ABSTRACT
Heat acclimation (HA) protocols repeatedly expose individuals to heat stress. As HA is typically performed close to the pinnacle event, it is essential that the protocol does not compromise immune status, health, or wellbeing. The purpose of this study was to examine the effect of HA on resting salivary immunoglobulin-A (s-IgA) and salivary cortisol (s-cortisol), self-reported upper-respiratory tract symptoms, and self-reported wellness parameters. Seventeen participants (peak oxygen uptake 53.2 ± 9.0 mL·kg−1·min−1) completed a 10-day controlled-hyperthermia HA protocol, and a heat stress test both before (HST1) and after (HST2) HA (33°C, 65% relative humidity). Resting saliva samples were collected at HST1, day 3 and 7 of the HA protocol, HST2, and at 5 ± 1 days post-HA. Upper-respiratory tract symptom data were collected weekly from one week prior to HA until three weeks post HA, and wellness ratings were reported daily throughout HA. HA successfully induced physiological adaptations, with a lower end-exercise rectal temperature and heart rate and higher whole-body sweat rate at HST2 compared to HST1. In contrast, resting saliva flow rate, s-IgA concentration, s-cortisol concentration, and s-cortisol secretion rate remained unchanged (n = 11–14, P = 0.10–0.48). Resting s-IgA secretion rate increased by 39% from HST1 to HST2 (n = 14, P = 0.03). No changes were observed in self-reported upper respiratory tract symptoms and wellness ratings. In conclusion, controlled-hyperthermia HA did not negatively affect resting s-IgA and s-cortisol, self-reported upper-respiratory tract symptoms, and self-reported wellness parameters in recreational athletes.
KEYWORDS: Athletes, heat stress, controlled-hyperthermia, adaptation, exercise, upper-respiratory tract, immunosuppression, cortisol, mucosal immunity
Introduction
Athletes and military personnel are frequently exposed to stressors that can suppress immune function and increase the risk of illness, such as high training loads, travel, sleep deprivation, psychological stress, and environmental extremes [1,2]. Episodes of upper respiratory tract symptoms (URTS; e.g. sore throat, headache, runny nose, and cough) are the most common illnesses reported in both military and athlete populations [1,3]. URTS diminish the athlete’s training availability, performance, and success [3–5]. URTS also have negative implications for military personnel, accounting for a high proportion of non-combat related medical visits and missed service days [1,6]. Thus, in these high-performance occupations, it is vital to keep individuals healthy [7].
Measurement of salivary biomarkers has been used as a tool to monitor URTS risk in athletes [2,8–10]. Mucosal surfaces, for example in the oral cavity, are protected by mucosal secretions that act as “the first line of defense” against infectious pathogens. Defense factors in saliva include alpha-amylase, lactoferrin, lysozyme, and immunoglobulins, with immunoglobulin-A being the most abundant secretory antibody [9,11]. Salivary immunoglobulin-A (s-IgA) has been studied extensively and research suggests that s-IgA availability diminishes with intensified training periods, resulting in elevated URTS risk [12,13]. Indeed, a recent systematic review identified both increased training intensity and reduced s-IgA as risk factors for the development of clinically diagnosed upper respiratory tract infection [10]. It should be noted, however, that URTS do not necessarily have an infective origin, but can also result from factors such as allergies or asthma [14], clouding the relationship between s-IgA and URTS. Some studies showed that high URTS incidences around intense training periods were preceded by relatively low s-IgA concentrations [8,15,16], although this is not a consistent finding [17–19].
Exercise increases the activity of the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis, resulting in elevated levels of circulating stress hormones [20]. It has been suggested that elevated HPA axis activity during periods of intensified training causes s-IgA to decrease through inhibition of IgA synthesis and transport [21]. When exercise is performed in hot and/or humid conditions, the stress response may be exaggerated, with increased activation of the HPA axis and sympathetic nervous system in hot versus thermoneutral conditions [20,22]. This could potentially induce s-IgA reduction and heightened URTS risk during a training period in the heat.
Repeated training bouts in the heat are commonly performed when preparing for an event in hot and/or humid conditions, a strategy called heat acclimation (HA). HA can be executed in various ways, but it has been recommended that exercise sessions in the heat should last for at least 60 minutes, performed on at least 8 consecutive days [23,24]. This strategy elicits various physiological adaptations that result in a lower thermal strain during exercise at a given workload, usually reflected by a lower core temperature, heart rate and skin temperature, higher whole-body sweat rate, and improved thermal comfort [24,25]. These adaptations aid improvement of exercise performance in the heat, and mitigation of heat illness risk [24,25].
Notwithstanding the benefits of HA, it should be considered that this protocol may act as a stressor on the body. That is, physiological adaptations will only occur when the body’s homeostasis is disrupted repeatedly [26]. Previous research showed that HA can indeed cause fatigue, in this case neuromuscular fatigue, in recreationally active males [27]. In addition, it was shown that a 5-day HA protocol reduced self-reported sleep quality and increased the duration participants were awake at night [28]. This may lead to higher URTS risk, as poor sleep efficiency and short sleep duration were associated with a higher susceptibility to respiratory illness following viral exposure [29]. On the other hand, studies that have investigated the effect of HA on immune function showed that both short-term (4–7 days) [30–32] and medium-term (9–10 days) [33–35] HA protocols did not influence the resting levels of circulating cytokines in physically active individuals. However, it is unclear whether the exercise-induced cytokine response influences URTS risk, and it has been questioned whether circulating cytokine concentrations are relevant for infection risk, as cytokine concentrations increase substantially when exposed to an infectious agent [3,12]. To our knowledge, there are no studies that evaluated the effect of HA on s-IgA and self-reported URTS.
Therefore, the purpose of the present study was to examine the effect of heat acclimation on resting salivary immunoglobulin-A and salivary cortisol, self-reported upper-respiratory tract symptoms, and self-reported wellness parameters. We hypothesized that HA would induce a reduction in s-IgA, an increase in s-cortisol, a higher prevalence of self-reported upper-respiratory tract symptoms and a deterioration of wellness parameters such as sleep quality.
Materials and methods
Participants
Seventeen healthy participants (11 males, 6 females; age 30 ± 7 years; height 182.5 ± 8.8 cm; body mass 76.8 ± 11.1 kg; body fat 20 ± 6%; peak oxygen uptake 53.2 ± 9.0 mL·kg−1·min−1; habitual training volume 399 ± 159 min·week−1) volunteered for this study. Participants had not resided in a warm environment (> 25°C air temperature) for longer than 7 days within the 3 months prior to the study. They did not smoke, had no history of heat-related illnesses or cardiovascular complications, and did not have any known issues with thermoregulation. Participants did not report any diseases, viral infections, asthma, or allergy symptoms at the start of the study. Eleven participants reported regular intake of supplements that may influence the immune system, such as vitamins, magnesium, and omega-3. They were asked to consistently maintain habitual supplement intake over the course of the study. Four females used oral contraceptives, and the remaining two reported natural menstrual cycles of regular duration (25–35 days). Participants reported their habitual training program (considering the two months preceding the study), from which habitual training volume was calculated. Procedures were approved by the ethics committee of the Faculty of Behavioral and Movement Sciences of the Vrije Universiteit Amsterdam (VCWE-2018-160R1), and conform to the standards set out by the Declaration of Helsinki. Prior to the study, participants were informed about the procedures and provided verbal and written consent.
Study design
The study design is presented in Figure 1. During the first visit to the laboratory, participants were familiarized with the saliva collection method. Then, they completed a graded exercise test in temperate conditions to determine peak oxygen uptake and subsequently, after a short break, were familiarized with the heat stress test (HST) [36] Body composition was assessed using a whole-body dual-energy X-ray absorptiometry scan. Approximately 7 days after the participants first reported to the laboratory, they completed the first HST (HST1). The next day, participants commenced a 10-consecutive-day controlled-hyperthermia HA program. To evaluate adaptive responses, participants performed a second HST (HST2), scheduled 48 h after the last HA session. All HST and HA sessions were administered in an environmental chamber (b-Cat B.V., Tiel, The Netherlands), with air temperature 33°C, relative humidity 65%, and minimal air flow. Resting saliva samples were taken ~30 min prior to both HSTs, HA sessions 3 and 7, and following the completion of HA (i.e. 5 ± 1 days after HST2). URTS data were collected pre-HA (7 days), throughout HA (2 × 6 days, i.e. HApart1 and HApart2) and during the 3 weeks post-HA (3x7 days). On the last day of each week, participants completed a retrospective URTS questionnaire to rate their illness symptoms of the preceding week. A daily wellness questionnaire was administered at the familiarization session (pre-HA), throughout HA and 5 ± 1 days after HST2. This study was conducted during winter time (the Netherlands; Jan–Apr) to avoid heat acclimatization prior to the experiment.
Figure 1.
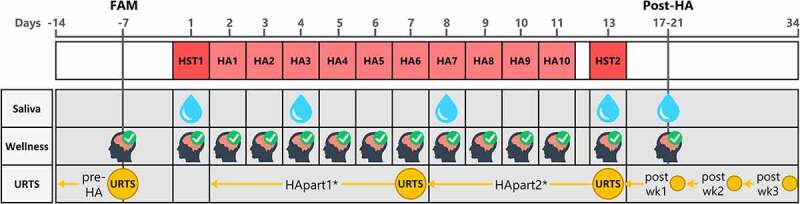
Study design illustrating the timepoints of data collection. First row = HA schedule; second row = saliva collection; third row = daily wellness questionnaire; fourth row = weekly retrospective upper respiratory tract symptoms (URTS) questionnaire. Day-numbers are denoted in gray at the top of the figure. *Weeks for HApart1 and HApart2 were 6 days instead of 7 days. Abbreviations: FAM, familiarization; HA, heat acclimation; HST, heat stress test; wk, week.
Heat stress tests
Testing took place throughout the day, but each participant completed his/her own two HSTs at the same time of day. Participants were instructed to refrain from caffeine and alcohol consumption, to avoid strenuous exercise, and to report and replicate food and beverage intake during the 24 h preceding the HSTs. To encourage euhydration, participants were asked to drink 500 mL of water the evening before and 10 mL·kg body mass−1 of water during the 3 hours prior to the HSTs. Upon arrival at the laboratory, participants provided a urine sample, from which urine-specific gravity was measured using a handheld refractometer (PAL-10S, Atago Co. Ltd, Tokyo, Japan). A urine-specific gravity value ≤ 1.025 was required before the HST commenced [37]. During the HSTs, participants cycled (Excalibur Sport, Lode B.V., Groningen, The Netherlands) for 35 min at 1.5 W·kg body mass−1, followed by a 5-min resting period, during which they consumed a standardized volume of water (3 mL·kg body mass−1). Participants then performed a graded exercise test, starting at a power output of 1.5 W·kg body mass−1 with subsequent increments of 25 W·min−1 until volitional exhaustion [36]. During the HSTs, we continuously measured heart rate (Polar Vantage-M, Kempele, Finland) and rectal temperature (10 cm past anal sphincter; MSR, Seuzach, Switzerland; or Yellow Springs Instruments, Yellow Springs, OH, USA; aimed to keep the type of probe consistent within participants). Whole-body sweat rate was calculated as the difference between pre- and post-session nude body mass, divided by exposure time (g·h−1; Platform scale, SATEX 34 SA-1 250, Weegtechniek Holland B.V., Zeewolde, The Netherlands).
Heat acclimation
Every participant performed 10 controlled-hyperthermia HA sessions at approximately the same time of day (at least within circa 3 h of HST time). Prior to each session, a urine sample was collected to monitor hydration status over the course of HA. The controlled-hyperthermia protocol served to increase rectal temperature to 38.5°C in approximately 35 min and subsequently maintain rectal temperature slightly above 38.5°C for 60 min. To increase rectal temperature, participants cycled at a power output expected to cause an increase in rectal temperature to 38.5°C within the set time window (Excalibur Sport, Lode B.V., Groningen, The Netherlands; or Wattbike Pro, Wattbike B.V., Duivendrecht, The Netherlands). To keep rectal temperature at 38.5°C, power output was adjusted, and resting periods were introduced when necessary. Participants were allowed to drink ad libitum during all HA sessions. During the HA sessions, we continuously measured power output, heart rate and rectal temperature. Whole-body sweat rate was calculated as the difference between pre- and post-session nude body mass plus drinking volume, divided by exposure time.
Saliva collection and storage
Prior to saliva sample collection, participants were instructed to rinse their mouth with water (tap water; temperature 17–20°C). Participants provided an unstimulated whole saliva sample by passively drooling into a pre-weighed plastic container for 4–6 minutes with minimal orofacial movement and head tilted slightly forward [9]. Saliva samples were re-weighed to measure saliva volume. Assuming a saliva density of 1 g·mL−1 [38], saliva flow rate (mL·min−1) was calculated by dividing saliva volume by the collection duration. Saliva samples were then transferred into 1.5 mL Eppendorf tubes and immediately stored at −20°C for 2–3 months. Thereafter, samples were transferred to −80°C where they were stored for approximately two years until analysis.
Analysis of salivary biomarkers
Prior to analysis, samples were thawed and centrifuged at 17,000 × g for 2 min to extract the supernatant. Then, s-IgA and s-cortisol concentrations were analyzed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (SLV-4636/SLV-2930, DRG Instruments GmbH, Marburg, Germany) according to the manufacturer’s instructions. s-IgA samples were diluted by a factor 1:1000 and then analyzed against a standard curve ranging from 0 to 400 ng·mL−1, with a sensitivity of 0.5 µg·mL−1. s-cortisol samples were not diluted and were analyzed against a standard curve ranging from 0 to 30 ng·mL−1, with a sensitivity of 0.09 ng·mL−1. All samples were analyzed in duplicate. When the absorbance in one or both wells was outside the range of the standard curve, the sample was removed from the dataset. The intra-assay coefficients of variation for s-IgA and s-cortisol were 3.0 ± 2.8% and 11.2 ± 10.2%, respectively. For both s-IgA and s-cortisol, results were expressed as absolute concentration (s-IgA, µg·mL−1; s-cortisol, ng·mL−1), and secretion rate (s-IgA, µg·min−1; s-cortisol, ng·min−1). Secretion rate was calculated by multiplication of biomarker concentration and saliva flow rate.
URTS and wellness questionnaires
A weekly retrospective questionnaire was administered to assess the occurrence of URTS during the preceding week. Symptoms included sore throat, mucus in throat, runny nose, cough, repetitive sneezing, fever, persistent muscle soreness, joint aches and pains, weakness, headache, and loss of sleep [12]. When a symptom was present, participants rated the severity as light, moderate, or severe. For analysis, these severities were numerically scored as 1, 2, or 3, whereafter a weekly URTS score was calculated as the sum of all severities reported for that week. A single URTS episode was defined as a period for which the total URTS score was ≥12 for one or multiple subsequent weeks. This questionnaire was a modified version of the questionnaire validated by Jackson et al. [39], as previously reported [12,19]. A daily questionnaire was administered over the course of HA to assess wellness parameters [40]. Participants rated their perceived fatigue, general muscle soreness, stress levels, mood and sleep quality on a five point-scale (1-point increments), with 1 indicating poor wellness and 5 indicating very good wellness. An overall wellness score was calculated as the sum of the five individual ratings [40]. Sleep quantity (hours) was also reported.
Data analysis
All data were synchronized and formatted using MATLAB (R2019a, The MathWorks Inc., Natick, MA, USA). Statistical analysis was performed using R software (version 4.1.1, R Foundation for Statistical Computing, Vienna, Austria) in the Rstudio environment (version 2021.09.0, Rstudio, Inc., Boston, MA, USA). The level of statistical significance was set at P < 0.050. Data were reported as mean ± standard deviation (in case of normal distribution) or median [first quartile, third quartile] (in case of non-normal distribution).
Thermoregulatory adaptations to HA were assessed by comparison of HST1- and HST2- values using paired t tests. Normality of the HST1-HST2 differences was confirmed by the Shapiro-Wilk test (P > 0.05). The Shapiro-Wilk test showed that data for s-IgA and s-cortisol (per cell of the design) did not follow a normal distribution (P < 0.05). We used the non-parametric Friedman test to evaluate the effect of time on salivary biomarkers, wellness indicators and overall wellness, and weekly URTS score. For salivary biomarkers, we included 5 timepoints (HST1, HA3, HA7, HST2, post-HA), for wellness parameters 14 timepoints (pre-HA, HST1, HA1-HA10, HST2, post-HA), and for weekly URTS score 6 time periods (pre-HA, HApart1, HApart2, post-HA weeks 1–3). Kendall’s W was used as an effect size estimate, with value 0 indicating no relationship to 1 indicating a perfect relationship. If a significant effect of time was observed, post-hoc pairwise comparisons were done using the Wilcoxon signed-rank test with Bonferroni correction. Associations between parameters were assessed using the Spearman’s rank-order correlation coefficient rs. The association between s-IgA concentrations and s-cortisol concentrations at all timepoints was evaluated, as well as the association between pre-to-post-HA changes (HST2-HST1) in s-IgA and s-cortisol. We investigated whether individual characteristics (peak oxygen uptake, habitual training volume and body mass) were associated with pre-to-post-HA (HST2-HST1) changes in s-IgA and s-cortisol.
Results
All participants successfully completed the HA protocol. For both s-IgA and s-cortisol, data from three participants were removed due to missing saliva flow rate data. Data from another three participants were removed from the s-cortisol dataset because their concentrations were consistently below the lower limit of the standard curve. One participant did not complete all URTS questionnaires. Hence, the analytical cohort was n = 14 (10 males, 4 females) for s-IgA, n = 11 (9 males, 2 females) for s-cortisol, and n = 16 (10 males, 6 females) for URTS.
Heat acclimation and heat stress tests
HA sessions lasted for 97 ± 4 min. Cycling during HA was performed at an average power output of 94 ± 17 W, heart rate of 132 ± 14 bpm (72 ± 7% of peak heart rate), and whole-body sweat rate of 1261 ± 433 g·h−1. Average urine-specific gravity was 1.012 ± 0.006. Resting rectal temperature was not significantly different between HST1 (37.54 ± 0.30°C) and HST2 (37.39 ± 0.38°C; t(16) = 1.90, P = 0.08), while end-exercise rectal temperature after 35 min of steady state cycling was lower in HST2 (38.08 ± 0.35°C) than in HST1 (38.24 ± 0.32°C; t(16) = 2.9, P = 0.01). End-exercise heart rate after 35 min of steady state cycling was lower in HST2 (139 ± 17 bpm) than in HST1 (147 ± 19 bpm; t(16) = 4.7, P < 0.001). Whole-body sweat rate during HST2 (1338 ± 549 g·h−1) was higher than during HST1 (1072 ± 370 g·h−1; t(16) = −4.9, P < 0.001). For more detailed heat acclimation data, the reader is referred to our previous work [36,41].
Resting salivary biomarkers
There was no effect of time on saliva flow rate (0.55 [0.28, 0.73] mL·min−1; χ2(4) = 7.3, P = 0.12, W = 0.13), s-IgA concentration (63.3 [43.9, 82.8] µg·mL−1; χ2(4) = 6.3, P = 0.18, W = 0.12), s-cortisol concentration (1.97 [0.99, 4.05] ng·mL−1; χ2(4) = 7.7, P = 0.10, W = 0.18), or s-cortisol secretion rate (0.74 [0.42, 1.89] ng·min−1; χ2(4) = 3.5, P = 0.48, W = 0.08). There was an effect of time on s-IgA secretion rate (30.7 [15.3, 46.6] µg·min−1; χ2(4) = 12.5, P = 0.01, W = 0.22). Post-hoc pairwise comparisons revealed that the s-IgA secretion rate at HST2 (44.7 [18.5, 51.4] µg·min−1) was significantly higher than at HST1 (20.8 [11.7, 36.9] µg·min−1, P = 0.03). Figure 2 shows the biomarker values for each timepoint, expressed as percentage change from HST1 (with HST1 as 100%). There was no significant association between absolute s-IgA and s-cortisol concentrations (n = 55, rs = −0.05, P = 0.7), or between the pre-to-post HA (HST2-HST1) change in s-IgA and s-cortisol concentrations (n = 11, rs = −0.2, P = 0.6). The pre-to-post HA change in s-IgA and s-cortisol concentrations and secretion rates were not significantly associated with individual characteristics.
Figure 2.
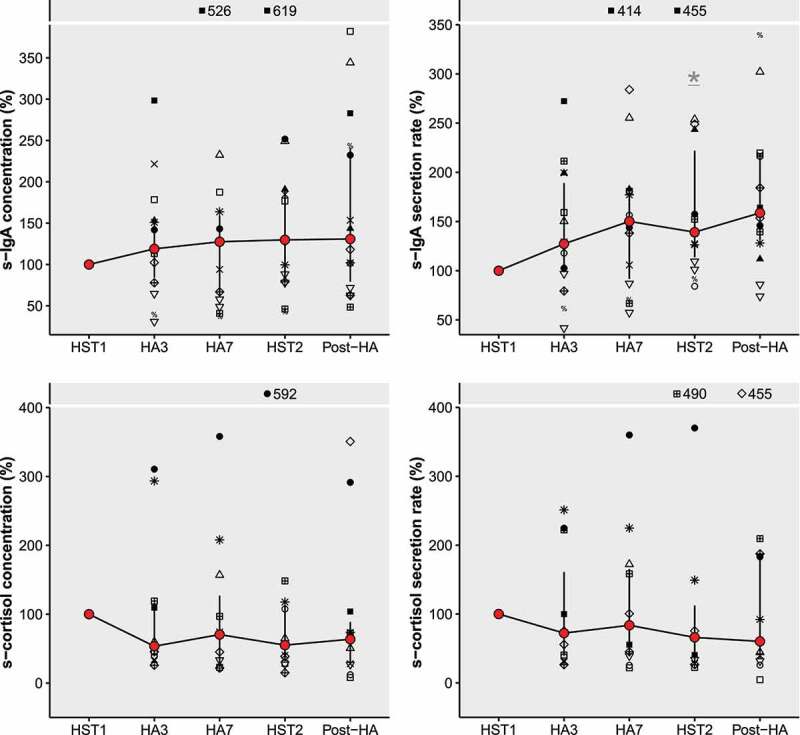
Salivary immunoglobulin-A (s-IgA) and salivary cortisol (s-cortisol) concentrations and secretion rates expressed as percentage change from HST1, with HST1 = 100%. Connected red points represent medians, with error bars ranging from Q1 to Q3. Symbols represent individual participant datapoints, where female datapoints have black fill. Outliers that fell out of the plot window were displayed with their percentage at the top of all panels. * Significantly different from HST1 (statistical testing was done using the absolute values). Abbreviations: HST, heat stress test; HA, heat acclimation.
Upper-respiratory tract symptoms
In total, 15 URTS episodes were reported over the study period (Table 1), with a weekly URTS score of 25 [18,35] and a duration of 2 [1, 3.5] weeks. Twelve participants had 1 or 2 URTS episodes (75%), while 4 participants did not experience a URTS episode (25%). There was no effect of time on weekly URTS score (χ2(5) = 3.3, P = 0.65, W = 0.04; Figure 3). Most reported symptoms were loss of sleep (20% of all symptom occurrences) and runny nose (17%), while least reported symptoms were joint aches and pains (2%) and fever (1%).
Table 1.
Upper respiratory tract symptom (URTS) episode onsets over the study period.
| Time period | Number of URTS episode onsets (proportion of total) |
|---|---|
| Pre-HA | 3 (20%) |
| HApart1 | 5 (33%) |
| HApart2 | 2 (13%) |
| Post-HAwk1 | 3 (20%) |
| Post-HAwk2 | 0 (0%) |
| Post-HAwk3 | 2 (13%) |
| Total | 15 (100%) |
HA, heat acclimation; wk, week.
Figure 3.
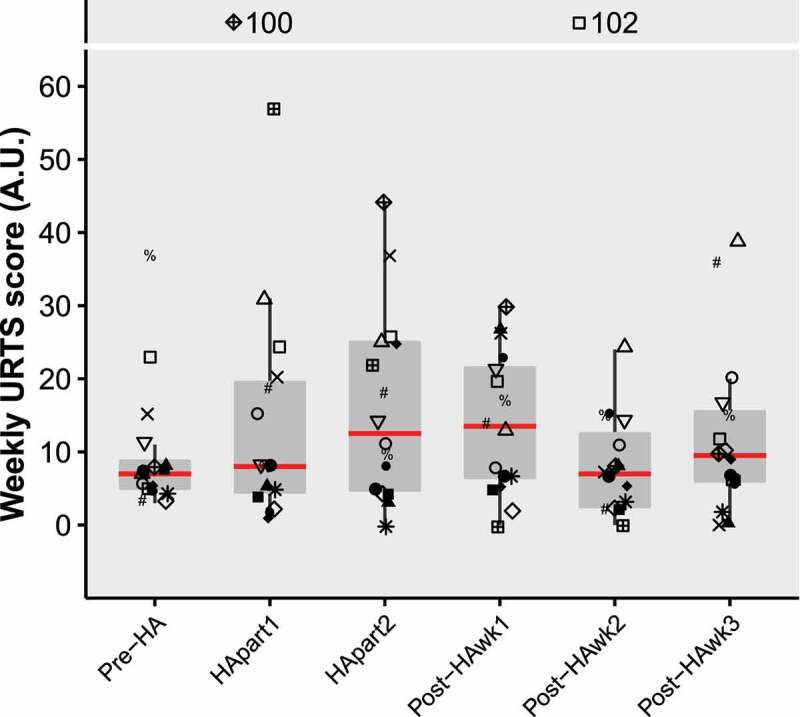
Boxplots of weekly URTS scores over time. Red lines represent medians. Symbols represent individual participant datapoints, where female datapoints have black fill. Outliers that fell out of the plot window were displayed with their value at the top of the figure. Abbreviations: URTS, upper-respiratory tract symptoms; HA, heat acclimation; wk, week.
Wellness
The overall wellness score was not affected by time (χ2(13) = 21.1, P = 0.07, W = 0.14; Figure 4). There was no effect of time on fatigue, muscle soreness, mood, sleep quantity, or overall wellness score (P = 0.10–0.81, W = 0.04–0.09; Figure 5). There was a significant effect of time on both stress levels (χ2(13) = 27.4, P = 0.01, W = 0.12) and sleep quality (χ2(13) = 30.5, P = 0.004, W = 0.14), but no significant post-hoc pairwise comparisons were observed (Figure 5).
Figure 4.
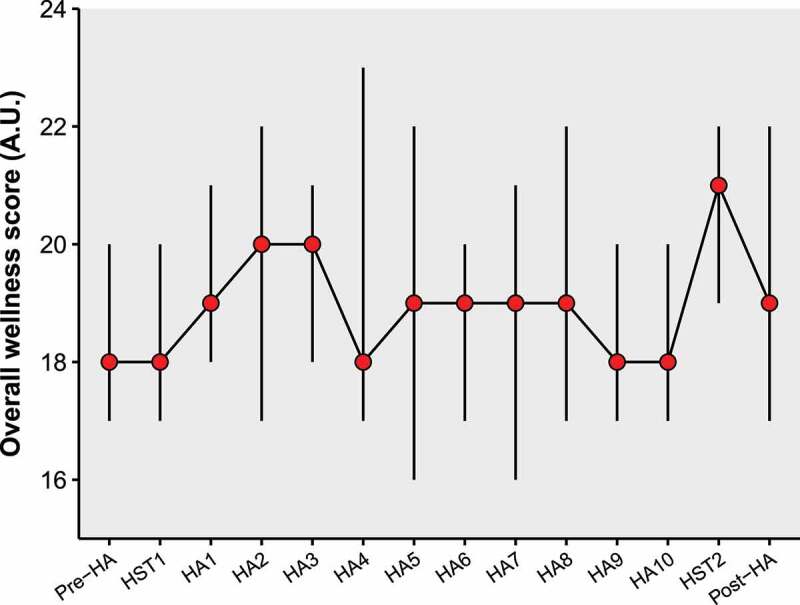
Overall wellness score over time. Red points represent the median, with error bars ranging from Q1 to Q3. Abbreviations: HA, heat acclimation; HST, heat stress test.
Figure 5.
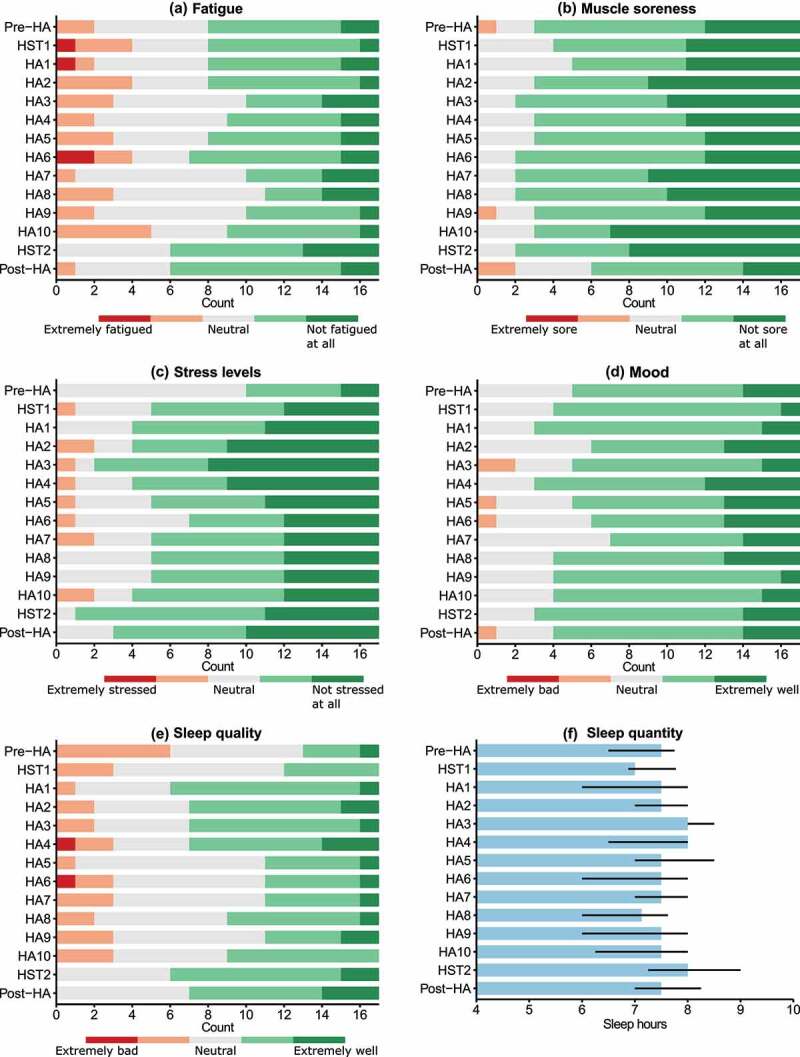
A-E, counts of individual wellness scores. F, median sleep quantity (hours) with error bars ranging from Q1 to Q3. Abbreviations: HA, heat acclimation; HST, heat stress test.
Discussion
Our 10-day controlled-hyperthermia heat acclimation protocol successfully induced heat adaptations, but did not negatively affect resting salivary immunoglobulin-A and salivary cortisol, self-reported upper-respiratory tract symptoms, and self-reported wellness parameters in recreational athletes. In fact, resting s-IgA secretion rate had increased by 39% at the end of HA (HST2) compared to the start (HST1), which is considered favorable for mucosal host defense. To our knowledge, this is the first study that investigated the impact of HA on mucosal immunity and self-reported illness symptoms.
HA did not negatively impact resting s-IgA, evidenced by an unchanged s-IgA concentration and elevated s-IgA secretion rate at HST2 compared to HST1. The unaffected s-IgA concentration reflects previous findings of unchanged cytokine levels following 9- to 10-day HA protocols [33–35]. The significant elevation in s-IgA secretion rate suggests that HA induced favorable changes in host defense. A recent study concluded that HA resulted in beneficial immune changes in sedentary individuals, based on a reduction in white blood cell count [42]. However, this reduction may primarily benefit clinical populations with chronic high white blood cell counts, rather than healthy individuals. It is difficult to compare our findings with these previous HA studies, as we have investigated different immune parameters with distinct function, but all studies agree that HA does not negatively impact the investigated immune parameters.
The elevation in s-IgA secretion rate was likely a combined effect of changes in s-IgA concentration and saliva flow rate. s-IgA secretion rate takes saliva flow rate into account and therefore may give a better indication of immune protection than s-IgA concentration, as it represents the availability of s-IgA on the mucosal surface [11,12]. Previous studies investigating the impact of training load on s-IgA have reported equivocal results, with s-IgA concentrations and secretion rates being suppressed [8,43] or unchanged [18,19] following intensified training periods. Less common is the observation that training load increases s-IgA. Nevertheless, Antualpa et al. [44] found an elevated s-IgA concentration (~35%) and secretion rate (~25%) following a 4-week intense training period in prepuberal gymnasts, which returned to baseline over the subsequent tapering period. Along with this s-IgA elevation, they did not observe changes in URTS [44]. Similarly, in our study, the increased s-IgA secretion rate was not accompanied by a reduction in URTS, suggesting that a 25–39% increase in s-IgA secretion rate may not reduce URTS risk.
The HA regimen in our study included daily HA sessions of 90 min at an intensity of ~72% of peak heart rate. It is unclear what features of an exercise protocol determine the influence on the immune system. Some consider chronic intense exercise training with prolonged sessions (i.e. >1.5 h/day) to be immunosuppressive, while others question whether any form of exercise can suppress immunity [14,22]. When considering chronic exercise as potentially immunosuppressive, factors such as intensity, frequency, and environmental conditions of the sessions may play a role, as well as the recovery time in between sessions. In this context, certain features of the controlled-hyperthermia HA protocol may elucidate our findings. First, the absence of high-intensity intervals in our HA sessions may explain the lack of immunosuppression. Intensive basketball training and competition periods (~540 min·week−1), involving high-intensity interval bouts, led to lower s-IgA secretion rates compared to recovery periods (~300 min·week−1) [43]. On the contrary, in our study, despite a similar increase in training volume (habitual training 399 ± 159 min·week−1 to HA ~679 min·week−1), s-IgA secretion rate increased. Perhaps, training periods with high-intensity intermittent exercise pose a greater risk for immunodepression than lower-intensity endurance-based exercise. Second, the recovery periods in our study, i.e. ~22 h after each HA session, was likely sufficient to recover from any transient immunosuppression. Indeed, Laing et al. [45] observed that s-IgA secretion rate decreased immediately following acute exercise in both hot (30°C, 80% relative humidity) and thermoneutral conditions but returned to pre-exercise values 2 h post exercise. Thus, the typical features of a controlled-hyperthermia exercise program may prevent chronic suppression of s-IgA.
There is ongoing debate as to whether exercise alone can suppress immune function, and researchers point toward a multifactorial cause [14]. Previous findings suggest that HA may induce concomitant non-exercise stressors, such as sleep reduction or fatigue [27,28]. However, we did not find any clear changes in ratings of fatigue, muscle soreness, stress levels, mood, sleep quality and sleep quantity over the course of HA. Our findings on sleep are in contrast with those of Skein et al. [28], who observed a reduction in self-reported sleep quality accompanied by increased awake-time at night after already 5 days of HA. In agreement with our findings, previous studies report no changes in self-reported moods (POMS questionnaire) and fatigue over the course of HA [28,46]. Hence, in our study, HA was not accompanied by non-exercise stressors such as sleep reduction and psychological stress, which may in part explain the absence of immunosuppression.
We considered HA as a substantial stressor, evidenced by the physiological adaptations that occur due to repeated disruption of the body’s homeostasis [26]. However, we did not observe increases in s-cortisol. This coincides with previous studies that observed no changes in resting levels of plasma cortisol during similar 9- and 10-day HA regimens [34,35]. The stable cortisol levels throughout HA may be explained by the moderate exercise intensity, sufficient recovery time and limited psychological stress.
Limitations
S-IgA co-operates with other antimicrobial proteins to protect mucosal surfaces against infectious pathogens, and is therefore not solely responsible for protection against URTS. He et al. [43] showed that an intensified training period may suppress both s-IgA and lactoferrin, but research into the effect of training programs on antimicrobial proteins beyond s-IgA is scarce. In the current study, we can therefore not make inferences about the effect of HA on the full range of mucosal defense factors. Furthermore, the relationship between s-IgA and URTS is debated, primarily because URTS does not have an infective origin per se [14]. We investigated self-reported URTS which were not clinically verified, thus the presence of an infection could not be confirmed. In addition, s-IgA is known to be highly variable between- and within individuals [8,16,19], which complicates interpretation of this biomarker. In our study, this variability may be increased by two specific confounders. First, it is difficult to fully control food and fluid intake during a 12-day heat acclimation study, and the lack thereof may have enlarged variability in saliva secretion [9]. Second, four females were included in the s-IgA analysis, of which two used oral contraceptives. Menstrual cycle phase in non-users of contraceptives may not influence the exercise-induced s-IgA response [47], while oral contraceptive phase could [48]. In our study, it is unlikely that variation in (synthetic) menstrual cycle phase introduced a systematic bias, as menstrual cycle phases during the HSTs were randomly distributed over the four participants. However, inclusion of female participants may have increased the variability in our data. Notwithstanding this limitation, inclusion of female participants should be viewed as a strength, given the paucity of female data on this topic. Seven previous HA studies investigating immune and/or wellness parameters included in total 129 male participants and two females [28,30–35], illustrating the need for female-oriented studies in the future. Rectal temperature data were collected using two different probe types, while aiming for consistent probe use within participants. Due to logistical issues, four participants used different probe types at HST1 and HST2. However, a sensitivity analysis among consistent probe users only (n = 13) showed similar outcomes to the findings of the full analytical cohort (n = 17), highlighting that it is unlikely that changes in probe type affected our outcomes.
Conclusion
The present study showed that a 10-day controlled-hyperthermia program did not negatively affect salivary immunoglobulin-A, salivary cortisol, self-reported upper-respiratory tract symptoms, and self-reported wellness ratings in recreational athletes.
Practical implications
Given the timing of HA, i.e. close to the pinnacle event to prevent adaptations to decay, it is essential that immunity, health, and wellbeing are not compromised. Therefore, the main focus of our paper was on potential negative effects of HA. We did find an elevated s-IgA secretion rate following HA, but it remains unclear whether this 39% gain could reduce the risk of upper respiratory tract infection and/or symptoms. Our results suggest that controlled hyperthermia HA can be safely used in preparation for events such as athletic competitions or military missions. It should be noted that these findings were obtained using laboratory experiments. In a real-life situation, HA may be combined with additional stressors such as travel, poor nutrition, and sleep deprivation, which could elevate the neuroendocrine response and thereby increase the risk for immunosuppression [2]. On the other hand, HA is mainly performed by elite athletes and highly trained military personnel, who may be less vulnerable to exercise-induced immunosuppression than our recreationally active participants [10,22,49]. Based on our findings, HA can be used to successfully induce heat adaptations in the weeks leading up to athletic competition or military deployment, without negatively affecting s-IgA availability, URTS risk and wellness.
Acknowledgments
The authors thank the following people for their assistance during the data collection: Lisa Klous, Mireille Folkerts, Giulio Tan, Romin Gaalman, Mae El Hamoud, Iris Dijkstra, Amber Berlijn, Michelle van Delden and Emma Wiedenmann. We thank Marina Celan for assisting with the ELISA analyses. Special thanks are given to the participants for their dedication to this study.
Funding Statement
This work was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek under Grant [P16-28]; and ZonMw under Grant [2019-30601].
List of abbreviations
HAHeat acclimation
HPA axisHypothalamic-pituitary-adrenal axis
HSTHeat stress test
s-cortisol Salivary cortisol
s-IgA Salivary immunoglobulin-A
URTSUpper respiratory tract symptoms
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Korzeniewski K, Nitsch-Osuch A, Konior M, et al. Respiratory tract infections in the military environment. Respir Physiol Neurobiol. 2015;209:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keaney LC, Kilding AE, Merien F, et al. The impact of sport related stressors on immunity and illness risk in team-sport athletes. J Sci Med Sport. 2018;21(12):1192–1199. [DOI] [PubMed] [Google Scholar]
- [3].Gleeson M, Pyne DB.. Respiratory inflammation and infections in high-performance athletes. Immunol Cell Biol. 2016;94(2):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Raysmith BP, Drew MK. Performance success or failure is influenced by weeks lost to injury and illness in elite Australian track and field athletes: a 5-year prospective study. J Sci Med Sport. 2016;19(10):778–783. [DOI] [PubMed] [Google Scholar]
- [5].Van Tonder A, Schwellnus M, Swanevelder S, et al. A prospective cohort study of 7031 distance runners shows that 1 in 13 report systemic symptoms of an acute illness in the 8–12 day period before a race, increasing their risk of not finishing the race 1.9 times for those runners who started the race: SAF. Br J Sports Med. 2016;50(15):939–945. [DOI] [PubMed] [Google Scholar]
- [6].Warr BJ, Heumann KJ, Dodd DJ, et al. Injuries, changes in fitness, and medical demands in deployed national guard soldiers. Mil Med. 2012;177(10):1136–1142. [DOI] [PubMed] [Google Scholar]
- [7].Keaney LC, Kilding AE, Merien F, et al. Keeping athletes healthy at the 2020 Tokyo summer games: considerations and illness prevention strategies. Front Physiol. 2019;10:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Neville V, Gleeson M, Folland JP. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc. 2008;40(7):1228–1236. [DOI] [PubMed] [Google Scholar]
- [9].Papacosta E, Nassis GP. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J Sci Med Sport. 2011;14(5):424–434. [DOI] [PubMed] [Google Scholar]
- [10].Derman W, Badenhorst M, Eken M, et al. Risk factors associated with acute respiratory illnesses in athletes: a systematic review by a subgroup of the IOC consensus on ‘acute respiratory illness in the athlete. Br J Sports Med. 2022. bjsports-2021-104795. DOI: 10.1136/bjsports-2021-104795. [DOI] [PubMed] [Google Scholar]
- [11].Bishop NC, Gleeson M. Acute and chronic effects of exercise on markers of mucosal immunity. Front Biosci (Landmark Ed). 2009;14(12):4444–4456. [DOI] [PubMed] [Google Scholar]
- [12].Gleeson M, Bishop N, Oliveira M, et al. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012;22(3):410–417. [DOI] [PubMed] [Google Scholar]
- [13].Gleeson M, Pyne DB, Elkington LJ, et al. Developing a multi-component immune model for evaluating the risk of respiratory illness in athletes. Exerc Immunol Rev. 2017;23:52–64. [PubMed] [Google Scholar]
- [14].Simpson RJ, Campbell JP, Gleeson M, et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- [15].Gleeson M, Pyne DB, Austin JP, et al. Epstein-Barr virus reactivation and upper-respiratory illness in elite swimmers. Med Sci Sports Exerc. 2002;34(3):411–417. [DOI] [PubMed] [Google Scholar]
- [16].Cunniffe B, Griffiths H, Proctor W, et al. Mucosal immunity and illness incidence in elite rugby union players across a season. Med Sci Sport Exercise. 2011;43(3):388–397. [DOI] [PubMed] [Google Scholar]
- [17].Tiollier E, Gomez-Merino D, Burnat P, et al. Intense training: mucosal immunity and incidence of respiratory infections. Eur J Appl Physiol. 2005;93(4):421–428. [DOI] [PubMed] [Google Scholar]
- [18].Moreira A, de Moura NR, Coutts A, et al. Monitoring internal training load and mucosal immune responses in futsal athletes. J Strength Cond Res. 2013;27(5):1253–1259. [DOI] [PubMed] [Google Scholar]
- [19].Keaney LC, Kilding AE, Merien F, et al. Predictors of upper respiratory tract symptom risk: differences between elite rugby union and league players. J Sports Sci. 2021;39(14):1594–1601. [DOI] [PubMed] [Google Scholar]
- [20].Walsh NP, Whitham M. Exercising in environmental extremes. Sports Med. 2006;36(11):941–976. [DOI] [PubMed] [Google Scholar]
- [21].Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- [22].Walsh NP, Oliver SJ. Exercise, immune function and respiratory infection: an update on the influence of training and environmental stress. Immunol Cell Biol. 2016;94(2):132–139. [DOI] [PubMed] [Google Scholar]
- [23].Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Br J Sports Med. 2015;49(18):1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Daanen HAM, Racinais S, Périard JD. Heat acclimation decay and re-induction: a systematic review and meta-analysis. Sports Med. 2018;48(2):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Périard JD, Eijsvogels TMH, Daanen HAM. Exercise under heat stress: thermoregulation, hydration, performance implications, and mitigation strategies. Physiol Rev. 2021;101(4):1873–1979. [DOI] [PubMed] [Google Scholar]
- [26].Taylor NAS. Human heat adaptation. Compr Physiol. 2014;4(1):325–365. [DOI] [PubMed] [Google Scholar]
- [27].Wingfield GL, Gale R, Minett GM, et al. The effect of high versus low intensity heat acclimation on performance and neuromuscular responses. J Therm Biol. 2016;58:50–59. [DOI] [PubMed] [Google Scholar]
- [28].Skein M, Wingfield G, Gale R, et al. Sleep quantity and quality during consecutive day heat training with the inclusion of cold-water immersion recovery. J Therm Biol. 2018;74:63–70. [DOI] [PubMed] [Google Scholar]
- [29].Cohen S, Doyle WJ, Alper CM, et al. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Barberio MD, Elmer DJ, Laird RH, et al. Systemic LPS and inflammatory response during consecutive days of exercise in heat. Int J Sport Med. 2015;36(3):262–270. [DOI] [PubMed] [Google Scholar]
- [31].Guy JH, Pyne DB, Deakin GB, et al. Acclimation training improves endurance cycling performance in the heat without inducing endotoxemia. Front Physiol. 2016;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Willmott AGB, Hayes M, Waldock KAM, et al. Short-term heat acclimation prior to a multi-day desert ultra-marathon improves physiological and psychological responses without compromising immune status. J Sports Sci. 2017;35(22):2249–2256. [DOI] [PubMed] [Google Scholar]
- [33].Yamada PM, Amorim FT, Moseley P, et al. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol. 2007;103(4):1196–1204. [DOI] [PubMed] [Google Scholar]
- [34].Costello JT, Rendell RA, Furber M, et al. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine. 2018;110:277–283. [DOI] [PubMed] [Google Scholar]
- [35].Willmott AGB, Hayes M, James CA, et al. Once- and twice-daily heat acclimation confer similar heat adaptations, inflammatory responses and exercise tolerance improvements. Physiol Rep. 2018;6(24):e13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alkemade P, Gerrett N, Eijsvogels TMH, et al. Individual characteristics associated with the magnitude of heat acclimation adaptations. Eur J Appl Physiol. 2021;121(6):1593–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kenefick RW, Cheuvront SN. Hydration for recreational sport and physical activity. Nutr Rev. 2012;70(Suppl 2):S137–42. [DOI] [PubMed] [Google Scholar]
- [38].Chicharro JL, Lucía A, Pérez M, et al. Saliva composition and exercise. Sports Med. 1998;26(1):17–27. [DOI] [PubMed] [Google Scholar]
- [39].Jackson GGEE, Dowling HF, Spiesman IG, et al. Transmission of the common cold to volunteers under controlled conditions: i. The common cold as a clinical entity. AMA Arch Intern Med. 1958;101(2):267–278. [DOI] [PubMed] [Google Scholar]
- [40].Buchheit M, Racinais S, Bilsborough JC, et al. Monitoring fitness, fatigue and running performance during a pre-season training camp in elite football players. J Sci Med Sport. 2013;16(6):550–555. [DOI] [PubMed] [Google Scholar]
- [41].Gerrett N, Alkemade P, Daanen H. Heat reacclimation using exercise or hot water immersion. Med Sci Sports Exerc. 2021;53(7):1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rivas E, Crandall CG, Suman OE, et al. Exercise heat acclimation causes post-exercise hypotension and favorable improvements in lipid and immune profiles: a crossover randomized controlled trial. J Therm Biol. 2019;84:266–273. [DOI] [PubMed] [Google Scholar]
- [43].C-S H, Tsai M-L, M-H K, et al. Relationships among salivary immunoglobulin A, lactoferrin and cortisol in basketball players during a basketball season. Eur J Appl Physiol. 2010;110(5):989–995. [DOI] [PubMed] [Google Scholar]
- [44].Antualpa K, Aoki MS, Moreira A. Intensified training period increases salivary IgA responses but does not affect the severity of upper respiratory tract infection symptoms in prepuberal rhythmic gymnasts. Pediatr Exerc Sci. 2018;30(2):189–197. [DOI] [PubMed] [Google Scholar]
- [45].Laing SJ, Gwynne D, Blackwell J, et al. Salivary IgA response to prolonged exercise in a hot environment in trained cyclists. Eur J Appl Physiol. 2005;93(5):665–671. [DOI] [PubMed] [Google Scholar]
- [46].Willmott AGB, Hayes M, and James CA, et al. Heat acclimation attenuates the increased sensations of fatigue reported during acute exercise-heat stress. Temperature. 2020;7(2):178–190. doi: 10.1080/23328940.2019.1664370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gillum T, Kuennen M, Miller T, et al. The effects of exercise, sex, and menstrual phase on salivary antimicrobial proteins. Exerc Immunol Rev. 2014;20:23–38. [PubMed] [Google Scholar]
- [48].Hayashida H, Dolan NJ, Hounsome C, et al. Salivary SIgA responses to acute moderate-vigorous exercise in monophasic oral contraceptive users. Appl Physiol Nutr Metab. 2015;40(9):863–867. [DOI] [PubMed] [Google Scholar]
- [49].Schwellnus M, Soligard T, Alonso J-M, et al. How much is too much? (Part 2) international olympic committee consensus statement on load in sport and risk of illness. Br J Sports Med. 2016;50(17):1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]


