Abstract
Background:
Breast cancer is the most common cancer and the leading cause of death among Latinas in the United States. The Multi-level Intervention to Increase Participation in Mammography Screening study (¡Fortaleza Latina!) is a partnership among research institutions, a Latino-serving community-based primary care clinic organization, and a cancer treatment center. The study will assess the efficacy of a clinic- and patient-level program to increase breast cancer screening among Latinas in Western Washington.
Methods/design:
The intervention is a multi-level breast cancer screening program in four participating primary care clinics. The study is a parallel randomized controlled trial of 600 Latino women aged 42–74 who are non-compliant with breast cancer screening guidelines. Participants will be randomized within clinic using block randomization to: (1) a control arm (usual care); and (2) a theory-based counseling program consisting of a ‘promotora’ or community health worker-led home-based intervention to encourage breast cancer screening. At the clinic-level, two clinics will offer additional mammography services provided by a mobile mammography unit operated by the Seattle Cancer Care Alliance. The primary endpoint is the rate of mammography uptake over the 1-year follow-up period.
Discussion:
This multi-level intervention aims to raise rates of participation in breast cancer screening among Latino women. If effective, the program may improve rates of early detection of breast cancer in Latino women.
Keywords: Breast cancer, Mammogram, Latino women, Hispanic women, Cancer disparities, Multi-level intervention
1. Introduction
Latinas in the United States have a high age-adjusted incidence of breast cancer compared to non-Latina white women. In 2012, there were an estimated 17,100 new cases and 2400 deaths from breast cancer among Latinas [1]. In 2012, breast cancer surpassed heart disease to become the leading cause of death among Latinas in the United States [1].The disproportionate burden of breast cancer among Latinas is reflected in a higher proportion of incident cases detected in advanced stages; data from the Surveillance, Epidemiology and End Results program from 2012 show that 42% of incident breast cancer cases in Latinas were detected in regional or distant stages, compared to 35% in non-Latina whites [1]. The advanced stage of disease detection is thought to be attributable in part to both higher occurrence of poor prognosis subtypes of breast cancer and lower rates of screening mammography. Data from the National Health Interview Survey from 2010 show that 69% of Latino women ages 50–74 had had a mammogram in the past two years compared with 74% of non-Latino white women [2].
Socio-demographic factors associated with non-adherence to breast cancer screening in this population include low income, lack of health insurance, limited access to healthcare services, lack of healthcare provider recommendation, length of residency in the United States, limited English language proficiency, level of acculturation, and lack of awareness of risks associated with non-participation in breast cancer screening programs [3–5].
The aims of the Multi-level Intervention to Increase Participation in Mammography Screening study (¡Fortaleza Latina!) are threefold: (1) to determine the effects of a patient-level intervention (high-intensity, culturally appropriate promotora-based intervention) and a clinic-level intervention (low-cost mammography services) on mammogram utilization, vs. control, in a sample of Latino women in Western Washington (WA) State; (2) to assess the cost effectiveness of the intervention program; and, secondarily, (3) to assess the influence of neighborhood-level characteristics on the program effect.
The study will be implemented with the aid of the two community based partners, Sea Mar Community Health Centers, which operates a network of 22 federally sponsored health clinics in Western Washington specializing in the delivery of primary care services to low-income Latinos; and the Seattle Cancer Care Alliance, which provides comprehensive cancer care and will provide a mobile mammography unit for women to undergo mammography screening, as well as specialty care for women who receive an abnormal mammogram result. (See Fig. 1.)
Fig. 1.
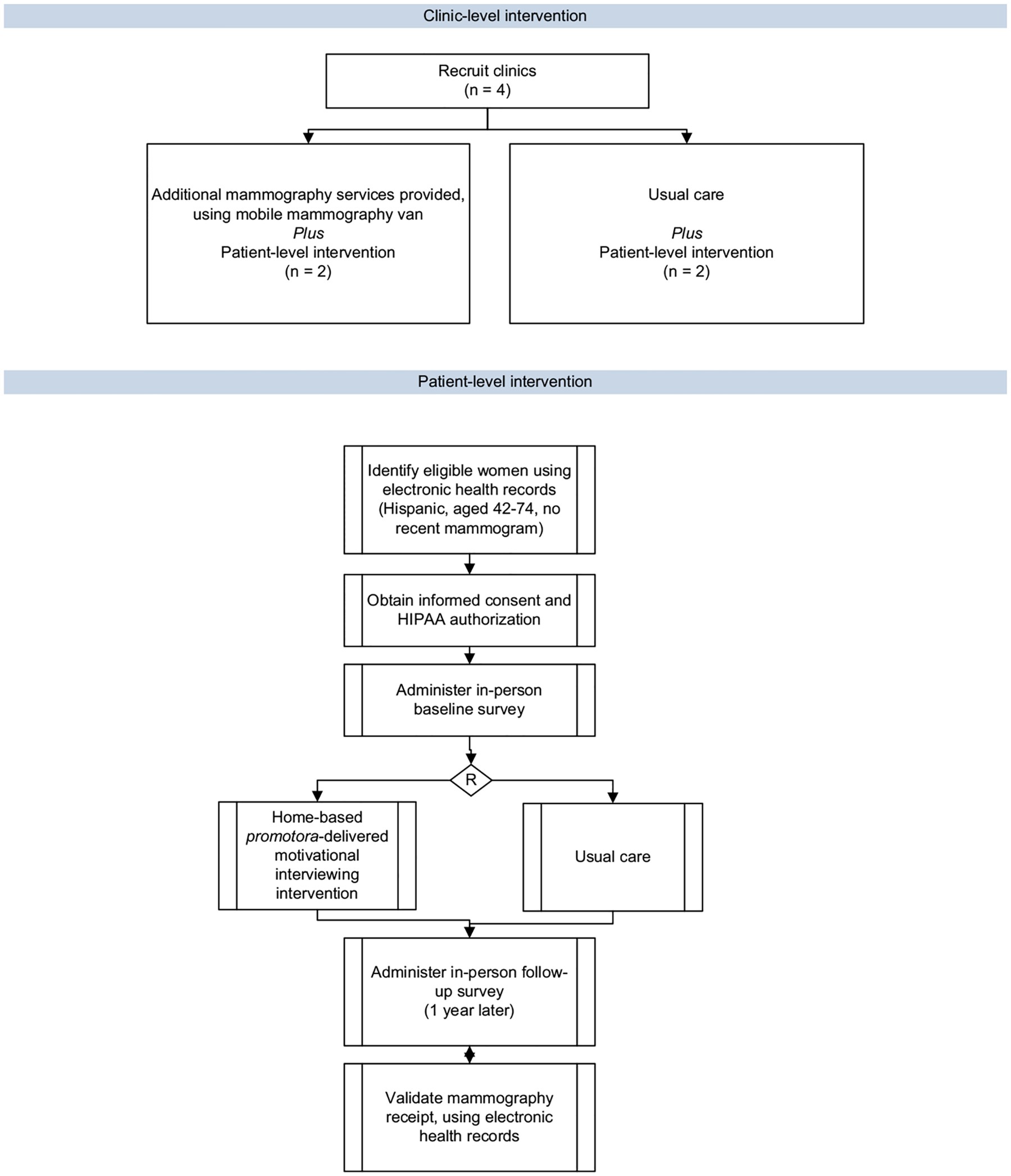
Fortaleza Latina study design.
2. Methods/design
2.1. Setting
In Washington State, the Latino population represents 12% of the total population. Latinos are the fastest growing population in the state, having increased by 71% (or 314,281 individuals) between the 2000 and 2010 census [6]. The four participating clinics are located in King (2 clinics), Snohomish, and Skagit counties, where Census estimates from 2010 show Latinos representing 9%, 10% and 34% of the total county population, respectively.
2.2. Participants
The study aims to recruit 600 Latinas who have been seen by a community health partner clinic (Sea Mar Community Health Centers) within the past 5 years, and who are out of compliance with current recommendations for mammography screening (i.e., have not had a mammogram within the previous two years). Eligible women will be between 42 and 74 years of age. While US Preventive Services Task Force recommendations call for informed decision-making with a healthcare provider for women aged 40–49 and biennial mammography beginning at age 50, we opted to include women aged 42–49 for two reasons: first, the Breast, Cervical, and Colon Health Program in King County, the program that serves many Sea Mar patients, provides reimbursements for screening among average risk women beginning at age 40. Second, the Preventive Health Mandate of the Affordable Care Act requires that all health insurance plans cover mammography screening beginning at age 40 at no cost sharing. Participants may be English or Spanish speakers.
2.3. Recruitment of participants
We will use recruitment strategies that were successfully used in our other studies involving community based clinics [7]. We will use computerized records to identify Latinas who are eligible, who have been seen in any of the four participating Sea Mar clinics in the past five years, and who are non-compliant with breast cancer screening recommendations. Sea Mar staff will invite eligible women to participate in the study in person. Interested women will be told that they will be randomized to one of two arms in the project. The women will be assured that refusal to participate will not have any implications on their ability to receive care at the clinic, asked to sign an informed consent to participate in the project and to sign a HIPAA authorization for release of medical record information for the purposes of verifying their use of breast cancer screening. Once consent is obtained, a study staff member will telephone or go to the home of the participant to complete a baseline survey. The baseline questionnaire includes items on breast-cancer-screening knowledge and attitudes as well as intention to be screened, acculturation, neighborhood characteristics, and socio-demographic variables.
2.4. Randomization
Participants will be allocated, within clinic, to the intervention or control arms via a computerized program, using randomized blocks to ensure equal distribution in age groups [40–49 and 50–74] across study arms. The randomization sequence has been generated by a statistician who is not involved with the implementation of the study. Medical record reviewers will be blinded to the randomization status of the participants.
2.5. Interventions
The control arm is usual care and the intervention arm is promotora outreach, as described below. Our intervention design and analysis were guided by the Health Disparities Framework developed by Warneke et al. [8].
2.6. Control arm
Participants allocated to the control arm (‘Usual Care’) will not receive any motivational messages or intervention materials from study staff. Usual care includes any information on the importance of regular mammograms for breast cancer early detection that is provided routinely by clinic staff to all women at community clinics.
2.7. Patient-level intervention (promotora outreach)
Before developing our culturally-appropriate promotora program, we gathered formative data from patients and providers [9,10]. These data were used to design our theory-based patient-level intervention; that is, an in-person promotora-based intervention that used motivational interviewing techniques. Promotoras are identified leaders in their communities and often serve as liaisons between their community, health professionals, and human and social service organizations for community-based health promotion efforts [11]. Because promotoras are from the communities they serve, they understand local social networks and are able to incorporate cultural strategies to promote health.
We will select promotoras from the community clinic partner organizations to work collaboratively with clinic staff and ensure sustainability of the program. Patients randomized to the intervention will receive a home visit from the promotora who will engage them in a discussion about breast-cancer prevention using principles of motivational interviewing. Motivational interviewing is an approach offered in various clinical settings, and has been found to be successful in interventions among Latinas [12,13]. Motivational interviewing is a patient-centered counseling approach that is considered culturally responsive because it allows counselors to incorporate into discussion issues related to social context. Motivational interviewing relies on four primary skills: 1) the ability to ask open ended questions, 2) the ability to provide affirmations, 3) the capacity for reflective listening, and 4) the ability to periodically provide summary statements to the patient [14]. Two weeks after the home visit, the promotora will make a telephone call to the woman to review any planned action steps, and assess readiness to schedule a mammogram.
Promotoras will be recruited from the community by Sea Mar Community Health Centers and will be provided with an initial training session lasting three days that will address procedures for approaching households and delivering the intervention, breast cancer screening facts, and tracking and documentation procedures. Other studies indicate promotora outreach to be a successful approach for Latinas [15,16].
2.8. Clinic-level intervention (additional mammography services via mobile mammography unit)
A non-randomized quasi-experimental approach will be used for the clinic-level intervention. The Seattle Cancer Care Alliance will provide additional screening mammography services through its state-of-the-art digital mobile mammography unit at two of the four participating clinics. Thus, at the clinic-level, there will be two intervention clinics and two control clinics. The mammography services will be offered free to women who are enrolled in the Washington State Breast, Cervical, and Colon Health Program or who are uninsured.
2.9. Primary outcome
The primary outcome is completion of a mammogram within 1 year after randomization. Participants will be tracked via medical record review at Sea Mar and mammography completion verified by a record entry. Differences in mammography rates in intervention and control arms will be evaluated, adjusting for clinic-level differences.
2.10. Secondary outcomes
The cost analysis will estimate the fixed and variable costs of delivering the multi-level intervention as well as the costs associated with screening mammography. We will assess the incremental cost-effectiveness of the intervention; that is, the difference in costs over the difference in effectiveness of the new intervention versus usual care. In addition, a secondary outcome is differences in neighbourhood-level characteristics that may influence the efficacy of the intervention. Neighborhood-level characteristics will be gathered both from publicly available census data and from participant self-report. Self-reported questions will assess neighborhood safety, social capital, social cohesion, and use of public services. Census data obtained from the American Community Survey will include education, income, and Hispanic density.
2.11. Sample size
The primary endpoint, i.e. increased adherence with breast cancer screening, will be used to assess power for our sample size. Patient-level compliance probabilities are assumed to range from 0.20 to 0.30 for usual care, 0.30 to 0.40 for the intervention arm, with at least a 0.125 difference between the intervention and control groups. We calculated the number needed pre arm for differential screening rates attributable to the promotora-level intervention adjusted for clinic-level effects employing a logistic regression model for a given number of randomized women. The number need per arm is estimated based on 80% power for a two-tailed test at alpha = 0.05 based on the assumption of at 12.5% difference in individual-level differences in rates of mammography screening between intervention and control arms and a 10% loss to follow-up. Based on these assumptions, 270 women are needed in each arm. About 540 women will be randomized (anticipated 270 per arm to each of 2 arms: control, intervention) into the study.
2.12. Statistical analysis
The primary end-point (mammogram screening in the past year) will be coded as a binary variable. Evaluation of the success of the intervention will be based only on mammogram compliance at follow-up. The intent-to-treat analysis will use chi square tests of 2 × 2 tables (screening Yes/No by arm Control vs. Intervention) to assess whether intervention arm affects the probability of screening at follow-up. We will use linear mixed modeling to adjust for clinic-level effects. Separate descriptive analysis will be conducted to compare the intervention arm to the control arm by clinic assignment to intervention condition (additional mammography services provided by the mobile mammography van) or control condition (no additional mammography services). Subsequent logistic regression analysis will include adjustment of the primary analysis by potential confounding characteristics such as age and income to account for potential biases in the randomization. The outcome measure will be determination of receipt of mammogram within 1 year of randomization, based on blinded medical record review and, secondarily, by self-report.
3. Discussion
¡Fortaleza Latina! has potential to identify strategies to reduce health disparities in breast cancer screening and thereby increase early detection of breast cancer in Latinas. The analysis will report the independent and combined effect of a clinic-level (additional mammography services) and a patient-level intervention on rates of guideline-consistent screening. If successful, the program may be an effective and cost-effective method of reducing cancer morbidity and mortality.
Acknowledgments
Funding:
This study was supported through funding from the National Cancer Institute grant R25 CA92408 and the Center for Population Health and Health Disparities: 5 P50 CA148143.
Abbreviation
- HIPAA
Health Insurance Portability and Accountability Act of 1996
Footnotes
Clinicaltrials.gov Registration Number: NCT02010008.
References
- [1].American Cancer Society. Cancer facts & figures for Hispanics/Latinos 2012–2014. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- [2].National Center for Health Statistics. Health, United States. With special feature on emergency care. Catalog number: 76–641496. 2013. Hyattsville, MD; 2012. [PubMed] [Google Scholar]
- [3].Rosales M, Gonzalez P. Mammography screening among Mexican, Central-American, and South-American women. J Immigr Minor Health 2013;15(2):225–33. [DOI] [PubMed] [Google Scholar]
- [4].Watson-Johnson LC, DeGroff A, Steele CB, Revels M, Smith JL, Justen E, et al. Mammography adherence: a qualitative study. J Women’s Health (2002) 2011;20(12):1887–94. [DOI] [PubMed] [Google Scholar]
- [5].Lubetkin EI, Santana A, Tso A, Jia H. Predictors of cancer screening among low-income primary care patients. J Health Care Poor Underserved 2008;19(1):135–48. [DOI] [PubMed] [Google Scholar]
- [6].Washington State Office of Financial Management. Office of financial management: Get county & city data; 2014. 1–18.
- [7].Puschel K, Coronado G, Soto G, Gonzalez K, Martinez J, Holte S, et al. Strategies for increasing mammography screening in primary care in Chile: results of a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2010;19(9):2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 2008;98(9):1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coronado GD, Gutierrez JM, Jhingan E, Angulo A, Jimenez R. Patient and clinical perspectives on changes to mammography screening guidelines. Breast J Jan-Feb 2014;20(1):105–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Martinez-Gutierrez J, Jhingan E, Angulo A, Jimenez R, Thompson B, Coronado GD. Cancer screening at a federally qualified health center: a qualitative study on organizational challenges in the era of the patient-centered medical home. J Immigr Minor Health 2013;15(5): 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rhodes SD, Foley KL, Zometa CS, Bloom FR. Lay health advisor interventions among Hispanics/Latinos: a qualitative systematic review. Am J Prev Med 2007;33(5):418–27. [DOI] [PubMed] [Google Scholar]
- [12].Corsino L, Rocha-Goldberg MP, Batch BC, Ortiz-Melo DI, Bosworth HB, Svetkey LP. The Latino Health Project: pilot testing a culturally adapted behavioral weight loss intervention in obese and overweight Latino adults. Ethn Dis 2012;22(1):51–7. [PMC free article] [PubMed] [Google Scholar]
- [13].Rocha-Goldberg MP, Corsino L, Batch B, Voils CI, Thorpe CT, Bosworth HB, et al. Hypertension Improvement Project (HIP) Latino: results of a pilot study of lifestyle intervention for lowering blood pressure in Latino adults. Ethn Health 2010;15(3):269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levensky ER, Forcehimes A, O’Donohue WT, Beitz K. Motivational interviewing: an evidence-based approach to counseling helps patients follow treatment recommendations. Am J Nurs 2007;107(10): 50–8. [DOI] [PubMed] [Google Scholar]
- [15].O’Brien MJ, Halbert CH, Bixby R, Pimentel S, Shea JA. Community health worker intervention to decrease cervical cancer disparities in Hispanic women. J Gen Intern Med 2010;25(11):1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Livaudais JC, Coronado GD, Espinoza N, Islas I, Ibarra G, Thompson B. Educating Hispanic women about breast cancer prevention: evaluation of a home-based promotora-led intervention. J Women’s Health (2002) 2010;19(11):2049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


