Abstract
Decades after the eradication of smallpox and the discontinuation of routine smallpox vaccination, over half of the world's population is immunologically naïve to variola virus and other orthopoxviruses (OPXVs). Even in those previously vaccinated against smallpox, protective immunity wanes over time. As such, there is a concomitant increase in the incidence of human OPXV infections worldwide. To identify novel antiviral compounds with potent anti-OPXV potential, we characterized the inhibitory activity of PAV-866 and other methylene blue derivatives against the prototypic poxvirus, vaccinia virus (VACV). These compounds inactivated virions prior to infection and consequently inhibited viral binding, fusion and entry. The compounds exhibited strong virucidal activity at non-cytotoxic concentrations, and inhibited VACV infection when added before, during or after viral adsorption. The compounds were effective against other OPXVs including monkeypox virus, cowpox virus and the newly identified Akhmeta virus. Altogether, these findings reveal a novel mode of inhibition that has not previously been demonstrated for small molecule compounds against VACV. Additional studies are in progress to determine the in vivo efficacy of these compounds against OPXVs and further characterize the anti-viral effects of these derivatives.
Keywords: Vaccinia virus, Antiviral, Poxviruses, Methylene blue, Novel inhibitor, PAV-866
1. Introduction
With 300–500 million deaths in the 20th century alone, variola virus (VARV), the causative virus of smallpox, is one of the most devastating human pathogens in history (Holmes KK, 2017). VARV is a member of the genus orthopoxvirus (OPXV), which contains several viruses of public health importance including monkeypox virus (MPXV), cowpox virus (CPXV) and vaccinia virus (VACV), among others (Damon, 2014). Human OPXV infections persist in different regions globally: MPXV primarily in Africa, CPXV in Europe, and VACV in South America (Moussatche et al., 2008). Since the cessation of routine smallpox vaccination, herd immunity against smallpox and other OPXVs has all but vanished due to a growing population of unvaccinated individuals and waning protective immunity in previously vaccinated persons (Rotz et al., 2001). The lack of pre-existing immunity against these pathogenic viruses is a significant public health risk, one that can be mitigated through effective prophylactic and therapeutic interventions.
Stittelaar et al. previously demonstrated that antivirals are more effective for the post-exposure treatment of lethal MPXV infection in cynomolgus monkeys than vaccination (Stittelaar et al., 2006). Accordingly, there is a growing interest in developing novel antivirals as post-exposure interventions for OPXV infection. Recently, tecovirimat (ST-246, TPOXX®) was approved by the FDA and is currently the first and only approved antiviral for the treatment of smallpox (Chan-Tack et al., 2019; Merchlinsky et al., 2019). ST-246 is highly effective against several OPXVs in multiple animal models, and has an impressive safety profile (Grosenbach et al., 2018). However, mutations in the target gene of ST-246, the virus encoded gene F13L, may lead to antiviral resistance as observed in a recent progressive vaccinia case (Lederman et al., 2012). The possibility of antiviral resistance emphasizes the need for additional therapeutic candidates that target different proteins or pathways than ST-246.
We previously identified PAV-866 as an effective inhibitor of rabies virus (RABV) based on an in vitro screen measuring viral assembly (Lingappa et al., 2013a). PAV-866 is an analog of methylene blue, a photoactive phenothiazine dye with antimicrobial activity against a broad range of pathogens (Floyd et al., 2004; Schirmer et al., 2003). Light-activated methylene blue inhibits several viruses including HSV1 and HSV2 (Lewin et al., 1980; Schnipper et al., 1980), yellow fever virus (Faddy et al., 2019), AIDS-related Kaposi sarcoma virus (Tardivo et al., 2006), West Nile virus (Papin et al., 2005), as well as the bacterium Staphylococcus aureus (Zolfaghari et al., 2009). We found that the presence of PAV-866 during the intracellular stage of the viral life cycle, and not after viral release, is critical for its anti-RABV efficacy. Our findings also suggest that the anti-RABV activity of PAV-866 is linked to its interactions with a host multi-protein complex involved in catalysis of RABV capsid formation (Lingappa et al., 2013a).
In this study, we investigated the antiviral properties of PAV-866 and its structural derivatives against the prototypic poxvirus, VACV. We demonstrate that these compounds inactivate viral particles causing a marked decrease in viral binding, fusion, entry and gene expression. The complete inactivation of input virus, as indicated by lower virus yields than those with the well-characterized replication inhibitor cytosine arabinoside (AraC), reveals a novel mechanism of poxviral inhibition that involves the direct and irreversible inhibition of VACV virions prior to infection. We also observe broad-spectrum inhibition of PAV-866 derivatives against multiple OPXVs besides VACV, including MPXV, CPXV and Akhmeta virus. These preliminary findings shed light on a new class of small molecule inhibitors with antiviral activity against OPXVs and provide a basis for future studies that examine their targets and in vivo potential.
2. Materials and methods
2.1. Viruses and cells
VACV-WR-GFP/NLS (Earl et al., 2003), VACV-WR-A4-YFP (Senkevich and Moss, 2005), VACV-LUC (Townsley et al., 2006), MPXV WA clade, CPXV Brighton Red strain and Akhmeta virus (Doty et al., 2019) were propagated in BSC-40 cells and titered by plaque assay prior to use. BSC-40 and HeLa cells were cultured in DMEM supplemented with 10% FBS, containing L-glutamine, penicillin, and streptomycin. All virus infections were performed in DMEM supplemented with 2% FBS, L-glutamine, penicillin, and streptomycin (hereby referred to as DMEM-2).
2.2. Chemical synthesis of methylene blue derivatives
The compounds used in this study were prepared from the corresponding phenothiazin-5-ium tetraiodide hydrate intermediates synthesized using the published procedure (US patent #8785434B2). Structures of PAV-164 and PAV-174 provided below.
Detailed step-wise descriptions of synthesis schemes for PAV-164 and PAV-174, and the structures and synthesis schemes for the other derivatives described in this study are provided in Supplementary Text 1.
2.3. VACV spread inhibition assay
The VACV spread assay was performed as previously published (Cryer et al., 2017; Johnson et al., 2008; Priyamvada et al., 2020). Briefly, HeLa cells plated in 96-well clear bottom, black plates (Corning, 06-443-2) were infected with VACV-NLS-GFP at MOI 0.4 in the presence of serially diluted methylene blue derivatives. Cells were also treated with AraC (46 μg/mL) to measure %GFP+ cells after a singular round of infection. A virus only (no treatment) control was included in each plate. After a 24 h (h) infection, cells were fixed with 4% paraformaldehyde, washed and stained DAPI (4′,6-diamidino-2-phenylindole) (10 μg/mL; ThermoFisher Scientific, D1306) for 15 min. Cells were imaged and analyzed using the ArrayScan XTI High Content Screen (HCS) Reader and HCS Studio Cell Analysis software. The percentage of viral spread in the presence of the compounds was calculated per the formula below:
Viral spread data were analyzed using a non-linear regression (curve fit) with the Sigmoidal dose-response variable slope equation to determine the concentrations required for 50% inhibition (EC50) of viral spread relative to the no treatment control. Values were determined based on two or more replicates from two independent experiments.
2.4. Measuring early gene expression by luciferase assay
HeLa cells seeded overnight in 96-well plates were infected with VACV-LUC virus for 1 h at RT. Input virus was removed by washing with PBS, and cells were incubated for 2 h at 37 °C in the presence or absence of compounds (2 μM). Cells were lysed using Reporter Lysis Buffer (Promega Corporation, E4030) and luciferase activity was determined using the Luciferase Assay System (Promega Corporation, E1501) as per the manufacturer's instructions. Luciferase levels were measured using an EnSpire microplate reader (PerkinElmer).
2.5. Plaque assay for determination of virus yield
Virus yield was determined as previously described (Priyamvada et al., 2020). Briefly, BSC-40 cells were infected with virus samples serially diluted in DMEM-2 in the presence or absence of compounds (2 μM). After 1 h, input virus was removed and an overlay of DMEM-2 containing 0.5% methylcellulose was added to the cells. After a 72 h incubation at 37 °C, cells were fixed and stained using crystal violet containing 4% formalin. Plates were washed with water and air dried, and viral plaques were counted thereafter.
2.6. Cytotoxicity assay
Cellular toxicity in the presence of PAV-866 and its derivatives was measured using the LDH Cytotoxicity Assay Kit per the manufacturer's instructions (ThermoFisher Scientific, 88953). Briefly, HeLa cells were incubated with serially diluted concentrations of the compounds for 24 h at 37 °C. Supernatants were collected and mixed with the Reaction Mixture provided in the kit. After a 30 min incubation at RT, Stop Solution was added and the levels of extracellular lactate dehydrogenase (LDH) were quantified using an EnSpire multimode microplate reader (PerkinElmer).
2.7. Binding assay
VACV-WR-YFP was added to BSC40 cells in the presence or absence of serially diluted PAV-866 derivative PAV-164. The cells were incubated with the virus-inhibitor mixture at RT for 90 min, which allowed virus binding but not entry. After viral adsorption, cells were washed, trypsinized and fixed with 4% paraformaldehyde. The percentage of YFP+ cells was determined using the Attune Nxt flow cytometer (ThermoFisher Scientific).
2.8. Fusion assay
Viral fusion in the presence of the PA-866 derivative PAV-164 was assessed using a lipophilic tracer-labeled virus as previously described (Laliberte et al., 2011) with modifications. Briefly, VACV WR virus was labeled with DiO (Invitrogen D275) in 1x PBS containing 0.2% BSA for 20 min at RT, followed by three rounds of washing and centrifugation to remove excess DiO. BSC40 cells plated in two separate 96-well plates were incubated with DiO-labeled virus in the presence or absence of serially diluted compound PAV-164 at RT for 1 h to allow for virus binding, and then washed to remove unbound virus. Subsequently, one plate was transferred to 37 °C for 90 min to allow viral fusion while the other remained at RT to determine the level of background signal. Cells in both plates were then trypsinized and fixed with 4% paraformaldehyde. Following fixation, samples were analyzed by flow cytometry using the Attune Nxt instrument to determine the percentage of DiO+ cells.
2.9. Statistical analysis
Data were analyzed using GraphPad Prism software (version 8, GraphPad). Statistical significance was determined using either a one-way ANOVA test and a post-hoc Dunnet's multiple comparisons test, or a t-test with a post-hoc Holm-Sidak multiple comparisons test (alpha = 5.000%), as specified in the figure legends.
3. Results
3.1. Reduced viral yield and low cytotoxicity in the presence of PAV-866 and derivatives
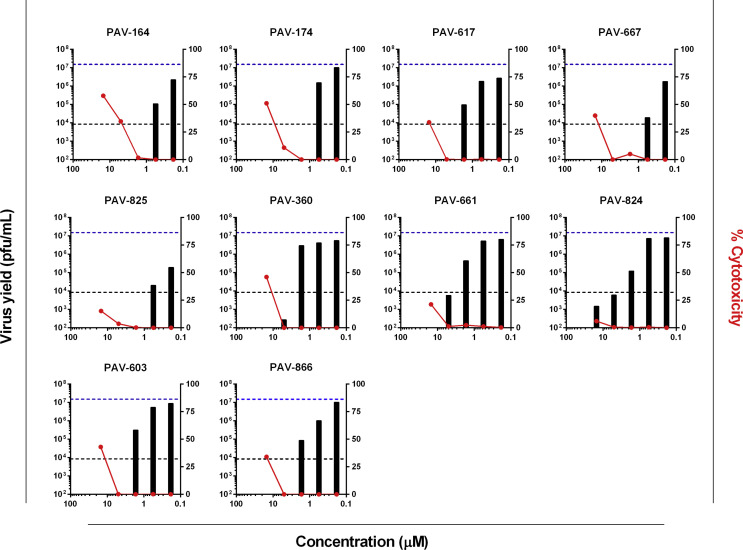
The antiviral activity of PAV-866 and its derivatives, namely PAV-164, PAV-174, PAV-617, PAV-667, PAV-825, PAV-360, PAV-661, PAV-824 and PAV-603, against VACV was tested by measuring virus yield and cytotoxicity at varying concentrations of the compounds. In the absence of any inhibitors, the total virus yield (dotted blue line) was nearly three logs higher than with AraC (dotted black line). The virus yield determined in the presence of AraC may correspond to the input virus present in the inoculum that failed to enter cells even after wash/removal after adsorption. In the presence of PAV-866 and its derivatives, a large reduction in virus yield was observed. At higher non-cytotoxic concentrations (right y-axis), virus yield was undetectable (less than limit of detection, 10 pfu/mL) for most PAV-866 derivatives and lower than AraC treatment (right y-axis, Fig. 1 ) suggesting an effect on input virus.
Fig. 1.
Treatment with PAV-866 and its derivatives decreases VACV yield with low cytotoxicity. HeLa cells were infected with VACV-NLS-GFP at MOI 3 and treated with different concentrations of PAV-866 and its 10 derivatives. After 24 h, cells were collected, and total virus yield was determined by plaque assay in the absence of inhibitors. Virus yields (pfu/mL) for each concentration tested are shown as filled black bars. The dotted lines in the graphs represent the total virus yield in the untreated control (blue, top) and in the presence of AraC (black, bottom). The right y-axis corresponds to percent cytotoxicity with respect to a lysed cell control, and the % cytotoxicity for each compound at the concentrations tested are shown as red circles. Values shown represent the mean of two or more replicates.
3.2. Inhibition of viral spread in the presence of methylene blue derived compounds
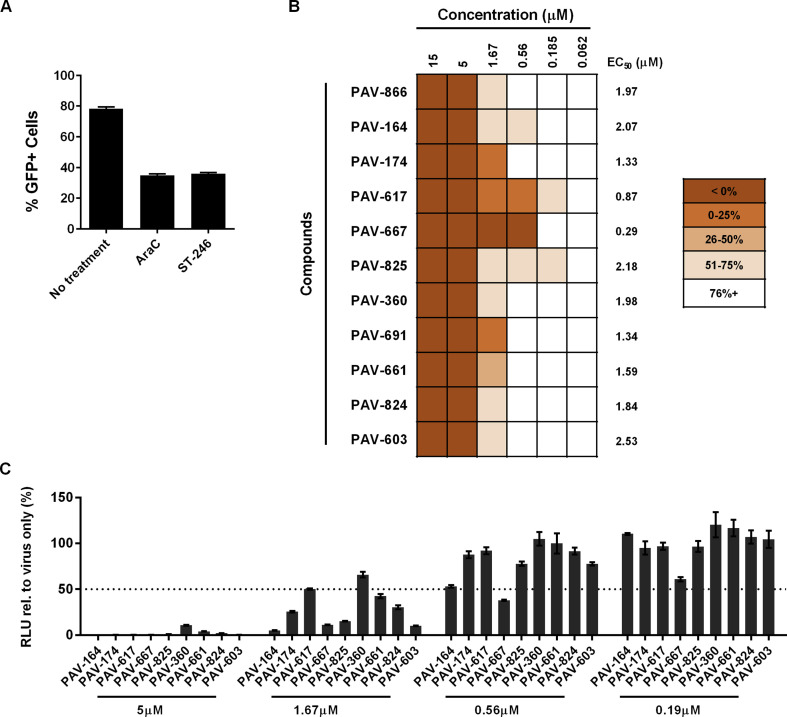
To determine whether PAV-866 and its derivatives exhibited similar patterns of antiviral activity, we examined the effects of the compounds on a late event (viral spread) and an early event (early gene expression) in the viral life cycle using a microscopy based assay. Spread was measured using VACV WR virus that expresses a nuclear localization signal-tagged GFP reporter gene controlled by a synthetic early/late promoter (Earl et al., 2003). As shown in Fig. 2 A, we first measured percent GFP expressing cells (%GFP+) in the presence of two compounds with known, distinct antiviral activities: AraC and ST-246. AraC is an inhibitor of viral DNA replication and blocks the generation of viral progeny, but does not affect early gene expression. ST-246 is an inhibitor of membrane wrapping and therefore does not impact early or late protein synthesis, but prevents virus spread. As expected, we observed that the percentage of GFP+ cells in the AraC and ST-246 treatment groups were comparable, owing to the fact that both treatments allow only one round of viral infection (Fig. 2A). Since AraC blocks viral replication, any GFP+ cells in excess to those in the presence of AraC would be the result of viral spread. We therefore used the AraC treatment as a threshold to quantitate viral spread in the presence of the compounds. We determined viral spread in the presence of a wide range of PAV-866 concentrations and found a sizeable reduction in %GFP+ cells even at the low micromolar level (EC50 = 1.97 μM). An inhibition of VACV spread was also observed in the presence of the 9 PAV-866 derived compounds with EC50 values ranging between 0.29 and 2.53 μM. At higher concentrations, no GFP expression was detected from the early gene promoter resulting in <0% spread (Fig. 2B). These results suggest that PAV-866 and its derivatives potentially impact earlier events in viral infection than AraC and corroborated our virus yield results (Fig. 1).
Fig. 2.
Reduction in VACV spread and early gene expression by PAV-866 and derivatives(A) Percentage of GFP+ cells in the presence of inhibitors AraC (DNA replication) and ST-246 (EV membrane wrapping). HeLa cells were infected with VACV-NLS-GFP at MOI 0.4 for 24 h in the absence and presence of inhibitors. Samples were fixed and stained with DAPI to visualize total number of cells. The percentage of infected cells was determined as the ratio of GFP+ nuclei to total number of nuclei. Values represent the mean of two or more replicates±SEM. (B) Percent VACV spread in the presence of serially diluted PAV-866 and derivatives. Shading of cells corresponds to degree of viral inhibition (darkest cells indicate low viral spread or high viral inhibition). Corresponding EC50 values also shown. C) HeLa cells were infected with VACV-LUC virus at MOI 3 in the presence or absence of four concentrations of 10 PAV-866 analogs. After 2 h, cells were lysed and luciferase activity was measured as a surrogate for early gene expression. The values plotted represent the percent relative luminescence units (RLU) of each treatment with respect to cells infected with virus alone. The dotted line represents the 50% RLU value relative to the virus only treatment. The mean of two or more replicates is shown±SEM.
3.3. Decreased VACV early gene expression in presence of PAV-866 and derivatives
To quantitate VACV early gene expression, we infected cells with VACV-LUC virus in the presence or absence of varying concentrations of the compounds. VACV-LUC contains a firefly luciferase reporter gene controlled by a synthetic early/late promoter, which allows for the use of luciferase expression as a surrogate for early protein synthesis. Most compounds substantially inhibited luciferase expression at 5 μM and at 1.67 μM. A few were also highly effective at 0.56 μM (Fig. 2C). These data show that at their respective effective concentrations, the presence of PAV-866 derivatives blocks early gene expression of VACV. For a majority of the compounds tested, this reduction of luciferase expression was not affected by the presence of the free radical quencher, glutathione (Supplementary Figure 1) (Masella et al., 2005). These data suggest that the antiviral activities of the compounds may not depend upon activation by UV-induced reactive oxygen species, as typically observed with methylene blue (Wagner, 2002).
Since all 9 derivatives exhibited similar inhibitory phenotypes, PAV-164 and PAV-174 were chosen for subsequent experiments (based on compound availability) to further characterize the methylene blue derivatives and the specific steps affected by their antiviral properties.
3.4. PAV-866 derivatives directly inactivate viral particles prior to infection
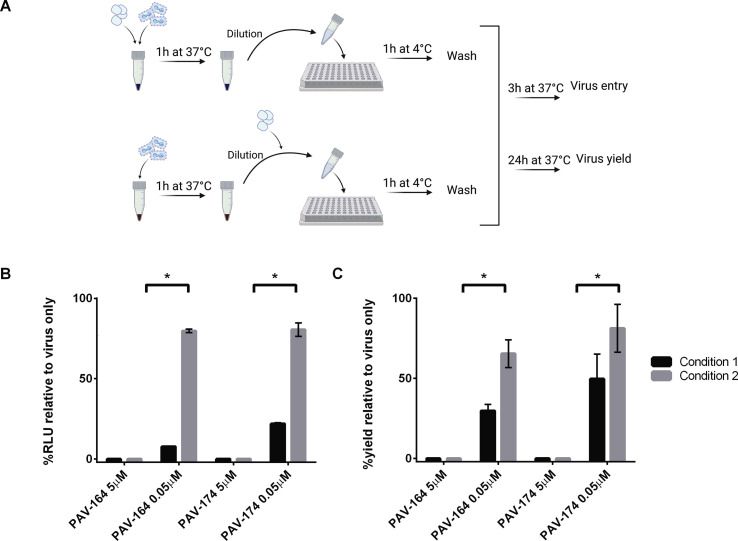
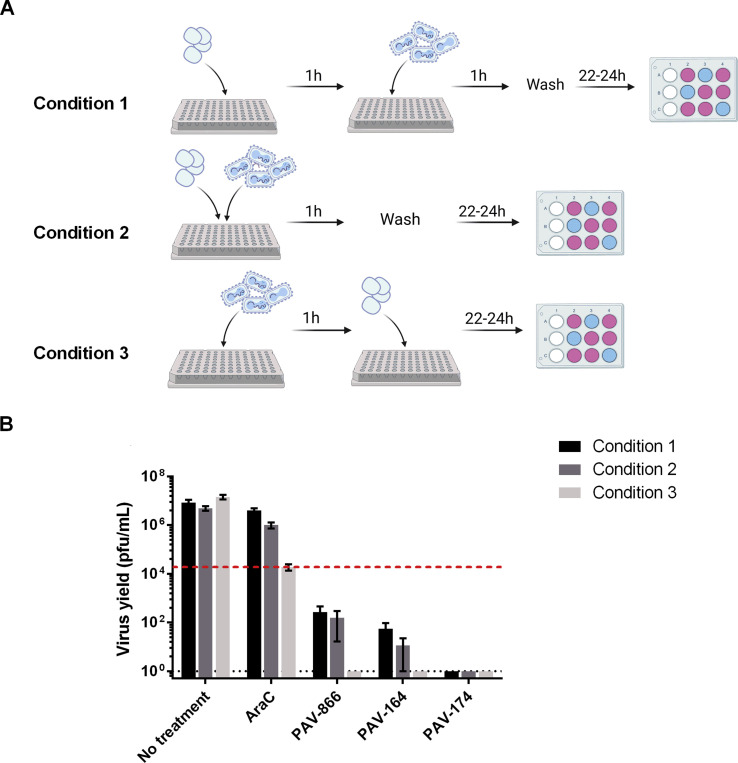
Based upon the reduction in early gene expression (luciferase assay) and <0% spread values observed in the presence of the compounds, we hypothesized that PAV-866 and its derivatives acted directly on viral particles to reduce viral infectivity and decrease entry. The experiment performed to test this hypothesis is illustrated in Fig. 3 A. As shown, we mixed VACV-LUC (equivalent to 3 MOI) with compounds PAV-164 and PAV-174 at two different concentrations (high and low treatments), both of which were inhibitory in low volume (Condition 1). After incubation at 37 °C for 1 h, the samples were diluted and added to cells to obtain final compound concentrations of 5 μM and 0.05 μM (for high and low treatments, respectively), the latter concentration falling below the inhibitory range of both compounds. As a control for direct inhibition of viral particles with high and low treatments, we incubated concentrated virus alone at 37 °C for 1 h, and added compounds at final concentrations of 5 μM and 0.5 μM directly at the infection step (Condition 2). After adsorption at 4 °C for 1 h, the inoculums in both conditions were removed, cells were washed and incubated without inhibitors at 37 °C, and harvested at 3h and 24h to measure virus entry and yield, respectively.
Fig. 3.
PAV-866 derivatives act directly on viral particles to decrease infectivity. A) Concentrated VACV-LUC (equivalent to 3 MOI) was incubated in the presence (Condition 1) or absence (Condition 2) of concentrated PAV-164 or PAV-174 in a small volume at 37 °C for 1h. Both groups were diluted 75-fold, and compounds were added to the virus-only tubes resulting in two treatments (final concentrations 5 μM and 0.05 μM) for each compound in each condition. Both conditions included virus-only controls. The diluted virus-compound mixtures were added to cells for 1 h at 4 °C to allow viral adsorption. After removing the inoculum and washing, cells were incubated at 37 °C until harvest. Each treatment was tested in triplicate. B) Virus entry measured by luciferase assay. Bars represent the mean±SEM percent RLU of compound treatments with respect to virus only. C) Virus yield determined by plaque assay. Values plotted represent the mean±SEM percent virus yield of compound treatments with respect to virus only. For both B and C, black and gray bars represent Conditions 1 and 2 respectively. Statistical significance determined using the Holm-Sidak method, with alpha = 5.000%. Asterisks indicate p values < 0.05.
As shown in Fig. 3B, at a final concentration of 0.05 μM, PAV-164 and PAV-174 were unable to inhibit viral entry when present only during the adsorption step (Condition 2, 0.05 μM). This was an expected result as a concentration of 0.05 μM lies outside the effective inhibitory range of these compounds. However, the pre-incubation of concentrated virus with the compounds prior to dilution to 0.05 μM and adsorption (Condition 1) was sufficient to substantially inhibit viral entry. These data demonstrate that the compounds inhibit viral entry by acting directly on virions in the absence of cells. This phenotype is mirrored by virus yield data in Fig. 3C, where a large reduction in virus yield is observed at a concentration of 0.05 μM in Condition 1 but not 2 for both PAV-164 and PAV-174.
3.5. Addition of compounds before, during or after viral adsorption inhibits VACV infection
We evaluated the inhibitory activity of the PAV-866 derivatives prior to, during and after the addition of virus using three experimental conditions as outlined in Fig. 4 A. Condition 1 involved pre-treating the cells with compounds for 1 h and washing the compounds away before viral infection for 24 h. Condition 2 involved the simultaneous incubation of cells with virus and compounds for 1 h, followed by a 24 h infection. Lastly, Condition 3 involved virus adsorption for 1 h, followed by infection in the presence of the compounds for 24 h. When AraC was added to cells pre or during viral adsorption and removed before the subsequent 24 incubation, it did not considerably decrease viral yield (Fig. 4B). In contrast, PAV-866, PAV-164 and PAV-174 all inhibited VACV yield in all three experimental conditions (Fig. 4B). The presence of the compounds at the time of virus addition was not essential for their virucidal activity.
Fig. 4.
Pre-treatment of cells with PAV-866 derivative can block VACV infection. (A) Schematic representation of the three treatment conditions tested. The incubation of cells with virus for 1 h at RT represents the viral adsorption step, and compounds (2 μM) were added at different time points pre and post adsorption as illustrated. (B) Virus yield (pfu/mL) at 24 h for all three treatments measured by plaque assay. The red line represents the input virus titer determined based upon AraC treatment titers from Condition 3. Bars represent average of two or more replicates±SEM.
3.6. Reduced virus binding and membrane fusion in the presence of PAV-866 derivative
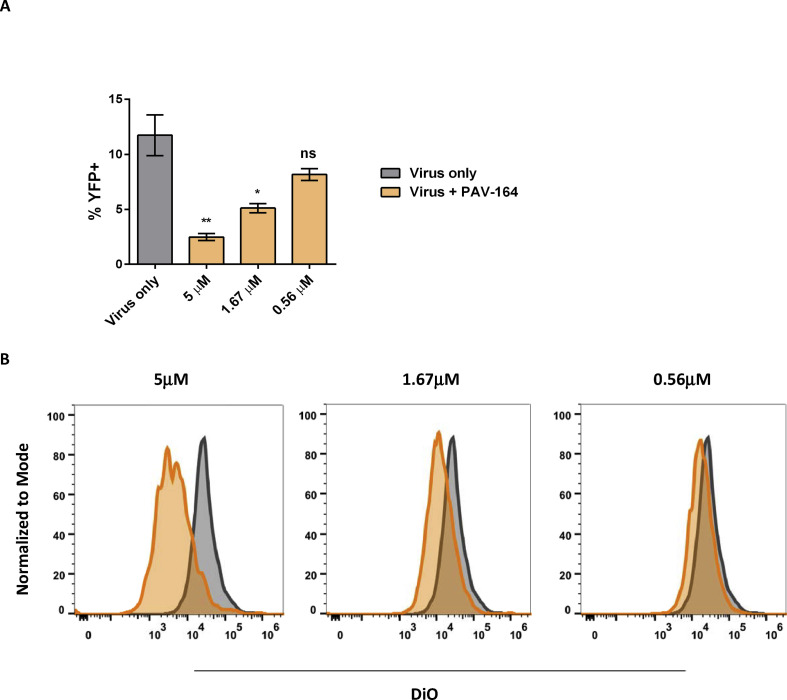
As PAV-866 derivatives have a direct effect on virus infectivity (Fig. 3), early steps in the viral life cycle including viral binding and fusion should also decrease in their presence. To determine whether the compounds decreased viral binding to cells, we used a VACV WR virus that expresses an A4-YFP fusion protein. A4 is one of the most abundant core proteins and its fusion with YFP allows for the detection of virus-bound cells by flow cytometry. Compared to the no treatment control, fewer YFP+ cells were measured in the presence of the compound PAV-164 (Fig. 5 A). The compound exerted the strongest effect on viral binding at 5 μM, and this effect was decreased at lower concentrations of PAV-164.
Fig. 5.
Reduced virus binding and membrane fusion of VACV in presence of PAV-866 derivative. (A) Percent YFP+ cells in the presence or absence of compound PAV-164 quantified by flow cytometry. Bars colored based upon treatment group: virus only (gray) and PAV-164 (orange). Values represent the average of two replicates±SEM. Statistical significance measured using one-way ANOVA and post-hoc Dunnet's multiple comparisons test. P values indicated within graph: * = p < 0.05, ** = p < 0.005 and ns = not significant. (B) Fusion of viral and cell membranes in the presence or absence of compound PAV-164 evaluated by flow cytometry-based detection of DiO+ cells. Histogram plots showing overlay of DiO intensity peaks in the presence (orange) and absence (gray) of three different concentrations of PAV-164. Peaks normalized to mode (y-axis), and the x-axis represents the intensity of DiO staining.
The next step in virus infection is the fusion of viral and cellular membranes to release the viral core into cytoplasm of infected cells. We determined whether PAV-164 affected the membrane fusion activity of VACV particles by incubating DiO-labeled virus with cells at 37 °C for 2 h. The number of DiO+ cells in the presence or absence of PAV-164 were measured by flow cytometry. We observed an inverse relationship between PAV-164 concentration and the peak fluorescence intensity of infected cells (Fig. 5B). Taken together, these data further support that PAV-866 derivatives can inhibit virus prior to the entry step.
3.7. PAV-866 derivative inhibits several OPXVs
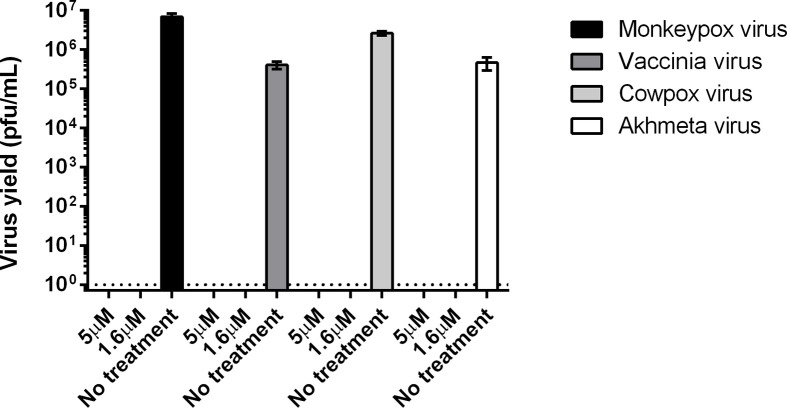
The genus OPXV includes several species with significant genetic identity. Given the public health benefits of a broadly protective antiviral, we were interested in determining whether PAV-866 derivatives could inhibit other OPXVs besides VACV. To this end, we tested the effect of compound PAV-164 on the replication of MPXV, CPXV and Akhmeta virus, a newly identified old-world OPXV, by plaque assay (Fig. 6 ). We observed complete inhibition of all three viruses at both concentrations tested, 5 μM and 1.6 μM (Fig. 6). The mechanism of inhibition of PAV-164 is therefore likely common between a wide range of OPXVs, as no virus (including input) was detected by plaque assay upon incubation with PAV-164.
Fig. 6.
PAV-866 derivative exhibits broad OPXV antiviral activity. HeLa cells were infected with four different OPXVs: VACV, CPXV, MPXV and Akhmeta virus for 24 h in the absence and presence of compound PAV-164. Virus yields (pfu/mL) determined by plaque assay. Bars represent the mean of three replicates±SEM.
4. Discussion
In this study, we evaluated the antiviral potential of PAV-866 (Lingappa et al., 2013a) and its structural derivatives PAV-164 and PAV-174 against the prototypic poxvirus VACV. Treatment with PAV-866 and its derivatives resulted in complete inhibition of virus yield at 24 h after incubation. In the presence of the DNA replication inhibitor AraC, we observed residual virus titers which were likely attributable to infectious viral particles that were bound to but had not entered the cells. The virus that had entered cells in the presence of AraC would express only early genes and due to the inhibition of DNA replication, would not be infectious during titer determination by plaque assay. Conversely, PAV-866 and its derivatives showed negative spread values and virus yields lower than AraC. These data suggested complete inhibition of input virus regardless of whether particles had entered the cells. We tested this hypothesis by incubating virus with compounds in low volume, and diluting the mixture beyond the effective inhibitory range of the compounds prior to infection. The pre-incubation step of virus with compounds was sufficient to inhibit viral entry and replication, demonstrating that the compounds are virucidal. To our knowledge, this is a novel mode of inhibition not observed with previously characterized anti-poxviral compounds.
While a specific cellular receptor for VACV has not yet been identified, viral entry can occur through macropinocytosis, low pH-mediated endocytosis or direct fusion at the plasma membrane (Laliberte and Moss, 2009; Mercer and Helenius, 2008). Vaccinia virus binding and entry are mediated by nearly 15 proteins present on the mature virus membrane which organize into distinct pathways that enable efficient viral entry (Gray et al., 2019; Moss, 2012). As the compounds inactivated virions prior to entry, we expected lower virus binding and fusion in the presence of the compounds. We measured virus binding and fusion in the presence of PAV-164 using VACV A4-YFP virus (Bengali et al., 2009) and DiO-labeled viral particles (Laliberte et al., 2011) respectively. Increasing concentrations of compounds inversely affected virus binding and fusion, further supporting our earlier observations of the virucidal effects of the compounds. Additional studies are required to determine whether the compounds remain effective if introduced post viral entry, and if so, which specific steps in the viral life cycle they target.
Our pre-treatment experiment indicated that incubating cells with compounds prior to the addition of the virus was sufficient to inhibit infection. Despite the absence of the compounds at the time of viral adsorption, considerably lower virus yields were observed for PAV-164 and PAV-174 compared to the no compound control suggesting a potential secondary effect on cells. One caveat of this experiment is the possibility of residual compounds in the wells at the time of virus addition. However, since the cells were washed prior to adding virus, the residual compound present at the time of virus addition, if any, would be highly diluted and potentially outside its effective concentration range. PAV-866, PAV-174, and PAV-164 are all members of a larger class of compounds termed assembly modulators, initially identified through cell-free protein synthesis and assembly (CFPSA) systems (Lingappa et al., 2013b). In at least some cases (Lingappa et al., 2013a), the targets of these compounds are host multi-protein complexes involved in catalysis of viral assembly. Whether the compounds in our study also affect host processes, either due to an effect on secondary targets or through feedback mechanisms, awaits further studies. We hypothesize that assembly modulation is an important and previously largely unexplored molecular basis for homeostasis, impinging on multiple feedback loops, which would be consistent with the data shown here. Our previous studies with RABV identified the ATP-binding cassette family E1 (ABCE1) protein, also known as the RNase L inhibitor (Bisbal et al., 1995), as a putative target for the anti-RABV activity of PAV-866 (Lingappa et al., 2013a). A similar affinity chromatography-based approach could be applied here to identify the specific viral targets for PAV-866, PAV-164 and PAV-174.
The only antiviral drug currently available for treatment of smallpox is the recently approved ST-246 (Chan-Tack et al., 2019; Merchlinsky et al., 2019). ST-246 targets the virus egress step, directly acting on VACV encoded protein F13 to inhibit MV membrane wrapping required for extracellular virus formation (Yang et al., 2005). Given the possibility of antiviral resistance developing against ST-246 during treatment, there is a need to identify and characterize additional antivirals with other viral or cellular targets. To this end, we have identified a new class of inhibitors that potently decrease VACV infectivity by inhibiting early steps in viral infection. The potent inhibition of PAV-866 derivative PAV-164 against VACV, MPXV, CPXV and Akhmeta virus further highlights the potential of these inhibitors for development as broad-range anti-poxviral drugs. We are currently exploring the structure-activity relationship of a larger panel of PAV-866 derivatives. Such a study may not only improve potency and therapeutic index of these compounds, but may also reveal subseries within the methylene blue derivative family that target steps in the multi-step lifecycle of poxviruses.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: VRL is the CTO and Co-CEO of Prosetta Biosciences, Inc. MBW and AK were employed by Prosetta Biosciences at the time of this study.
Acknowledgements
We thank Bernie Moss for kindly providing us recombinant vaccinia viruses. The study was supported by CDC Intramural Research funding, Biomedical Advanced Research and Developmental Authority (BARDA) funding, and an ORISE Postdoctoral Fellowship (LP, JB). Schematic representations of experimental assays were created on BioRender.com. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2021.105086.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bengali Z., Townsley A.C., Moss B. Vaccinia virus strain differences in cell attachment and entry. Virology. 2009;389:132–140. doi: 10.1016/j.virol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbal C., Martinand C., Silhol M., Lebleu B., Salehzada T. Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2-5A pathway. J. Biol. Chem. 1995;270:13308–13317. doi: 10.1074/jbc.270.22.13308. [DOI] [PubMed] [Google Scholar]
- Chan-Tack K.M., Harrington P.R., Choi S.Y., Myers L., O'Rear J., Seo S., McMillan D., Ghantous H., Birnkrant D., Sherwat A.I. Assessing a drug for an eradicated human disease: US Food and Drug Administration review of tecovirimat for the treatment of smallpox. Lancet Infect. Dis. 2019;19:e221–e224. doi: 10.1016/S1473-3099(18)30788-6. [DOI] [PubMed] [Google Scholar]
- Cryer M., Lane K., Greer M., Cates R., Burt S., Andrus M., Zou J., Rogers P., Hansen M.D., Burgado J., Panayampalli S.S., Day C.W., Smee D.F., Johnson B.F. Isolation and identification of compounds from Kalanchoe pinnata having human alphaherpesvirus and vaccinia virus antiviral activity. Pharm. Biol. 2017;55:1586–1591. doi: 10.1080/13880209.2017.1310907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon I. In: sixth ed. Knipe D.M., Howley P.M., editors. vol. 2. Lippincott, Williams and Wilkins; Philadelphia, pA, USA: 2014. Poxviruses; p. 2160. (Fields Virology). [Google Scholar]
- Doty J.B., Maghlakelidze G., Sikharulidze I., Tu S.L., Morgan C.N., Mauldin M.R., Parkadze O., Kartskhia N., Turmanidze M., Matheny A.M., Davidson W., Tang S., Gao J., Li Y., Upton C., Carroll D.S., Emerson G.L., Nakazawa Y. Isolation and characterization of Akhmeta virus from wild-caught rodents (Apodemus spp.) in Georgia. J. Virol. 2019;93 doi: 10.1128/JVI.00966-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Moss B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 2003;77:10684–10688. doi: 10.1128/JVI.77.19.10684-10688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy H.M., Fryk J.J., Hall R.A., Young P.R., Reichenberg S., Tolksdorf F., Sumian C., Gravemann U., Seltsam A., Marks D.C. Inactivation of yellow fever virus in plasma after treatment with methylene blue and visible light and in platelet concentrates following treatment with ultraviolet C light. Transfusion. 2019;59:2223–2227. doi: 10.1111/trf.15332. [DOI] [PubMed] [Google Scholar]
- Floyd R.A., Schneider J.E., Jr., Dittmer D.P. Methylene blue photoinactivation of RNA viruses. Antivir. Res. 2004;61:141–151. doi: 10.1016/j.antiviral.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Gray R.D.M., Albrecht D., Beerli C., Huttunen M., Cohen G.H., White I.J., Burden J.J., Henriques R., Mercer J. Nanoscale polarization of the entry fusion complex of vaccinia virus drives efficient fusion. Nat Microbiol. 2019;4:1636–1644. doi: 10.1038/s41564-019-0488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenbach D.W., Honeychurch K., Rose E.A., Chinsangaram J., Frimm A., Maiti B., Lovejoy C., Meara I., Long P., Hruby D.E. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 2018;379:44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes Kk B.S., Bloom B.R., Jha P. third ed. 2017. Major Infectious Diseases: Key Meassages from Disease Control Priorities. (Chapter 1) [PubMed] [Google Scholar]
- Johnson M.C., Damon I.K., Karem K.L. A rapid, high-throughput vaccinia virus neutralization assay for testing smallpox vaccine efficacy based on detection of green fluorescent protein. J. Virol Methods. 2008;150:14–20. doi: 10.1016/j.jviromet.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Laliberte J.P., Moss B. Appraising the apoptotic mimicry model and the role of phospholipids for poxvirus entry. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17517–17521. doi: 10.1073/pnas.0909376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberte J.P., Weisberg A.S., Moss B. The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman E.R., Davidson W., Groff H.L., Smith S.K., Warkentien T., Li Y., Wilkins K.A., Karem K.L., Akondy R.S., Ahmed R., Frace M., Shieh W.J., Zaki S., Hruby D.E., Painter W.P., Bergman K.L., Cohen J.I., Damon I.K. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J. Infect. Dis. 2012;206:1372–1385. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A.A., Schnipper L.E., Crumpacker C.S. Photodynamic inactivation of herpes simplex virus by hematoporphyrin derivative and light. Proc Soc Exp Biol Med. 1980;163:81–90. doi: 10.3181/00379727-163-40727. [DOI] [PubMed] [Google Scholar]
- Lingappa U.F., Wu X., Macieik A., Yu S.F., Atuegbu A., Corpuz M., Francis J., Nichols C., Calayag A., Shi H., Ellison J.A., Harrell E.K., Asundi V., Lingappa J.R., Prasad M.D., Lipkin W.I., Dey D., Hurt C.R., Lingappa V.R., Hansen W.J., Rupprecht C.E. Host-rabies virus protein-protein interactions as druggable antiviral targets. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E861–E868. doi: 10.1073/pnas.1210198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V.R., Hurt C.R., Garvey E. Capsid assembly as a point of intervention for novel anti-viral therapeutics. Curr. Pharmaceut. Biotechnol. 2013;14:513–523. doi: 10.2174/13892010113149990201. [DOI] [PubMed] [Google Scholar]
- Masella R., Di Benedetto R., Vari R., Filesi C., Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Mercer J., Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M., Albright A., Olson V., Schiltz H., Merkeley T., Hughes C., Petersen B., Challberg M. The development and approval of tecoviromat (TPOXX((R))), the first antiviral against smallpox. Antivir. Res. 2019;168:168–174. doi: 10.1016/j.antiviral.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4:688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatche N., Damaso C.R., McFadden G. When good vaccines go wild: feral Orthopoxvirus in developing countries and beyond. J Infect Dev Ctries. 2008;2:156–173. doi: 10.3855/jidc.258. [DOI] [PubMed] [Google Scholar]
- Papin J.F., Floyd R.A., Dittmer D.P. Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo. Antivir. Res. 2005;68:84–87. doi: 10.1016/j.antiviral.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Priyamvada L., Alabi P., Leon A., Kumar A., Sambhara S., Olson V.A., Sello J.K., Satheshkumar P.S. Discovery of retro-1 analogs exhibiting enhanced anti-vaccinia virus activity. Front. Microbiol. 2020;11:603. doi: 10.3389/fmicb.2020.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotz L.D., Dotson D.A., Damon I.K., Becher J.A., Advisory Committee on Immunization P. Vaccinia (smallpox) vaccine: recommendations of the advisory committee on immunization practices (ACIP), 2001. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2001;50:1–25. quiz CE21-27. [PubMed] [Google Scholar]
- Schirmer R.H., Coulibaly B., Stich A., Scheiwein M., Merkle H., Eubel J., Becker K., Becher H., Muller O., Zich T., Schiek W., Kouyate B. Methylene blue as an antimalarial agent. Redox Rep. 2003;8:272–275. doi: 10.1179/135100003225002899. [DOI] [PubMed] [Google Scholar]
- Schnipper L.E., Lewin A.A., Swartz M., Crumpacker C.S. Mechanisms of photodynamic inactivation of herpes simplex viruses: comparison between methylene blue, light plus electricity, and hematoporhyrin plus light. J. Clin. Invest. 1980;65:432–438. doi: 10.1172/JCI109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T.G., Moss B. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 2005;79:4744–4754. doi: 10.1128/JVI.79.8.4744-4754.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stittelaar K.J., Neyts J., Naesens L., van Amerongen G., van Lavieren R.F., Holy A., De Clercq E., Niesters H.G., Fries E., Maas C., Mulder P.G., van der Zeijst B.A., Osterhaus A.D. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- Tardivo J.P., Del Giglio A., Paschoal L.H., Baptista M.S. New photodynamic therapy protocol to treat AIDS-related Kaposi's sarcoma. Photomed Laser Surg. 2006;24:528–531. doi: 10.1089/pho.2006.24.528. [DOI] [PubMed] [Google Scholar]
- Townsley A.C., Weisberg A.S., Wagenaar T.R., Moss B. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J. Virol. 2006;80:8899–8908. doi: 10.1128/JVI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S.J. Virus inactivation in blood components by photoactive phenothiazine dyes. Transfus. Med. Rev. 2002;16:61–66. doi: 10.1053/tmrv.2002.29405. [DOI] [PubMed] [Google Scholar]
- Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Buller R.L., Touchette E., Waller K., Schriewer J., Neyts J., DeClercq E., Jones K., Hruby D., Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J. Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari P.S., Packer S., Singer M., Nair S.P., Bennett J., Street C., Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.