Abstract
In this study, a rapid method for the detection of Central and West Africa clades of Monkeypox virus (MPXV) using recombinase polymerase amplification (RPA) assay targeting the G2R gene was developed. MPXV, an Orthopoxvirus, is a zoonotic dsDNA virus, which is listed as a biothreat agent. RPA was operated at a single constant temperature of 42°C and produced results within 3 to 10 minutes. The MPXV-RPA-assay was highly sensitive with a limit of detection of 16 DNA molecules/μl. The clinical performance of the MPXV-RPA-assay was tested using 47 sera and whole blood samples from humans collected during the recent MPXV outbreak in Nigeria as well as 48 plasma samples from monkeys some of which were experimentally infected with MPXV. The specificity of the MPXV-RPA-assay was 100% (50/50), while the sensitivity was 95% (43/45). This new MPXV-RPA-assay is fast and can be easily utilised at low resource settings using a solar powered mobile suitcase laboratory.
Keywords: Recombinase polymerase amplification assay, Monkeypox Virus, mobile suitcase, point of need, rapid detection system
1. Introduction
Monkeypox virus (MPXV) belongs to the genus Orthopoxvirus (OPXV, subfamily Chordopoxvirinae, family Poxviridae), which is an enveloped double stranded DNA virus (Parker et al., 2007). It is subdivided into two clades: the West African and the Congo Basin clades. The latter is more pathogenic (Likos et al., 2005) and the clinical signs of MPXV infections are similar to that of smallpox but in a milder form and with lower mortality (1 to 10%). The majority of deaths occurs at a young age due to the lack of immunization (Khodakevich et al., 1988). Rodents (Squirrels and Gambian rats) are the primary hosts (Falendysz et al., 2015, Falendysz et al., 2017), which can transmit the virus to monkeys and humans through direct contact with blood and bodily fluids (Nolen et al., 2015). The handling and consumption of infected monkeys and squirrels were documented as major infection sources in Africa (Cantlay et al., 2017). Furthermore, human-to-human transmission can occur through exposure to fomites and air droplets (Fleischauer et al., 2005). A specific vaccine for use in humans is not available, but cross protection in humans vaccinated against smallpox has been documented (Rimoin et al., 2010). This protection however, has been waning because when smallpox was declared eradicated in 1980, nationwide vaccination against smallpox has stopped (Breman and Henderson, 2002). The antiviral tecovirimat for treatment of accidental smallpox infections has been shown to reduce symptoms and to improve survival of MPXV infected macaques if applied up to 5 days post infection (Russo et al., 2018).
Human MPXV infections are endemic in West and Central Africa (McCollum and Damon, 2014). The first MPXV outbreak outside Africa was reported in 2003 in the USA after the shipment of animals from Ghana (Di Giulio and Eckburg, 2004). The latest outbreak was in Nigeria with 113 laboratory confirmed cases and seven deaths from September 2017 until August 2018 (Yinka-Ogunleye et al., 2018). Two recent zoonotic MPVX infections imported in the UK highlight ongoing MPXV activity in Nigeria (Vaughan et al., 2018).
Several diagnostic methods for the detection of MPXV are established with real-time PCR as the gold standard because of its high sensitivity and specificity (Li et al., 2006). To use this diagnostic tool, a highly equipped laboratory and specialized technicians are needed, which are not available in areas where MPXV infections occur. Therefore, an easy to handle simple molecular diagnostic method would improve the detection and surveillance of MPXV. Isothermal amplification methods have been proven to be an alternative to real-time PCR. Recombinase polymerase amplification (RPA) is one of these methods, in which an enzymatic based DNA amplification can be achieved at a temperature range of 37 to 42°C within 15 minutes (Piepenburg et al., 2006). The amplification is initiated by a primer-recombinase-complex. This complex invades the DNA double strand at the homologues sequences of the primer, where single-strand-binding proteins stabilize the reaction. Then, a strand-displacing polymerase DNA conducts the extension step. For real-time detection, a fluorophore/quencher-probe is used. Since RPA reagents are freeze-dried, the RPA kit can be stored at room temperature for several months. This allows the use of the RPA assay at point of need making them even more versatile through a mobile suitcase laboratory (Abd El Wahed et al., 2015a).
In this study, we have developed a rapid detection method specific for both clades of MPXV using a recombinase polymerase amplification (RPA) assay targeting the tumor necrosis factor (TNF) binding protein gene, which is present in duplicate as ORF G2L and G2R in the inverted terminal repeats of the MPXV genome.
2. Materials and Methods
2.1. Molecular MPXV DNA Standard and RPA Oligonucleotide
For assay validation, a molecular DNA standard based on 300 bp of the TNF binding protein gene (ORF: G2R, Accession number: DQ011153, nucleotides: 195915 - 196964), was synthesized by GeneArt (Regensburg, Germany). Three forward primers (FP), three reverse primers (RP) and one exo-probe were designed (Fig. S1). All oligos were synthesized by TIB MOLBIOL GmbH (Berlin, Germany).
2.2. RPA Assay Conditions
The TwistAmp exo kit (TwistDx Ltd, Cambridge, UK) was used. Per reaction, 29.5 μl rehydration buffer, 10.7 μl H2O, 2.1 μl of each primer (10 μM) and 0.6 μl of 10 μM exo-probe were added into the lid of the reaction tube containing the freeze-dried pellet. After adding 2.5 μl of 280 mM magnesium acetate and 1 μl template, the reaction mixture was centrifuged, mixed, centrifuged and placed immediately into the tube scanner ESEQuant (QIAGEN Lake Constance GmbH, Stockach, Germany). The reaction was incubated at 42°C for 15 minutes. To increase the sensitivity, a mixing and centrifugation step was performed after 230 seconds of starting the measurement. A positive result was measured by the FAM channel of the ESEQuant tube scanner and analysed with the Tubescanner studio software (version 2.07.06, QIAGEN Lake Constance GmbH, Stockach, Germany).
2.3. MPXV RPA Assay Analytical Sensitivity
In total, nine primer combinations were tested with the MPXV DNA standard with concentration of 105 DNA molecules/μl. The best combination, which produced the earliest and highest fluorescence signal, was selected for further assay validation. The ability of the selected primer combination to amplify 104 to 1 DNA molecules/μl of the MPXV standard DNA was checked in order to test the analytical sensitivity and to determine the limit of detection.
2.4. MPXV RPA assay cross reactivity
The specificity of the MPXV-RPA-assay was tested with DNA of viruses of the two MPXV clades, six other poxviruses and other pathogens of clinical importance, see Table 1 .
Table 1.
Reactivity of the MPXV_RPA assay to the genome of poxviruses and other pathogens. MPXV_RPA assay detected both clades of MPXV, but not other poxviruses and pathogens.
| Pathogen | Clade/ Source | Concentration [ng/μl] |
RPA | Real-time PCR |
|---|---|---|---|---|
| Monkeypox | Central Africa | + | + | |
| Monkeypox | West Africa | + | + | |
| Vaccinia | Elstree | 7.6 | - | - |
| Cowpox | 2 | 3.6 | - | - |
| Camelpox | - | 18 | - | - |
| Sheeppox | Russia | 4.6 | - | - |
| Goatpox | India | 3.1 | - | - |
| Orf | Burghessler | 3 | - | - |
| Calpox virus | - | 6.1 | - | - |
| Herpes-simplex-Virus 1 | Quality Control for Molecular Diagnostics (QCMD) | 1.7 | - | - |
| Herpes-simplex-Virus 2 | 3.6 | - | - | |
| Varicella-zoster Virus | 3.1 | - | - | |
| Staphylococcus aureus | DSMZ ID: 1104 | 4.2 | - | - |
| Clostridium perfringes | DSMZ ID: 756 | 40.2 | - | - |
| Enterococcus faecialis | DSMZ ID: 20478 | 35.2 | - | - |
| Plasmodium falciparum | University of Ibadan, Nigeria | 2.8 | - | - |
| Rickettsia rickettsia | BNITM Hamburg, Germany | 4.7 | - | - |
| Rickettsia africae | 4.3 | - | - |
2.5. Clinical samples
The MPXV-RPA-assay performance was validated with plasma samples of infected (n=25) and uninfected (n=23) monkeys. The animals were looked after by experienced personnel from the German Primate Center and kept according to the German Animal Welfare Act, which is in compliance with the European Union Guidelines on the use of non-human primates for biological research and the Weatherall report. Sampling from MPXV-infected monkeys was approved by the Lower Saxony State Office of Consumer Production and Food Safety with the project license 33.9.42502-04/019/07, that from uninfected animals with the project license 33.9.42502-04-15/1769. In addition, 20 positive (4 whole blood, 16 serum) and 27 negative (8 whole blood, 19 serum) human samples from the recent MPXV outbreak in Nigeria (November 2017) were tested with the RPA-MPXV-assay. The samples were collected for diagnostics purposes and handled anonymously. The DNA from these samples was isolated using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer instructions.
2.6. Real-time PCR
For comparison, the molecular DNA standards as well as clinical samples were tested with a reference MPXV real-time PCR assay targeting the same gene region of the developed RPA assay (Li et al., 2010). The G2R-G real-time PCR assay detects both MPXV clades and the real-time PCR reaction was performed as described previously (Kissenkotter et al., 2018) using the LightCycler DNA-Master HybProbe kit and the LightCycler 480 (Roche Mannheim, Germany).
2.7. Statistical Analysis
The limit of detection of the MPXV-RPA-assay was calculated by performing a probit regression analysis on the data set of eight RPA assay using STATISTICA software (StatSoft, Hamburg, Germany) in order to determine the number of DNA molecules/μl, which were detected in 95% of the cases. Furthermore, the detection time was calculated by performing a semi-logarithmic regression on the same data set with GraphPad PRISM 7 software (GraphPad Software Inc., San Diego, California).
3. Result
3.1. Selection of RPA Primers and Probe
In order to select sensitive RPA oligonucleotides, all possible primer combinations were tested using a MPXV DNA molecular standard at a concentration of 105 DNA molecules/μl. As a result, the primer combination FP3 + RP3 (Table 2) produced the best amplification curves (Fig. S1) and was selected for further assay validation.
Table 2.
RPA primers and exo-probe combination, yielding the earliest and highest signal in the MPXV RPA assay. QTF are sites of the quencher and fluorophore in the following order BHQ1-dt (Q), Tetrahydrofuran (T) and Fam-dT (F).
| Name | Sequence (5´ to 3´) |
|---|---|
| MPXV RPA P1 | ACAGAAGCCGTAATCTATGTTGTCTATCGQTFCCTCCGGGAACTTA |
| MPXV RPA FP3 | AATAAACGGAAGAGATATAGCACCACATGCAC |
| MPXV RPA RP3 | GTGAGATGTAAAGGTATCCGAACCACACG |
3.2. Analytical Sensitivity and Specificity
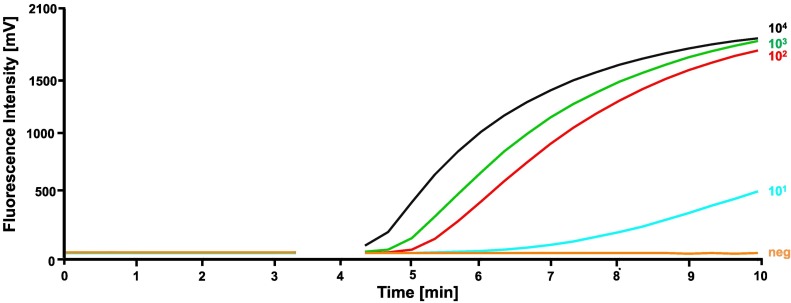
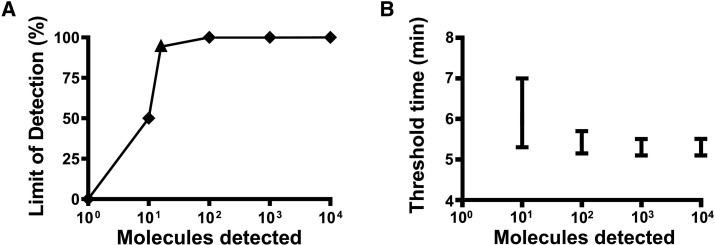
To determine the analytical sensitivity, the performance of the best primer combination FP3 and RP3 was evaluated with a tenfold dilution range of the MPXV DNA standard (104 to 1 DNA molecules/μl, Fig. 1 ) in eight replicates. The MPXV-RPA-assay detected the molecular MPXV DNA standard with the concentration from 104 to 102 molecules/μl in all eight RPA runs and the concentration of 101 molecules/μl in four runs, while no amplification was observed in the tube containing one molecule/μl. With this data set, a probit regression analysis was performed and revealed a detection limit of 16 DNA molecules/μl in 95% of the cases (Fig. 2 ). Seven minutes is the maximum time needed to amplify as low as 10 DNA molecules by the MPXV RPA assay (Fig. 3 ). FP3 and RP3 primers were able to amplify the two clades of MPXV but did not detect high concentration DNA of related poxviruses or other pathogens (Table 1).
Fig. 1.
Analytical sensitivity of the MPXV-RPA-assay tested with a tenfold dilution of the molecular DNA standard (104 – 100 DNA molecules/μl). The primer combination FP3 + RP3 detected the concentration 104 – 101 DNA molecules/μl. After 230 seconds a mixing step was performed.
Fig. 2.
Probit regression analysis of the dataset of the eight repetitions of the analytical sensitivity test of the MPXV-RPA-assay for the determination of the detection limit (A) and semi-logarithmic regression of the detection time (B). Performing the probit regression analysis on the dataset revealed a detection limit of 16 DNA molecules/μl in 95% of the cases (A). Using Prism Software, a semi-logarithmic regression of the data from the eight runs on a dilution range of the molecular DNA standard (104-100 DNA molecules/reaction) were performed. The lowest concentration of 101 DNA molecules/μl was detected after a maximum of seven minutes (B).
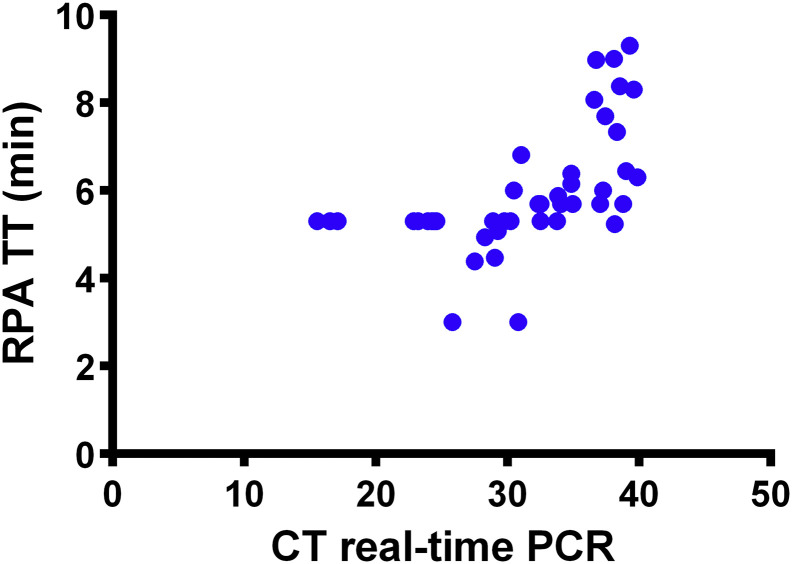
Fig. 3.
Screening of 45 blood, plasma or serum samples from MXPV infected macaques and humans by real-time PCR and RPA assays. Linear regression analysis of real-time RT-PCR cycle threshold values (Ct) and RPA threshold time in minutes (TT) were determined. No correlation was found between TT and Ct values since the RPA is much faster than the real-time PCR. Diagnostic sensitivity of real-time PCR assay was 100%, while that of MPXV-RPA-assay was 95 %(43/45).
3.3. Clinical Samples
All collected samples were screened in parallel with both real-time PCR and the RPA assays. Employing the real-time PCR assay, all 45 samples tested positive, while by the RPA assay 43/45 were identified as positive. Fifty samples (23 monkey plasma and 27 human serum and whole blood samples) were negative in both methods. With this data, the clinical specificity and sensitivity of the MPXV-RPA-assay could be calculated as 100 and 95%, respectively.
4. Discussion
Infection with MPXV occurs in West Africa and the Congo Basin (McCollum and Damon, 2014). The most affected regions suffer from limited resources, infrastructure and diagnostic capacities, beside insufficient accessibility to remote and conflict areas. Thus, identification of MPXV infected cases is difficult (Thomassen et al., 2013). Therefore, a simple point of need diagnostic test is crucial in order to limit the spread of MPXV and control the outbreaks.
Applying the MPXV-RPA-assay both the West Africa and the Congo Basin clade were detected within seven minutes with a detection limit of 16 DNA molecules/μl. The RPA oligonucleotides target the TNF receptor gene as no mismatch between both MPXV clades was identified and thus cover the currently known diversity of MPXV, while between 13-31 mismatches were identified when this sequence was compared to those of other poxviruses (Fig. S3). The number of mismatches between the targeted MPXV gene sequence and the sequences of closely related poxviruses was the key to a specific RPA assay. Two samples were negative in the RPA assay but weakly positive in real-time PCR (CT: 38.8 and 39.97). Eight samples with CT values around 38-39 and eight samples with CT values 35-37 were scored positive in the RPA. All these samples had low DNA levels and lack of positive scoring of two samples in the RPA lay within the probability of missing weak positives as shown by the probit anaylsis.
Real-time PCR assays for MPXV detection need at least 90 minutes and highly sophisticated thermal cycler (Li et al., 2010). Although freeze-dried PCR reagents are slowly becoming available (Babonneau et al., 2015), they are as yet not in widespread use, whereas the RPA kits per se are freeze-dried and stable under different environmental conditions including temperatures above 30°C (Abd El Wahed et al., 2013). This is a huge advantage in areas where highly equipped laboratories are not available. When comparing the performance of the MPXV-RPA-assay with the real-time PCR assay on clinical samples with linear regression analysis, no correlation was found between TT and Ct values since the RPA is much faster than the real-time PCR (Fig. 3). One reason for this observation for several RPA assays (Abd El Wahed et al., 2013, Abd El Wahed et al., 2015b, Patel et al., 2016) is that the RPA reaction is optimized for maximal enzymatic activity at one temperature leading to very dynamic non linear amplification (Piepenburg et al., 2006), whereas the real-time PCR reaction depends on different temperature steps for denaturation, annealing and amplification yielding a close to exponential amplification (Deepak et al., 2007).
Another isothermal amplification assay based on loop-mediated isothermal amplification for the detection of MPXV is available (Iizuka et al., 2009). This assay has a clinical sensitivity of 72%. However, our MPXV-RPA-assay proved to be more sensitive (95 % sensitivity). The LAMP MPXV assay requires 6 primers to amplify the MPXV DNA in around 60 minutes, while RPA uses two primers and one probe producing a result within 15 minutes.
The MPXV-RPA-assay appears an appropriate assay for the point of need detection of active MPXV cases as RPA is fast, highly sensitive and specific as well as utilizing cold-chain independent reagents.
The following are the supplementary data related to this article.
MPXV_RPA _assays amplicon as well as primer and probe sequences. MPXV-RPA-assay oligonucleotides were placed at nucleotides 195962-196146; Genbank accession number: DQ011153. Three forward and three reverse primers as well as one exo-probe were screened to select the combination with higher RPA analytical sensitivity. RC: reverse complementary sequence.
Testing of all possible primer combination of the MPXV-RPA-assay. All nine primer combination were tested with the molecular DNA standard with a concentration of 105 DNA molecules/μl. A mixing step was conducted after 230 sec. The combination FP3 + RP3 showed the earliest and highest fluorescence signal and was therefore chosen for further assay validation.
Alignment of the MPXV-RPA-assays amplicon with the Congo Basin clade and other Chordopoxvirinae of interest. Using Geneious (Version: 11.1.2, Biomatters Limited, New Zealand), the target sequence of the G2R gene of the monkeypox West African virus (Genebank accession number: DQ011153, nucleotides: 195962 – 1969143) was compared with monkeypox Congo Basin virus (accession number: NC_003310, nt: 194120 – 194301), variola virus (accession number: NC_001611, nt: 182618 - 182749), vaccinia virus (accession number: NC_006998, nt: 189299 – 189472), camelpox virus (accession number: NC_003391, nt: 201497 – 201678), cowpox virus (accession number: NC_003663, nt: 219885 – 220071), sheeppox virus (accession number: NC_004002, nt: 112967 – 113171) and goatpox virus (accession number: NC_004003, nt: 112695 – 112892). Between 1 to 31 mismatches could be identified in the primers and probe sequences.
References
- Abd El Wahed A., El-Deeb A., El-Tholoth M., Abd El Kader H., Ahmed A., Hassan S., et al. A Portable Reverse Transcription Recombinase Polymerase Amplification Assay for Rapid Detection of Foot-and-Mouth Disease Virus. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El Wahed A., Weidmann M., Hufert F.T. Diagnostics-in-a-Suitcase: Development of a portable and rapid assay for the detection of the emerging avian influenza A (H7N9) virus. J Clin Virol. 2015;69:16–21. doi: 10.1016/j.jcv.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El Wahed A., Patel P., Faye O., Thaloengsok S., Heidenreich D., Matangkasombut P., et al. Recombinase Polymerase Amplification Assay for Rapid Diagnostics of Dengue Infection. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babonneau J., Bernard C., Marion E., Chauty A., Kempf M., Robert R., et al. Development of a dry-reagent-based qPCR to facilitate the diagnosis of Mycobacterium ulcerans infection in endemic countries. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman J.G., Henderson D.A. Diagnosis and management of smallpox. N Engl J Med. 2002;346:1300–1308. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- Cantlay J.C., Ingram D.J., Meredith A.L. A Review of Zoonotic Infection Risks Associated with the Wild Meat Trade in Malaysia. EcoHealth. 2017;14:361–388. doi: 10.1007/s10393-017-1229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak S., Kottapalli K., Rakwal R., Oros G., Rangappa K., Iwahashi H., et al. Real-Time PCR: Revolutionizing Detection and Expression Analysis of Genes. Curr Genomics. 2007;8:234–251. doi: 10.2174/138920207781386960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falendysz E.A., Lopera J.G., Lorenzsonn F., Salzer J.S., Hutson C.L., Doty J., et al. Further Assessment of Monkeypox Virus Infection in Gambian Pouched Rats (Cricetomys gambianus) Using In Vivo Bioluminescent Imaging. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falendysz E.A., Lopera J.G., Doty J.B., Nakazawa Y., Crill C., Lorenzsonn F., et al. Characterization of Monkeypox virus infection in African rope squirrels (Funisciurus sp.) PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischauer A.T., Kile J.C., Davidson M., Fischer M., Karem K.L., Teclaw R., et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40:689–694. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- Iizuka I., Saijo M., Shiota T., Ami Y., Suzaki Y., Nagata N., et al. Loop-Mediated Isothermal Amplification-Based Diagnostic Assay for Monkeypox Virus Infections. J Med Virol. 2009;81:1102–1108. doi: 10.1002/jmv.21494. [DOI] [PubMed] [Google Scholar]
- Khodakevich L., Jezek Z., Messinger D. Monkeypox Virus - Ecology and Public-Health Significance. Bull World Health Organ. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- Kissenkotter J., Hansen S., Bohlken-Fascher S., Ademowo O.G., Oyinloye O.E., Bakarey A.S., et al. Development of a pan-rickettsial molecular diagnostic test based on recombinase polymerase amplification assay. Anal Biochem. 2018;544:29–33. doi: 10.1016/j.ab.2017.12.018. [DOI] [PubMed] [Google Scholar]
- Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- McCollum A.M., Damon I.K. Human Monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- Nolen L.D., Osadebe L., Katomba J., Likofata J., Mukadi D., Monroe B., et al. Introduction of Monkeypox into a Community and Household: Risk Factors and Zoonotic Reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93:410–415. doi: 10.4269/ajtmh.15-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S., Nuara A., Buller R.M., Schultz D.A. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- Patel P., Abd El Wahed A., Faye O., Pruger P., Kaiser M., Thaloengsok S., et al. A Field-Deployable Reverse Transcription Recombinase Polymerase Amplification Assay for Rapid Detection of the Chikungunya Virus. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. DNA detection using recombination proteins. PLoS Biol. 2006;4:1115–1121. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd-Smith J.O., Kisalu N.K., Kinkela T.L., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A.T., Grosenbach D.W., Brasel T.L., Baker R.O., Cawthon A.G., Reynolds E., et al. Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques. J Infect Dis. 2018;218:1490–1499. doi: 10.1093/infdis/jiy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen H.A., Fuller T., Asefi-Najafabady S., Shiplacoff J.A.G., Mulembakani P.M., Blumberg S., et al. Pathogen-Host Associations and Predicted Range Shifts of Human Monkeypox in Response to Climate Change in Central Africa. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al. Two cases of monkeypox imported to the United Kingdom. September 2018 Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinka-Ogunleye A., Aruna O., Ogoina D., Aworabhi N., Eteng W., Badaru S., et al. Reemergence of Human Monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MPXV_RPA _assays amplicon as well as primer and probe sequences. MPXV-RPA-assay oligonucleotides were placed at nucleotides 195962-196146; Genbank accession number: DQ011153. Three forward and three reverse primers as well as one exo-probe were screened to select the combination with higher RPA analytical sensitivity. RC: reverse complementary sequence.
Testing of all possible primer combination of the MPXV-RPA-assay. All nine primer combination were tested with the molecular DNA standard with a concentration of 105 DNA molecules/μl. A mixing step was conducted after 230 sec. The combination FP3 + RP3 showed the earliest and highest fluorescence signal and was therefore chosen for further assay validation.
Alignment of the MPXV-RPA-assays amplicon with the Congo Basin clade and other Chordopoxvirinae of interest. Using Geneious (Version: 11.1.2, Biomatters Limited, New Zealand), the target sequence of the G2R gene of the monkeypox West African virus (Genebank accession number: DQ011153, nucleotides: 195962 – 1969143) was compared with monkeypox Congo Basin virus (accession number: NC_003310, nt: 194120 – 194301), variola virus (accession number: NC_001611, nt: 182618 - 182749), vaccinia virus (accession number: NC_006998, nt: 189299 – 189472), camelpox virus (accession number: NC_003391, nt: 201497 – 201678), cowpox virus (accession number: NC_003663, nt: 219885 – 220071), sheeppox virus (accession number: NC_004002, nt: 112967 – 113171) and goatpox virus (accession number: NC_004003, nt: 112695 – 112892). Between 1 to 31 mismatches could be identified in the primers and probe sequences.