Abstract
In this study, we identified a receptor for the K88ad fimbrial adhesin of Escherichia coli in neutral glycosphingolipid preparations from intestinal epithelial cells of K88ad-adhesive pigs, which was absent in preparations from K88ad-nonadhesive pigs. Neither K88ab nor K88ac adhesin variants bound to this neutral glycosphingolipid. Because this receptor is an intestinal glycosphingolipid that binds K88ad adhesin, it has been designated IGLad. Carbohydrate compositional analysis of a partially purified preparation of IGLad identified galactose, glucose, and N-acetylglucosamine in a ratio of 1.5:1.0:0.5 as the major monosaccharides. Preliminary characterization experiments using lectins showed that IGLad contains the terminal glycanic structure Galβ1-4GlcNAc. Removal of terminal β-linked galactose residues from IGLad decreased the recognition of IGLad by the K88ad adhesin, indicating that terminal β-linked galactose is an essential component of the K88ad adhesin recognition site on IGLad. Studies with purified glycosphingolipid standards demonstrated that K88ad adhesin binds to neolactotetraosylceramide (nLc4Cer) (Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1Cer), lactotriosylceramide (GlcNAcβ1-3Galβ1-4Glcβ1-1Cer) and lactotetraosylceramide (Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ1-1Cer). Based on these studies, IGLad appears to be nLc4Cer.
Newborn and weaned piglets are particularly susceptible to diarrhea induced by colonization of their small intestine by enterotoxigenic Escherichia coli (ETEC) strains that express K88 fimbrial adhesins (37, 41). The initial step in the colonization of a host by bacteria involves the interaction of bacterial adhesins with host cell receptors (23). Using an in vitro adherence test, Sellwood et al. (35) demonstrated the existence of two phenotypes of piglets relative to attachment of K88+ E. coli to intestinal brush border preparations. Ability to bind K88+ E. coli, and thus presumably expression of a receptor for K88 adhesins, was found to be inherited in a simple Mendelian fashion, with susceptibility to K88+ E. coli adhesin (receptor presence) dominant over resistance to adhesion (receptor absence) (15). In more recent studies, other investigators have found that the three antigenic variants of K88 adhesin (K88ab, K88ac, and K88ad) differ in their porcine enterocyte brush border binding specificities (5, 31). Results published by several research groups suggest the presence of up to six porcine phenotypes in nature, relative to the binding of the three K88 adhesin variants to brush borders (4, 5, 31). These phenotypes and the K88 variants that bind to their brush borders are A (K88ab, K88ac, K88ad); B (K88ab, K88ac); C (K88ab, K88ad); D (K88ad); E (no binding); and F (K88ab).
Differences in the adhesiveness of intestinal brush border membranes from pigs of each of the six phenotypes are due to the presence of phenotype-specific K88 fimbrial adhesin receptors. We have defined phenotype-specific receptors as those which fulfill the following three criteria. (i) The receptor must display specificity for the particular K88 fimbrial adhesin variant. (ii) The receptor must be detectable exclusively in brush borders phenotyped as adhesive for that particular K88 fimbrial adhesin variant. (iii) The receptor must be expressed in multiple animals of the same adhesive phenotype (6). Many other research groups have reported the identification of putative K88 fimbrial adhesin receptors. Of the reported K88 receptors, only two brush border receptors have been identified which fulfill the aforementioned criteria for phenotype-specific receptors. These receptors are (i) intestinal mucin-type sialoglycoproteins IMTGP-1 and IMTGP-2, which bind K88ab and K88ac fimbrial adhesins (6, 10, 11, 34) and (ii) intestinal transferrin, which binds K88ab fimbrial adhesin (16). No phenotype-specific receptors for the K88ad fimbrial adhesin have been identified.
Knowledge of the receptor specificity of the K88 fimbrial adhesins is essential for understanding the molecular mechanism of K88+ ETEC adhesion. Several researchers have investigated the molecular interaction of K88 fimbrial adhesin with erythrocytes, intestinal mucus, and intestinal epithelial cells (10, 11, 14, 16, 28, 29, 37, 40). It has been clearly demonstrated that all three K88 adhesin variants recognize carbohydrate structures expressed on host cell glycoconjugates. However, previous studies to determine the carbohydrate specificity of the K88 adhesins have not been definitive. Data from monosaccharide blocking studies indicate that N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), N-acetylmannosamine (2), and d-galactosamine (36) may be involved. Results from glycoprotein blocking studies indicate that terminal GlcNAc, GalNAc, and galactose (Gal) may play a role in the interaction of the K88 adhesin with brush border receptors (2, 14). Also, Gal has been reported to be an important residue in the recognition of putative intestinal mucus receptors and glycosphingolipids by the K88ab adhesin (7, 30). Recently, we determined that β-linked Gal is an essential component in recognition of IMTGP-1 and IMTGP-2 by K88ac adhesin (17). Studies aimed at determining the differences in carbohydrate specificity between the K88 adhesin variants have not been completed.
The aim of the present study was to identify and begin to characterize phenotype-specific receptors for K88 adhesin variants among intestinal glycolipids. We identified a phenotype-specific receptor (IGLad) for the K88ad adhesin in intestinal neutral glycosphingolipid preparations from two K88ad-adhesive phenotypes, A and D. No phenotype-specific receptors for K88ab and K88ac adhesins were detected among the intestinal glycolipids. We characterized IGLad as being Galβ1-GlcNAcβ1-3Galβ1-4Glcβ1-1Cer (nLc4Cer). Exoglycosidase studies demonstrated that terminal β-linked Gal is an essential component of the site on IGLad recognized by K88ad adhesin.
MATERIALS AND METHODS
Materials.
Organic solvents, glycolipid standards (lactocerebroside [Lc2Cer] [Galβ1-4Glcβ1-1Cer], globoside [Gb4Cer] [GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1Cer], and globopentaglycosylceramide [Gb5Cer] [GalNAcα1-3GalNAcβ1-3Galα1-4Galβ1-4Glcβ1-1Cer]), DEAE-cellulose, orcinol, β-galactosidase from E. coli, biotinylated monoclonal antibody (MAb) to human Lex (CD15), peroxidase-conjugated antiserum to mouse immunoglobulin G (IgG), and polyvinylpyrrolidone 40 (PVP-40) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Precoated high-performance thin-layer chromatography (HPTLC) plates (Silica Gel 60) with aluminum backing, florisil (60 to 100 mesh), and silicic acid (150 mesh) were obtained from E. Merck AG (Darmstadt, Federal Republic of Germany). Biotinylated vegetable lectins (concanavalin A lectin [ConA], soybean agglutinin [SBA], peanut agglutinin [PNA], Sambucus nigra agglutinin [SNA], Bandeira simplicifolia lectin [BSL II], Datura stramonium lectin [DSL], Jacalin, wheat germ agglutinin [WGA], Ulex europaeus agglutinin [UEA], Ricinus communis agglutinin [RCA120], Maackia amurensis lectin I and II [MAL I and MAL II], Erythrina cristagalli lectin [ECL], Vicia villosa agglutinin [VVA]) were obtained from Vector Laboratories, Inc. (Burlingame, Calif.). MAb against blood group antigens A, B, and H were obtained from Dako Corporation (Carpinteria, Calif.).
The purified glycosphingolipids were prepared as follows. nLc4Cer and Lc3Cer (GlcNAcβ1-3Galβ1-4Glcβ1-1Cer) were obtained sequentially by acid catalyzed desialosylation and β-galactosidase treatment of bovine erythrocyte IV3NeuAc(Gc)nLc4Cer as described by Levery et al. (26). Lc4Cer (Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ1-1Cer) was obtained by partial conversion of Lc3Cer with a recombinant human β1,3-galactosyltransferase as described in reference 1. Lex [Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4Glcβ1-1Cer] was obtained from human adenocarcinoma (18, 19, 42). V3FucnLc6Cer [Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1Cer] was obtained from human granulocytes (13, 22, 39). VI2FucnLc6Cer (Fucα1-2Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1Cer) was obtained from human O erythrocytes (25). The Lea glycosphingolipid [Galβ1-3(Fucα1-4)GlcNAcβ1-3Galβ1-4Glcβ1-1Cer] was isolated from a mixture of Folch lower-phase glycosphingolipids extracted from pooled liver and colonic adenocarcinoma (19) and was kindly provided by Mark R. Stroud (Department of Cell Surface Biochemistry, Northwest Hospital, Seattle, Wash. and Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Wash.).
Extraction of brush border glycolipids.
Brush border vesicles were prepared from frozen adult porcine small intestines from a number of pigs of different phenotypes (16 of phenotype A, 1 of phenotype B, 1 of phenotype C, 3 of phenotype D, and 2 of phenotype E) as previously described (10). Total lipids were extracted from brush border vesicles (0.5 to 1.7 g [dry weight]) by dissolving the lyophilized brush borders in 50 volumes (based on dry weight) of methanol at room temperature. The undissolved solids were removed by filtration through a Buchner funnel with a fritted disc (porosity, 10 to 15 μm) and then were mixed with 50 volumes of methanol and incubated for 30 min at 40°C. The undissolved solids were again removed by filtration through a Buchner funnel. This extraction procedure was repeated on the undissolved solids by using methanol-chloroform (2:1 [vol/vol]) and methanol-chloroform (1:2 [vol/vol]) as the extracting solvent. All of the clarified extracts (total lipid extract) were pooled and dried by evaporation. Further processing of the total lipid extract was accomplished using the procedure described by Schnaar (32).
Removal of nonglycolipid contaminants.
Dried total lipid extract was suspended in 0.1 N NaOH in methanol (150 ml) and heated for 2 h at 30°C. The pH of the solution was then brought back to neutrality by adding 15 ml of 1 M sodium phosphate (pH 7.0) and 15 ml of aqueous 1 M HCl. The neutralized solution was evaporated to dryness. The residue was suspended in water, dialyzed against tap water for 72 h at 4°C, and evaporated to dryness. This residue was dissolved in chloroform and loaded onto a silicic acid column (1.5 by 15 cm) equilibrated with chloroform. Cholesterol, triglycerides, phospholipids, and sphingomyelin contaminants were eluted using 10 column volumes of chloroform followed by 10 column volumes of chloroform-methanol (98:2 [vol/vol]) and discarded. Glycolipids were eluted using 10 column volumes of chloroform-methanol (1:3 [vol/vol]) followed by 10 column volumes of methanol and were dried by evaporation.
Separation of neutral and acidic glycolipids by anion exchange chromatography.
The total glycolipid fraction was dissolved in 10 ml of chloroform-methanol-water (30:60:8 [vol/vol/vol]) and loaded onto a column (1.5 by 15 cm) of DEAE-Sephadex A25 in acetate form (Pharmacia, Piscataway, N.J.). The flow through the column was stopped, and the sample was allowed to incubated on the column for 24 h at room temperature. The neutral glycosphingolipids were eluted with 10 volumes of chloroform-methanol-water (30:60:8 [vol/vol/vol]), evaporated to dryness, and then further dried for 16 h in a dessicator containing P2O5. The acidic fraction (gangliosides) was recovered by eluting the column with 5 volumes of 0.5 M sodium acetate in methanol. The acidic fraction was then dried and desalted on a Sep-Pak C18 cartridge (Waters-Millipore Co., Milford, Mass.) as described by Ledeen and Yu (24). The gangliosides were then dried under nitrogen and stored at −20°C.
Acetylation and deacetylation.
Neutral glycosphingolipids were acetylated by dissolving the dried glycosphingolipids in 9 ml of chloroform-pyridine-acetic anhydride (1:1:1 [vol/vol/vol]) and then incubating this solution in the dark at room temperature for 16 h. The solvents were removed by evaporation in the presence of an excess of toluene. The acetylated neutral glycosphingolipids were dissolved in dichloroethane (DCE) and loaded onto a florisil column (1.5 by 15 cm) equilibrated with DCE. Cholesterol and free ceramide were removed by washing the column with 10 column volumes of DCE and were discarded. Acetylated neutral glycosphingolipids were eluted with 10 column volumes of DCE-acetone (9:1 [vol/vol]), 10 column volumes of DCE-acetone (1:1 [vol/vol]) and 10 column volumes of acetone. The eluants were combined and evaporated to dryness. Deacetylation was accomplished by dissolving the dried acetylated glycosphingolipids in 20 ml of 0.1 N NaOH in methanol followed by incubation at 37°C for 2 h. This solution was neutralized by adding 2 ml of 0.1 N HCl. It was then evaporated to dryness, dissolved in 2 ml of 0.2 M sodium acetate-methanol (1:1 [vol/vol]), desalted using Sep-Pak C18 cartridges (8), and again evaporated to dryness.
Removal of nonpolar contaminants.
Neutral glycosphingolipids were dissolved in chloroform-methanol (2:1 [vol/vol]), and loaded onto a DEAE-cellulose column (0.5 by 10 cm) equilibrated in the same solvent. The flow through the column was stopped, and the sample was allowed to incubate on the column for 24 h at room temperature. Neutral glycosphingolipids were then eluted using two column volumes of chloroform-methanol (2:1 [vol/vol]) followed by two column volumes of methanol. The eluted neutral glycosphingolipids were evaporated to dryness, solubilized in chloroform-methanol (98:2 [vol/vol]), and loaded on a silicic acid column (0.5 by 10 cm) equilibrated in the same solvent. The nonpolar contaminants were eluted using five column volumes of chloroform-methanol (98:2 [vol/vol]). Neutral glycosphingolipids were eluted using five column volumes of chloroform-methanol (1:3 [vol/vol]) followed by five column volumes of methanol and evaporated to dryness. The neutral glycosphingolipids were dissolved in a minimum volume of chloroform-methanol (2:1 [vol/vol]) and dried under a stream of nitrogen. The amount of neutral glycosphingolipid was determined as lipid-bound neutral sugar by using the phenol-sulfuric acid method (9).
HPTLC overlay binding assay.
Glycolipids were separated on HPTLC plates as described previously (33). HPTLC was performed using solvents containing chloroform-methanol-water (60:30:5 [vol/vol/vol]) for the intestinal neutral glycosphingolipids (20) and chloroform-methanol-water (50:47:14 [vol/vol/vol]) containing 0.038% CaCl2 for purified glycosphingolipids. Typically, two chromatograms were developed in parallel on the same HPTLC plate. One was sprayed with orcinol-sulfuric acid reagent to detect glycosphingolipids (33), and the other was overlaid with biotinylated K88 adhesins (prepared as previously described by Erickson et al. [10]), biotinylated vegetable lectins, or anti-blood group MAb. After separation, the dried chromatogram was sprayed with either buffer A (0.01 M Na2HPO4, 0.14 M NaCl, 2% PVP-40 [pH 7.2]), which was used for overlay assays with bacterial adhesins, or buffer B (0.1 mM CaCl2, 0.15 NaCl, 0.01 M HEPES, 2% PVP-40 [pH 7.5]) which was used for biotinylated lectins and MAbs. The plates were then immersed in the appropriate buffer for 1.5 h at room temperature with gentle stirring (20 rpm). The plates were removed from the buffer solution, and the excess buffer was allowed to drip off the plate. The plates were placed silica side down into buffered solutions containing K88 adhesins, lectins, or MAb (10 μg/ml) and were incubated for 2 h at room temperature with gentle stirring (20 rpm). Unbound molecules were then removed by washing the plate two times for 10 min each in buffer A or B. Bound biotinylated K88 adhesins, lectins, or antibodies were detected by incubating the plate with horseradish peroxidase conjugated to streptavidin (1 μg/ml diluted in appropriate buffer) for 1 h at room temperature with stirring. Bound MAb to blood groups A and H were detected by incubating the plate with peroxidase-conjugated antibodies to mouse IgG (diluted 1:5,000 in buffer B) for 1 h at room temperature. After two washes, the bound peroxidase activity was detected by using 3,3′-diaminobenzidine in the presence of CoCl2 as previously described (21).
Exoglycosidase treatment.
Exoglycosidase digestions were performed on 1.30 mg of glycolipid (based on amount of neutral sugar) in 200 μl (final digestion volume) of 0.2 M sodium citrate (pH 5.0) containing 0.1% sodium taurodeoxycholate. Either 4 U of β-galactosidase or 0.2 U of α-l-fucosidase (Boehringer Mannheim Corp., Indianapolis, Ind.) dissolved in 0.2 M sodium citrate (pH 5.0) was added to the digestion mixture. The digestion mixture was incubated at 37°C for 16 h. After digestion, a chloroform-methanol-water mixture was added to obtain a final ratio of 2:43:55 (vol/vol/vol), and the solution was desalted on Sep-Pak C18 cartridges prior to separation on HPTLC plates.
Monosaccharide analysis.
IGLad was partially purified using preparative silica gel HPTLC plates which had been prerun with chloroform-methanol-water (50:50:15 [vol/vol/vol]) to remove any contaminants. The crude intestinal glycosphingolipid extract from phenotype A pigs corresponding to 845 μg of neutral sugars was applied in the 2-cm linear band on the preparative silica gel plate. On the same plate, 100 μg of crude intestinal glycosphingolipid extract and the glycosphingolipid standards were loaded. This plate was run in the solvent system containing chloroform-methanol-water 60:30:5 (vol/vol/vol). The portion of the plate which contained the glycosphingolipid standards and the 100 μg of crude extract was cut and stained by orcinol-sulfuric acid reagent. The stained plate was matched with the plate containing 845 μg of separated crude extract, and the silica in the region of the plate containing IGLad was scraped from the support. It was placed in a solvent containing chloroform-methanol-water 10:10:1 (vol/vol/vol) and incubated overnight with stirring. The silica was removed by centrifugation at 1,000 × g for 10 min, and the supernatant containing IGLad was dried under nitrogen. Carbohydrate compositional analysis was performed on this sample at the Complex Carbohydrate Research Center at the University of Georgia, Athens, Ga. Trimethylsilylated methyl glycosides were prepared by methanolysis, N-(re)acetylation and trimethylsilylation as described by Merkle and Poppe (27). These trimethylsilylated methylglycosides were separated and quantified on a Hewlett-Packard 5890 gas chromatograph coupled to a 5970 mass spectrometer using a 50-m Quadrex methyl silicone capillary column.
RESULTS
Identification of K88 adhesin receptors among intestinal glycolipids.
To determine if any of the three K88 adhesin variants bind to intestinal glycolipids, we prepared both gangliosides and neutral glycosphingolipids from the small intestine of a phenotype A pig. These glycolipids were separated on HPTLC plates and overlaid with one of the three biotinylated K88 adhesin variants. None of the three K88 adhesin variants bound to any bands in the ganglioside preparation (data not shown). K88ad fimbrial adhesin bound intensely to a number of neutral glycosphingolipids, while neither the K88ab nor the K88ac variant bound intensely to any neutral glycosphingolipids (data not shown). Based on these results, we focused our experiments on the K88ad adhesin variant. Biotinylated K88ad adhesin was used to detect potential receptors in neutral glycosphingolipid preparations from five different phenotypes of pigs (see Introduction for discussion of phenotypes) (Fig. 1, panel II, lanes 2 to 6). One neutral glycosphingolipid migrating slightly slower than Lc2Cer was recognized by the K88ad adhesin in glycosphingolipid preparations from phenotype A, E, B, and D pigs (Fig. 1, panel II). Because this molecule is detected in preparations from non-K88ad-adhesive phenotypes of pigs (E and B), it is not likely a phenotype-specific K88ad receptor. Another neutral glycosphingolipid migrating between the Gb4Cer and Gb5Cer was recognized by the K88ad adhesin in glycosphingolipid preparations from two K88ad-adhesive phenotypes (A and D) of pigs, but not in preparations from nonadhesive phenotypes (E or B) of pigs (Fig. 1, panel II). Since this neutral glycosphingolipid is the only K88ad adhesin-binding molecule detected exclusively in K88ad adhesive phenotypes of pigs, we have designated this putative phenotype-specific K88ad adhesin receptor as intestinal glycosphingolipid-ad (IGLad). Since the initial identification of IGLad, we have individually tested brush border preparations from 16 phenotype A, 1 phenotype B, 1 phenotype C, 3 phenotype D and 2 phenotype E animals for the presence of IGLad. IGLad was found in all phenotype A and D animals and not in any phenotype B, C, or E animals.
FIG. 1.
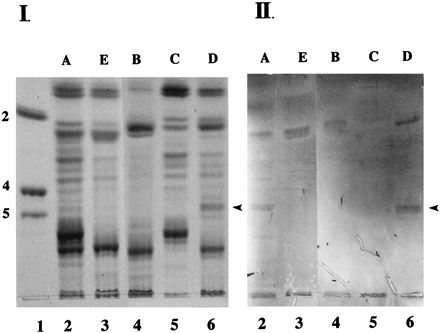
Binding of K88ad adhesin to neutral glycosphingolipids from adhesive and nonadhesive phenotypes of pigs. Neutral glycosphingolipids (100 μg) from five phenotypes of pigs (A [lane 2], E [lane 3], B [lane 4], C [lane 5], and D [lane 6]) were separated on HPTLC plates as described in Materials and Methods. Chromatograms were either stained with orcinol-sulfuric acid reagent (panel I) or incubated with biotinylated K88ad adhesin (panel II) as described in Materials and Methods. Lane 1 contains glycolipid standards Lc2Cer (2), Gb4Cer (4), and Gb5Cer (5). The arrowheads indicate the positions of IGLad.
Preliminary characterization of IGLad.
To determine the monosaccharide composition of IGLad, we used preparative HPTLC to prepare sufficient amounts of IGLad for carbohydrate compositional analysis (Fig. 2). We then determined the monosaccharide composition of this partially purified IGLad preparation (Table 1). Based on the expected presence of a single glucosyl residue in mammalian glycosphingolipids (38), we normalized the molar ratio of the glucosyl residues to 1.00. The major monosaccharides detected in the IGLad preparation were Gal, glucose (Glc), and GlcNAc in an approximate ratio of 1.5:1.0:0.5. Much lower levels of fucose (Fuc) (0.17) and GalNAc (0.15) were also found in this preparation, suggesting that it contains more than one type of glycosphingolipid.
FIG. 2.
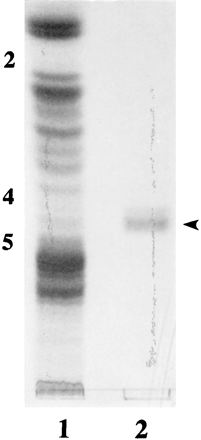
Purification of IGLad. IGLad was purified using preparative HPTLC as described in Materials and Methods. Crude intestinal neutral glycosphingolipids (100 μg of neutral sugars) from phenotype A (lane 1) and purified IGLad (lane 2) were separated by HPLTC as described in Materials and Methods. Chromatograms were stained with orcinol-sulfuric acid reagent. The numbers on the left correspond to the glycolipid standards Lc2Cer (2), Gb4Cer (4) and Gb5Cer (5). The arrowhead indicates the position of IGLad.
TABLE 1.
Monosaccharide composition of partially purified IGLad
| Monosaccharide | Molar ratioa |
|---|---|
| Fuc | 0.17 |
| Gal | 1.46 |
| Glc | 1.00 |
| GalNAc | 0.15 |
| GlcNAc | 0.51 |
Molar ratio normalized to Glc = 1.00.
To further characterize the structure of the carbohydrate moiety of IGLad, we separated intestinal neutral glycosphingolipids by HPTLC and overlaid them with 13 different biotinylated lectins with known carbohydrate specificities (Table 2). ECL, RCA120, and UEA lectins were shown to bind intensely, and MAL-I weakly, in the region of IGLad migration. Strong reactivity with RCA120 and UEA indicates that both galactosyl and fucosyl residues are found in terminal positions on IGLad or on glycosphingolipids with similar HPTLC mobilities as IGLad. Strong reactivity with ECL which is specific for Galβ1-4GlcNAc and weak reactivity with MAL-I which is specific for Galβ1-3-GlcNAc indicates that IGLad and/or glycosphingolipids with HPTLC mobilities similar to that of IGLad contain predominantly Galβ1-4GlcNAc and not Galβ1-3GlcNAc (Table 2). In addition to lectin studies, we performed studies using anti-blood group MAb (Fig. 3). We found that anti-Lex, anti-B, and anti-A MAb (Fig. 3B, D, and E), but not anti-H MAb (Fig. 3C), bound to glycosphingolipids which migrate in the same region as IGLad. The band detected in the anti-B panel is much lighter than those detected in the anti-Lex and anti-A panels and may represent cross-reactivity of the anti-B MAb with blood group A or Lex-containing glycosphingolipids (Fig. 3D, lanes 2 and 3). These results indicate that IGLad or glycosphingolipids with similar HPTLC mobilities as IGLad contain the following: (i) Lex antigen [Galβ1-4(Fucα1-3)GlcNAc], (ii) blood group A antigen [GalNAcα1-3(Fucα1-2)Gal], and possibly (iii) blood group B antigen [Galβ1-3(Fucα1-2)Gal]. These results are consistent with those of the monosaccharide composition and lectin-binding studies described above.
TABLE 2.
Determination of the binding of selected biotinylated lectins
| Lectin | Carbohydrate specificity | Binding to glycosphingolipids in the region of IGLada of phenotype:
|
|
|---|---|---|---|
| A | D | ||
| RCA120 | Terminal β-linked Gal | +++b | +++ |
| UEA | α-l-Fuc | ++ | ++ |
| MAL I | Galβ1-3GlcNAc | + | + |
| ECL | Galβ1-4GlcNAc | +++ | +++ |
Intestinal neutral glycosphingolipids (100 μg of neutral sugars) from phenotypes A and D of pigs were chromatographed in a solvent system with chloroform-methanol-water (60:30:5 [vol:vol:vol]) and incubated with the desired lectin as described in Materials and Methods.
+++, high-intensity binding; ++, medium-intensity binding; and +, low-intensity binding. The following biotinylated lectins did not react with any glycosphingolipids in the region of IGLad: ConA, SBA, PNA, SNA, MAL II, BSL II, DSL, WGA, and Jacalin.
FIG. 3.
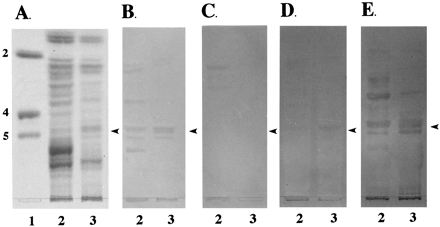
Binding of anti-blood group MAb to neutral glycosphingolipids. Neutral glycosphingolipids (100 μg) from phenotypes A (lane 2) and D (lane 3) pigs were separated on HPTLC plates as described in Materials and Methods. Chromatograms were stained with orcinol-sulfuric acid reagent (A) or incubated with the anti-Lex (B), anti-H (C), anti-B (D), and anti-A (E) MAb as described in Materials and Methods. Lane 1 contains glycolipid standards Lc2Cer (2), Gb4Cer (4) and Gb5Cer (5). The arrowheads indicate the positions of IGLad.
Glycosphingolipids like IGLad that migrate between the Gb4Cer and Gb5Cer standards likely contain four or five monosaccharide units. Antibody studies suggest that the region that IGLad migrates on HPTLC contains glycosphingolipids with Lex and blood group A antigens (see Fig. 3). Both of these structures contain Fuc. We know from the monosaccharide composition that Fuc and N-acetylgalactosamine residues are found in lower quantities than Gal, GlcNAc, and Glc residues (Table 1), indicating that Lex and blood group A containing glycosphingolipids are not the major component found in the region of IGLad. The major component in this region likely contains only Gal, GlcNAc, and Glc. One structure that consists of these monosaccharides in approximately the proportions indicated by compositional analysis and is consistent with the lectin and antibody studies presented in this paper is nLc4Cer.
Determination of the carbohydrate residue on IGLad recognized by the K88ad adhesin.
To determine the binding specificity of K88ad adhesin, we used two exoglycosidases, β-galactosidase (Fig. 4) and α-fucosidase (Fig. 5), to remove terminal monosaccharides on IGLad and then evaluated the effect of their removal on K88ad adhesin binding activity. The effectiveness of these digestions in removing the appropriate carbohydrates from IGLad was determined by overlaying the treated and untreated glycosphingolipids with an appropriate biotinylated lectin. RCA120 was used to assess the effectiveness of the β-galactosidase treatment (Fig. 4C), and UEA was used for α-fucosidase treatment (Fig. 5C). Removal of terminal β-linked galactosyl residues decreased recognition of IGLad by RCA120 and K88ad adhesin (Fig. 4B and C). In contrast, removal of fucosyl residues decreased UEA recognition of IGLad but did not decrease recognition of IGLad by K88ad adhesin (Fig. 5B and C). These results demonstrate that β-linked Gal and not Fuc is an essential component of the site on IGLad recognized by K88ad adhesin and verify that IGLad contains a terminal β-linked Gal.
FIG. 4.
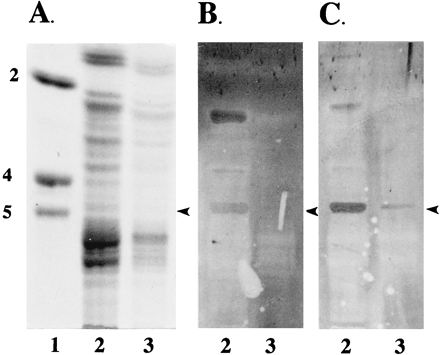
Binding of K88ad adhesin to intestinal neutral glycosphingolipids after β-galactosidase treatment. Neutral glycosphingolipids from a phenotype A animal were treated with β-galactosidase as described in Materials and Methods. Both treated (lane 3) and untreated (lane 2) glycosphingolipids (100 μg) were separated on HPTLC plates as described in Materials and Methods. Chromatograms were stained with orcinol-sulfuric acid reagent (A), or incubated with biotinylated K88ad adhesin (B) or RCA120 (C) as described in Materials and Methods. Lane 1 contains glycolipid standards Lc2Cer (2), Gb4Cer (4) and Gb5Cer (5). The arrowheads indicate the positions of IGLad.
FIG. 5.
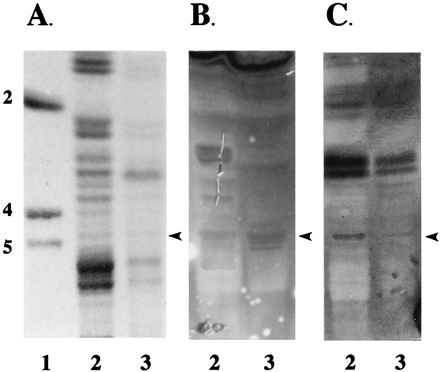
Binding of K88ad adhesin to intestinal neutral glycosphingolipids after α-fucosidase treatment. Neutral glycosphingolipids from a phenotype A animal were treated with α-fucosidase as described in Materials and Methods. Both treated (lane 3) and untreated (lane 2) glycosphingolipids (100 μg) were separated on HPTLC plates as described in Materials and Methods. Chromatograms were stained with orcinol-sulfuric acid reagent (A) or incubated with biotinylated K88ad adhesin (B) or UEA (C) as described in Materials and Methods. Lane 1 contains glycolipid standards Lc2Cer (2), Gb4Cer (4) and Gb5Cer (5). The arrowheads indicate the positions of IGLad.
Determination of K88ad binding specificity using purified glycosphingolipids.
To further characterize the glycanic structure recognized by the K88ad adhesin, we tested the ability of seven purified glycosphingolipids of known structure to bind the K88ad adhesin (Fig. 6). The results of this study indicated that K88ad adhesin interacts with Lc3Cer, Lc4Cer, and nLc4Cer (Fig. 6, lanes 1 to 3). Glycosphingolipids expressing Lex and Lea, which are fucosylated forms of nLc4Cer and Lc4Cer, respectively, did not bind K88ad adhesin, indicating that fucosylation of GlcNAc interferes with the receptor recognition site on the glycosphingolipids (Fig. 6, lanes 4 and 5). This observation was further demonstrated by the lack of reactivity of K88ad adhesin with VI2FucnLc6Cer and V3FucnLc6Cer (Fig. 6, lanes 6 and 7). These results are in agreement with the exoglycosidase experiments described above which demonstrated that β-linked Gal, but not Fuc, is involved in recognition of IGLad by the K88ad adhesin (Fig. 4 and 5). Of the glycosphingolipids tested, only three (Lc4Cer, nLc4Cer, and Lea) migrate between the Gb4Cer and Gb5Cer standards which is where IGLad migrates. Only two of these (Lc4Cer and nLc4Cer) were found to bind K88ad adhesin, and only nLc4Cer contains the structure (Galβ1-4GlcNAc) that is so strongly recognized in the region of IGLad migration by the ECL lectin. In addition, IGLad and nLc4Cer were found to migrate in similar positions on HPTLC plates (Fig. 7), indicating that IGLad may be nLc4Cer.
FIG. 6.

Binding of K88ad adhesin to purified neutral glycosphingolipids. Two micrograms of Lc3Cer (lane 1), Lc3Cer plus Lc4Cer (lane 2), nLc4Cer (lane 3), III4FucLc4Cer or Lea (lane 4), III3FucnLc4Cer or Lex (lane 5), V3FucnLc6Cer (lane 6), and VI2FucnLc6Cer (lane 7) were separated on HPTLC. These glycosphingolipids were either stained with orcinol-sulfuric acid reagent (A) or incubated with biotinylated K88ad adhesin (B) as described in Materials and Methods.
FIG. 7.
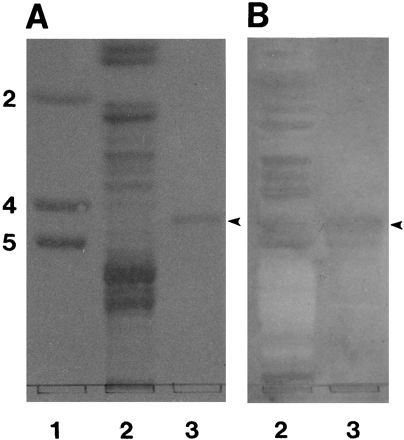
Comigration of IGLad and nLc4Cer on HPTLC plates. Neutral glycosphingolipids from a phenotype A animal (100 μg, lane 2), and nLc4Cer (2 μg, lane 3) were separated on HPTLC. These glycosphingolipids were either stained with orcinol-sulfuric acid reagent (A) or incubated with biotinylated K88ad adhesin (B) as described in Materials and Methods. Lane 1 contains glycolipid standards (Lc2Cer [2], Gb4Cer [4], and Gb5Cer [5]). The arrowheads indicate the position of IGLad.
DISCUSSION
The K88 fimbrial adhesin variants are lectins that recognize specific carbohydrate structures on the surface of porcine intestinal epithelial cells. Each of the three K88 variants has a different carbohydrate binding specificity as evidenced by the existence of multiple phenotypes of pigs whose intestinal brush borders bind different combinations of K88 adhesin variants. As with any binding molecule, K88 adhesins could potentially interact with a number of different types (proteins, glycoproteins, or glycolipids) and sizes of molecules. Determining which of these molecules is an essential receptor for the colonization of porcine small intestine by K88+ ETEC is a difficult task. We have developed a receptor identification strategy in which we look for phenotype-specific receptors (see Introduction for list of criteria) for each of the K88 variants in porcine intestinal brush border preparations. Previously, we have searched for glycoproteins that serve as K88 adhesin receptors and have reported the identification and characterization of two mucin-type sialoglycoproteins designated IMTGP-1 and IMTGP-2 which meet the criteria as phenotype-specific receptors for K88ab and K88ac adhesins (10, 11). Recently, we have demonstrated that the presence of the IMTGPs is more closely correlated to susceptibility of pigs to K88ab and K88ac ETEC infections than results from the previously used brush border adherence assay (12).
In the present study, we evaluated intestinal brush border glycolipid preparations for the presence of phenotype-specific K88 adhesin receptors. No phenotype-specific receptors for K88ab and K88ac adhesins were detected among the intestinal glycolipids. However, we did identify a neutral glycosphingolipid (IGLad) which meets the criteria as a phenotype-specific receptor for the K88ad adhesin. This receptor, IGLad, binds the K88ad adhesin. This result meets the requirements of the first criterion mentioned at the beginning of this paper, which states that the receptor must display specificity for the particular K88 fimbrial adhesin variant. In addition, IGLad was detected in brush border preparations from adhesive phenotypes (A and D) of pigs, and not in brush border preparations from nonadhesive phenotypes (B and E) of pigs. These results meet the requirements of the second criterion, which states that the receptor must be detectable exclusively in brush borders phenotyped as adhesive for that particular K88 fimbrial adhesin variant. An important aspect of this criterion is that the receptor must not be found in any brush borders that are nonadhesive for that particular K88 fimbrial adhesin variant. This criterion does not require that all adhesive phenotypes or even all animals within a particular adhesive phenotype have the receptor. Interestingly, we have found that, like IGLad, the IMTGPs are not found in brush border preparations from phenotype C pigs (6), which are adhesive for both the K88ab and K88ad adhesins. Finally, IGLad was found in brush border preparations from all phenotype A (n = 16) and phenotype D (n = 3) animals tested. These results meet the requirements of the third criterion, which states that the receptor must be expressed in multiple animals of the same adhesive phenotype. This criterion requires that more than one animal, but not all animals, of the same adhesive phenotype must have the receptor. An example of the importance of this criterion is that not all phenotype A animals possess the IMTGPs, and only those pigs that possess the IMTGPs are susceptible to K88ab and K88ac ETEC infections (6, 12).
Based on its migration position relative to the standards on HPTLC separation, the carbohydrate moiety of IGLad consists of four or five monosaccharides. Carbohydrate compositional analysis indicates that a partially purified preparation of IGLad contains predominantly Gal, Glc, and GlcNAc, and a lower proportion of Fuc and GalNAc. Lectin studies indicate that IGLad or glycosphingolipids with similar HPTLC mobilities as IGLad contain terminal Fuc and β-linked Gal along with the structure, Galβ1,4GlcNAc. Studies using anti-blood group MAbs indicated that glycosphingolipids expressing the Lex [Gal β1-4(Fuc α1-3)GlcNAc] and blood group A [GalNAcα1-3(Fucα1-2)Gal] antigens migrate in the same region as IGLad. Overall, these results indicate that the region in which IGLad migrates contains three or more glycosphingolipids including (i) a Lex-containing glycosphingolipid, (ii) a blood-group-A-containing glycosphingolipid, and (iii) nLc4Cer. Studies with glycosphingolipid standards indicate that K88ad adhesin only binds to one of these structures, nLc4Cer. In addition, IGLad and nLc4Cer migrate with similar mobilities on HPTLC plates. These results strongly suggest that IGLad is nLc4Cer. Studies to prepare sufficient amounts of highly purified IGLad from intestinal brush borders of K88ad adhesive pigs for full chemical characterization of this glycosphingolipid are currently underway.
Both Lc4Cer and nLc4Cer are precursor chains which become substituted with various monosaccharides, like Fuc, to form mature glycosphingolipids. Lc4Cer and nLc4Cer are known as type 1 and type 2 precursor chains, respectively. Recently, Bäcker et al. (3) evaluated the non-acid glycosphingolipids isolated from intestinal epithelial cells of a single blood group A pig. In this study, they found that the type 1 chain (Lc4Cer), not type 2 chain (nLc4Cer), was the predominantly expressed core glycosphingolipid. This result does not exclude the possibility that IGLad is nLc4Cer, since phenotypic variation in the carbohydrates expressed on porcine intestinal epithelial cells is known to occur as evidenced by the five different phenotypes of pigs based on K88 adhesin variant binding. One possibility is that pigs that express IGLad on their intestinal epithelial cells may express type 2 precursor chains while those that do not have IGLad may express type 1, not type 2, precursor chains as reported by Bäcker et al. (3).
Removal of the terminal β-linked Gal residue from IGLad using β-galactosidase decreased the recognition of IGLad by the K88ad adhesin, while removal of Fuc using α-fucosidase did not affect recognition of IGLad by K88ad adhesin. These results indicate that terminal β-linked Gal is an essential component of the K88ad adhesin recognition site on IGLad. This result is consistent with previously published studies that indicate that Gal residues play an important role in the recognition of glycolipids by the K88ad adhesin (7, 30) and IMTGPs by K88ac adhesin (17). β-Linked Gal appears to be an important component of the recognition site for all three K88 adhesin variants. But because each variant recognizes different receptors, the recognition site for each variant must be more complex then simply β-linked Gal. Other monosaccharides, particularly the penultimate monosaccharide which is β-linked to Gal, are likely to be important constituents of the K88 adhesin recognition site. Results in the current study indicate that the penultimate sugar in IGLad is GlcNAc. The fact that K88ad adhesin binds to Lc3Cer, which is nLc4Cer minus the terminal β1-4-linked Gal, indicates that GlcNAc likely plays an important role in the recognition of nLc4Cer by K88ad adhesin. In addition, fucosylation of the penultimate GlcNAc residue results in loss of the ability of nLc4Cer and Lc4Cer to bind K88ad adhesin, which is a further indication that this GlcNAc is important in recognition by the K88ad adhesin.
It is interesting to note that K88ad adhesin appears to preferentially bind to glycolipids, while K88ab and K88ac adhesins preferentially bind to glycoproteins (6, 10, 11, 16). This observation suggests that the lipid and protein moieties of the K88 receptors have some role in receptor recognition by the K88 adhesin variants. The role that the protein and lipid moieties play in the interaction between the adhesin and the receptor has not been clearly defined but may include (i) being an integral part of the K88 recognition structure, (ii) serving as a site for a secondary interaction between adhesin and receptor that stabilizes the adhesin-carbohydrate interaction, or (iii) presenting the carbohydrates in the proper conformation for recognition by the K88 adhesin. Previously, it has been demonstrated that the level of hydroxylation of the lipophilic moiety of glycosphingolipids affects the conformation of the carbohydrate moiety which may in turn affect the recognition of the glycosphingolipid by the adhesin (30).
We have previously proposed a three-receptor model to explain the presence of multiple porcine phenotypes (6). The three receptors proposed in this model are (i) receptor bcd, which binds all three variants and is found in phenotype A pigs, (ii) receptor bc, which binds K88ab and K88ac and is found in phenotype A and B pigs, and (iii) receptor d, which binds K88ad and is found in phenotype C and D pigs. We believe that receptor bc is the intestinal mucin-type sialoglycoproteins, IMTGP-1 and IMTGP-2. Receptor d may be IGLad based on the fact that it only binds K88ad adhesin. The bcd receptor has yet to be identified. If receptor d is IGLad, the three-receptor model needs to be modified in two ways. (i) In addition to being found in phenotype D animals, receptor d (IGLad) is also found in phenotype A animals, and (ii) receptor d is not present in phenotype C animals, indicating that there may be a fourth K88 adhesin receptor, possibly a bd receptor that binds both K88ab and K88ad adhesin and is found exclusively in phenotype C animals.
One of our long-term goals is to determine the differences in glycoconjugate specificity between the K88 adhesin variants. With the identification of IGLad, we have now identified intestinal phenotype-specific receptors for all three K88 variants (IGLad for K88ad, IMTGPs for K88ab and K88ac [10, 11] and intestinal transferrin for K88ab [16]). Future studies will be directed at determining the glycanic structure of the oligosaccharides recognized by the K88 adhesin variants on each of these receptors. Determination of these structures will make it possible to begin to understand the changes in receptor specificity that occur during evolutionary development of new adhesin variants, and to synthesize structural analogues of the receptor recognition sequence which can be used to prevent and treat K88+ ETEC-induced disease in pigs.
ACKNOWLEDGMENTS
We gratefully acknowledge USDA grant 94-02419, NSF grant OSR-9452894, the South Dakota Future Fund, and the South Dakota Agricultural Experiment Station for providing financial assistance. This research was supported in part by the National Institutes of Health (NIH)-funded Resource Center for Biomedical Complex Carbohydrates (NIH grant 2-P41-RR05351-06) to the Complex Carbohydrate Research Center.
We thank David Benfield and Larry Holler for critical review of the manuscript.
Footnotes
South Dakota Agricultural Experiment Station paper no. 3083.
REFERENCES
- 1.Amado M, Almeida R, Carneiro F, Levery S B, Holmes E H, Nomoto M, Hollingsworth M A, Hassan H, Schwientek T, Neilsen P, Bennett E, Clausen H. A family of human β3-galactosyltransferases. Characterization of four members of a UDP-galactose:β-N-acetyl-glucosamine/β-N-acetyl galactosamine β-1,3-galactosyltransferase family. J Biol Chem. 1998;273:12770–12778. doi: 10.1074/jbc.273.21.12770. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M J, Whitehead J S, Kim Y S. Interaction of Escherichia coli K88 antigen with porcine intestinal brush border membranes. Infect Immun. 1980;29:897–901. doi: 10.1128/iai.29.3.897-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäcker A E, Breimer M E, Samuelson B E, Holgersson J. Biochemical and enzymatic characterization of blood group ABH and related histo-blood group glycosphingolipids in the epithelial cells of porcine small intestine. Glycobiology. 1997;7:943–953. doi: 10.1093/glycob/7.7.943. [DOI] [PubMed] [Google Scholar]
- 4.Baker D, Billey L O, Francis D H. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet Microbiol. 1997;54:123–132. doi: 10.1016/s0378-1135(96)01277-1. [DOI] [PubMed] [Google Scholar]
- 5.Bijlsma I G W, de Nijs A, van der Meer C, Frik J F. Different pig phenotypes affect adherence of Escherichia coli to jejunal brush borders by K88ab, K88ac, or K88ad antigen. Infect Immun. 1982;37:891–894. doi: 10.1128/iai.37.3.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billey L O, Erickson A K, Francis D H. Multiple receptors on porcine intestinal epithelial cells for the three variants of Escherichia coli K88 fimbrial adhesin. Vet Microbiol. 1997;59:203–212. doi: 10.1016/s0378-1135(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 7.Blomberg L, Krivan H C, Cohen P S, Conway P L. Piglet ileal mucus contains protein and glycolipid (galactosylceramide) receptors specific for Escherichia coli K88 fimbriae. Infect Immun. 1993;61:2526–2531. doi: 10.1128/iai.61.6.2526-2531.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouhours D, Bouhours J F. Developmental changes of the lipidic part of the neutral glycosphingolipids of the rat stomach. J Biol Chem. 1985;260:2172–2177. [PubMed] [Google Scholar]
- 9.Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Biochem. 1956;28:350–356. [Google Scholar]
- 10.Erickson A K, Willgohs J A, McFarland S Y, Benfield D A, Francis D H. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these glycoproteins with the adhesive phenotype. Infect Immun. 1992;60:983–988. doi: 10.1128/iai.60.3.983-988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson A K, Baker D R, Bosworth B T, Casey T A, Benfield D A, Francis D H. Characterization of porcine intestinal receptors for the K88ac fimbrial adhesin of Escherichia coli as mucin-type sialoglycoproteins. Infect Immun. 1994;62:5404–5410. doi: 10.1128/iai.62.12.5404-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis D H, Grange P A, Zeman D H, Baker D R, Sun R, Erickson A K. Expression of mucin-type glycoprotein K88 receptors strongly correlates with piglet susceptibility to K88+ enterotoxigenic Escherichia coli, but adhesion of this bacterium to brush borders does not. Infect Immun. 1998;66:4050–4055. doi: 10.1128/iai.66.9.4050-4055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda M N, Dell A, Oates J E, Wu P, Klock J C, Fukuda M. Structures of glycosphingolipids isolated from human granulocytes. The presence of a series of linear poly-N-acetyllactosaminylceramide and its significance in glycolipids of whole blood cells. J Biol Chem. 1985;260:1067–1082. [PubMed] [Google Scholar]
- 14.Gibbons R A, Jones G W, Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a hemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975;86:228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons R, Sellwood R, Burrows M, Hunter P. Inheritance of resistance to neonatal diarrhea in the pig: examination of the genetic system. Theor Appl Genet. 1977;51:65–70. doi: 10.1007/BF00299479. [DOI] [PubMed] [Google Scholar]
- 16.Grange P A, Mouricout M A. Transferrin associated with the porcine intestinal mucosa is a receptor specific for K88ab fimbriae of Escherichia coli. Infect Immun. 1996;64:606–610. doi: 10.1128/iai.64.2.606-610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grange P A, Erickson A K, Anderson T J, Francis D H. Characterization of the carbohydrate moiety of intestinal mucin-type sialoglycoprotein receptors for the K88ac fimbrial adhesin of Escherichia coli. Infect Immun. 1998;66:1613–1621. doi: 10.1128/iai.66.4.1613-1621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakomori S, Nudelman E, Levery S B, Solter D, Knowles B B. The hapten structure of a developmentally regulated glycolipid antigen (SSEA-1) isolated from human erythrocytes and adenocarcinoma: a preliminary note. Biochem Biophys Res Commun. 1981;109:36–44. doi: 10.1016/0006-291x(81)90699-9. [DOI] [PubMed] [Google Scholar]
- 19.Hakomori S, Nudelman E, Kannagi R, Levery S B. The common structure in fucosyllactosaminolipids accumulating in human adenocarcinomas, and its possible absence in normal tissue. Biochem Biophys Res Commun. 1982;109:36–44. doi: 10.1016/0006-291x(82)91562-5. [DOI] [PubMed] [Google Scholar]
- 20.Hakomori S. Chemistry of glycosphingolipids. In: Kanfer J N, Hakomori S-I, editors. Handbook of lipid research. Sphingolipid biochemistry. New York, N.Y: Plenum Press; 1983. pp. 1–165. [Google Scholar]
- 21.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 22.Kannagi R, Nudelman E, Levery S B, Hakomori S. A series of human erythrocyte glycosphingolipids reacting to the monoclonal antibody directed to a developmentally regulated antigen, SSEA-1. J Biol Chem. 1982;257:14865–14874. [PubMed] [Google Scholar]
- 23.Karlsson K-A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- 24.Ledeen R W, Yu R K. Gangliosides: structure, isolation and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- 25.Levery S B, Nudelman E D, Andersen N H, Hakomori S. 1H-NMR analysis of glycolipids possessing mono- and multi-meric X and Y haptens: characterization of two novel extended Y structures from human adenocarcinoma. Carbohydr Res. 1986;151:311–328. doi: 10.1016/s0008-6215(00)90351-3. [DOI] [PubMed] [Google Scholar]
- 26.Levery S B, Holmes E H, Harris D D, Hakomori S. 1H-NMR studies of a biosynthetic lacto-ganglio hybrid glycosphingolipid: confirmation of structure, interpretation of “anomalous” chemical shifts, and evidence for interresidue amide-amide hydrogen bonding. Biochemistry. 1992;31:1069–1080. doi: 10.1021/bi00119a016. [DOI] [PubMed] [Google Scholar]
- 27.Merkle R K, Poppe I. Carbohydrate composition analysis of glycoconjugates by gas-liquid chromatography/mass spectrometry. Methods Enzymol. 1994;118:3–40. doi: 10.1016/0076-6879(94)30003-8. [DOI] [PubMed] [Google Scholar]
- 28.Metcalfe J W, Krogfelt K A, Krivan H C, Cohen S C, Laux D C. Characterization and identification of a small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991;59:91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson G, Svensson S. The role of the carbohydrate portion of glycolipids for the adherence of Escherichia coli K88+ to pig intestine. In: Chester M A, Heinegard D, Lundblad A, Svensson S, editors. Proceedings of the 7th International Symposium on Glycoconjugates. Lund-Ronneby, Sweden. 1983. pp. 637–638. [Google Scholar]
- 30.Payne D, O’Reilly M, Williamson D. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to β1-linked galactosyl residues in glycosphingolipids. Infect Immun. 1993;61:3673–3677. doi: 10.1128/iai.61.9.3673-3677.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapacz J, Hasler-Rapacz H. Polymorphism and inheritance of swine small intestinal receptors mediating adhesion of three serological variants of Escherichia coli producing K88 pilus antigen. Anim Genet. 1986;17:305–321. doi: 10.1111/j.1365-2052.1986.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 32.Schnaar R L. Isolation of glycosphingolipids. Methods Enzymol. 1994;230:348–370. doi: 10.1016/0076-6879(94)30024-0. [DOI] [PubMed] [Google Scholar]
- 33.Schnaar R L, Needham L. Thin-layer chromatography of glycosphingolipids. Methods Enzymol. 1994;230:371–389. doi: 10.1016/0076-6879(94)30025-9. [DOI] [PubMed] [Google Scholar]
- 34.Seignole D, Grange P, Duval-Iflah Y, Mouricout M. Characterization of O-glycan moieties of the 210 and 240 kDa pig intestinal receptors for Escherichia coli K88ac fimbriae. Microbiology. 1994;140:2467–2473. doi: 10.1099/13500872-140-9-2467. [DOI] [PubMed] [Google Scholar]
- 35.Sellwood R, Gibbons R, Jones G, Rutter J. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush border: the existence of two pig phenotypes. J Med Microbiol. 1975;8:405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- 36.Sellwood R. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim Biophys Acta. 1980;632:326–335. doi: 10.1016/0304-4165(80)90090-2. [DOI] [PubMed] [Google Scholar]
- 37.Staley T E, Wilson I B. Soluble pig intestinal cell membrane components with affinities for E. coli K88+ antigen. Mol Cell Biochem. 1983;52:177–189. doi: 10.1007/BF00224926. [DOI] [PubMed] [Google Scholar]
- 38.Stults C L M, Sweeley C C, Macher B A. Glycosphingolipids: structure, biological source, and properties. Methods Enzymol. 1989;179:167–214. doi: 10.1016/0076-6879(89)79122-9. [DOI] [PubMed] [Google Scholar]
- 39.Symington F W, Hedges D L, Hakomori S. Glycolipid antigens of human polymorphonuclear neutrophils and the inducible HL-60 myeloid leukemia line. J Immunol. 1985;134:2498–2506. [PubMed] [Google Scholar]
- 40.Willemsen P T J, de Graaf F K. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 1992;12:367–375. doi: 10.1016/0882-4010(92)90099-a. [DOI] [PubMed] [Google Scholar]
- 41.Wilson R A, Francis D H. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am J Vet Res. 1986;47:213–217. [PubMed] [Google Scholar]
- 42.Yang H-J, Hakomori S. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose III. J Biol Chem. 1971;246:1192–1200. [PubMed] [Google Scholar]


