, and the agent could also be used as a bioterrorist weapon.
Both smallpox and monkeypox viruses spread from sick to healthy persons in the form of respiratory droplets, usually over short distances (Fenner et al., 1986, Fenner et al., 1988). However, in one well-documented case, variola virus spread from a single patient throughout a three-story hospital, resulting in 20 cases and four deaths (Wehrle et al., 1970). It is in this highly infectious aerosol form that orthopoxviruses are most likely to be encountered if they are used as biological weapons. Small-particle (1–5 μm) aerosols are distributed throughout the entire respiratory tree, including terminal bronchioles and alveolar spaces, a target area measuring approximately 75 m2 in adult humans (Byron and Patton, 1994). The lack of mucociliary clearance from alveolar spaces provides time for virus to encounter susceptible cells, increasing the efficiency of infection.
The few antiviral medications available in the 1970s were only weakly active against variola virus (De Clercq, 2001). Even had effective drugs been available, they would have played a minimal role in the global eradication campaign, since at that time all governments still performed extensive smallpox vaccination, adequate supplies of vaccine were available to immunize exposed populations in remaining endemic regions, and vaccination was only contraindicated for rare individuals. Thirty years later, the situation has changed: most of the world's population (essentially all persons under 25) have never been immunized, only limited stockpiles of vaccine are available, and the emergence of the human immunodeficiency virus has ruled out vaccination for millions of people. If smallpox were to re-appear, there would clearly be an urgent need for an antiviral medication that could help to curtail an epidemic by blocking the initiation of infection, mitigating illness, reducing secondary transmission and preventing death.
The acyclic cytidine analog cidofovir (HPMPC, Vistide®) is currently under consideration as a potential therapeutic agent for smallpox or monkeypox infection. Cidofovir is in clinical use in the USA for the treatment of cytomegalovirus (CMV) retinitis complicating the acquired immune deficiency syndrome (Hitchcock et al., 1996; De Clercq, 2001). The drug has potent activity in vitro against a range of orthopoxviruses, including variola; its in vivo efficacy has been demonstrated in murine models of vaccinia and cowpox infection and in a cynomolgus monkey model of monkeypox infection (Neyts and De Clercq, 1993; Naesens et al., 1997; Bray et al., 2000; Smee et al., 2000; Bray and Huggins, unpublished data). In contrast to some compounds used to treat herpesvirus infections, cidofovir undergoes phosphorylation in uninfected cells. The end-product, cidofovir diphosphate, forms an adduct with choline and has an intracellular half-life of several days, permitting treatment at 1–2 week intervals (Soike et al., 1991; Ho et al., 1992). This has important implications for the emergency prophylaxis or early post-exposure therapy of virulent orthopoxvirus infections: even if an individual is only treated once, the drug will be retained in tissues and continue to act for a week or more. In mice, for example, a single treatment 1 week before exposure prevented death from an otherwise lethal cowpox virus challenge (Bray et al., 2000).
Although cidofovir thus appears to be ideally suited for orthopoxvirus therapy, it has some limitations. It is poorly absorbed when taken by mouth, and causes localized fibrosis if injected (Wachsman et al., 1996). Current therapeutic guidelines therefore require the drug to be administered intravenously (i.v.). In addition, because cidofovir is actively transported into renal proximal tubular cells more rapidly than it is secreted, it may accumulate to toxic levels, and has occasionally caused renal insufficiency (Cundy, 1999). It is therefore necessary to increase hydration and co-administer probenecid to prevent nephrotoxicity. These therapeutic requirements would significantly limit the usefulness of cidofovir under the disruptive conditions of a large-scale biowarfare attack or in the limited medical facilities of rural Africa. It is therefore a priority to develop simpler and safer methods of treating severe orthopoxvirus infections.
One approach would be to create modified forms of cidofovir, with reduced nephrotoxicity, that could be taken by mouth. None are yet available. A form of cidofovir in which the sugar component is cyclized (cyclic HPMPC) is less nephrotoxic than cidofovir, apparently because it is more readily excreted (Bischofberger et al., 1994; Cundy et al., 1999). cHPMPC is converted to cidofovir intracellularly, giving tissue levels similar to those produced by equivalent doses of cidofovir. Unfortunately, cHPMPC has low oral bioavailability. To overcome the requirement for i.v. administration, modified forms of cidofovir and cHPMPC bearing lipophilic side chains have been synthesized; these have not yet undergone clinical evaluation (Oliyai et al., 1999). Even were such compounds available, it is not known whether an orally administered drug that is efficiently excreted by the kidneys could achieve high enough tissue levels to protect against a virulent aerosolized orthopoxvirus.
An alternative approach would be to deliver cidofovir directly to the same cells in the respiratory tract that are the targets of infection. Once taken up, the drug would create a barrier against the initiation or spread of infection in the lung. High systemic doses and consequent nephrotoxicity could thus be avoided by delivering cidofovir only where it is needed. The drug's remarkably long intracellular half-life would mean that a single low dose delivered to the lung could provide protection for a week or longer. Along this line, Smee et al. have shown that intranasally administered cidofovir was protective against an intranasal cowpox virus challenge, and suggested that the aerosolized drug would be protective against aerosolized virus (Smee et al., 2000). We decided to use our murine model of aerosol cowpox infection to test the hypothesis that a single dose of aerosolized cidofovir would be more effective than the same amount of drug injected s.c. We reported previously that a small-particle aerosol of cowpox virus causes uniformly lethal bronchopneumonia in weanling BALB/c mice, and that the disease can be prevented or treated with one s.c. injection of cidofovir (Bray et al., 2000; Martinez et al., 2000). In the present study, we infected groups of mice with aerosolized cowpox virus and treated them with aerosolized or s.c. cidofovir, either the day before, 2 h after, or 1 or 2 days after virus challenge, and measured a number of parameters of illness for three weeks postinfection, as described below.
2. Materials and methods
2.1. Viruses, cells and antiviral compounds
The Brighton strain of CPV was provided by Dr J. Esposito, Centers for Disease Control and Prevention, Atlanta, GA. The virus was prepared for aerosolization as described (Bray et al., 2000; Martinez et al., 2000). Vero C1008 (ATCC CRL 1586) monkey kidney cells were propagated in Eagle's minimal essential medium with Earle's salts (EMEM), nonessential amino acids, 10% fetal bovine serum (FBS), glutamine, penicillin, and streptomycin at 37 °C in a 5% CO2 atmosphere. Cidofovir [(S-1-[3-hydroxy-2(phosphonylmethoxy) propyl] cytosine] (HPMPC, Vistide®) and cyclic HPMPC [(1-[(S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl] cytosine)] (cHPMPC) were provided by Dr Norbert Bischofberger, Gilead Sciences Inc., Foster City, CA.
2.2. Experimental design
Female weanling (9–11 g) BALB/c mice were obtained from the National Cancer Institute, Frederick, MD, transferred to a Biosafety Level 3 containment area, housed in filtertop microisolator cages and given commercial mouse chow and water ad libitum. Five experiments were performed, in which groups of mice infected with aerosolized cowpox virus were treated with aerosolized or s.c. cidofovir or cHPMPC dissolved in phosphate-buffered saline solution (PBS), or with aerosolized PBS only (‘placebo’). Two different aerosol doses were produced by loading the nebulizer with a solutions of 10 mg/ml (‘high dose’) or 1 mg/ml (‘low dose’) of cidofovir or cHPMPC. Control groups were either exposed to aerosolized PBS, then treated with aerosolized cidofovir or cHPMPC (‘sham-infected’), or left uninfected and untreated (‘naïve’). Treatment was performed either 24 h before challenge (‘day −1’), 2 h after challenge (‘day 0’), or 24 or 48 h after challenge (‘day 1’ or ‘day 2’). All groups were weighed daily as a group and observed for 21 days postinfection for illness and death. [Weighing mice as a group did not allow statistical comparisons of weight change of different groups, but permitted observation of trends, as described below.] In some experiments, all surviving mice were observed for up to day 108 postinfection, to ensure that there were no delayed effects of infection or treatment. In two of the experiments, treatment and control groups consisted of 20–30 mice, of which a subgroup of ten was observed, while on every fourth day from day 4–20, four mice were randomly selected from the remainder of each group and killed, and their lungs were weighed, frozen, thawed and ground in a sterile mortar and the viral titers were determined (Bray et al., 2000). In a third experiment, these studies were performed on day 8 only. In two experiments, three mice were killed on day 8 and their lungs were fixed in formalin for pathology study (Martinez et al., 2000).
2.3. Generation of viral and therapeutic aerosols
Aerosols were generated using a Collison nebulizer (BGI Inc., Waltham, MA) contained within a Class III biocabinet (‘glove box’) (May, 1973). The unit was supplied with high efficiency particulate-filtered air at a pressure of 18 pounds per square inch, nebulizing the solution at a constant rate of 300 μl/min. Analysis with an aerodynamic particle sizer (APS 3320, TSI Instruments, St. Paul, MN) showed that the aerosol consisted of highly respirable particles with a mass mean aerodynamic diameter (MMAD) of 0.9 μm.
For each exposure, up to 40 mice were placed in groups of ten in four stainless steel mesh cages within a dynamic 0.01 m3 temperature- and humidity-controlled whole-body exposure chamber. Nebulized virus or drug was mixed with air to provide a flow rate through the chamber of 19.5±0.5 l/min, maintained at atmospheric pressure. The aerosol was continuously sampled using a 6 l/min all glass impinger (AGI; Ace Glass, Vineland, NJ) containing EMEM or PBS for virus or drug collection, respectively (Brachman et al., 1964). The samples were frozen, stored and analyzed as a batch after each exposure, as described below. Virus exposures were carried out for 10 min and drug exposures for 30 min. Because no more than 40 mice could be exposed at one time, several rounds of aerosolization were required to infect all mice on the day of challenge; the mean and standard deviation of the virus dose were calculated. Only one exposure was required to deliver aerosolized drug to each treatment group.
2.4. Assays of virus and drug
For viral exposures, the titers of the nebulizer suspension and of the AGI samples were determined by plaque assay (Bray et al., 2000). For drug exposures, the concentrations of cidofovir or cHPMPC in the nebulizer solution and in the AGI samples were determined by high-performance liquid chromatography (HPLC), using a Varian Dynamax HPLC system (Varian Analytical Instruments, Walnut Creek, CA) with a 4.6×250 mm Whatman Partisil 10 SAX analytical column (Whatman Inc., Newton, MA). The mobile phase consisted of a linear gradient of pH 3.5 10–40 mM potassium phosphate buffer. A standard curve was constructed using a series of dilutions of cidofovir and cHPMPC of known concentration. Drug peaks were identified on the basis of their elution time. Concentrations were determined by measuring the area under the curve (AUC), which varied linearly with concentration over the range of interest (not shown).
The quantity of virus or drug delivered to the mice over the course of exposure could not be directly measured, rather, the dose was estimated by multiplying the concentration of virus or drug in the aerosol (C A), in pfu/l or mg/l, respectively, by the total volume (V) of air respired by a mouse of given body weight over the exposure time t, using the formula determined by Guyton (1947), V m=2.1×(weight)0.75. For 10 g mice, V M=.0118 l/min. The ‘presented dose’ thus calculated somewhat overestimates the actual quantity of virus or drug taken up by the mice, since it assumes that all inhaled particles are retained in the respiratory tract.
The value of C A was determined in two different ways. The first used the characteristics of the exposure system to predict its value, using the equation
| CA=(concentrationofnebulizersolution, mg/ml)×(totalquantitynebulized, ml)(totalvolumeofaerosol, l) |
For virus infection, we used C A to calculate the dose per mouse in pfu; for drug treatment, the dose was expressed in mg/kg of body weight. Because this predicted presented dose is that which the mice would receive if all virus or drug introduced into the nebulizer were optimally aerosolized and completely taken up on inhalation, it represents the largest dose that the animals could receive, i.e. the upper boundary of the dose range.
CA was also determined by drawing a portion of the aerosol through an AGI at a constant rate throughout each exposure, measuring the amount of virus or drug collected in the sampling medium, and calculating
| CA=(concentrationofAGImedium, mg/ml)×(volumeofAGImedium, ml)(rateofairflowthroughAGI, l/min)×(time, min) |
This value was used to determine the measured presented dose in pfu of virus or mg/kg of drug.
For virus exposures, the predicted and measured doses were in close agreement. The predicted value of C A, based on a nebulizer concentration of 6×108 pfu/ml, was approximately 107 pfu/l, resulting in a presented dose of 106 pfu per mouse. The measured doses calculated in experiments 1–5 ranged from 5.4×105 to 1.2×106 pfu (Table 1 ), averaging 9×105 pfu per mouse. In Experiment 3, the virus was inadvertently prepared for nebulization at a lower concentration, which gave a measured dose of 5×104 pfu/mouse. The effect of this lower dose on the outcome of infection is described below.
Table 1.
Experimental conditions and outcomes of five experiments in which weanling BALB/c mice were infected with aerosolized cowpox virus and treated with the indicated doses of aerosolized or subcutaneously injected cidofovir (HPMPC) or cHPMPC
| Expt | Mean virus dose and wt. | Drug | ||||||
|---|---|---|---|---|---|---|---|---|
| Aerosol | S.c. | |||||||
| Day treated | Dose (mg/kg) | Survival | Day treated | Dose (mg/kg) | Survival | |||
| 1 | 9.2×105 pfu | Cidofovir | −1 | 0.5–5 | 10/10* | 0 | 10 | 0/10 |
| 9.4 g | −1 | 0.06–0.5 | 3/10 | 0 | 1 | 0/10 | ||
| 0 | 0.5–5 | 10/10* | ||||||
| 0 | 0.06–0.5 | 1/10 | ||||||
| 1 | 0.5–5 | 7/10* | ||||||
| 1 | 0.06–0.5 | 0/10 | ||||||
| Placebo | – | 0/10 | ||||||
| cHPMPC | −1 | 0.9–5 | 0/10 | 0 | 10 | 0/10 | ||
| −1 | 0.09–0.5 | 0/10 | 0 | 1 | 0/10 | |||
| 0 | 0.9–5 | 1/10 | ||||||
| 0 | 0.09–0.5 | 0/10 | ||||||
| 1 | 0.9–5 | 0/10 | ||||||
| 1 | 0.09–0.5 | 0/10 | ||||||
| 2 | 1.2×106 pfu | Cidofovir | −1 | 0.5–5 | 10/10* | −1 | 100 | 10/10* |
| 10.2 g | −1 | 0.06–.5 | 9/10* | −1 | 75 | 10/10* | ||
| Placebo | – | 0/10 | −1 | 50 | 10/10* | |||
| −1 | 25 | 8/10* | ||||||
| −1 | 10 | 6/10* | ||||||
| CHPMPC | −1 | 0.9–5 | 8/10* | −1 | 100 | 6/10* | ||
| −1 | 0.09–0.5 | 0/10 | −1 | 75 | 5/10* | |||
| Placebo | – | 0/10 | −1 | 50 | 2/10 | |||
| −1 | 25 | 0/10 | ||||||
| −1 | 10 | 2/10 | ||||||
| 3 | 5.2×104 pfu | Cidofovir | −1 | 0.5–5 | 10/10* | −1 | 100 | 10/10* |
| 10.5 g | −1 | 0.06–0.5 | 10/10* | 0 | 100 | 10/10* | ||
| 0 | 0.5–5 | 9/10* | ||||||
| 0 | 0.06–0.5 | 10/10* | ||||||
| Placebo | – | 3/10 | ||||||
| 4 | 5.4×105 | Cidofovir | 0 | 0.5–5 | 10/10* | 0 | 25 | 7/10* |
| pfu 8.9 g | 1 | 0.5–5 | 9/10* | 1 | 25 | 6/10* | ||
| 2 | 0.5–5 | 5/10* | 2 | 25 | 5/10* | |||
| Placebo | – | 0/10 | ||||||
| 5 | 8.5×105 | Cidofovir | −1 | 0.5–5 | 8/8* | −1 | 25 | 8/8* |
| pfu 9.0 g | −1 | 0.06–0.5 | 5/8* | 0 | 25 | 7/8* | ||
| 0 | 0.5–5 | 8/8* | ||||||
| 0 | 0.06–0.5 | 8/8* | ||||||
| Placebo | – | 0/8 | ||||||
The percent of mice surviving differs significantly from that of the placebo group (P<0.05), by Fisher's exact test.
For drug exposures, there was a difference between the predicted and measured doses. This did not result from an error in assaying cidofovir or cHPMPC, since analysis of samples of known concentration by HPLC showed that the AUC varied linearly with concentration (not shown). Rather, it appears that the AGI was less efficient in capturing drug than virus. Thus, the predicted value of C A based on a nebulizer concentration of 10 mg/ml was 160 μg/l, giving an average dose per mouse of approximately 50 μg, or 5 mg/kg. By contrast, the measured dose calculated from AGI samples was 4.7±1.2 μg, or approximately 0.5 mg/kg. For a nebulizer concentration of 1 mg/ml, the predicted dose was 0.5 mg/kg, but the mean measured dose was approximately 6 μg per mouse, or approximately 0.06 mg/kg. For cHPMPC, the predicted high and low doses were the same as for cidofovir, but the measured doses were 0.9 and 0.09 mg/kg. Since it is reasonable to assume that the mice were exposed to at least the measured dose, but less than the predicted dose, we concluded that the actual mean dose fell within the range of 0.5–5 mg/kg for ‘high dose’ and 0.06–0.5 mg/kg for ‘low dose’ cidofovir therapy (Table 1). For cHPMPC, these values were 0.9–5 and 0.09–0.5 mg/kg, respectively.
2.5. Statistical analysis
Analysis of data was performed using SAS Version 8.0 software (SAS Institute, Cary, NC). Because of small sample sizes, differences in group means were tested by two-sided non-parametric Wilcoxon analysis. Differences in group means of groups followed over time were compared by analysis of variance (ANOVA) at each time point, followed by multiple comparisons.
3. Results
3.1. Aerosolized cidofovir protects mice against aerosolized cowpox virus
Aerosolized cidofovir administered on day −1 or 0 was highly effective in preventing weight loss, illness and death. In experiment 1, placebo-treated mice began to lose weight on day 5, and all were dead by day 9 (Table 1, Fig. 1 A). By contrast, all mice treated with 0.5–5 mg/kg of aerosolized cidofovir on day −1 or day 0 continued to gain weight briskly and were still healthy on day 28. Treatment on day 1 was less effective. The lower aerosol dose slowed weight loss and delayed death (Fig. 1B), but most treated mice died. In contrast to the solid protection provided by 0.5–5 mg/kg of aerosolized cidofovir, 10 mg/kg s.c. did not prevent death (Table 1).
Fig. 1.
Experiment 1. (A) Percent change in mean body weight of groups of ten mice infected by aerosol with cowpox virus and treated with a 0.5–5 mg/kg of aerosolized cidofovir on day −1, 0 or 1, or with aerosolized PBS (placebo) on day 0, with respect to viral challenge. (B) Same experiment, 0.06–0.5 mg/kg.
Experiments 2–5 compared the efficacy of aerosolized cidofovir to larger s.c. doses. In experiment 2, infected mice treated with 0.5–5 mg/kg of cidofovir by aerosol on day −1 gained weight at almost the rate of sham-infected drug-treated mice, and slightly faster than the infected group treated with 100 mg/kg s.c. (Fig. 2 A). The weight loss of mice treated with the lower aerosol dose (0.06–0.5 mg/kg) was similar to that of the group given 25 mg/kg s.c. S.c. doses of 50 mg/kg or higher were required to achieve 100% survival (Table 1).
Fig. 2.
Experiment 2. (A) Percent change in mean body weight (g) of groups of ten mice infected by aerosol with cowpox virus and treated on day −1 with a 0.5–5 or 0.06–0.5 mg/kg of aerosolized cidofovir, or with 100 or 25 mg/kg injected s.c., or with aerosolized PBS (placebo) or mock-infected with PBS and treated with 0.5–5 mg/kg of aerosolized cidofovir (sham). (B) Same experiment, treatment with cHPMPC.
The effects of treatment on the development of lung disease were compared by killing subsets of mice at time points postexposure and examining the lungs. In experiment 2, although small group sizes (n=4) did not permit differences in mean lung weight to reach the level of statistical significance, trends were clearly evident (Fig. 3 A). Placebo-treated infected mice had heavier lungs than naïve animals on day 8. Treatment with 0.5–5 mg/kg of cidofovir by aerosol or with 100 mg/kg s.c. prevented the increase in lung weight. Those that received 0.06–0.5 mg/kg by aerosol had intermediate values, while those treated with 25 mg/kg s.c. did not differ from placebo controls. Placebo-treated mice had a mean lung viral titer of 1.2×107 pfu/g (Fig. 3B). Mice treated by aerosol with 0.5–5 mg/kg of cidofovir showed a greater than 100-fold reduction in mean viral titer. This difference was statistically significant (P=0.0286) by Wilcoxon non-parametric analysis. Those treated with 100 mg/kg s.c. also had significantly lower mean titers than the placebo group. Other groups had intermediate titers, which did not differ significantly from the placebo group. The pattern of viral titers among the various groups mirrored that of lung weights (Fig. 3A).
Fig. 3.
Experiment 2. (A) Lung weight (g) on day 8 postinfection of mice infected by aerosol with cowpox virus and treated by aerosol or s.c. with the indicated drug (each value is the mean of four animals). White bar: uninfected. Light stippling: 0.5–5 mg/kg (aerosol) or 100 mg/kg (s.c.). Heavy stippling: 0.06–0.5 mg/kg (aerosol) or 25 mg/kg (s.c.). Black bar: placebo. (B) Log10 of the geometric mean viral titer, in pfu/g, of the same groups.
In experiment 3, treatment on day −1 and day 0 produced a similar outcome; the results of cidofovir or placebo treatment on day −1 are presented. The 20-fold lower dose of virus administered in this experiment did not delay the onset of weight loss of placebo-treated mice (Fig. 4 A). All became severely ill, but their weight loss stabilized at about day 10. The last three placebo-treated mice still alive on day 16 were killed to collect lung tissues for pathology studies. Infected mice treated with 0.5–5 mg/kg of cidofovir by aerosol gained weight as rapidly as naive controls. By contrast, those treated with 100 mg/kg s.c. lost weight to approximately the same extent as those that received 0.06–0.5 mg/kg by aerosol. The higher aerosol dose was also the most effective in preventing an increase in lung weight. The ratio of lung weight to body weight proved to be a sensitive measure of disease progression (Fig. 4B). Mice treated with 0.5–5 mg/kg of cidofovir by aerosol had significantly lower mean ratios of lung to body weight than placebo-treated mice on days 8, 12 and 16 postinfection (P=0.0055, <0.0001 and <0.0001, respectively, by analysis of variance [ANOVA]). The mean ratios of mice treated with the lower aerosol dose or s.c. did not differ significantly from the placebo group at any time point. ‘High dose’ aerosol therapy was also the most effective in reducing viral replication (Fig. 4C). The mean viral titer of placebo-treated mice on day 8 (8×107 pfu/g) was more than 1000-fold higher than that of mice treated with 0.5–5 mg/kg by aerosol (3×104 pfu/g), and still differed significantly on day 12 (P<0.0001 for both days [ANOVA]). Mice treated by s.c. injection also had significantly lower mean lung viral titers on day 8 (P<0.0001); titers of those treated with the lower dose by aerosol did not differ significantly from placebo controls.
Fig. 4.
Experiment 3. (A) Percent change in mean body weight of groups of ten mice treated on day −1 with 0.5–5 or 0.06–0.5 mg/kg of aerosolized cidofovir, or with 100 mg/kg s.c., or with aerosolized PBS (placebo), or left uninfected and untreated (naive). (B) Ratio of lung weight to body weight (percent) over the course of infection of mice infected by aerosol with cowpox virus and treated by aerosol or s.c. on day −1 with the indicated drug (each value is the mean of four animals). (C) Log10 of the geometric mean viral titer, in pfu/g, of the specimens in Fig. 4B.
The result of experiment 4 is discussed below. Experiment 5 confirmed earlier findings. Aerosol or s.c. treatment on day −1 and day 0 again produced similar results (Table 1; other data not shown). An aerosol dose of 0.5–5 mg/kg was much more effective than 25 mg/kg s.c. in maintaining the normal increase in body weight, preventing an increase in lung weight, and restricting viral replication, while the lower aerosol dose was less effective.
3.2. Aerosolized cidofovir prevents bronchopneumonia
In experiment 2, microscopic examination of the lungs on day 8 revealed significant differences among the groups (not shown). Placebo-treated mice showed severe bronchiolitis and incipient bronchopneumonia. Mice treated with 100 mg/kg of cidofovir s.c. showed minimal bronchiolitis, while those treated with 0.5–5 mg/kg by aerosol developed slightly more prominent bronchiolar changes, to the same degree as mice treated with 25 mg/kg s.c.; none developed bronchopneumonia. The lungs of mice treated with the lower dose of aerosolized cidofovir resembled those of placebo controls.
In experiment 3, the lungs of mice treated on day −1 or day 0 showed similar changes; results from day −1 treatment are shown. The lungs of mice treated with 0.5–5 mg/kg of aerosolized cidofovir showed no significant lesions (Fig. 5 A), while mice treated with 0.06–0.5 mg/kg by aerosol (Fig. 5B) developed severe, necrotizing bronchopneumonia with peribronchiolar hemorrhage, similar to the placebo group (Fig. 5D). S.c. treatment with 100 mg/kg resulted in mild bronchiolitis, without bronchopneumonia (Fig. 5C). The results thus differed slightly from experiment 2, in which 100 mg/kg s.c. was more effective than 0.5–5 mg/kg by aerosol in preventing the development of bronchiolitis.
Fig. 5.
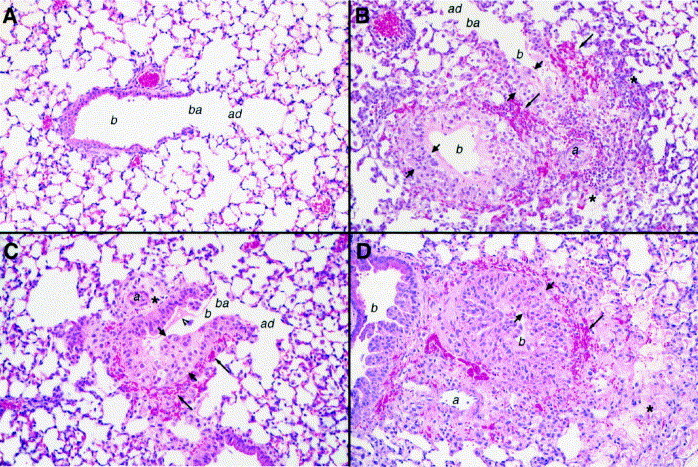
Experiment 3. Representative fields from sections of lung tissue collected on day 8 postinfection from the mice described in Fig. 4, which were treated on day −1 with 0.5–5 or 0.06–0.5 mg/kg of aerosolized cidofovir, or with 100 mg/kg s.c., or with aerosolized PBS (placebo). In all figures, short arrows indicate thickened bronchiolar mucosal epithelium; arrowhead, edema fluid in airway; long arrows, peribronchiolar hemorrhage; asterisk, inflammatory cells and edema in peribronchovascular and alveolar spaces; b, bronchiole; ba, bronchoalveolar junction; ad, alveolar duct; a, bronchiolar artery. Hematoxylin and eosin. Original magnification 200×. (A) Aerosol treatment with 0.5–5 mg/kg of cidofovir: no lesions are observed. (B) Aerosol treatment with 0.06–0.5 mg/kg: bronchopneumonia and extensive pulmonary disease. (C) S.c. treatment with 100 mg/kg: mild bronchiolitis. (D) Placebo: bronchopneumonia and extensive pulmonary disease.
3.3. Aerosolized cidofovir is most effective when given prophylactically
Experiment 4 examined the efficacy of aerosolized cidofovir for post-exposure therapy by treating mice with 0.5–5 mg/kg by aerosol or 25 mg/kg s.c. on day 0, 1 or 2 (Fig. 6 A,B, Table 1). Aerosol therapy on day 0 was the only regimen that resulted in continuous weight gain and the survival of all mice. Aerosol treatment on day 1 or 2 and s.c. treatment on day 0, 1 or 2 all resulted in the survival of at least half of the animals. Treatment on day 0 was most effective in minimizing loss of body weight, while delaying therapy to day 1 or 2 resulted in a progressive decrease in benefit (Fig. 7 A). The same pattern of loss of efficacy with delay in treatment was observed for the ratio of lung to body weight (Fig. 7B). This ratio was significantly lower for mice treated on day 0 by aerosol (P=0.0079) or s.c. (P=0.0159) than for the placebo group (Wilcoxon nonparametric analysis). By contrast, mice treated by either route on day 1 or 2 had lung/body weight ratios that did not differ significantly from the placebo group. The pattern of lung viral titers resembled the pattern of the ratio of lung weight to body weight (Fig. 7C). The mean titer of mice treated by aerosol on day 0 was significantly lower than that of the placebo group (P=0.0079), as was that of mice treated s.c. on day 0 (P=0.0079) or by aerosol on day 1 (P=0.0079). For all three parameters studied there was a small advantage of aerosol over s.c. therapy for each treatment day.
Fig. 6.
Experiment 4. (A) Percent change in mean body weight of cowpox-infected mice treated with 0.5–5 mg/kg of aerosolized cidofovir on day 0, 1 or 2 postinfection. (B) Percent change in mean body weight of cowpox-infected mice treated with 25 mg/kg of cidofovir s.c. on day 0, 1 or 2 in the same experiment.
Fig. 7.
Experiment 4. (A) Percent change in mean body weight of groups of five mice from the same experiment as Fig. 6 on day 8 postinfection. White bar: uninfected. Light stippling: aerosol treatment. Dark stippling: s.c. treatment. Black bar: placebo. (B) Mean ratio of lung weight to body weight (per cent) of four animals per group in the same experiment on day 8 postinfection. (C) Log10 of the geometric mean lung viral titer (pfu/g) of the same specimens as in (B).
3.4. cHPMPC is less protective than cidofovir
Because the molecular weights of cidofovir and cHPMPC are almost identical, the efficacy of the two drugs can be compared on a weight-for-weight basis. In experiment 1, treatment with 0.9–5 mg/kg of aerosolized cHPMPC increased the mean time to death, but all treated mice died (Table 1). Groups treated with the lower aerosol dose, or with 10 mg/kg s.c., did not differ from placebo controls. In experiment 2, mice treated with 0.9–5 mg/kg of cHPMPC lost weight as rapidly as those that received 0.06–0.5 mg/kg of cidofovir, while those that received 0.09–0.5 mg/kg of cHPMPC did not differ from placebo controls (Fig. 2B). Even 100 mg/kg of cHPMPC s.c. did not give 100% survival. cHPMPC was also less effective than cidofovir in preventing increase in lung weight and restricting pulmonary viral replication postinfection (Fig. 4A, B).
3.5. Aerosolized cidofovir is not toxic to the lung
In all experiments, cowpox-infected mice treated with the higher dose of aerosolized cidofovir or cHPMPC fared better than those that received the lower dose of the same drugs, consistent with a lack of drug toxicity. In those experiments in which uninfected mice were treated with aerosolized cidofovir or cHPMPC, they gained weight as rapidly as uninfected mice treated with aerosolized PBS. In experiment 3, three uninfected mice treated with 0.5–5 mg/kg of aerosolized cidofovir and three treated with aerosolized PBS were killed on day 9 for pathology studies. The lungs of the former showed no changes suggestive of drug toxicity. In experiment 2, all mice surviving on day 21 were retained for study through day 108. All groups remained healthy and gained weight and no deaths were observed. On day 108, three mice per group were killed for pathology studies. Microscopic examination revealed residual chronic inflammatory cells in the peribronchiolar tissues of infected, treated mice, but there was no evidence of fibrosis or persistent infection. Uninfected mice treated with aerosolized cidofovir or cHPMPC showed no pulmonary changes indicative of drug toxicity.
4. Discussion
As hypothesized, cidofovir was much more potent when delivered by aerosol than when injected s.c. in preventing the initiation and early spread of pulmonary cowpox virus infection. A dose of 0.5–5 mg/kg on day −1 or 0 was much more potent than 10 mg/kg s.c. (experiment 1), more efficacious than 25 mg/kg s.c. (experiments 2, 4 and 5) and either nearly as effective or even more effective than 100 mg/kg s.c. (experiments 2 and 3) in ensuring survival, limiting loss of body weight and increase in lung weight, restricting pulmonary viral replication, and preventing the development of bronchiolar lesions. cHPMPC was less protective than cidofovir. We had expected that the two compounds would give similar results, since cHPMPC is converted to cidofovir intracellularly (Bischofberger et al., 1994). The outcome suggests that HPMPC may be taken up less efficiently than cidofovir by pulmonary cells.
Our findings indicate that cidofovir is most potent when delivered directly to target tissues of infection before or soon after the arrival of virus. They mirror the results of Smee et al., who found that intranasally administered cidofovir was highly protective against a intranasal cowpox challenge (Smee et al., 2000). They are also consistent with earlier studies in animal models of pulmonary viral infection (respiratory syncytial virus [RSV], CMV and influenza), which have shown that aqueous or dry-powder aerosols of antiviral medications result in higher drug concentrations in the lung than systemic administration and are therapeutically effective (Wilson et al., 1980; Debs et al., 1988; Gilbert and Wyde, 1988; Gilbert et al., 1993; Sudo et al., 1999). Such work has led to the introduction of two aerosol medications for human antiviral therapy: ribavirin and zanamivir. Ribavirin is used to treat RSV infection in hospitalized infants and immunocompromised adults. Its short half-life necessitates repeated and prolonged administration as an aqueous mist (Knight and Gilbert, 1988; Mills, 1999). Zanamivir, by contrast, is self-administered as a dry powder aerosol from a metered-dose inhaler. The recommended dose of 10 mg twice daily for four days produces significant decreases in the severity and duration of influenza symptoms, and reduces secondary transmission (Monto et al., 1999; Hayden et al., 2000).
This initial study serves as a ‘proof of concept’ that aerosolized cidofovir would be highly efficacious for pre-exposure or immediate post-exposure prophylaxis of aerosolized smallpox or monkeypox infection. The method employed is not intended to be a model for human therapy, since it required the mice to be exposed to an aqueous aerosol for 30 min. For human use, treatment would probably take the form of a self-administered dry-powder aerosol, resembling the zanamivir inhaler. In contrast to zanamivir, the long intracellular half-life of cidofovir might permit single-dose treatment. Once taken up by cells in the respiratory tract, cidofovir would restrict viral replication, limit the severity of lung disease, reduce infectivity and provide time for the immune system to mobilize a protective response. The aerosol route was much more effective than s.c. injection in mice treated on day −1 or 0, but the difference diminished when treatment was deferred to day 1 or 2 (experiments 1 and 4). This suggests that aerosolized cidofovir would be most useful for pre- or early post-exposure prophylaxis for aerosolized orthopoxvirus infections, while i.v. cidofovir would be more appropriate if therapy is begun later in infection.
The fact that aerosol treatment was equally protective on day −1 and day 0 (experiments 1 and 3) suggests that a significant fraction of the inhaled drug was taken up and retained by pulmonary cells, with a long half-life. We did not attempt to study the pharmacokinetics of aerosolized cidofovir in these experiments, because our assay would not have been sensitive enough to measure low drug levels in blood and tissue samples over time. However, we are about to commence a pharmacokinetic study of aerosolized and s.c.-injected cidofovir in mice, using 14C-labeled drug, basing our work on a previously reported study of the parmacokinetics of i.v.- and s.c.-injected cidofovir in rats (Cundy et al., 1996). We also plan to compare the efficacy of aqueous and dry-powder aerosols as we move forward to evaluate aerosolized cidofovir in large animal models of virulent aerosolized orthopoxvirus infection.
Acknowledgements
The excellent technical assistance of Assaf Hazan, Scott Lewis, Ralph Tammariello, Linda Young and Michael Zimmerman is greatly appreciated. We thank James Boles, John Huggins and Louise Pitt for useful discussions. The views, opinions, and/or findings contained herein are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation. The investigators adhered to the ‘Guide for the Care and Use of Laboratory Animals,’ prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (National Institutes of Health Publication No. 86-23, revised 1996), and used facilities fully accredited by the American Association for Accreditation of Laboratory Animal Care.
References
- Bischofberger N., Hitchcock M., Chen M., et al. 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine, an intracellular prodrug for (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine with improved therapeutic index in vivo. Antimicrob. Agents. Chemother. 1994;38:2387–2391. doi: 10.1128/aac.38.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman P.S., Erlich E., Eichenwald H.F., et al. Standard samplier for assay of airborne microorganisms. Science. 1964;144:1295. [Google Scholar]
- Bray M., Martinez M., Smee D., Kefauver D., Thompson E., Huggins J.W. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 2000;181:10–19. doi: 10.1086/315190. [DOI] [PubMed] [Google Scholar]
- Byron P.R., Patton J.S. Drug delivery via the respiratory tract. J. Aerosol Med. 1994;7:49–75. doi: 10.1089/jam.1994.7.49. [DOI] [PubMed] [Google Scholar]
- Cundy K. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinet. 1999;36:127–143. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- Cundy K.C., Bidgoo A.M., Lynch G., Shaw J.P., Griffin L., Lee W.A. Pharmacokinetics, bioavailability, metabolism and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug. Metab. Dispos. 1996;24:745–752. [PubMed] [Google Scholar]
- Cundy K.C., Barditch-Crovo P., Petty B., Ruby A., Redpath M., Jaffe H., Lietman P.S. Clinical pharmacokinetics of 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine in hunman immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 1999;43:271–277. doi: 10.1128/aac.43.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debs R.J., Montgomery A.B., Brunette E.N., DeBruin M., Shanley J.D. Aerosol administration of antiviral agents to treat lung infection due to murine cytomegalovirus. J. Infect. Dis. 1988;157:327–331. doi: 10.1093/infdis/157.2.327. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 2001;14:382–397. doi: 10.1128/CMR.14.2.382-397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. Smallpox and its Eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- Fenner F., Wittek R., Dumbell K.R. The Orthopoxviruses. Academic Press; New York: 1986. [Google Scholar]
- Gilbert B.E., Wyde P.R. Pharmacokinetics of ribavirin aerosol in mice. Antimicrob. Agents Chemother. 1988;32:117–121. doi: 10.1128/aac.32.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert B.E., Wyde P.R., Wilson S.Z., Meyerson L.R. SP-303 small-particle aerosol treatment of influenza A virus infection in mice and respiratory syncytial virus infection in cotton rats. Antivir. Res. 1993;21:37–45. doi: 10.1016/0166-3542(93)90065-q. [DOI] [PubMed] [Google Scholar]
- Guyton A.C. Measurement of the respiratory volumes of laboratory animals. Am. J. Physiol. 1947;150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- Hayden F., Gubareva L., Monto A., et al. Inhaled zanamavir for the prevention of influenza in families. N. Engl. J. Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- Henderson D.A. Bioterrorism as a public health threat. Emerg. Infect. Dis. 1998;4:488–492. doi: 10.3201/eid0403.980340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock M., Jaffe H.S., Martin J.C., Stagg R.J. Cidofovir, a new agent with potent anti-herpesvirus activity. Antivir. Chem. Chemother. 1996;7:115–127. [Google Scholar]
- Ho H.T., Woods K.L., Bronson J.J., De Boeck H., Martin J.C., Hitchcock M.J. Intracellular metabolism of the antiherpes agent (S)-1-[3-hydroxy-2-(phosphonylmethoxy) propyl]cytosine. Mol. Pharmacol. 1992;41:197–202. [PubMed] [Google Scholar]
- Hutin Y., Williams R., Malfait P., et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996–1997. Emerg. Infect. Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight V., Gilbert B. Antiviral therapy with small particle aerosols. Eur. J. Clin. Microbiol. Infect. Dis. 1988;7:721–731. doi: 10.1007/BF01975037. [DOI] [PubMed] [Google Scholar]
- Martinez M., Bray M., Huggins J.W. A mouse model of aerosol-transmitted orthopoxviral disease. Arch. Pathol. Lab. Med. 2000;124:362–371. doi: 10.5858/2000-124-0362-AMMOAT. [DOI] [PubMed] [Google Scholar]
- May K.R. The collison nebulizer: description, performance and application. Aerosol Sci. 1973;4:235–243. [Google Scholar]
- Mills J. Prevention and treatment of respiratory syncytial virus infections. Adv. Exp. Med. Biol. 1999;458:39–53. doi: 10.1007/978-1-4615-4743-3_5. [DOI] [PubMed] [Google Scholar]
- Monto A.S., Fleming D.M., Henry D., et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J. Infect. Dis. 1999;180:254–264. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- Neyts J., De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 1993;41:242–246. doi: 10.1002/jmv.1890410312. [DOI] [PubMed] [Google Scholar]
- Naesens L., Snoeck R., Andrei G., Balzarini J., Neyts J., De Clercq E. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 1997;8:1–23. [Google Scholar]
- Oliyai R., Shaw J.P., Sueoika-Lennen C., Cundy K., Arimilli M., Jones R., Lee W. Aryl ester prodrugs of cyclic HPMPC. I: physicochemical characterization and in vitro biological stability. Pharmacol. Res. 1999;16:1687–1693. doi: 10.1023/a:1018945713623. [DOI] [PubMed] [Google Scholar]
- Orent, W., 1998. Escape from Moscow. The Sciences May/June 1998, 26–31.
- O'Toole T. Smallpox: an attack scenario. Emerg. Inf. Dis. 1999;5:540–546. doi: 10.3201/eid0504.990416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D.F., Bailey K.W., Sidwell R.W. Treatment of cowpox virus respiratory infections in mice with ribavirin as a single agent or followed sequentially by cidofovir. Antivir. Chem. Chemother. 2000;11:303–309. doi: 10.1177/095632020001100406. [DOI] [PubMed] [Google Scholar]
- Soike K.F., Huang J.L., Zhang J.Y., Bohm R., Hitchcock M.J., Martin J.C. Evaluation of infrequent dosing regimens with (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine (S-HPMPC) on simian varicella infection in monkeys. Antivir. Res. 1991;16:17–28. doi: 10.1016/0166-3542(91)90055-v. [DOI] [PubMed] [Google Scholar]
- Sudo K., Watanabe W., Konno K., et al. Efficacy of RD3-0028 aerosol treatment against respiratory syncytial virus infection in immunosuppressed mice. Antimicrob. Agents Chemother. 1999;43:752–757. doi: 10.1128/aac.43.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman M., Petty B.G., Cundy K.C., et al. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antivir. Res. 1996;29:153–161. doi: 10.1016/0166-3542(95)00829-2. [DOI] [PubMed] [Google Scholar]
- Wehrle P.F., Posch P.J., Richter K.H., Henderson D.A. An airborne outbreak of smallpox in a German hospital and its significance with respect to other recent outbreaks in Europe. Bull. World Health Org. 1970;43:669–679.4. [PMC free article] [PubMed] [Google Scholar]
- Wilson S.Z., Knight V., Wyde P.R., Drake S., Couch R.B. Amantadine and ribavirin aerosol treatment of influenza A and B infection in mice. Antimicrob. Agents Chemother. 1980;17:642–648. doi: 10.1128/aac.17.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]


