ABSTRACT
Adoptive transfer of γδ T cells is a novel immunotherapeutic approach to glioblastoma. Few recent studies have shown the efficacy of γδ T cells against glioblastoma, but no previous studies have identified the ligand–receptor interactions between γδ T cells and glioblastoma cells. Here, we identify those ligand–receptor interactions and provide a basis for using γδ T cells to treat glioblastoma. Vγ9Vδ2 T cells were generated from peripheral blood mononuclear cells of healthy donors using artificial antigen presenting cells. MICA, ULBP, PVR and Nectin-2 expression in 10 patient-derived glioblastoma (PDG) cells were analyzed. The in vitro cytokine secretion from the γδ T cells and their cytotoxicity toward the PDG cells were also analyzed. The in vivo anti-tumor effects were evaluated using a U87 orthotopic xenograft glioblastoma model. Expression of ligands and cytotoxicity of the γδ T cells varied among the PDG cells. IFN-γ and Granzyme B secretion levels were significantly higher when γδ Tcells were co-cultured with high-susceptible PDG cells than when they were co-cultured with low-susceptible PDG cells. Cytotoxicity correlated significantly with the expression levels of DNAM-1 ligands of the PDG cells. Blocking DNAM-1 resulted in a decrease in γδ T cell–mediated cytotoxicity and cytokine secretion. Intratumoral injection of γδ T cells showed anti-tumor effects in an orthotopic mouse model. Allogenic γδ T cells showed potent anti-tumor effects on glioblastoma in a DNAM-1 axis dependent manner. Our findings will facilitate the development of clinical strategies using γδ T cells for glioblastoma treatment.
KEYWORDS: Γδ T cells, glioblastoma, adoptive cell transfer, PVR (CD-155), Nectin-2, DNAM-1 receptor (CD266)
Introduction
Glioblastoma is the most common primary brain malignancy and has the most lethal prognosis; the median overall survival is still less than 2 years, and 5-year survival rates are lower than 10% despite aggressive multimodal standard treatments that include maximal surgical resection and chemoradiation followed by 6 cycles of chemotherapy.1,2 In the past two decades, the survival outcomes of several cancers, such as melanoma, non-small cell lung cancer, and leukemias, have improved dramatically as the result of targeted therapy and immunotherapy.3–5 However, the prognosis of glioblastoma has not improved much.6,7 Recent phase III clinical trials using an immune checkpoint inhibitor (ICI) for recurrent glioblastoma failed to improve survival outcomes.7 As an alternative approach, adoptive transfer of immune cells, such as T cells, natural killer (NK) cells, or γδ T cells, with or without genetic engineering has recently been tried on ICI-resistant cancers, including glioblastoma.8–10
Human γδ T cells are a small subset of T cells and have been relatively unstudied compared with conventional αβ T cells.11,12 Their immune surveillance function and ability to recognize transformed cells in a major histocompatibility complex-unrestricted manner has been considered an advantage to their use in anti-tumor therapy.13 Along with the γδ T cell receptor, various NK receptors are also involved in regulating γδ T cell activation and its anti-tumor function.14–16 For example, NKG2D, which binds to MICA, MICB, and ULBP is known to activate γδ T cells, along with DNAM-1, which binds to poliovirus receptor (PVR, CD155) and Nectin-2.14,16 In human peripheral blood, Vγ9Vδ2 T cells, which constitute 1–5% of peripheral blood lymphocytes,15 are the main subtype of γδ T cells. Vγ9Vδ2 T cells can be expanded easily using phosphoantigens or artificial antigen presenting cells (aAPCs).17,18 Therefore, adoptive transfer of γδ T cells has been suggested as a new immunotherapeutic approach for various cancers.19 A few recent studies have suggested that activated human Vγ9Vδ2 T cells can kill glioma and glioblastoma cells in vitro and in vivo.20–24 However, among the different ligand-receptor interactions, which ligand-receptor interaction plays an important role in the cytotoxicity of γδ T cells has not yet been defined.
In this study, we demonstrate that allogenic human γδ T cells activated by aAPCs can kill patient-derived glioblastoma (PDG) cells in vitro and in vivo. Interestingly, we found that the ligand expression of glioblastoma cells varied among patients and that among them the expression of DNAM-1 ligands could be significantly related to the anti-tumor effects of γδ T cells in glioblastoma. The potent efficacy of γδ T cells against PDG cells shown here and our identification of specific targets on glioblastoma could facilitate the development of clinical γδ T cell immunotherapy for glioblastoma treatment.
Materials and methods
Generation of γδ T cells
γδ T cells were cultured as previously described.17 Briefly, peripheral blood mononuclear cells from five healthy donors were stimulated with 3 μM zoledronic acid (Daewoong Pharmaceutical, Korea) and 1000 U/mL of IL-2 (Proleukin, Clinigen, United Kingdom). After 7 days, the cells were treated weekly with irradiated (100 Gy) aAPCs in a T cell: aAPC ratio of 2:1 in the presence of 1000 U/mL of IL-2. The cells were cultured until day 14 or 21 and then frozen using a controlled-rate freezer (Thermo Fisher Scientific, MA) and cryopreserved in liquid nitrogen until further use. For the in vitro and in vivo assays, the frozen γδ T cells were thawed and rested overnight in the presence of IL-2 (1000 U/mL). The detailed information of generation of our γδ T cells were presented in a previous study.17
Cells
All tissues were obtained from Seoul St. Mary’s Hospital under the guidance of our institutional review boards. After freshly resected samples were obtained from glioblastoma patients, the tumor tissue was chopped and suspended in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, MA) supplemented with 20% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Thermo Fisher Scientific, MA). All cells were maintained at 37°C in humidified air with 5% CO2. The clinical characteristics of the PDG cells are provided in the supplementary table.
Glioma cell lines (T98G, U87, U138, and U373) were purchased from the American Type Culture Collection and cultured in high-glucose DMEM (Thermo Fisher Scientific, MA) supplemented with 10% FBS (Thermo Fisher Scientific, MA) and 10,000 μg/ml of penicillin and streptomycin (Thermo Fisher Scientific, MA). For luciferase expression in the mouse model, U87-luciferase cells were transduced using a lentiviral vector containing a luciferase gene.
Flow cytometry analysis
The expression of ligands on tumor cells was analyzed with flow cytometry. PDG cells were harvested when they reached optimal cell density with 0.25% trypsin-EDTA and PBS containing 2%FBS was used as a staining buffer. After staining with antibodies for 30 minutes at 4°C, cells were washed. Data acquisition was performed on BD FACS Canto (Becton Dickinson BD Biosciences, San Jose, CA, USA), and FlowJo software (v10.0.7, FlowJo LLC, Ashland, OR, USA) was used to analyze the data. The following antibodies were used for flow cytometry: human MICA PE-conjugated antibody (clone 159227) and human ULBP-2/5/6 APC-conjugated antibody (clone 165903), which were purchased from R&D Systems; and APC/Fire 750 anti-human CD155 (PVR) antibody (clone SKII.4) and APC anti-human CD112 (Nectin-2) antibody (clone TX31), which were purchased from BioLegend. Isotype controls for each antibody were used according to each manufacturer’s instructions.
Cytotoxicity and cytokine secretion
Cytotoxicity assay was performed using Calcein-AM (Invitrogen, MA). Target cells were stained with 5 μM of Calcein-AM and incubated for 20 minutes at 37°C with 5% CO2 in the dark. After incubation, the target cells were washed twice with phosphate-buffered saline (PBS) and suspended in DMEM with 20% FBS at 1 × 105 cells/mL. Effector cells and target cells were co-cultured in a 96-well V-bottomed plate at an E:T ratio of 20:1. For maximum release, Triton X-100 was added to a final concentration of 2%. For spontaneous fluorescence, only target cells were incubated. After 4 hours of incubation, the plates were centrifuged (2500rpm, 5 min), and the supernatants were carefully transferred to an opaque black 96-well plate. The fluorescence was measured with a microplate reader (Synergy H1, Biotek, VT). Specific release was calculated as (Experimental fluorescence) – (Spontaneous fluorescence)/(Maximum fluorescence) – (Spontaneous fluorescence) × 100. For the cytokine secretion analysis, 1 × 105 γδ T cells and 5 × 104 target cells were co-cultured for 24 hours, and then the supernatants were analyzed with a LEGENDplex Human CD8/NK panel (Biolegend, CA) according to the manufacturer’s protocol. Cytokine concentration was calculated as (cytokine secretion from γδ T cells co-cultured with tumor cell lines)-(cytokine secretion from tumor cells alone). For blocking assays, γδ T cells were treated with mouse IgG1 κ isotype control antibody (clone MOPC-21), anti-human CD226 (DNAM-1) antibody (clone 11A8), or anti-human CD314 (NKG2D) antibody (clone 1D11) at the concentration of 2.5 μg/mL for 15 min at room temperature before adding the target cells. For blocking PVR and Nectin-2, anti-human CD155 (PVR) antibody (clone SKII.4) and anti-human CD112 (Nectin-2) antibody (clone TX31) was used at the concentration of 20ug/mL.
3D spheroid generation and viability assay
3D spheroids of the U87 cell line and PDG cells were generated by seeding 5 × 103/50 μl of cells to a low-adherent 96-well plate. On day 4, 2 × 106 γδ T cells or culture medium was added to a final volume of 100 μl. After 24 hours of co-culture, the viability of the 3D spheroids was analyzed using CellTiter-Glo (Promega, Madison, WI) following the manufacturer’s protocol. Briefly, 100ul of CellTiter-Glo reagent was added to the wells after acclimating the spheroid and reagent to room temperature for 30 minutes. Wells were thoroughly mixed to lyse viable cells and luminescence was measured with Synergy H1 microplate reader (BioTek, VT). The luminescence of the 3D spheroids treated with γδ T cells was calculated as (luminescence of 3D spheroids with γδ T cells)-(luminescence of γδ T cells alone).
Animals and adoptive γδ T cell transfer
All procedures for the mouse experiments and determination of endpoints were carried out in accordance with guidelines approved by the animal institutional review boards of our institution. For the orthotopic intracranial glioblastoma-bearing mice, 7.5 × 104 U87 cells were stereotactically inoculated with 3 μl of PBS into the right frontal lobes of NSG mice (NOD.Cg-Prkdc<scid> Il2rg<t. m1Wjl>/SzJ, Jackson Laboratory) 2 mm lateral and 1 mm anterior to the bregma at 2.5 mm depth from the skull base using a Hamilton syringe (Hamilton Company) and a micro-infusion pump (Harvard Apparatus). After tumor implantation, tumor size was monitored in vivo by luciferase bioluminescence imaging using an IVIS Lumina XRMS (PerkinElmer), and the mice were randomly allocated into treatment arms. In the early model, 2 × 106 human γδ T cells were injected on day 4 intracranially. In the advanced model, 2 × 106 human γδ T cells were injected on day 7 and day 14 intracranially. In the control group, same volume of PBS was injected at the same site. We chose intracranial injection over intravenous injection according to previous studies showing the superior anti-tumor effects of intracranial injection of T cells in a glioblastoma orthotopic mouse model.10,25 Tumor size and survival were monitored every week. They were euthanized when they showed predetermined signs of neurologic deficits (failure to ambulate, weight loss >20% body mass, lethargy, hunched posture).
Immunofluorescence
To verify tumor size using a histologic analysis, the mice were sacrificed on day 10 after tumor implantation. The mouse brains were perfused with 4% paraformaldehyde under deep anesthesia. Then, it was fixed within 100% methanol (chilled to −20°C) at room temperature for 5 min. Fixed tissues were cryo-sectioned (14-µm sections) and stained with hematoxylin and eosin without antigen retrieval steps. For immunofluorescence staining, we used the following antibodies: for γδ TCR (clone 5A6.E9, Thermo Fisher Scientific, MA), for PVR (clone D8A5G, Cell Signaling Technology, MA), Nectin-2 (clone AF2229, R&D Systems, MN), Alexa 488-conjugated goat anti-rat IgG (A-11006, I Thermo Fisher Scientific, MA), Alexa 546-conjugated goat anti-mouse IgG (A-11010, Thermo Fisher Scientific, MA), and Alexa 546-conjugated goat anti-rabbit IgG (A-11057, Thermo Fisher Scientific, MA). Nuclei were counterstained with 40, 6-diamidino-2-phenylindole (DAPI; Sigma). Fluorescence images were acquired using an LSM900 confocal microscope (Carl Zeiss, Oberkochen, Germany).
RNA sequencing
All PDG cells were thawed and washed with PBS. Messenger RNA sequencing analysis was performed with 1 × 10^6 viable cells from Macrogen, Inc (Seoul, South Korea). RNA sequencing was performed using the TruSeq stranded RNA protocol on Illumina. Reads were trimmed based on the Fastp package and quality was checked by FASTQC. Trimmed reads were aligned on a Homo sapiens reference sequence (GRCh38) using HISAT2. Read counts were normalized and analyzed by StringTie. Differentially expressed genes were defined by DESeq2 in R v 4.1.3. Normalized data were visualized using the plotPCA function.
Statistical analysis and software
The statistical significance of differences in cytotoxicity and cytokine secretion was assessed by paired t-testing. Correlations between ligand expression levels and cytotoxicity were analyzed by Pearson’s correlation. The survival curve was calculated with the Kaplan-Meier equation, and the statistical significance of survival differences between groups was assessed by the log-rank test. All statistical analyses were performed and graphs produced using the R version 1.4.3 software program (R Foundation for Statistical Computing, Vienna, Austria) and Prism Software (GraphPad 9.0).
Results
Different susceptibility of patient-derived glioblastoma cells to γδ T cells
To identify whether human glioma cell lines express ligands that bind to the activating receptors of γδ T cells, we analyzed MICA, ULBP, PVR and Nectin-2 expression (Supplementary Figure 1). MICA was rarely expressed in the U87, U138, and U373 cell lines (0.8%, 1.51%, and 0.91% respectively). ULBP was highly expressed in U138 and U373 cells (91.4% and 98.6%, respectively), but ULBP expression in U87 cells was relatively low (32.8%). PVR and Nectin-2 were highly expressed in all those cell lines (94.5% and 89.0% for U87, 99.2% and 70.8% for U138, and 65.7% and 94.8% for U373, respectively) (Figure 1a). We next evaluated the cytotoxic effects of γδ T cells toward the human glioma cell lines. U87 had the highest susceptibility to γδ T cell–mediated cytotoxicity, with 60.2% ± 15.0 (average ± SD) of specific lysis, followed by U138 and U373 at 43.8% ± 6.9 and 33.2% ± 5.0, respectively (Figure 1b). γδ T cells also showed cytotoxic effects on the U87 3D spheroid models (Supplementary Figure 2A, B).
Figure 1.
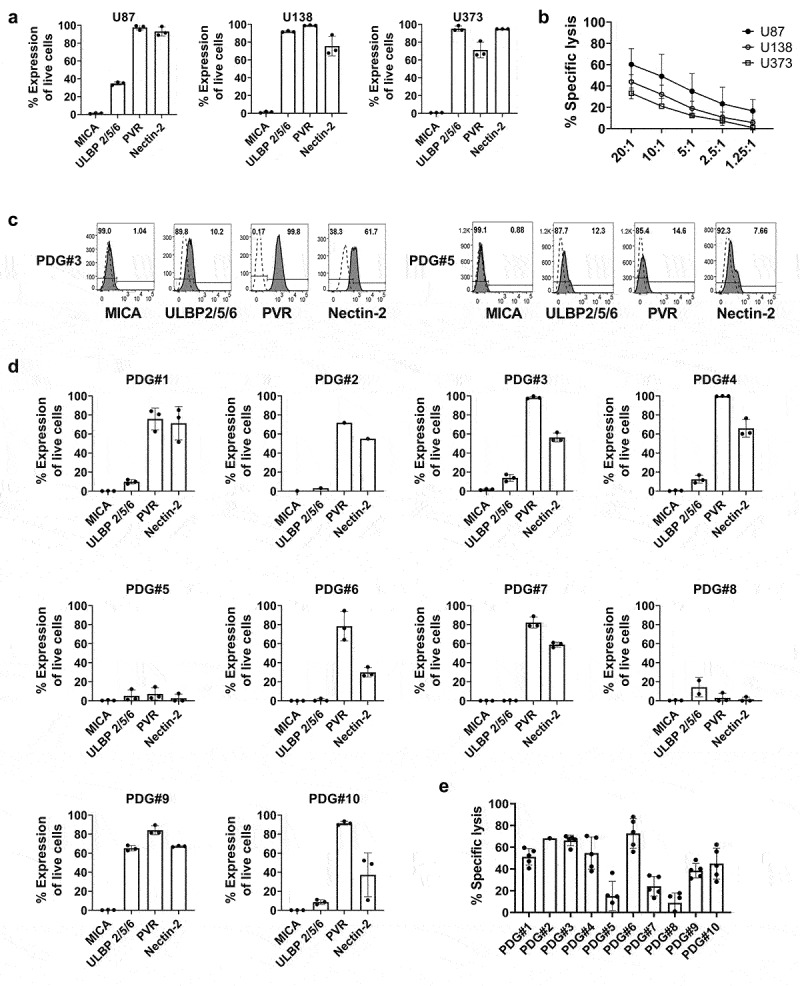
Expression of various ligands on glioblastoma tumor cell lines and γδ T cell–mediated cytotoxicity. (a) The expression of MICA, ULBP 2/5/6, PVR, and Nectin-2 on the glioblastoma cell lines U87, U373, and U138 was analyzed three times by flow cytometry. (b) The cytotoxic effect of γδ T cells toward glioblastoma cell lines were analyzed. Expanded γδ T cells were co-cultured with Calcein-AM-labeled tumor cell lines for 4 hours at indicated E:T ratio. The % specific lysis was calculated as [(Experimental Release-Spontaneous Release)/(Maximum Release-Spontaneous Release)] × 100. Experiments were performed in triplicate with γδ T cells derived from five different healthy donors. (c, d) PDG cells were cultured and the expression of MICA, ULBP 2/5/6, PVR and Nectin-2 was analyzed by flow cytometry. Experiments were performed three times independently. Representative flow cytometry data of the expression of ligands on PDG#3 and PDG#5 are shown. (e) Cytotoxicity of γδ T cells toward PDG cells was analyzed by Calcein-AM assay in duplicate, using γδ T cells from five healthy donors at the ratio of E:T=20:1. Dots represent data from each γδ T cell. Bars and error bars indicate mean and SD.
Ligand expression levels were also analyzed in the 10 PDG cells. MICA was rarely expressed in the PDG cells (range 0.08 to 1.04%). The expression levels of ULBP, PVR, and Nectin-2 varied among the PDG cells. The mean expression level across all PDG cells of ULBP was 14.10% (range, 0.63% to 68.2%), that of PVR was 72.4% (range, 8.45% to 99.8%), and that of Nectin-2 was 40.6% (range, 7.66% to 66.5%) (Figure 1c and d). The cytotoxicity of γδ T cells also varied among the PDG cells (Figure 1e). The highest tumor cell lysis (76.9% ± 11.3) was in PDG#6, and the lowest lysis (9.6% ± 8.1) was in PDG#8. γδ T cells were also cytotoxic to the PDG#4- and PDG#6-derived 3D spheroids (Supplementary Figure 2C). Therefore, human allogenic γδ T cells can kill human glioma cells and PDG cells, but the ligand expression levels and susceptibility to γδ T cell cytotoxicity vary by cell line.
Cytokine secretion from γδ T cells according to the susceptibility of PDG cells
Next, we sought to investigate whether cytokine secretion patterns from γδ T cells varied upon co-culture with different PDG cells. The PDG cells were classified into two groups according to their susceptibility to γδ T cells; PDG cells with specific lysis above 50% were classified as the high-susceptibility group, and those with specific lysis lower than 50% were considered the low-susceptibility group. Then, two PDG cells were selected from each group, and γδ T cells from five different healthy donors were co-cultured with those tumor cells. As expected, the secretion levels of cytokines showed different patterns according to the susceptibility of the PDG cells (Figure 2). Specifically, the secretion levels of IFN-γ and granzyme B were significantly higher in the high-susceptibility group than the low-susceptibility group.
Figure 2.
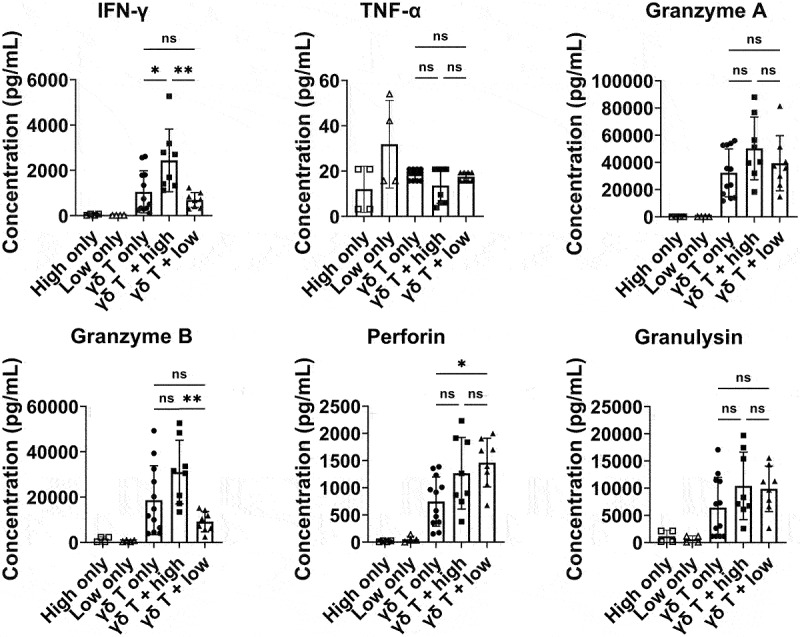
Cytokine secretion patterns of γδ T cells according to the target PDG cells. Cytokine secretion patterns were analyzed upon co-culture with high-susceptibility or low-susceptibility patient GBM PDG cells. Two high-susceptibility (PDG#3 and PDG#4) and two low-susceptibility PDG cells (PDG#5 and PDG#8) were co-cultured with or without expanded γδ T cells from 5 healthy donors at an E:T ratio of 2:1. After 24 hours of co-culture, the supernatants were harvested, and cytokine secretion levels were analyzed with a LEGENDplex Human CD8/NK panel according to the manufacturer’s protocol. Cytokine concentration was calculated as (cytokine concentration from γδ T cells co-cultured with tumor cell lines)-(cytokine concentration from tumor cells alone) and cytokine secretion level from only tumor cells is also presented. Cyokine secretion levels from high-susceptibility PDG cells are denoted as “High only” and those from low-susceptibility PDG cells are denoted as “Low only”. Experiments were performed in duplicate using γδ T cells from five different healthy donors. Bars and error bars indicate the mean and SD, respectively. Paired t test; *P < .05, **P < .005.
Expression of DNAM-1 ligands on PDG cells correlates with susceptibility to γδ T cells
Because the PDG cells showed varying ligand expression levels and susceptibility to γδ T cell–mediated cytotoxicity, we analyzed the correlations between the ligand expression levels and γδ T cell–mediated cytotoxicity (Figure 3). Ligands which were investigated were MICA, ULBP2/5/6, PVR and Nectin-2 in which the former two ligands bind to the receptor NKG2D and the latter two to the receptor DNAM-1. Therefore, the correlations between ligand expression levels and γδ T cell–mediated cytotoxicity were grouped according to the receptors. MICA and ULBP 2/5/6 were grouped as NKG2D ligands while PVR and Nectin-2 were grouped as DNAM-1 ligands. Interestingly, the expression of DNAM-1 ligands by PDG cells correlated significantly with the degree of γδ T cell cytotoxicity (r = 0.5090, p = 0.0219), but the expression of NKG2D ligand did not correlate with the cytotoxicity of γδ T cells. In other words, γδ T cells efficiently killed PDG cells with high expression of DNAM-1 ligands.
Figure 3.
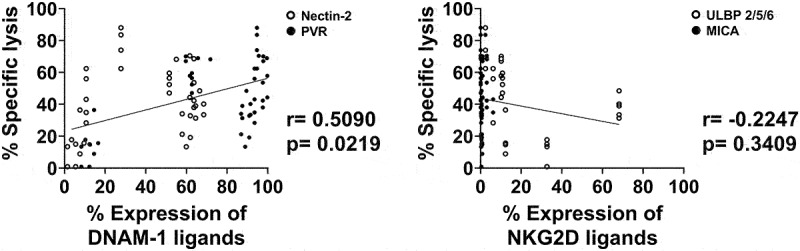
Correlation between the cytotoxic effects of γδ T cells and ligand expression on patient GBM tumor PDG cells. Correlations were analyzed between the cytotoxicity of γδ T cells toward PDG cells at a 20:1 E:T ratio and the ligand expression of the patient GBM cell lines. The ligands were grouped according to the receptors they bind to. PVR (black circle) and Nectin-2 (white circle) were grouped as DNAM-1 ligands (left panel) while MICA (black circle) and ULBP2/5/6 (white circle) were grouped as NKG2D ligands (right panel). Experiments were done with GBM tumor cells PDG cells from 10 patients and γδ T cells from 5 healthy donors and cytotoxicity assays were done as triplicates. Data were analyzed by Pearson’s correlation.
DNAM-1 axis is crucial to γδ T cell–mediated lysis of PDG cells
Next, we examined whether the DNAM-1 axis played a crucial role in the anti-tumor effects of γδ T cells. Four PDG cells with high DNAM-1 ligand expression were co-cultured with expanded γδ T cells in the presence of an isotype control antibody or a DNAM-1 blocking antibody. The cytotoxicity of the γδ T cells was significantly reduced in the presence of the DNAM-1 blocking antibody (Figure 4a). In addition, when PVR and Nectin-2, ligands of DNAM-1 were blocked simultaneously, the cytotoxicity of γδ T cells was significantly reduced (Supplementary Figure 3). To determine whether the secretion levels of cytokines from γδ T cells change on blocking DNAM-1 signaling, representative cytokines involved in the cytotoxicity of γδ T cells such as IFN- γ, TNF-α, Granzyme and Perforin were analyzed. When cytokine secretion levels were analyzed, IFN-γ, Granzyme B, Perforin, and Granulysin secretion levels also decreased significantly in the presence of the DNAM-1 blocking antibody (Figure 4b). This data demonstrates the role of DNAM-1 signaling on the secretion of various cytokines involved in cytotoxicity of γδ T cells. In sum, the DNAM-1 axis plays a critical role in γδ T cell–mediated cytotoxicity toward glioblastoma cells.
Figure 4.
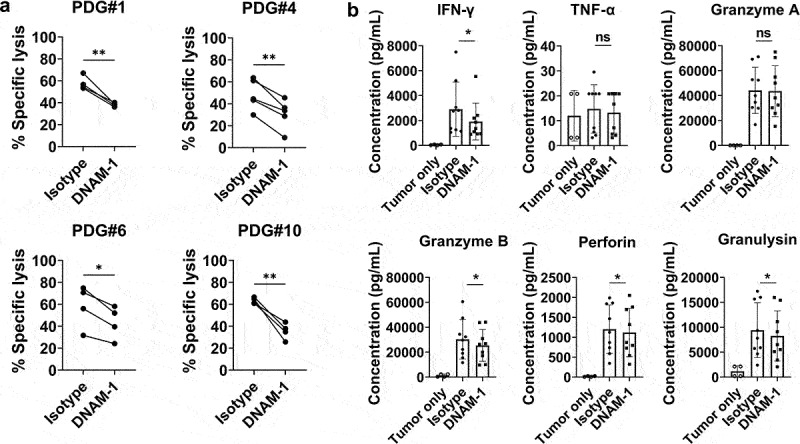
Blocking DNAM-1 significantly inhibits the cytotoxic effects and cytokine secretion of γδ T cells. (a) Expanded γδ T cells were co-cultured with Calcein-AM-stained target cells for 4 hours at an E:T ratio of 20:1 in the presence of an isotype control or DNAM-1 blocking antibody. The % specific lysis was calculated as [(Experimental Release-Spontaneous Release)/(Maximum Release-Spontaneous Release)]×100. Experiments were carried out in duplicates using γδ T cells derived from 5 healthy donors. (b) The level of cytokine secretion from γδ T cells was analyzed after co-culture with two tumor PDG cells with an isotype control antibody or DNAM-1 blocking antibody. Cytokine secretion from only tumor cells was subtracted from the data and is also demonstrated separately. Experiments were carried out in duplicate using γδ T cells derived from five healthy donors and two PDG cells. Bars and error bars indicate mean and SD, respectively, paired t-test, *P < .05, **P < .005.
When NKG2D was blocked, the cytotoxicity of γδ T cells toward PDG#4 cells and IFN-γ was reduced, and Granzyme B secretion levels were also reduced (Supplementary Figure 4). However, γδ T cell cytotoxicity was not affected by the tumor cell transcriptome (Supplementary Figure 5) and DNAM-1 ligand expression level measured by FACS in PDG cells did not correlate with mRNA expression (Supplementary Figure 6)
Intratumoral injection of human γδ T cells elicits anti-tumor effects in orthotopic glioblastoma model
To investigate whether an intratumoral injection of human γδ T cells show anti-tumor effect against glioblastoma cell lines expressing DNAM-1 ligand in vivo, an orthotopic NSG mouse model was established. First, an early tumor model was established to verify the anti-tumor effect of γδ T cells toward glioblastoma in vivo. U87 cells with high DNAM-1 ligand expression (Figure 1) were injected intracranially on day 0. Then PBS (control group) or 2 × 106 γδ T cells (γδ T cell group) were injected intratumorally on day 4 (experiment 1) after tumor engraftment (Figure 5a). When tumor growth and survival were analyzed weekly, the γδ T cell group showed reduced tumor size and significantly longer survival compared with the control group (Figure 5c, 5d, and 5e). Next, to verify the anti-tumor effect of γδ T cells in an advanced glioblastoma model, PBS or 2 × 106 γδ T cells were injected intratumorally on days 7 and 14 (experiment 2) after tumor engraftment (Figure 5b). The advanced tumor model also showed reduced tumor size and significantly better survival in the γδ T cell group (Figure 5f, 5g, and 5h). γδ T cell infiltration within the tumors (Figure 5i) and reduced PVR expression were shown in the immunofluorescence results (Figure 5j). These findings demonstrate that an intratumoral injection of human γδ T cells show anti-tumor effects on DNA-1 ligand expressing glioblastoma cells in vivo.
Figure 5.
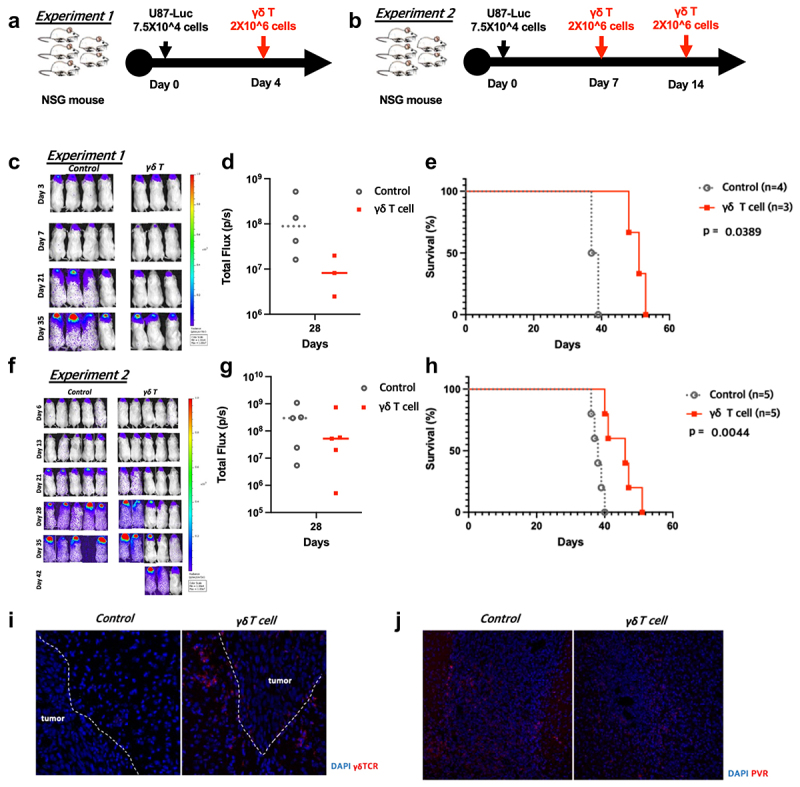
In vivo anti-tumor effects of human γδ T cells in an intracranial U87 NSG mouse model. (a,b) Intracranial injections of 5 × 104 U87 cells were done on day 0, and the mice were randomized into two groups on the treatment day. We performed intratumoral injections of PBS (control group) or 2 × 106 human γδ T cells (γδ T cell group) following two schedules (experiment 1: on day 4, experiment 2: on days 7 and 14). (c,f) Tumor growth was weekly monitored by IVIS and the average radiance per mouse were analyzed, following treatments. (d,g) Total photons were calculated by IVIS Lumina XRMS. (e,h) A Kaplan-Meier survival curve of intracranial glioblastoma–bearing mice intratumorally treated with PBS (control) or human γδ T cells (γδ T cell) is presented. (i) Infiltrating γδ T cells within tumors from each group are shown from day 10 after tumor implantation. (j) PVR expression in each group on day 10 after tumor implantation.
Discussion
Our findings demonstrate that human allogenic γδ T cells expanded and activated by aAPCs can kill PDG cells in vitro. Interestingly, we found that the anti-tumor effects of the human γδ T cells correlated significantly with the DNAM-1 ligand expression on the glioblastoma cells. Blocking the DNAM-1 receptor or ligands decreased the cytotoxicity of γδ T cells to glioblastoma cells and decreased the cytokine secretion levels. However, γδ T cell cytotoxicity was not affected by tumor cell transcriptome (Supplementary Figure 2) and DNAM-1 ligand expression level measured by FACS in PDG cells did not correlate with mRNA expression (Supplementary Figure 3). Most reports on mRNA and protein abundance showed a weak correlation, and this discrepancy could be influenced by some biological factors such as regulatory proteins, translational efficiency, and protein half-lives.26 These findings may imply that DNAM-1 ligand expression on the surface of glioblastoma cells could affect the cytotoxicity of γδ T cells, rather than PVR RNA expression levels. Glioblastoma is regarded to be a heterogeneous disease that can be broadly divided into different subtypes based on the molecular characteristics of the tumor cells. When the associations between DNAM-1 ligand expression in PDG cells and the subtype of GBM based on Verhaak’s classification were analyzed, no significant correlations were found (data not shown). Further studies on the heterogeneous expression of DNAM-1 ligands between PDG cells are needed.
In addition to in vitro experiments, we showed that intracranial injection of γδ T cells reduced tumor size and improved overall survival in both early and advanced tumor models. However, in the advanced model, the anti-tumor effect seemed to be similar to that in the early tumor model despite additional injection of γδ T cells. When comparing the early and advanced models, the degree of cancer progression at the point of injection seemed to be an important factor in the anti-tumor effect of γδ T cells.
Previously, several preclinical studies suggested that human Vγ9Vδ2 T cells show anti-tumor immunity against glioblastoma cells.20–24 In a recent in vitro study, Vγ9Vδ2 T cells stimulated with (E)-4-hydroxy-3-methyl-but-enyl pyrophosphate were activated by glioblastoma cells and had cytotoxic effects.24 In another recent preclinical study, stereotaxic administration of human Vγ9Vδ2 T cells in orthotopic glioma–bearing mice treated with zoledronic acid showed anti-tumor effects.22 However, no one had yet determined which of the various possible ligands for γδ T cell receptors expressed in glioblastoma cells play an important role in γδ T cell cytotoxicity. To the best of our knowledge, our findings are the first to show that DNAM-1 axis could be the most important immunologic interaction in γδ T cell therapy and that glioblastoma cells with high levels of DNAM-1 ligands could potentially be eliminated by an adoptive transfer of human γδ T cells. Our findings are consistent with previous studies demonstrating DNAM-1 receptor mediated tumor cell killing of γδ T cells.27–29 Specifically, DNAM-1 expression level on γδ T cells and its interaction with its ligands were significantly related to the cytotoxicity of γδ T cells against neuroblastoma and hepatocellular carcinoma models.27,29 In addition to solid tumors, DNAM-1-mediated γδ T cell lysis of tumor cells was reduced in acute myeloid leukemic blasts when DNAM-1 was blocked.28
PVR, a member of the Nectin-like family of proteins, has been known to have two critical roles in glioblastoma. It was originally known to be related to cellular adhesion processes, like other members of its family.30 Cancer cells with high PVR expression tend to have more aggressive cellular invasion, migration, and progression than those with low PVR expression.31,32 In addition, PVR has an immunomodulatory function because it can bind to both DNAM-1 (activating) and TIGIT (inhibitory).33 PVR overexpression has been extensively identified in numerous cancers, including glioblastomas, and it induces immune escape by binding to the inhibitory receptors of host T cells.34,35 Thus, PVR is an emerging target in cancer immunotherapy. Several studies have already demonstrated the presence of PVR in glioblastoma and other pediatric and adult gliomas.35,36 In addition, recent clinical trials using recombinant oncolytic poliovirus, which can target the PVR of glioblastoma cells, showed promising therapeutic potential.37 Furthermore, a TIGIT-inhibitor, which reduces the inhibitory effects of PVR in the immune system, enhanced the anti-tumor effects of a PD-1 inhibitor in an in vivo mouse glioblastoma model.38
Our study has several limitations. First, our data about PVR and Nectin-2 expression in the PDG cells might not be representative of whole tumor tissues, although several studies have shown frequent PVR expression in glioblastoma specimens.34,35 Further studies are needed to validate PVR and Nectin-2 expression in whole tumor specimens. Second, the importance of other interactions, including those of the NKG2D ligands and their receptors cannot be overlooked. In our study, only one out of ten PDG cells showed ULBP expression exceeding 50%, and the average expression level was 15.2% ± 20. The MICA expression levels were even lower than that, with an average expression of 0.5% ± 0.3. Low expression of MICA and ULBP have also been reported in other studies. Hypoxia in the tumor microenvironment and miRNA targeting NKG2D ligands were found to be involved in the low expression of those ligands.39 This phenomenon is one mechanism by which GBM evades immunity. Furthermore, MICA and ULBP2 expression decrease as the WHO grade of malignancy increases.40 Although expression of the NKG2D ligands is low, a partial decrease in the cytotoxicity of γδ T cells toward GBM cell lines expressing NKG2D ligands has been reported.23 Also, in this study, blocking NKG2D decreased the cytotoxicity of γδ T cells and the secretion levels of IFN-γ and Granzyme B upon co-culture with ULBP-expressing PDG#4 (Supplementary Figure 4). Third, the immune suppressive environment of glioblastoma was not investigated. In our findings, IFN-γ was the only cytokine that showed a significant increase upon co-culture with high-susceptibility PDG cells. Moreover, IFN-γ and Granzyme B from γδ T cells tended to decrease upon co-culture with low-susceptibility PDG cells compared to baseline secretion. This strongly suggests the immunosuppressive effect of GBM and the reverse correlation with DNAM-1 ligand expression, which should further be studied. Also, additional study of the strong immunosuppressive effect of low-susceptibility GBM tumor cells (e.g. expression of checkpoint inhibitors, inhibitory cytokine secretion) will help to maximize the anti-tumor effects of γδ T cells. Fourth, since immunodeficient mice were used in this study, the immunoediting process and tumor microenvironment could not be assessed directly. Further in vivo experiments using a large number of humanized mice with DNAM-1 inhibition could demonstrate these processes and the importance of PVR-DNAM-1 axis in detail.
Taken together, our study results indicate that adoptive cell transfer of human Vγ9Vδ2 T cells can successfully eliminate glioblastoma cells expressing high levels of DNAM-1 ligands. Because PVR overexpression in glioblastoma has been established in several studies,35,36 the administration of allogenic human Vγ9Vδ2 T cells may provide clinical benefits for glioblastoma patients. In addition, combination treatments with a TIGIT inhibitor, which targets the PVR-TIGIT axis, could increase the therapeutic potential of human Vγ9Vδ2 T cell therapy. However, because not all glioblastoma cells express abundant DNAM-1 ligands, further strategies, including redirecting γδ T cells to new targets and producing γδ T cells that express a chimeric antigen receptor, are needed. When considering clinical trials of γδ T cells that mainly target DNAM-1 ligands, safety validation is also essential. Although PVR and Nectin-2 is rarely expressed in normal brain parenchyma,30 further studies are warranted to validate the safety of the intratumoral administration of γδ T cells for glioblastoma.
Supplementary Material
Funding Statement
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01055575) and by the Research Fund of Seoul St. Mary’s Hospital, Catholic University of Korea (ZC21CISI0060). The funders have no role in this study.
Availability of data and material
Data are available only on request due to privacy/ethical restrictions.
Consent to participate
The requirement for informed consent to participate was met according to the policy of the IRB of our institution.
Ethics approval
The Institutional Review Board of Seoul St. Mary’s Hospital approved this study design (ethical code: KC21TISI0793, KC22SISI0079).
Disclosure statement
Nothing to declare.
Author contributions
Drafting the manuscript: Stephen Ahn and Haeyoun Choi
Acquisition, analysis, or interpretation of data for this study: Haeyoun Choi, Yunkyung Lee, Soon A Park, Ji Hyeon Lee, Junseong Park, Jang Hyun Park, and Heung Kyu Lee.
Conceptualization and supervision of this study: Stephen Ahn, Haeyoun Choi, Tai-Gyu Kim, and Sin-Soo Jeun.
All authors approved the final version of the manuscript for publication.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2022.2138152.
References
- 1.Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS.. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(Supplement_3):iii1–10. doi: 10.1093/neuonc/noab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, Van Den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpten J, Craig D, Byron S, Aldrich J, Borad M, Bryce A, Barrett M, Vasmatzis G, Stewart K. Targeted therapies for cancer. Google Patents; 2021.
- 6.Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- 7.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr O. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018;78(4):1031–1043. doi: 10.1158/0008-5472.CAN-17-1788. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Kim H-J, Kim CW, Kim HC, Jung Y, Lee H-S, Lee Y, Ju YS, Oh JE, Park S-H. Tumor hypoxia represses γδ T cell-mediated antitumor immunity against brain tumors. Nat Immunol. 2021;22(3):336–346. doi: 10.1038/s41590-020-00860-7. [DOI] [PubMed] [Google Scholar]
- 11.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279(5357):1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 12.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro S, Ribot J, Silva-Santos B. Five layers of receptor signaling in γδ T-cell differentiation and activation. Front Immunol. 2015;6:15. doi: 10.3389/fimmu.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human Vγ9Vδ2 T cell antitumor cytotoxicity through protein kinase Cθ-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol. 2010;185(1):55–63. doi: 10.4049/jimmunol.1000373. [DOI] [PubMed] [Google Scholar]
- 15.Davodeau F, Peyrat MA, Hallet MM, Houde I, And HV, Bonneville M. Peripheral selection of antigen receptor junctional features in a major human γδ subset. Eur J Immunol. 1993;23(4):804–808. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- 16.Correia DV, Lopes AC, Silva-Santos B. Tumor cell recognition by γδ T lymphocytes: t-cell receptor vs. NK-cell receptors. Oncoimmunology. 2013;2(1):e22892. doi: 10.4161/onci.22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi H, Lee Y, Hur G, Lee S-E, Cho H-I, Sohn H-J, Cho BS, Kim H-J, Kim T-G. γδ T cells cultured with artificial antigen-presenting cells and IL-2 show long-term proliferation and enhanced effector functions compared with γδ T cells cultured with only IL-2 after stimulation with zoledronic acid. Cytotherapy. 2021;23(10):908–917. doi: 10.1016/j.jcyt.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Cho HW, Kim SY, Sohn DH, Lee MJ, Park MY, Sohn HJ, Cho HI, Kim TG. Triple costimulation via CD80, 4‐1BB, and CD83 ligand elicits the long‐term growth of Vγ9Vδ2 T cells in low levels of IL‐2. J Leukocyte Biol. 2016;99(4):521–529. doi: 10.1189/jlb.1HI0814-409RR. [DOI] [PubMed] [Google Scholar]
- 19.Deniger DC, Maiti SN, Mi T, Switzer KC, Ramachandran V, Hurton LV, Ang S, Olivares S, Rabinovich BA, Huls MH. Activating and propagating polyclonal gamma delta T cells with broad specificity for malignancies. Clin Cancer Res. 2014;20(22):5708–5719. doi: 10.1158/1078-0432.CCR-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazawa T, Nakamura M, Park YS, Motoyama Y, Hironaka Y, Nishimura F, Nakagawa I, Yamada S, Matsuda R, Tamura K. Cytotoxic human peripheral blood-derived γδT cells kill glioblastoma cell lines: implications for cell-based immunotherapy for patients with glioblastoma. J Neuro Oncol. 2014;116(1):31–39. doi: 10.1007/s11060-013-1258-4. [DOI] [PubMed] [Google Scholar]
- 21.Nakazawa T, Nakamura M, Matsuda R, Nishimura F, Park YS, Motoyama Y, Hironaka Y, Nakagawa I, Yokota H, Yamada S. Antitumor effects of minodronate, a third-generation nitrogen-containing bisphosphonate, in synergy with γδT cells in human glioblastoma in vitro and in vivo. J Neuro oncol. 2016;129(2):231–241. doi: 10.1007/s11060-016-2186-x. [DOI] [PubMed] [Google Scholar]
- 22.Jarry U, Chauvin C, Joalland N, Léger A, Minault S, Robard M, Bonneville M, Oliver L, Vallette FM, Vié H. Stereotaxic administrations of allogeneic human Vγ9Vδ2 T cells efficiently control the development of human glioblastoma brain tumors. Oncoimmunology. 2016;5(6):e1168554. doi: 10.1080/2162402X.2016.1168554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chitadze G, Lettau M, Luecke S, Wang T, Janssen O, Fürst D, Mytilineos J, Wesch D, Oberg -H-H, Held-Feindt J. NKG2D-and T-cell receptor-dependent lysis of malignant glioma cell lines by human γδ T cells: modulation by temozolomide and A disintegrin and metalloproteases 10 and 17 inhibitors. Oncoimmunology. 2016;5(4):e1093276. doi: 10.1080/2162402X.2015.1093276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosso DA, Rosato M, Iturrizaga J, González N, Shiromizu CM, Keitelman IA, Coronel JV, Gómez FD, Amaral MM, Rabadan AT. Glioblastoma cells potentiate the induction of the Th1-like profile in phosphoantigen-stimulated γδ T lymphocytes. J Neuro Oncol. 2021;153(3):403–415. doi: 10.1007/s11060-021-03787-7. [DOI] [PubMed] [Google Scholar]
- 25.Brown CE, Aguilar B, Starr R, Yang X, Chang W-C, Weng L, Chang B, Sarkissian A, Brito A, Sanchez JF. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther. 2018;26(1):31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13(4):227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, Desille M, de La Pintière CT, Daniel P, Bouet F. DNAX accessory molecule‐1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vγ9Vδ2 T cells. Eur J Immunol. 2009;39(5):1361–1368. doi: 10.1002/eji.200838409. [DOI] [PubMed] [Google Scholar]
- 28.Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A, Charbonnier A, Collette Y, Vey N, Olive D. Human Vγ9Vδ2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188(9):4701–4708. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Mou W, Han W, Xi Y, Chen X, Zhang H, Qin H, Wang H, Ma X, Gui J. Diminished cytolytic activity of γδ T cells with reduced DNAM-1 expression in neuroblastoma patients. Clin Immunol. 2019;203:63–71. doi: 10.1016/j.clim.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag LL. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4(1):1–14. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res. 2005;65(23):10930–10937. doi: 10.1158/0008-5472.CAN-05-1890. [DOI] [PubMed] [Google Scholar]
- 32.Enloe BM, Jay DG. Inhibition of Necl-5 (CD155/PVR) reduces glioblastoma dispersal and decreases MMP-2 expression and activity. J Neuro Oncol. 2011;102(2):225–235. doi: 10.1007/s11060-010-0323-5. [DOI] [PubMed] [Google Scholar]
- 33.Kučan Brlić P, Lenac Roviš T, Cinamon G, Tsukerman P, Mandelboim O, Jonjić S. Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell Mol Immunol. 2019;16(1):40–52. doi: 10.1038/s41423-018-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JB, Hong MH, Park SY, Chae S, Hwang D, Ha S-J, Shim HS, Kim HR, Distler JHW, Šenolt L. Overexpression of PVR and PD-L1 and its association with prognosis in surgically resected squamous cell lung carcinoma. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandramohan V, Bryant JD, Piao H, Keir ST, Lipp ES, Lefaivre M, Perkinson K, Bigner DD, Gromeier M, McLendon RE. Validation of an immunohistochemistry assay for detection of CD155, the poliovirus receptor, in malignant gliomas. Archiv Pathol Lab Med. 2017;141(12):1697–1704. doi: 10.5858/arpa.2016-0580-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson EM, Brown M, Dobrikova E, Ramaswamy V, Taylor MD, McLendon R, Sanks J, Chandramohan V, Bigner D, Gromeier M. Poliovirus receptor (CD155) expression in pediatric brain tumors mediates oncolysis of medulloblastoma and pleomorphic xanthoastrocytoma. J Neuropathol Exp Neurol. 2018;77(8):696–702. doi: 10.1093/jnen/nly045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, Kim E, Wu A, Xia Y, Garzon-Muvdi T. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7(8):e1466769. doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ou Z-L, Luo Z, Wei W, Liang S, Gao T-L, Lu Y-B. Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: role of circ_0000977/miR-153 axis. RNA Biol. 2019;16(11):1592–1603. doi: 10.1080/15476286.2019.1649585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-β and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129(9):2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available only on request due to privacy/ethical restrictions.


