Abstract
Background: The skin is a major target organ for extrahepatic manifestations of liver diseases, and dermatologic abnormalities are common in patients with hepatic disorders. Clinical examination of the skin, nails and hair can allow for appropriate recognition, early diagnosis and treatment of liver diseases, and improvement in the quality of life and life expectancy of affected patients.
Methods: We searched 3 databases (Pubmed,Medline and Embase) and selected studies about cirrhosis related skin manifestations and their pathophysiology.
Results: A total of 73 articles were included in the review. Studies displayed the spectrum of cutaneous manifestations related to hormonal and vascular changes as well as nail and hair changes in patients with cirrhosis and/or portal hypertension.
Conclusion: Cutaneous alterations are important clues or potential indications in the diagnosis of liver cirrhosis. Familiarity with skin conditions can be promptly diagnosed and appropriate management initiated.
KEY MESSAGES
Manifestations of the liver and skin disorders are interrelated in various ways. Cutaneous changes may be the first clue that a patient has liver disease.
The skin is a major target organ for extrahepatic manifestations of liver diseases. A broad range of cutaneous alterations can be present in patients with cirrhosis, such as vascular, nail, hair, hormonal changes, etc.
Recognizing these signs is crucial so that potential underlying diseases including liver disease can be promptly diagnosed and appropriate management timely initiated.
Keywords: Liver cirrhosis, palmar erythema, spider angioma, Terry’s nails, skin manifestations, nail diseases
Introduction
The liver is the second largest organ in the body and is involved in carbohydrate, protein, lipid homeostasis, bile production and storage and filtration of blood [1]. Liver impairment affects almost all body systems, and skin manifestations are common and sometimes the most noticeable aspect of the physical examination and/or symptoms. The majority of cutaneous conditions are asymptomatic requiring no specific therapy, but the diagnosis of cutaneous conditions can often help unmask undiagnosed liver disease and facilitate earlier treatment. Despite modern technological advances, physical examination by a care provider remains the cornerstone of medical diagnosis and management.
Efficient and timely bedside diagnosis of chronic liver disease, especially cirrhosis is particularly relevant, as cirrhosis is the eleventh most common cause of death globally according to a recent report in 2019 [2,3]. In the US, an increase in cirrhosis incidence is also associated with increased mortality, hospitalization and cost in the past decade [4–6].
Therefore, general practitioners as well as hepatology and dermatology specialists should be familiar with cutaneous manifestations of liver disease to facilitate early diagnosis and treatment of liver cirrhosis. In this synopsis, we reviewed the association between hormonal, vascular, nail and hair changes as well as other cutaneous manifestations related to liver disease, focusing on cirrhosis and/or portal hypertension. We will also review the pathophysiologic mechanism underlying these cutaneous conditions in patients with cirrhosis and/or portal hypertension, relevant differential diagnoses (Tables 1–3) and management.
Table 1.
Dermatologic vascular manifestations associated with liver and non-liver conditions.
| Cutaneous condition | Figure | Characteristics of skin lesions | Associated liver conditions | Prevalence | Pathogenesis of cirrhosis related skin conditions | Associated non-liver conditions |
|---|---|---|---|---|---|---|
| Hemodynamic and vascular changes | ||||||
| Palmar erythema | Figure 1(a) | Blanchable redness in the skin of the thenar eminence and fingertips | Cirrhosis | 23% | Changes in peripheral hemodynamics increased free oestrogen levels leading to vasodilation of surface capillaries | Rheumatoid arthritis Hyperthyroidism Diabetes Female of childbearing age |
| Spider angioma | Figure 1(b,c) | Central arteriole surrounded by capillaries radiating peripherally above the line joining the nipples | Alcohol-associated cirrhosis Hepatopulmonary syndrome |
33% | Healthy young adolescent Pregnancy Rheumatoid arthritis Hypertrophic osteoarthropathy |
|
| Arteriovenous haemangiomas | Figure 1(d–f) | Bluish erythematous papules or nodules with a diameter of 0.5–1.0 cm in the head and neck | Cirrhosis | NA | Elevated oestrogen levels | Cardiac arteriovenous haemangioma Composite hemangioendothelioma |
| Caput medusa | Figure 1(h) | Distended veins that radiate from the umbilicus across the abdominal wall resembling a ‘caput medusa’ | Cirrhosis Schistosomiasis mansoni Sinus venous thrombosis |
NA | Portal hypertension leading to paraumbilical veins that have closed since birth to reopen | Developmental venous anomaly |
NA: Data unavailable.
Table 2.
Nail changes associated with liver and non-liver conditions.
| Cutaneous condition | Figure | Characteristics of skin lesions | Associated liver conditions | Prevalence | Pathogenesis of cirrhosis related skin conditions | Associated non-liver conditions |
|---|---|---|---|---|---|---|
| Terry’s nails | Figure 2(a–c) | White nail bed A distal brown to pink transverse band of 0.5–2.0 mm in width Absence of lunulae |
Cirrhosis | 25.6% | Decrease in vascularity and increase in connective tissue in the nail bed | Congestive heart failure Adult-onset diabetes mellitus Renal failure Human immunodeficiency disease Ageing |
| Muehrcke’s nail | One or more pale transverse white bands across the nail plate Parallel to the lunula Disappear on pressure |
Cirrhosis | NA | Hypoalbuminemia Oedema of the nail bed causing abnormalities in the vasculature of the nail bed |
Nephrotic syndrome Chemotherapy Renal insufficiency Cushing’s syndrome |
|
| Leukonychia | Figure 2(e) | Diffuse ground glass opacity White in the nail bed |
Alcohol-associated cirrhosis | 2% | Abnormalities in nail bed vascularization | Hereditary leukonychia totalis Onychopapilloma Psoriasis Chemotherapy HIV infection |
| Red and blue lunula | Red/blue discolouration of the lunula | Cirrhosis Wilson’s disease |
NA | Unknown | Systemic lupus erythematosus Lichen planus Erythonychia Rheumatoid arthritis Alopecia areata Chemotherapy |
|
| Clubbing | Figure 2(g) | Increased curvature of the nails Lovibond’s angle Schamroth sign |
Cirrhosis Hepatopulmonary syndrome |
7% | Elevated platelet-derived growth factor and vascular endothelial growth factor | Cyanotic congenital heart disease Pulmonary fibrosis Inflammatory bowel disease Lung cancer Sarcoidosis HIV infection Tuberculosis Hypersensitivity pneumonitis |
| Onycholysis | Figure 2(f) | Detachment of the nail bed from the overlying nail plate | Cirrhosis | NA | Hypoalbuminemia | Psoriasis Pemphigus vulgaris Connective tissue diseases Thyroid diseases Hyperthyroidism Diabetes mellitus |
| Brittle nail | Figure 2(h) | Onychoschizia, onychorrhexis, superficial granulation of keratin and worn-down nail | Chronic liver disease Primary biliary cholangitis |
10% | Impairment of peripheral circulation leading to reduced nail matrix vascularization with production of a thin nail plate. | Drug therapy Nutritional deficiencies Psoriasis Pregnancy |
| Longitudinal striations | Figure 2(i) | Embossed crista on the nail surface accompanied by nail thinning and fracture | Cirrhosis Chronic liver disease |
17% | Unknown | Psoriasis Chronic kidney disease Vitiligo |
| Koilonychia | Figure 2(j) | Concave centrally and raised laterally nail plates | Cirrhosis Hemochromatosis |
NA | Nail matrix changes due to blood flow abnormalities | Iron deficiency anaemia Psoriasis Familial koilonychia Trauma |
| Onychomycosis | Figure 2(k,l) | Nail discolouration, nail separation, brittleness and thickening | Cirrhosis Primary biliary cholangitis |
49% | Fungal infection | Trauma Senility Tinea pedis Diabetes Immunosuppression Malignancies Pemphigus vulgaris |
NA: Data unavailable.
Table 3.
Hair, hormonal and other changes associated with liver and non-liver conditions.
| Cutaneous condition | Figure | Characteristics of skin lesions | Associated liver conditions | Prevalence | Pathogenesis of cirrhosis related skin conditions | Associated non-liver conditions |
|---|---|---|---|---|---|---|
| Hair | ||||||
| Loss of pubic hair or beard | Figure 3(a) | Loss of pubic hair | Cirrhosis | NA | Elevated oestrogen level | Trichotillomania Hypothalamic dysfunction XYY-male Sheehan’s syndrome Menopause Treatment with glucocorticoids |
| Hormonal changes | ||||||
| Gynaecomastia | Figure 3(b) | Breast enlargement | Cirrhosis | 44% | Elevated oestrogen level | Extreme obesity Hypogonadism Adrenal disease Tumours of the adrenal glands, pituitary, lungs and testes Use of digoxin, thiazides, oestrogens, phenothiazines and theophylline Use of methotrexate, alkylating agent, imatinib and vinca alkaloids Use of marijuana Transexual woman Thyroid disorders |
| Others | ||||||
| Pruritus | Cholestatic liver diseases Cirrhosis |
40-60% | Bile salts, endogenous opioids, serotonin, progesterone metabolites and lysophosphatidic acid | Medications Allergies Atopic dermatitis Psoriasis Kidney disease |
||
| Xanthelasma | Figure 3(e) | Pale yellow, planar or slightly bulged, soft plaques around the eyelids | Cholestatic liver disease | NA | Hypercholesterolaemia | Autosomal dominant form of hereditary hypercholesterolaemia Hypercholesterolaemia |
| Pigmentation | Figure 3(f–h) | Blotchy or diffuse muddy gray coloured hyperpigmentation in the pretibial area | Cirrhosis | 46.9% | Red blood cells extravasation and deposition of hemosiderin due to lower extremity edoema and obstruction of venous reflux | Physical dermatosis Inflammatory dermatoses Immunologic dermatoses Allergic/hypersensitivity Cardiac abnormalities |
| Leg ulcer | Figure 3(i) | Necrotic tissue adhering to basilar part and raised edge | Cirrhosis | NA | Hypoxia-ischemia induced by lower extremity edoema and obstruction of venous reflux | Chronic venous insufficiency Peripheral arterial occlusive disease Vasculitis Diabetes Pyoderma gangrenosum Martorell hypertensive leg ulcer |
| Coagulation defects | Skin petechiae, ecchymosis, or mucosal bleeding | Cirrhosis | NA | Thrombocytopenia, hyperfibrinolysis, reduction in most factors and inhibitors of clotting and fibrinolytic systems |
Haematological diseases Trauma Diabetes Uraemia Anticoagulant therapy for thrombotic diseases |
|
NA: Data unavailable.
Ethical approval and considerations
This study was carried out in accordance with the International Conference on Good Clinical Practice Standards and the Declaration of Helsinki, and was approved by the Institutional Review Board of The Second Affiliated Hospital of Xi’an Jiaotong University (2018059; Shaanxi, China). The authors obtained written informed consent from all participants in the study. And all participants agreed to publish their de-identified image data.
Vascular alterations
Hemodynamic changes and palmar erythema
Liver cirrhosis is characterized by splanchnic and peripheral vasodilation, hyperdynamic circulation and local differences in peripheral circulation between the upper and lower limbs as well as the torso, and these hemodynamic differences predispose to the development of skin vascular abnormalities, including spider veins, palmar erythema and warm hands [7–9].
Palmar erythema (Figure 1(a)), also known as liver palms, is characterized by capillary dilatation in the skin of the thenar eminence and fingertips and manifested by blanchable redness of the skin. This condition is caused by elevated serum oestrogen levels and changes in peripheral hemodynamics [8,10]. Though palmar erythema occurs in about two-thirds of patients with liver cirrhosis [10], it is non-specific and can also occur in patients with rheumatoid arthritis, hyperthyroidism, diabetes, juvenile dermatomyositis and pregnancy [10,11].
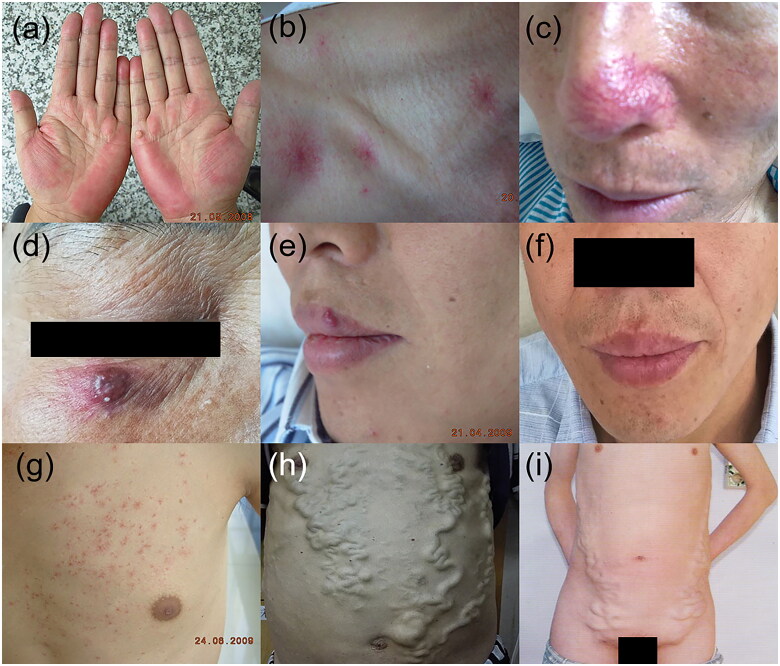
Figure 1.
Vascular alterations in patients with chronic liver disease. (a) Palmar erythema, (b, c) Spider angioma, (d) Arteriovenous haemangioma, (e, f) arteriovenous haemangioma on the upper lip of a 38-year-old cirrhotic male with HBV infection, with remission after 10 years of antiviral treatment. (g) Paper money skin, (h) caput medusa, (i) abdominal varicose veins in inferior vena cava obstruction syndrome.
Spider angioma
Spider angioma (Figure 1(b)) is a small telangiectatic lesion consisting of a central arteriole from which capillaries radiate peripherally [12]. This entity occurs in 10–15% of healthy individuals, especially adolescents, pregnant women and women who use oestrogen for contraception. However, when multiple spider angiomas appear, they may be a skin manifestation of liver disease, especially alcoholic cirrhosis and hepatopulmonary syndrome [13,14]. Approximately 33% of patients with liver cirrhosis have spider angiomas [15]. Spider angiomas usually range from a few millimetres to a few centimetres in diameter. These lesions are commonly observed in the area of the superior vena cava above the line joining the nipples [13,15]. Spider angiomas localized on the nose (Figure 1(c)) should be differentiated from erythematotelangiectatic rosacea (ETTR). In contrast to painless spider angiomas, ETTR leads to sensitive skin prone to irritation upon contact with skin care products [16].
Younger age as well as increased levels of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) were also found to be independent predictors of spider naevus in patients with liver cirrhosis [17]. The high incidence of spider angiomas in patients with alcoholic liver cirrhosis may occur because ethanol induces VEGF expression and promotes angiogenesis [13,18].
Arteriovenous haemangioma
Arteriovenous haemangiomas (AVH) (Figure 1(d)) are benign and acquired vascular tumours characterized by bluish erythematous papules or nodules with a diameter of 0.5–1.0 cm. Lesions present as single erythematous plaques in the head and neck in most cases and are more prevalent in middle-aged men [19], which can regress as liver function improves (Figure 1(e,f)). Histopathological features include well-circumscribed dome-shaped lesions in the upper and mid-reticular dermis with dilated thick- and thin-walled endothelium-lined spaces resembling arteries and veins, respectively. In addition, there are also a small number of inflammatory cells infiltrating interstitial cells, while elastic staining indicates the absence of an internal elastic lamina in these vessels, suggesting the dominance of the venous components [20]. The mechanism of AVH is unclear and may be related to elevated oestrogen levels, though Lee et al. found no oestrogen receptors in the biopsy of AVH in patients with liver cirrhosis [19].
Paper money skin
Paper money skin (Figure 1(g)) is an atypical spider angioma, which manifests as numerous threadlike small blood vessels scattered randomly throughout the skin and disappears under pressure. The distribution is similar to spider angiomas. Lesions are commonly found in the neck, upper torso and upper limbs and resemble security threads on paper money. The mechanism is the same as that of spider angioma [21].
Caput medusa
Severe portal hypertension of various aetiologies including cirrhosis promotes collateral circulation leading to oesophageal, gastric, abdominal and rectal varices. Abdominal varicose veins can be manifested in the superficial veins of the chest and abdomen presenting a net shape, or abdominal veins with obvious varicose beads or masses. Severe abdominal varicose veins appear as distended veins that radiate from the umbilicus across the abdominal wall resembling a ‘caput medusa’ because the affected veins resemble the snake-like hair of Medusa, a gorgon from Greek mythology (Figure 1(h)) [22].
Caput medusa should be differentiated from vena cava obstruction syndrome (Figure 1(i)). In the latter, abdominal varicose veins are located on the right or bilateral abdomen [23]. The blood flow direction of the abdominal varicose veins of the superior vena cava obstruction syndrome is from top to bottom, and that of the inferior vena cava obstruction syndrome is from bottom to top [24].
Superficial abdominal varicose veins are usually asymptomatic and are found during physical examination or abdominal imaging in patients with advanced liver cirrhosis. However, cases of severe superficial abdominal variceal bleeding have been reported in the literature [25]. Local treatment, such as pressure dressings or sutures, may temporarily control bleeding, but it recurs easily due to unrelieved portal hypertension. Transjugular intrahepatic portosystemic shunt (TIPS) may be effective in treating abdominal variceal haemorrhage and reduces the recurrence rate [26,27].
Nail changes
Nail abnormalities are also common among patients with liver diseases. Salem et al. found that nail changes occurred in 68% of patients with chronic liver diseases [28]. The most common abnormalities were onychomycosis and longitudinal striations, but several other nail changes are also associated with liver cirrhosis and will be reviewed below. Although non-specific in most cases, nail changes can help provide diagnostic clues to liver cirrhosis.
Terry’s nails
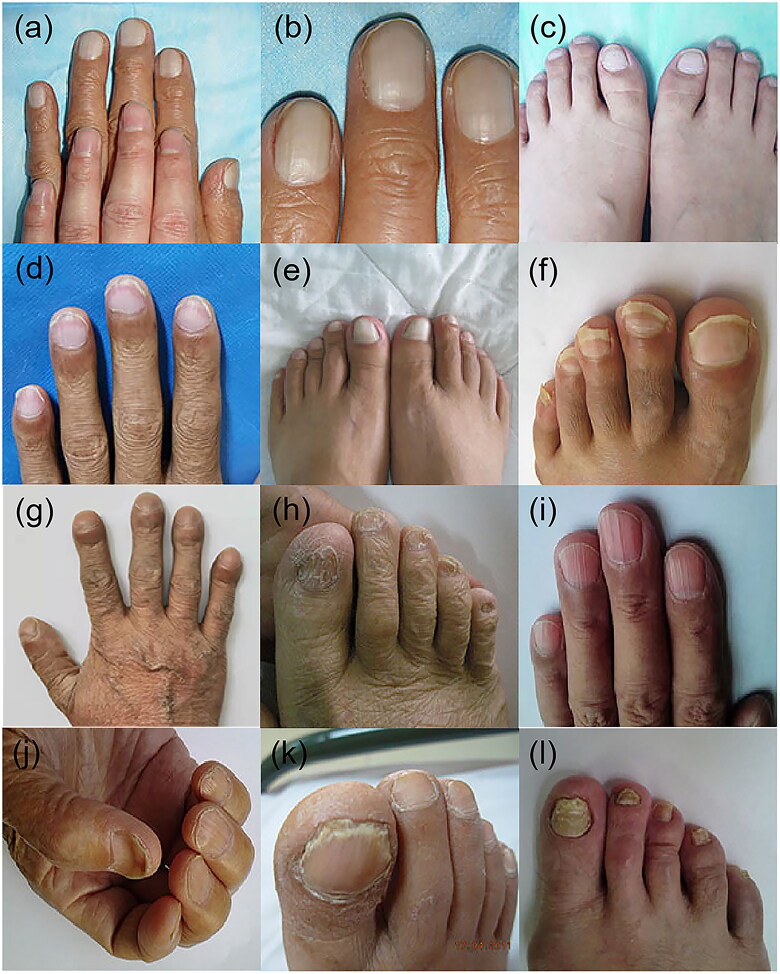
Terry’s nails (Figure 2(a–c)) are characterized by the presence of a white nail bed, a distal brown to pink transverse band of 0.5–2.0 mm in width, and the absence of lunulae [29–31]. First reported by Terry in 1954, this condition is a cardinal sign of hepatic cirrhosis [29,32]. However, Holzberg and Walker found a strong correlation between Terry’s nails and congestive heart failure as well as adult-onset diabetes mellitus in addition to cirrhosis in their study of 512 hospitalized patients [33]. Hyperthyroidism, malnutrition, renal failure and Reiter syndrome have also been associated with Terry’s nails [15,34]. In addition, Terry’s nails are associated with ageing in populations without known systemic diseases [33].
Figure 2.
Nail changes in patients with chronic liver disease. (a–c) Terry’s nails, (d) Lindsay’s nails, (e) leukonychia, (g) clubbing nails, (f) onycholysis, (h) brittle nails, (i) longitudinal striations, (j) koilonychia, (k, l) onychomycosis.
The pathogenesis of Terry’s nails is poorly understood but is thought to be related to telangiectasia [33]. Meanwhile, Lindsay’s nails (Figure 2(d)), the most common differential diagnosis of Terry’s nails, are characterized by the distal extension of the lunula and a pink or reddish-brown distal transverse band, and this condition is closely linked to chronic renal diseases [35].
Muehrcke’s nail
Muehrcke’s nail is a type of leukonychia in which the second, third and fourth fingernails have one or more pale transverse white bands across the nail plate, parallel to the lunula, which disappear on pressure [36]. This condition indicates hypoalbuminemia secondary to cirrhosis, nephrotic syndrome, pellagra and sprue and can resolve by increasing serum levels of albumin [37]. Muehrcke’s lines constitute an abnormality of the vascular nail bed that does not shift as the nails grow [38]. This abnormality is also associated with chemotherapy [39,40].
Leukonychia
Leukonychia (Figure 2(e)), or whitening of the nail plate, also known as ongchostoma sima, is a common disease that was first described in 1919. Leukonychia is classified as acquired or congenital and may be due to an abnormality of the nail bed (pseudoleukonychia) or nail plate (true leukonychia) [41]. True leukonychia showed diffuse ground glass opacity and white colour of the nail bed, which was different from Terry’s nail in that there was no distal pink or brown band.
Red and blue lunula
Red lunula refers to the blanchable red discolouration of the lunula and occurs in patients with cirrhosis, cardiac failure, systemic lupus erythematosus, chronic urticaria, psoriasis and carbon monoxide poisoning [42]. The azure lunula is the blue discolouration of the lunula that occurs in Wilson’s disease, a hereditary disorder of copper metabolism [43].
Clubbing and hypertrophic osteoarthropathy
Clubbing (Figure 2(g)) is the incrassation of the soft organization beneath the proximal nail plate, leading to increased curvature of the nails. Diagnostic findings include Lovibond’s angle, and the Schamroth sign [44]. This condition may indicate cyanotic congenital heart disease, pulmonary fibrosis, bronchial carcinoma, inflammatory bowel disease, cirrhosis and thyroid acropachy. Although the underlying mechanism remains elusive, several hypotheses have been proposed, including neurocirculatory reflex, growth hormone and megakaryocyte/platelet clump [45]. It is important to differentiate between clubbing and hypertrophic osteoarthropathy, which may resemble clubbing but is distinguished from clubbing by the presence of a painful nail bed, while clubbing is asymptomatic. Hypertrophic osteoarthropathy is associated with the paraneoplastic syndrome of several malignancies, including primary liver cancer [46].
Onycholysis
Onycholysis (Figure 2(f)) is the detachment of the nail bed from the overlying nail plate and may be due to trauma, manicuring and photodermatitis. While psoriasis is the most prevalent disease leading to onycholysis [47], there is also a significant association between liver cirrhosis and onycholysis, which have been reported to resolution following liver transplant [48].
Brittle nail syndrome
Brittle nail syndrome (Figure 2(h)) is characterized by increased fragility of the nail plate. The main clinical features are onychoschizia, onychorrhexis, superficial granulation of keratin and worn-down nail. This condition affects up to 20% of the population, especially women over 50 years of age. Brittle nails can be either inherited or acquired and are associated with systemic diseases including liver cirrhosis, drug therapy, nutritional deficiencies, trauma, infections and nail dehydration [49,50].
Longitudinal striations
Longitudinal striations (Figure 2(i)) are embossed crista on the nail surface, accompanied by nail thinning and fracture. The presence of longitudinal striations is a common form of nail dystrophy, which is caused by a deficiency in vitamins and calcium. The condition is termed 20-nail dystrophy in cases involving all nails [40,51].
Koilonychia
Koilonychia (Figure 2(j)) (spoon-shaped nails) is a disorder in which nail plates are concave centrally and raised laterally. Water droplets can converge on the hollow nail plate and can serve as a useful diagnostic tool [52]. Chinazzo et al. performed a prospective observational study and found that the prevalence of koilonychia in healthy newborns was 32.7% [53]. While the abnormality is a natural variant, it is associated with iron deficiency anaemia, hemochromatosis, coronary disease, hypothyroidism and cirrhosis, giving diagnostic clues to these conditions when present in an appropriate context [43].
Onychomycosis
Onychomycosis (Figure 2(k,l)) is a common nail disease caused by dermatophytes, nondermatophytes and yeast. Clinical manifestations include nail discolouration, nail separation, brittleness and thickening that may worsen over time. It may cause local pain, paresthaesia, difficulty performing activities of daily living, and social isolation. Predisposing factors include trauma, senility, tinea pedis, diabetes, immunosuppression and cirrhosis [54,55]. The prevalence of onychomycosis in primary biliary cholangitis (PBC) patients is as high as 49% [55,56].
Hair and hormonal changes
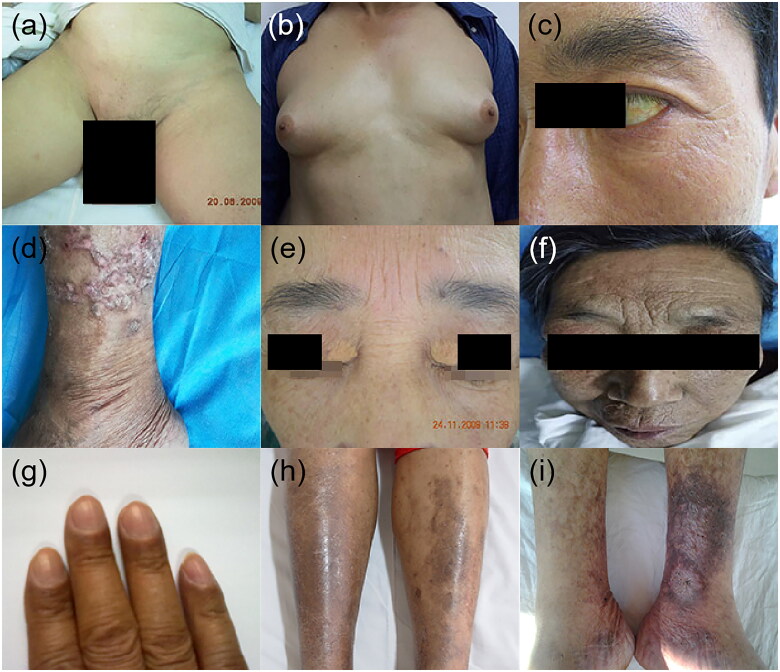
Chronic liver diseases, especially end-stage liver disease such as decompensated cirrhosis, are associated with loss of axillary, arm and pubic hair (Figure 3(a)). In men, liver cirrhosis is linked with a decrease in the growth rate of facial hair, female pubic hair pattern, loss of libido, testicular atrophy, oligospermia and gynaecomastia (Figure 3(b)). Gynaecomastia is the first symptom of liver cirrhosis in some cases and should be differentiated from the gynaecomastia induced by hormone therapy for prostate cancer [57,58]. Commonly-used medications in patients with cirrhosis and ascites such as spironolactone can exacerbate symptoms of gynaecomastia and an alternate dose or therapy may be required [59].
Figure 3.
Hair, hormonal and other dermatologic manifestations in patients with chronic liver disease. (a) Loss of pubic hair, (b) gynaecomastia, (c) jaundice, (d) prurigo nodularis, (e) xanthelasmas, (f–h) pigmentation, (i) leg ulcer.
Other dermatologic manifestations
Jaundice
Jaundice (Figure 3(c)) describes discolouration of the skin, sclera and mucous membranes that is attributable to the accumulation of bilirubin and its metabolites in the tissues. Jaundice generally begins to become visible when the concentration of serum bilirubin surpasses about 2 mg/dL (34 mmol/L). The colour of the skin varies from lemon yellow to apple green, gradually evolving as the serum bilirubin level becomes elevated. According to the presence of conjugated or unconjugated components of bilirubin, we can classify the three major groups of underlying causes of jaundice: prehepatic, intrahepatic or post-hepatic. Prehepatic jaundice involves haemolytic anemias, which can also be seen in neonatal physiological jaundice and breast milk jaundice. Intrahepatic jaundice involves both conjugated and unconjugated hyperbilirubinemia resulting from liver failure-associated severe acute hepatitis or cirrhosis. Other intrahepatic causes of hyperbilirubinemia include intrahepatic cholestatic diseases, such as PBC and the various congenital genetic disorders involving bilirubin metabolism or transport such as Gilbert, Crigler–Najjar syndrome, or Dubin-Johnson syndrome. Post-hepatic jaundice involves conjugated hyperbilirubinemia arising from extrahepatic issues, such as biliary tract obstruction [1].
Pruritus
Pruritus is a sensation that induces persistent or intermittent itching and involuntary scratching. It can affect the whole body or be confined to the limbs, especially the footplate and palm, where more intensive itchiness may occur. It is one of the most common skin abnormalities that occur in liver disease, particularly in patients with cholestatic liver disease. As a frequent concomitant symptom without visible lesions of liver cirrhosis, pruritus is usually linked to cholestasis in PBC, primary sclerosing cholangitis, obstructive gallstone disease and carcinoma of the bile duct. It can also be the most prominent symptom in certain pregnancy-associated liver conditions such as intrahepatic cholestasis of pregnancy [60]. Viral hepatitis-related cirrhosis can lead to intense pruritus, accompanied by solid crusty nodules, which are called prurigo nodularis (Figure 3(d)). Usually distributed in the extremities, especially between the knee and ankle and forearm, the lesion is associated with the topical deposition of an immune complex consisting of HBV/HCV in the skin [1]. Data from a large cohort of patients with chronic liver disease (n = 1631) suggest that the overall prevalence of pruritus was about 40% overall, higher among those with cirrhosis and as high as 50% in those with PBC and 60% in those with autoimmune overlap syndrome [61].
Pruritus may be intermittent and minimally symptomatic but when persistent can lead to a dramatic reduction in quality of life, insomnia, depression and even suicidal attempts. The intensity or pruritus appears to follow a nyctohemeral rhythm, being most severe in the late evening [62,63]. While the health-related quality of life and severity of pruritus appears to be exacerbated by the presence of advanced diseases such as cirrhosis, the ailment development in PBC is not linked to the severity of pruritus [64,65].
The pathogenesis of pruritus in cholestasis is complicated and obscure. Bile salts, endogenous opioids, serotonin, progesterone metabolites and lysophosphatidic acid have all been considered contributory factors [66]. However, the precise role of this substance is unclear due to the lack of an observed relation between concentrations of these molecules and the intensity of the pruritus. Lysophosphatidic acid (commonly referred to as LPA), produced by the enzyme autotaxin (ATX), has also been characterized as an underlying pruritogen in cholestasis. ATX activity and serum LPA levels correlate with the severity of pruritus, suggesting the potential role of each as therapeutic target [67].
Xanthelasmas or xanthomas
Xanthelasma (Figure 3(e)) manifests as pale yellow, planar or slightly bulged, soft plaques around the eyelids, being essentially subcutaneous lipid deposits. The condition is associated with dyslipoproteinaemia secondary to liver diseases such as PBC and other forms of cholestatic liver disease. The condition is seen most frequently in females over 50 years old, and half of the cases present with comorbid dyslipidemia. Xanthelasmas associated with the autosomal dominant form of hereditary hypercholesterolaemia develop during childhood and the clinical profile includes xanthelasma, tendon xanthoma, increased low-density lipoprotein, arcus corneae and premature coronary artery disease [68]. One case of regression has been reported after liver transplantation in a patient with PBC [69].
Pigmentation
Patients with cirrhosis usually have abnormal pigmentation. The pigmentation on the face is manifested by a muddy gray complexion, called hepatic face (Figure 3(f)), which is induced by hormone metabolism turbulence. Pigmentation can also occur in terminals of the extremities (Figure 3(g)), presenting with black discolouration, especially common in patients with cirrhosis. When occurring in the tibial anterior (Figure 3(h)), pigmentation is caused by the extravasation of red blood cells and deposition of hemosiderin due to lower extremity edoema and obstruction of venous reflux [70].
Leg ulcer
Leg ulcer (Figure 3(i)) appears as a lesion in which necrotic tissue adheres to the basilar part and the edge is raised, often occurring on the tibial anterior. It is related to hypoxia-ischemia induced by lower extremity edoema and obstruction of venous reflux.
Coagulation defects
Cirrhosis is considered to be a thrombosis-prone state because venous stasis from portal hypertension and the inflammatory milieu promote the development of portal vein thrombosis [71]. However, coagulation defects can also be observed in cirrhosis [72,73]. In patients with cirrhosis, coagulation defects manifest as skin petechiae, ecchymosis, or mucosal bleeding, the causes being complex and multiple. Cirrhosis and portal hypertension are often marked with thrombocytopenia. While patients with cirrhosis typically have platelet counts adequate for the production of thrombin at a level equivalent to the rock bottom of the normal extent in a healthy population, the balance of fibrinolysis may be altered by acute events such as infection leading to hyperfibrinolysis, which increases the risk of haemorrhage. Moreover, in patients with cirrhosis, protein synthesis often is diminished, leading to a dramatic reduction in most factors and inhibitors of clotting and fibrinolytic systems, leading to further coagulation defects [72,73].
Conclusions
A broad range of cutaneous alterations can be present in patients with liver cirrhosis, portal hypertension and other chronic liver diseases. Vascular changes in the upper part of the body are important clues in the diagnosis of liver cirrhosis, and toenail changes and other skin signs may indicate potential chronic liver diseases, especially liver cirrhosis. While the majority of these cutaneous conditions are asymptomatic, some may require specific therapy, such as pruritus and all should be recognized as signs of potential underlying diseases, including liver cirrhosis, so that underlying conditions can be promptly diagnosed and appropriate management initiated.
Author contributions
Conception and design: Y.L., Y.Z., F.J., Z.L. and M.H.N.; literature search and investigation: Y.L., Y.Z., X.G. and L.J.; writing-original draft: Y.L., Y.Z. and F.J.; writing-review and editing: F.J., Y.H., Z.L. and M.H.N.
Funding Statement
This report was funded by the National Natural Science Foundation of China [81773327, 82170626]; Excellent Series of Teaching Materials for Graduate Students of Xi’an Jiaotong University in the ‘14th 5-year Plan’ [JC2020-10120].
Disclosure statement
No potential conflict of interest was reported by all the authors. All authors agree to be accountable for all aspects of the work. The funding source had no involvement in writing the report.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Hazin R, Abu-Rajab Tamimi TI, Abuzetun JY, et al. Recognizing and treating cutaneous signs of liver disease. Cleve Clin J Med. 2009;76(10):599–606. [DOI] [PubMed] [Google Scholar]
- 2.Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. [DOI] [PubMed] [Google Scholar]
- 3.Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68(6):1–77. [PubMed] [Google Scholar]
- 4.Tapper EB, Parikh ND.. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471–1482. [DOI] [PubMed] [Google Scholar]
- 6.Zou B, Yeo YH, Jeong D, et al. A nationwide study of inpatient admissions, mortality, and costs for patients with cirrhosis from 2005 to 2015 in the USA. Dig Dis Sci. 2020;65(5):1520–1528. [DOI] [PubMed] [Google Scholar]
- 7.Møller S, Bendtsen F.. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018;38(4):570–580. [DOI] [PubMed] [Google Scholar]
- 8.Okumura H, Aramaki T, Katsuta Y, et al. Regional differences in peripheral circulation between upper and lower extremity in patients with cirrhosis. Scand J Gastroenterol. 1990;25(9):883–889. [DOI] [PubMed] [Google Scholar]
- 9.Steele JD, Dillon JF, Plevris JN, et al. Hand skin temperature changes in patients with chronic liver disease. J Hepatol. 1994;21(6):927–933. [DOI] [PubMed] [Google Scholar]
- 10.Serrao R, Zirwas M, English JC.. Palmar erythema. Am J Clin Dermatol. 2007;8(6):347–356. [DOI] [PubMed] [Google Scholar]
- 11.Amoroso S, Pastore S, Tommasini A, et al. Palmar erythema: a diagnostic clue of juvenile dermatomyositis. J Paediatr Child Health. 2020;56(7):1159. [DOI] [PubMed] [Google Scholar]
- 12.Li CP, Lee FY, Hwang SJ, et al. Role of substance P in the pathogenesis of spider angiomas in patients with nonalcoholic liver cirrhosis. Am J Gastroenterol. 1999;94(2):502–507. [DOI] [PubMed] [Google Scholar]
- 13.Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function. Scand J Gastroenterol. 1999;34(5):520–523. [DOI] [PubMed] [Google Scholar]
- 14.Silvério AdO, Guimarães DC, Elias LFQ, et al. Are the spider angiomas skin markers of hepatopulmonary syndrome? Arq Gastroenterol. 2013;50(3):175–179. [DOI] [PubMed] [Google Scholar]
- 15.Ghosn SH, Kibbi AG.. Cutaneous manifestations of liver diseases. Clin Dermatol. 2008;26(3):274–282. [DOI] [PubMed] [Google Scholar]
- 16.Yigider AP, Kayhan FT, Yigit O, et al. Skin diseases of the nose. Am J Rhinol Allergy. 2016;30(3):83–90. [DOI] [PubMed] [Google Scholar]
- 17.Li CP, Lee FY, Hwang SJ, et al. Spider angiomas in patients with liver cirrhosis: role of vascular endothelial growth factor and basic fibroblast growth factor. World J Gastroenterol. 2003;9(12):2832–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu JW, Elam J, Sartin A, et al. Moderate levels of ethanol induce expression of vascular endothelial growth factor and stimulate angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R365–R372. [DOI] [PubMed] [Google Scholar]
- 19.Lee HW, Yun WJ, Choi JC, et al. Arteriovenous haemangioma in chronic liver diseases: case report and determination of oestrogen receptor status. J Eur Acad Dermatol Venereol. 2006;20(7):884–885. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama M, Inamoto N.. Arteriovenous haemangioma in chronic liver disease: clinical and histopathological features of four cases. Br J Dermatol. 2001;144(3):604–609. [DOI] [PubMed] [Google Scholar]
- 21.Smith KE, Fenske NA.. Cutaneous manifestations of alcohol abuse. J Am Acad Dermatol. 2000;43(1):1–16. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Zhi XT, Hu SY.. Congenital portal venous system aneurysms associated with caput medusae. Hepatology. 2011;53(3):1052–1053. [DOI] [PubMed] [Google Scholar]
- 23.Qi X, Han G.. Images in clinical medicine. Abdominal-wall varices in the Budd-Chiari syndrome. N Engl J Med. 2014;370(19):1829. [DOI] [PubMed] [Google Scholar]
- 24.Philips CA, Arora A, Shetty R, et al. A comprehensive review of portosystemic collaterals in cirrhosis: historical aspects, anatomy, and classifications. Int J Hepatol. 2016;2016:6170243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen PT, Tzeng HL, Wang HP, et al. Caput medusae bleeding. Am J Gastroenterol. 2020;115(10):1570. [DOI] [PubMed] [Google Scholar]
- 26.Sze DY, Magsamen KE, McClenathan JH, et al. Portal hypertensive hemorrhage from a left gastroepiploic vein caput medusa in an adhesed umbilical hernia. J Vasc Interv Radiol. 2005;16(2):281–285. [DOI] [PubMed] [Google Scholar]
- 27.Strauss C, Sivakkolunthu M, Ayantunde AA.. Recurrent and troublesome variceal bleeding from parastomal caput medusae. Korean J Gastroenterol. 2014;64(5):290–293. [DOI] [PubMed] [Google Scholar]
- 28.Salem A, Gamil H, Hamed M, et al. Nail changes in patients with liver disease. J Eur Acad Dermatol Venereol. 2010;24(6):649–654. [DOI] [PubMed] [Google Scholar]
- 29.Terry R. White nails in hepatic cirrhosis. Lancet. 1954;266(6815):757–759. [DOI] [PubMed] [Google Scholar]
- 30.Nia AM, Ederer S, Dahlem KM, et al. Terry’s nails: a window to systemic diseases. Am J Med. 2011;124(7):602–604. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Ji F, Deng H.. Terry’s nails. Braz J Infect Dis. 2012;16(3):311–312. [PubMed] [Google Scholar]
- 32.Nelson N, Hayfron K, Diaz A, et al. Terry’s nails: clinical correlations in adult outpatients. J Gen Intern Med. 2018;33(7):1018–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holzberg M, Walker HK.. Terry’s nails: revised definition and new correlations. Lancet. 1984;1(8382):896–899. [DOI] [PubMed] [Google Scholar]
- 34.Coskun BK, Saral Y, Ozturk P, et al. Reiter syndrome accompanied by terry nail. J Eur Acad Dermatol Venereol. 2005;19(1):87–89. [DOI] [PubMed] [Google Scholar]
- 35.Pitukweerakul S, Pilla S.. Terry’s nails and Lindsay’s nails: two nail abnormalities in chronic systemic diseases. J Gen Intern Med. 2016;31(8):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma V, Kumar V.. Muehrcke lines. Canadian Med Assoc J. 2013;185(5):E239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozaru T, Fukumoto T, Shirai T, et al. Muehrcke lines on fingers and toenails. Eur J Dermatol. 2019;29(1):87–88. [DOI] [PubMed] [Google Scholar]
- 38.Fawcett RS, Linford S, Stulberg DL.. Nail abnormalities: clues to systemic disease. Am Fam Physician. 2004;69:1417–1424. [PubMed] [Google Scholar]
- 39.Chen W, Yu YS, Liu YH, et al. Nail changes associated with chemotherapy in children. J Eur Acad Dermatol Venereol. 2007;21(2):186–190. [DOI] [PubMed] [Google Scholar]
- 40.Chang X, Zhen P, Zeng J.. Fingernails changes associated with chemotherapy in breast cancer: Muehrcke’s lines. Clin Case Rep. 2018;6(8):1653–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ates D, Kosemehmetoglu K.. Acquired leukonychia of the distal nail plate: a morphologic and proteomic analysis. Am J Dermatopathol. 2020;42(4):261–264. [DOI] [PubMed] [Google Scholar]
- 42.Wilkerson MG, Wilkin JK.. Red lunulae revisited: a clinical and histopathologic examination. J Am Acad Dermatol. 1989;20(3):453–457. [DOI] [PubMed] [Google Scholar]
- 43.Dzieżyc-Jaworska K, Litwin T, Członkowska A.. Clinical manifestations of Wilson disease in organs other than the liver and brain. Ann Transl Med. 2019;7(2):S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregoriou S, Argyriou G, Larios G, et al. Nail disorders and systemic disease: what the nails tell us. J Fam Pract. 2008;57(8):509–514. [PubMed] [Google Scholar]
- 45.Spicknall KE, Zirwas MJ, English JC. 3rd.. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005;52(6):1020–1028. [DOI] [PubMed] [Google Scholar]
- 46.Callemeyn J, Van Haecke P, Peetermans WE, et al. Clubbing and hypertrophic osteoarthropathy: insights in diagnosis, pathophysiology, and clinical significance. Acta Clin Belg. 2016;71(3):123–130. [DOI] [PubMed] [Google Scholar]
- 47.Zaias N, Escovar SX, Zaiac MN.. Finger and toenail onycholysis. J Eur Acad Dermatol Venereol. 2015;29(5):848–853. [DOI] [PubMed] [Google Scholar]
- 48.Gandhi V, Nagral A, Philip S, et al. Reversal of nail changes after liver transplantation in a child. Indian J Gastroenterol. 2009;28(4):154–156. [DOI] [PubMed] [Google Scholar]
- 49.Chessa MA, Iorizzo M, Richert B, et al. Pathogenesis, clinical signs and treatment recommendations in brittle nails: a review. Dermatol Ther. 2020;10(1):15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Kerkhof PC, Pasch MC, Scher RK, et al. Brittle nail syndrome: a pathogenesis-based approach with a proposed grading system. J Am Acad Dermatol. 2005;53(4):644–651. [DOI] [PubMed] [Google Scholar]
- 51.Solak B, Acikgoz SB, Sipahi S, et al. Cutaneuos findings in patients with predialysis chronic kidney disease. J Eur Acad Dermatol Venereol. 2016;30(9):1609–1613. [DOI] [PubMed] [Google Scholar]
- 52.Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30(11):1985–1991. [DOI] [PubMed] [Google Scholar]
- 53.Chinazzo M, Lorette G, Baran R, et al. Nail features in healthy term newborns: a single-Centre observational study of 52 cases. J Eur Acad Dermatol Venereol. 2017;31(2):371–375. [DOI] [PubMed] [Google Scholar]
- 54.Gupta AK, Versteeg SG, Shear NH.. Onychomycosis in the 21st century: an update on diagnosis, epidemiology, and treatment. J Cutan Med Surg. 2017;21(6):525–539. [DOI] [PubMed] [Google Scholar]
- 55.Lipner SR, Scher RK.. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80(4):835–851. [DOI] [PubMed] [Google Scholar]
- 56.Koulentaki M, Ioannidou D, Stefanidou M, et al. Dermatological manifestations in primary biliary cirrhosis patients: a case control study. Am J Gastroenterol. 2006;101(3):541–546. [DOI] [PubMed] [Google Scholar]
- 57.Michalopoulos NV, Keshtgar MR.. Images in clinical medicine. Gynecomastia induced by prostate-cancer treatment. N Engl J Med. 2012;367(15):1449. [DOI] [PubMed] [Google Scholar]
- 58.Neong SF, Billington EO, Congly SE.. Sexual dysfunction and sex hormone abnormalities in patients with cirrhosis: review of pathogenesis and management. Hepatology. 2019;69(6):2683–2695. [DOI] [PubMed] [Google Scholar]
- 59.Sehgal R, Singh H, Singh IP.. Comparative study of spironolactone and eplerenone in management of ascites in patients of cirrhosis of liver. Eur J Gastroenterol Hepatol. 2020;32(4):535–539. [DOI] [PubMed] [Google Scholar]
- 60.Glantz A, Reilly SJ, Benthin L, et al. Intrahepatic cholestasis of pregnancy: amelioration of pruritus by UDCA is associated with decreased progesterone disulphates in urine. Hepatology. 2008;47(2):544–551. [DOI] [PubMed] [Google Scholar]
- 61.Oeda S, Takahashi H, Yoshida H, et al. Prevalence of pruritus in patients with chronic liver disease: a multicenter study. Hepatol Res. 2018;48(3):E252–E262. [DOI] [PubMed] [Google Scholar]
- 62.Montagnese S, Nsemi LM, Cazzagon N, et al. Sleep-wake profiles in patients with primary biliary cirrhosis. Liver Int. 2013;33(2):203–209. [DOI] [PubMed] [Google Scholar]
- 63.Bergasa NV, Alling DW, Talbot T, et al. Effects of naloxone infusions in patients with the pruritus of cholestasis. A double-blind, randomized, controlled trial. Ann Intern Med. 1995;123(3):161–167. [DOI] [PubMed] [Google Scholar]
- 64.Talwalkar JA, Souto E, Jorgensen RA, et al. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2003;1(4):297–302. [PubMed] [Google Scholar]
- 65.Raszeja-Wyszomirska J, Wunsch E, Krawczyk M, et al. Assessment of health related quality of life in polish patients with primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. 2016;40(4):471–479. [DOI] [PubMed] [Google Scholar]
- 66.Shah RA, Kowdley KV.. Mechanisms and treatments of pruritus in primary biliary cholangitis. Semin Liver Dis. 2019;39(2):209–220. [DOI] [PubMed] [Google Scholar]
- 67.Carrion AF, Rosen JD, Levy C.. Understanding and treating pruritus in primary biliary cholangitis. Clin Liver Dis. 2018;22(3):517–532. [DOI] [PubMed] [Google Scholar]
- 68.Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(2):181–188. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt HH, Manns MP.. Images in hepatology. Regression of xanthelasmas in a patient with primary biliary cirrhosis after liver transplantation. J Hepatol. 1998;28(6):1077. [DOI] [PubMed] [Google Scholar]
- 70.Dogra S, Jindal R.. Cutaneous manifestations of common liver diseases. J Clin Exp Hepatol. 2011;1(3):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rugivarodom M, Charatcharoenwitthaya P.. Nontumoral portal vein thrombosis: a challenging consequence of liver cirrhosis. J Clin Transl Hepatol. 2020;28(8):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tripodi A, Mannucci PM.. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–156. [DOI] [PubMed] [Google Scholar]
- 73.Mammen EF. Coagulation defects in liver disease. Med Clin North Am. 1994;78(3):545–554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.