Abstract
Background
The COVID-19 pandemic has transformed and affected every aspect of health care. Like any catastrophic event, the stress on hospitals to maintain a certain level of function is immense. Acute surgical pathologies cannot be prevented or curtailed; therefore, it is important to understand patterns and outcomes during catastrophes in order to optimize care and organize the health care system.
Methods
In a single urban tertiary care center, a retrospective study examined the first complete lockdown period of Israel during the COVID-19 pandemic. This was compared to the same time period the previous year.
Results
During the pandemic, time to hospitalization was significantly decreased. There was also an overall reduction in surgical admissions yet with a higher percentage being hospitalized for further treatment (69.2% vs 23.5%). The patients admitted during this time had a higher APACHE-II score and Charlson comorbidity index score. During the pandemic, time to surgery was decreased, there were less laparoscopic procedures, and more RBC units were used per patient. There were no differences in overall complications, except when sub-analyzed for major complications (9.7% vs 6.3%). There was no significant difference in overall in-house mortality or morbidity. Length of hospitalization was significantly decreased in the elderly population during the pandemic.
Conclusion
During the COVID-19 pandemic, despite a significantly less number of patients presenting to the hospital, there was a higher percentage of those admitted needing surgical intervention, and they were overall sicker than the previous year.
Keywords: COVID-19, acute care surgery, pandemic
Introduction
The COVID-19 pandemic has affected every aspect of health care. Hospitals have quickly reorganized in order to accommodate the sudden needs of symptomatic COVID-19 patients, while struggling to maintain the routine surgical and medical requirements of their communities. Most countries from the Americas, Europe, and Asia either halted elective surgeries or placed heavy limitations on them.1–3 Other countries, such as Japan and Sweden, had less restrictive measures and continued with almost fully normal activity.4,5 The majority of countries that restricted surgical services, besides the acute care surgery (ACS) and trauma surgery, continued with a handful of oncological cases which had a narrow window for a therapeutic operation. The infrastructure of some countries and regions was so completely overwhelmed that they were forced to shut down all surgical services (emergent and elective) due to a limited number of respirators and staff. In Israel, the Ministry of Health ordered all elective surgical services to be suspended, with the exception of time-dependent oncological cases.6
A perfect level of preparedness for an unforeseeable event is never possible, yet lessons learned from past tragedies and patterns from similar events are what guide preparedness efforts and the early stages of response.7 For example, during Hurricane Katrina in the Gulf Coast region of the United States, “alternative-site” primary care and triage facilities were established to buffer the surge toward functioning emergency rooms (ERs) and hospitals.8 In addition, during this natural disaster, mobile surgical units were deployed to provide damage control surgery and ICU level of care.9 During the Ebola epidemic of 2014-2015 in Sierra Leone, surgical admission and operations fell drastically when compared to their preoutbreak numbers, with a large number due to the death of surgeons and the lack of personal protective equipment.10 Israel’s own experience with civilian hospital activity during a military conflict showed a significant decrease in elective surgical procedures while only performing oncologic surgeries and emergent surgery procedures.11
Understanding the patterns of presentation and outcomes of acute surgical illnesses during a disaster is vital in order to establish appropriate and adequate care on a national level. Such knowledge can allow hospitals to adequately prepare for a surge of patients by estimating the number of general surgeons, trauma surgeons, and operating room(OR)/ICU resources that must be allocated.6,12–15
There is paucity of data describing how war, natural disasters, or an epidemic/pandemic16 affect the presentation and treatment of emergent surgical illnesses. Here, we analyze our ACS patient population presenting to an Israeli tertiary hospital during a month of complete lockdown in our country due to the COVID-19 pandemic.
Methods
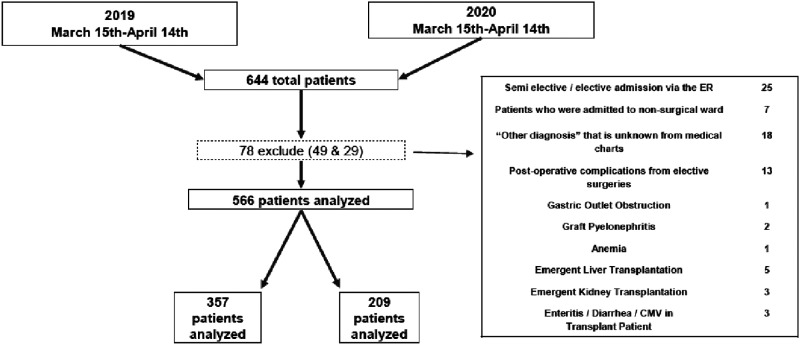
All patients who presented to the Tel Aviv Sourasky Medical Center (TASMC) emergency department (ED) and were admitted to the surgical division with an acute surgical pathology were included in this study. Tel Aviv Sourasky Medical Center is a 1500-bed, Level-1 trauma and tertiary care center serving downtown Tel Aviv and surrounding communities. The study period compared patients from March 15 to April 14, 2019 to the corresponding period in 2020, the specific dates of Israel’s full lockdown as ordered by the Ministry of Health, as a result of the COVID-19 pandemic. After receiving approval by our Internal Ethics Committee, retrospective data were obtained from the computerized medical record system. We reviewed patients' demographics, comorbidities, preoperative/preadmission diagnosis, imaging, intraoperative parameters, and postoperative course including complications, length of stay, and morbidity and mortality outcomes. The APACHE-II score17 was used to assess the severity of systemic illness at presentation. Postoperative morbidity was graded according to the Clavien-Dindo (CD) classification,18 and a major complication was defined as ≥ III. A total of 644 patients were admitted from the ED to the surgery department during these 2 periods. Out of this number, 78 patients were excluded, and therefore 566 patients were included in the study cohort. Excluded from this study are patients whose original admission order was canceled, admitted for semi-elective cases, incomplete medical records or unclear diagnosis, those for immediate transplant, nonsurgical diagnosis, and patients being admitted for a complication from a previous elective surgery. Figure 1 shows the outline of the study process. Intraoperative complications were classified according to the Classic Delphi study.19
Figure 1.
Outline of study process.
During the COVID-19 outbreak, the ED triage was based on clinical suspicion of COVID-19-related symptoms. All cases with even mild suspicion of COVID-19 were referred to a separated and isolated ED wing. A trained senior ED resident or attending was responsible for the triage checkpoint at all hours of the day or night. This was not only to maintain a high level of triage quality but also to directly discharge patients who would not benefit from ED workup, to identify critical patients that needed immediate airway intervention, and to immediately obtain samples for COVID-19 polymerase chain reaction (PCR) testing. COVID-19-related placement and triage was conducted based on the National Early Warning Score 2 (NEWS 2 score).20 Patients entering the COVID-19 isolated wing were further stratified as suspected with pending results or as having a diagnosed active COVID-19 infection. Polymerase chain reaction results for all patients were obtained within 6 hours. All personnel in the COVID-19 wing used full personal protective equipment. Senior emergency medicine and internal medicine residents and physicians were responsible for the care of these patients while in the isolation wing. Surgical consultations were provided upon request.
Statistical analysis was performed using the IBM SPSS v.26 statistics data editor. Continuous data were expressed as median values with the corresponding standard deviation. Student’s t-test was used for continuous data, and the Chi-square test was used for categorical data. Sub-analysis and post hoc analysis were performed by mean of intervention (surgical, percutaneous drainage, and endoscopic) as well as by age (above 70). A P-value of <.05 was considered statistically significant.
Results
Patient Demographics and Clinical Characteristics
Overall, 302 surgical patients admitted to the ED during the COVID-19 quarantine compared to 1519 patients in the same time frame of 2019. Of these patients admitted during the COVID-19 pandemic, a higher percentage were hospitalized for further treatment, 69.2% vs 23.5% (P = .003). Table 1 depicts their demographic and clinical characteristics. Median age was comparable in both groups 57 vs 62 years old (P = .33), respectively; the proportion of elderly patients (age >70 years) was also equal, 37% vs 33% (P = .44). Male gender was more common during the COVID-19 quarantine, 60% vs 54%; however, it did not significantly differ (P = .15). As shown in Table 1, the rates of non-Jewish citizensand tourists and immigrants admitted to surgery were significantly reduced during the pandemic, 5.9% vs1% and 6.2% vs 3.4%, respectively (P = .022). During the quarantine time period, overall patients had a higher mean Charlson comorbidity index 1.94 vs 1.33 (P = .004) and a higher APACHE-II score 6.69 vs 5.75 (P = .024). A higher APACHE-II score was also noticed amid the group of patients who underwent surgery during the quarantine as compared with the parallel time period in 2019, 6.15 vs 3.98, respectively (P = .003) (Table 2).
Table 1.
Demographics, Intervention, and Diagnosis.
| 2019 | 2020 | P-Value | ||
|---|---|---|---|---|
| ED admission | 1519 | 302 | – | |
| Hospitalization | 357 (23.5%) | 209 (69.2%) | .03 | |
| Age (median) | 56.34 ± 21.07 | 58.07 ± 21.03 | .35 | |
| Gender (male/female) | 193/164 | 126/83 | .15 | |
| Ethnicity (Jewish/minorities/tourists and foreigners) | 87.9%/5.9%/6.2% | 95.7%/1%/3.4% | .022 | |
| Charlson comorbidity index | 1.33 ± 2.23 | 1.94 ± 2.77 | .004 | |
| APACHE-II score | 5.75 ± 4.34 | 6.69 ± 4.19 | .024 | |
| Time to hospitalization | 10.12 ± 5.31 | 7.53 ± 4.24 | <.001 | |
| Type of intervention | Surgery | 158 (44.3%) | 72 (34.5%) | .12 |
| Drainage | 22 (6.2%) | 22 (10.5%) | .06 | |
| Endoscopy | 39 (10.9%) | 32 (15.3%) | .12 | |
| Angiography | 2 (.6%) | 0 (0%) | .29 | |
| Radiation | 1 (.3%) | 0 (0%) | .45 | |
| Differential diagnosis of hospitalized patients | Appendicitis | 52 (14.6%) | 26 (12.4%) | .51 |
| Diverticulitis | 13 (3.6%) | 11 (5.3%) | .37 | |
| Cholecystitis | 28 (7.8%) | 16 (7.7%) | .94 | |
| Gastrointestinal bleeding | 48 (13.5%) | 34 (16.3%) | .39 | |
| Hernia | 15 (4.2%) | 5 (2.4%) | .27 | |
| Bowel obstruction | 33 (9.2%) | 31 (14.8%) | .06 | |
| Perianal abscess | 39 (10.9%) | 15 (7.2%) | .16 | |
| Pancreatitis | 15 (4.2%) | 12 (5.7%) | .42 | |
| Cholangitis | 25 (7%) | 7 (3.4%) | .08 | |
| Trauma | 26 (7.3%) | 12 (5.7%) | .49 | |
| Other | 61 (17.1%) | 38 (18.2%) | .76 | |
| NA | 2 (.6%) | 2 (1%) | .59 | |
Abbreviations: ED, emergency department.
Table 2.
Patients Who Had Surgical Intervention.
| 2019 | 2020 | P-Value | ||
|---|---|---|---|---|
| Number of patients | 158 | 72 | .08 | |
| Age | 48.9 ± 21.4 | 49.36 ± 20.75 | .87 | |
| Gender (male/female) | 87/71 | 44/28 | .39 | |
| Charlson comorbidity index | .82 ± 1.59 | .96 ± 1.74 | .56 | |
| APACHE-II score | 3.98 ± 3.75 | 6.15 ± 4.64 | .003 | |
| Median time to hospitalization | 9:23 (6:14-13:18) | 6:56 (4:15-10:02) | .72 | |
| Median time to surgical intervention | 19:01 (10:22-42:25) | 11:00 (7:27-23:26) | <.001 | |
| Median duration of surgery | 1:04 (0:29-1:41) | 1:14 (0:42-2:10) | <.001 | |
| LOS from surgery | 5.69 ± 10.43 | 5.06 ± 6.6 | .588 | |
| Presence of attending | 70 (44.3%) | 41 (56.9%) | .031 | |
| Laparoscopic surgery | 84 (53.2%) | 30 (41.7%) | .011 | |
| Intraoperative complication (Delphi study) | 9 (5.7%) | 7 (9.7%) | .091 | |
| Postoperative | ||||
| Complications (Clavien-Dindo score) | 1 | 5 (3.2%) | 5 (6.9%) | .019 |
| 2 | 21 (13.3%) | 9 (12.5%) | .053 | |
| 3a | 1 (.6%) | 1 (2.8%) | .02 | |
| 3b | 3 (1.9%) | 3 (2.8%) | .047 | |
| 4 | 1 (.6%) | 1 (1.4%) | .044 | |
| 5 | 5 (3.2%) | 2 (2.8%) | .053 | |
| Major complication CD ≥ 3 | 10 (6.3%) | 7 (9.7%) | .03 | |
| Number of PRBC during hospitalization | .08 .42 | .42 1.58 | .013 | |
| LOS | 5.69 10.43 | 5.06 6.7 | .65 | |
| In-hospital mortality | 5 (3.1%) | 4 (5.5%) | .326 | |
| 30-day readmission | 10 (6.49%) | 6 (11.1%) | .273 | |
| 30-day mortality | 4 (2.53%) | 4 (5.5%) | .12 | |
Abbreviation: CD, Clavien-Dindo; LOS, length of stay; PRBC, packed red blood cells.
Comparing the two study periods, the specific common diagnoses for what patients were hospitalized for showed no significant difference (Table 1). The five most common diagnoses were the same in both 2019 and 2020—appendicitis, gastrointestinal bleeding, perianal abscess, bowel obstruction, and cholecystitis. Only the most prevalent of these diagnoses was different between the two study periods, with gastrointestinal hemorrhage being the most admitted diagnosis (14.3%) during the COVID-19 period, compared to the appendicitis (12.8% of admissions) during the previous year.
Treatment and Outcome Characteristics
For the entire cohort, the median time to hospitalization was significantly shorter during the quarantine period 7:53 hours vs 10:12 hours (P = <.001). During the lockdown, 72 (34.5%) had surgery, the majority for appendicitis. The rate of patients who had surgery during April 2019 was greater (44.5% vs 34.5%, P = .12) despite not being statistically significant. During the COVID-19 quarantine, there was also more endoscopic and percutaneous drainage intervention (15.3% vs 10.9%, P = .12 and 10.6% vs 6.2%, P = .06, respectively) (Table 1). In a sub-analysis of patients who underwent percutaneous drainage or endoscopy therapy, there were no significant differences in the APACHE-II score or any other clinical outcomes.
For the patients requiring surgery (Table 2), the time to hospitalization was not significantly shorter (P = .72); however, the median time from ED admission to surgery was significantly shorter during the COVID-19 quarantine month, 19:01 hours (range 10:22-42:25) vs 11:00 hours (7:27-23:26) (P = <.001). Also during this quarantine period, the rate of laparoscopic procedures was lower, 41.7% vs 53.2% (P = .011), an attending surgeon was present in more cases (56.9% vs 44.3%, P = .031), and the median duration of surgery was longer (1:14 hours vs 1:04 hours, P = <.001). The average number of RBC units consumed during surgery and postoperatively was significantly higher during the COVID-19 quarantine period (.42 vs .08, P = .013). During the quarantine time, intraoperative and postoperative complication rates did not significantly differ (9.7% vs 5.7%, P = .091 and 22.8% vs 29.2%, P = .37); however, major complications categorized as CD >3 were more common (9.7% vs 6.3%, P = .03). In any age-group, there were no statistical differences in length of stay, in-hospital or 30-day mortality rates, and readmission amid the groups of patients having surgery.
When analyzing only elderly patients, age>70, they exhibited similar demographics and trends in 2020 to the entire cohort by having similar Charlson comorbidity index scores (P = .044), and a shorter time to hospitalization (P = <.001), yet there was no difference with regard to the APACHE-II score (P = .47). In this elderly subgroup, the 30-day mortality rate was increased in 2020 (3.57% vs 11.3%, P = .045), with no significant change in in-hospital mortality (Table 3).
Table 3.
Elderly Patients Sub-Analysis.
| 2019 | 2020 | P-Value | |||
|---|---|---|---|---|---|
| N | 119 | 78 | |||
| Age | 80.34 ± 6.88 | 79.76 ± 6.87 | .563 | ||
| Gender (male/female) | 59/60 | 42/36 | .559 | ||
| Charlson comorbidity index | 2.39 ± 2.65 | 3.22 ± 3.04 | .044 | ||
| APACHE-II score | 9.66 ± 3.67 | 10.07 ± 3.42 | .466 | ||
| Time to hospitalization | 10.55 ± 5.41 | 7.51 ± 4.34 | <.001 | ||
| Patients requiring surgery | 36 | 15 | .084 | ||
| Time to surgical intervention | 53.91 ± 88.82 | 37.34 ± 65.67 | .461 | ||
| Duration of surgery | 1.89 ± 1.0 | 2.07 ± 1.47 | .154 | ||
| LOS from surgery | 12.4 ± 17.5 | 7.07 ± 7.74 | .143 | ||
| Presence of attending | 25 (69.4%) | 10 (66.7%) | 1.0 | ||
| Laparoscopic surgery | 15 (41.7%) | 6 (40%) | .97 | ||
| Intraoperative complications | 3 (8.3%) | 5 (31.3%) | .057 | ||
| Major complication CD≥3 | 6 (16.7%) | 5 (31.3%) | .215 | ||
| Number of PRBC during hospitalization | All patients | .24 ± .72 | .85 ± 1.53 | <.001 | |
| Patients undergoing surgery | .11 ± .40 | 1.0 ± 1.84 | .01 | ||
| LOS | All patients | 11.64 ± 13.6 | 6.41 ± 5.18 | <.001 | |
| Patients undergoing surgery | 14.75 ± 17.13 | 8.57 ± 7.62 | .085 | ||
| In-hospital mortality | 5 (4.2%) | 6 (7.6%) | .269 | ||
| 30-day readmission | 16 (13.45%) | 7 (8.97%) | .528 | ||
| 30-day mortality | All patients | 4 (3.4%) | 7 (9.0%) | .045 | |
| Patients undergoing surgery | 3 (2.5%) | 3 (3.8%) | .345 | ||
Abbreviation: LOS, length of stay; PRBC, packed red blood cells.
Only ten patients of the entire 2020 cohort were first triaged to the COVID-19 isolation wing of the ED. Of these patients, none were positive for COVID-19, four required emergent surgery, and three percutaneous drainage for intra-abdominal infections (Table 4). For the patients who entered the COVID-19 isolation wing, the average time to hospitalization was 8:00 hours (SD 7:30 hours), which was significantly less when compared to the non-isolated patients in 2020 with a time to hospitalization of 7:30 hours (SD 4:05 hours), P = .027. The average time to nonsurgical intervention and surgical intervention for the patients initially isolated due to COVID-19 protocol was 14:30 hours (SD 9:42 hours) and 31:08 hours (SD 15:15 hours). This was similar to those not isolated during the same period that were 39:25 hours (SD 43:34 hours, P-value .1) and 31:13 hours (SD 67:40 hours, P-value .5). There were no confirmed COVID-19 positive patients who had an acute surgical illness requiring surgery or hospitalization for surgical care.
Table 4.
10 Patients Originally Triaged as Potential COVID-19 Patients.
| Diagnosis | Number of Patients |
|---|---|
| Appendicitis | 1 |
| Diverticulitis | 1 |
| Cholecystitis | 3 |
| Gastrointestinal bleeding | 0 |
| Hernia | 1 |
| Bowel obstruction | 0 |
| Perianal abscess | 0 |
| Pancreatitis | 1 |
| Cholangitis | 1 |
| Trauma | 1 |
| Other (excluded) | Intestinal perforation |
| NA (excluded) | 0 |
| Total | 10 |
Discussion
During this period, we have shown that despite a significant decrease in the number of patients presenting to our ER, a majority of them presented with more severe disease when compared to the previous year. In general, the patients undergoing an operation were in worse condition than the patients without operation. Clearly, during this pandemic, or any other natural disaster or military campaign, there is limited ability to curtail the inevitable presentation of acute surgical pathologies or traumatic events. During challenging circumstances, hospitals must be prepared for and must be able to maintain emergent surgical services in order to avoid preventable deaths and mortality. The results of this study have shown the effect of the COVID-19 pandemic on our patient population suffering from acute general surgery illnesses.
There was no difference in the pathologies presenting between the two periods, which is an important statistical finding, yet must be taken in proper context. Depending on the type of catastrophe, the required mobilization of specific medical specialties and strain on the systems will be drastically different. During times of war or natural disasters, hospitals will require a larger ratio of orthopedic surgeons, general surgeons, surgical specialties, OR access, and ICU beds.14 In contrast, pathogen-derived epidemics/pandemics will require more specialists in the fields of internal medicine (ie, infectious disease, pulmonology, intensivist, and hematologist), ICU beds, and potential access to extracorporeal membrane oxygenation.15 As seen with our results, this primarily respiratory pandemic did not significantly change the acute surgical pathologies.
In regard to the younger patient population, there were several observations that were of interest yet not statistically significant. These patients appeared to present later in their course of disease, with a higher rate of complicated pathologies (ie, perforated appendix and gangrenous cholecystitis), postoperative complications, and blood transfusions. These younger patients were therefore more likely able to tolerate their symptoms at home for a longer period and therefore had a delay in diagnosis and treatment. In contrast, the older population, with their lower physiological reserve, appeared less able to delay medical treatment and came to the hospital regardless of fears and inconveniences caused by the pandemic restrictions.
The “fear” of presenting to the ER with severe illness has been documented from this current COVID-19 pandemic21 and from previously published literature during catastrophes. For example, during the SARS epidemic in 2002, Taiwan and Hong Kong showed a decrease in routine care not related to the severe acute respiratory syndrome.22 During this COVID-19 pandemic, one recent study reported the patterns of patients presenting to the ER of a busy level-1 trauma center in America. This group showed a global decrease in arrivals to the ER, along with a decreased proportion of patients presenting with abdominal pain when compared to other systemic complaints before the pandemic.23 This cannot be accurately compared to our data because we were only looking at acute surgical pathologies. Nevertheless, what was reported in this reference might also be a result of avoiding going to the hospital with abdominal pain during a pandemic that involves primarily respiratory symptoms. In Italy, toward the beginning of the pandemic, a series of children, without COVID-19-related illness, were shown to have presented late and in more critical condition, due to the fear of presenting to a hospital or lack of provisional care because of closure of health consults/centers.24 This corresponds with our overall results in general of sicker patients being admitted to the surgical ward.
The country where this study took place, Israel, also presents a unique confounding factor to these data. Our hospital is a public hospital within a socialized system where every citizen is born with universal health care coverage25; therefore, lacking health care is rarely a reason for not seeking medical attention.26 Compared to the rest of the world, our mortality has been comparatively low.27,28 Our “lockdown” measures and monitoring methods were considered aggressive, but the country also progressed in easing the regulations and opening its economy relatively fast. Nevertheless, the cooperation and coordination of our Ministry of Health, security sectors, and private industry played a pivotal role in preventing our hospital infrastructure from being completely overwhelmed. Our results showed a different demographic in terms of ethnicity presenting to the hospital during the COVID-19 pandemic. There was a significant increase in the ratio of Israeli-Jewish to Israeli-non-Jewish patients presenting to the ED and a smaller proportion of minorities, tourist, and foreign workers when compared to 2019. As mentioned above, the TASMC is located in the center of Tel Aviv, where 91% of the population is Jewish and 9% are non-Jewish. This difference might be explained by the lack of transport, hesitancy to travel distances, and fear of larger medical centers because of their treatment of COVID-19 patients.29 Hence, smaller local medical centers were potentially receiving more patients, regardless of appropriateness of level of care.
We found a low incidence of mis-triage of surgical patients into the COVID-19 isolation wing by our ER triage protocol. Out of the 209 patients admitted during this time, only 10 were initially placed in isolation. None of these 10 isolated patients had a positive COVID-19 test result. Despite being placed in triage, the average time to hospitalization, despite being statistically different, was only 30 minutes longer than the non-isolated group. When intervention (surgical and nonsurgical) was needed in the isolated group, there was no increase in time when compared to the non-isolated patients and, on average, even occurred more rapidly. With many surgical pathologies presenting with fever, it was difficult, especially at the beginning of the pandemic, not to over triage these patients and potentially delay intervention for surgical emergencies. This became especially relevant as evidence began to show that a possible presenting symptom of COVID-19 is fever with diarrhea.30 The significant reduction in time to hospitalization during the pandemic may be a confounding effect of both a decrease in volume to the ED and also sicker patients presenting to the ED, which would require quicker diagnosis and appropriate therapy. This decrease in hospitalization time happened despite a reduction in working staff.
Multiple studies have established that this elderly population is at an increased risk to clinically significant COVID-19.31 Our elderly population (>70 years old) during the pandemic had slightly more comorbidities and had a greater 30-day mortality than the previous year. Despite their increased comorbidities, they did not present to the hospital sicker than their counterparts the previous year. This trend might demonstrate the opposite of the “fear” phenomenon mentioned above for younger patients that sicker, elder patients present more easily to the hospital driven by the distress that something is wrong. With the elderly patients who did not undergo surgery, we were able to significantly reduce their hospitalization time. We have emphasized in our department not to neglect surgical pathologies during this time in this vulnerable population.
An interesting finding from the patients undergoing surgery during the pandemic is that they had a higher APACHE score, more complications, more blood given, yet more cases were open (verses laparoscopic), and there was an attending present more often. This seemingly contradicting findings might be a true indicator of how severely ill/complicated the patients were when compared to the previous year. Yet, this finding would need to be compared to other large tertiary centers in order to gather a significant conclusion. There are additional limitations to this study, including the fact that it is coming from a single urban referral center. These data may not be representative of other smaller and/or rural centers. Other limitations include the short study time and not having a cohort of surgical patients who were COVID-19 positive. The short study time was chosen in order to describe the initial effect of the pandemic and the most constricting limitations imposed by the government during the initial lockdown. Therefore, the initial month of true, full lockdown was taken and compared to the previous year. Clearly, during different stages and evolution of the pandemic, societies and medical systems have changed/adapted; therefore, the time when this study was conducted needs to be taken into consideration. There was no (knowingly) COVID-19-positive patient in our cohort and therefore no comparison between the differences in surgical pathology progression between positive and negative surgical patients. As there is more understanding of the COVID-19 pathophysiology, this would be an important patient population to analyze.
In conclusion, we have shown that during this COVID-19 pandemic, the patients presenting were sicker than the patients during the same time frame in the previous year despite there being fewer patients needing surgical admission. We were able to decrease the time until hospitalization and the length of hospitalization on the highly vulnerable elderly population. These data support the need for advocating and educating the public to present to hospitals without delay if there are symptoms of a possible acute surgical illness. Clearly, there are difficult times that may force the population to stay home (natural disasters and war), but fear alone should not delay the presentation of a patient with abdominal pain to the hospital. With well-organized systems and cooperation between governing and health sectors, hospitalization can be streamlined and physician/hospital workforce exposure can be minimized, while not compromising the level of care and treatment needed for acute surgical pathologies. Through understanding the surgical needs of a population during a catastrophic event, the public health sector may more rapidly and precisely distribute needed personnel, essential resources, and allocate certain medical centers as referral points for surgical emergencies.
Acknowledgment
The authors would like to thank Alexa Nicole Cárdenas Quiodettis for her spectacular editorial skills.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lisi G, Campanelli M, Spoletini D, Carlini M. The possible impact of COVID‐19 on colorectal surgery in Italy. Colorectal Dis. 2020;22:641-642. [DOI] [PubMed] [Google Scholar]
- 2.Diaz A, Sarac BA, Schoenbrunner AR, Janis JE, Pawlik TM. Elective surgery in the time of COVID-19. Am J Surg. 2020;219:900-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Personal Correspondence on May 5th, 2020, with the Chief of the Department of Surgery at Stanto Tomas Hospital. Panama City, Panama: Dra. Martha Quiodettis; 2020. [Google Scholar]
- 4.Iwasaki A, Grubaugh ND. Why does Japan have so few cases of COVID‐19? EMBO Mol Med. 2020;12(5):e12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensvik L, Skans O. COVID-19 Crisis Response Monitoring. Sweden: IZA Country Report; 2020. [Google Scholar]
- 6.The Israeli Ministry of Health information page on COVID-19. https://www.health.gov.il/Subjects/disease/corona/Pages/default.aspx.
- 7.Lancaster EM, Sosa JA, Sammann A, et al. Rapid response of an academic surgical department to the COVID-19 pandemic: Implications for patients, surgeons, and the community. J Am Coll Surg. 2020;230:1064-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eastman AL, Rinnert KJ, Nemeth IR, Fowler RL, Minei JP. Alternate site surge capacity in times of public health disaster maintains trauma center and emergency department integrity: Hurricane Katrina. J Trauma Inj Infect Crit Care. 2007;63(2):253-257. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T, Bosse M. Use of an innovative design mobile hospital in the medical response to Hurricane Katrina. Ann Emerg Med. 2007;49(5):580-588. [DOI] [PubMed] [Google Scholar]
- 10.Bundu I, Patel A, Mansaray A, Kamara TB, Hunt LM. Surgery in the time of Ebola: How events impacted on a single surgical institution in Sierra Leone. J Roy Army Med Corps. 2016;162(3):212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadary A, Schecter W, Embon OM, Einav S. Impact of military conflict on a civilian receiving hospital in a war zone. Ann Surg. 2009;249(3):502-509. [DOI] [PubMed] [Google Scholar]
- 12.https://hospitals.clalit.co.il/rabin/he/news/news_2020/Pages/corona_english.aspx.
- 13.Peleg K, Kellermann AL. Enhancing hospital surge capacity for mass casualty events. J Am Med Assoc. 2009;302(5):565-567. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz D, Glassberg E, Nadler R, Hirschhorn G, Marom OC, Aharonson-Daniel L. Injury patterns of soldiers in the second Lebanon war. J Trauma Acute Care Surg. 2014;76(1):160-166. [DOI] [PubMed] [Google Scholar]
- 15.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049-2055. [DOI] [PubMed] [Google Scholar]
- 16.Mack D, Rust GS, Baltrus P, et al. Using appendiceal perforation rates to measure impact of a disaster on healthcare system effectiveness. South Med J. 2013;106(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13(10):818-829. [PubMed] [Google Scholar]
- 18.Clavien PA, Barkun J, De Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal R, Hoffmann H, Clavien P-A, Bucher HC, Dell-Kuster S. Definition and classification of intraoperative complications (CLASSIC): Delphi study and pilot evaluation. World J Surg. 2015;39(7):1663-1671. [DOI] [PubMed] [Google Scholar]
- 20.Myrstad M, Ihle-Hansen H, Tveita AA, et al. National Early Warning Score 2 (NEWS2) on admission predicts severe disease and in-hospital mortality from Covid-19-A prospective cohort study. Scand J Trauma Resusc Emerg Med. 2020;28:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong Laura E, Hawkins Jessica E, Murrell Karen L. Where are all the Patients? Addressing Covid-19 Fear to Encourage Sick Patients to Seek Emergency Care. Massachusetts: NEJM Catalyst Innovations in Care Delivery; 2020. [Google Scholar]
- 22.Ichikawa M, Nakahara S, Wakai S, Hong-Jen C, Huang N. Lowered tuberculosis notifications and deterred health care seeking during the sars epidemic in Hong Kong. Am J Publ Health. 2005;95(6):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westgard BC, Morgan MW, Vazquez-Benitez G, Erickson LO, Zwank MD. An analysis of changes in emergency department visits after a state declaration during the time of COVID-19. Ann Emerg Med. 2020;76:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4(5):e10-e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soffer D, Klausner JM. Trauma system configurations in other countries: The Israeli model. Surg Clin. 2012;92(4):1025-1040. [DOI] [PubMed] [Google Scholar]
- 26.Poteat T, Millett G, Nelson LE, Beyrer C. Understanding COVID-19 risks and vulnerabilities among Black communities in America: The lethal force of syndemics. Ann Epidemiol. 2020;47:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itelman E, Wasserstrum Y, Segev A, et al. Clinical characterization of 162 COVID-19 patients in Israel: Preliminary report from a large tertiary center. Isr Med Assoc J. 2020;22(5):271. [PubMed] [Google Scholar]
- 28.Rhodes JM, Subramanian S, Laird E, Kenny RA. Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51(12):1434-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantica G, Riccardi N, Terrone C, Gratarola A. Non-COVID-19 visits to emergency departments during the pandemic: The impact of fear. Publ Health. 2020;183:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID-19 patients with mild disease severity: Clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80:e14-e18. [DOI] [PMC free article] [PubMed] [Google Scholar]