Abstract
Objective
To determine whether comorbidity is associated with clinical (relapses, disability worsening) and MRI outcomes in multiple sclerosis (MS) by conducting a secondary analysis of the CombiRx clinical trial.
Methods
CombiRx compared interferon beta-1a, glatiramer acetate, and the combination of these agents. For participants eligible for evaluation of 6-month confirmed disability worsening, we used medical history, concomitant medications, and adverse events to ascertain comorbidity status. Comorbid conditions evaluated included hypertension, dyslipidemia, diabetes mellitus, depression, anxiety disorders, and migraine. Clinical outcomes included disease activity consisting of protocol-defined relapses, disability worsening, and MRI activity. We summarized the prevalence of these comorbid conditions and their association with disease activity and its components using multivariable Cox regression.
Results
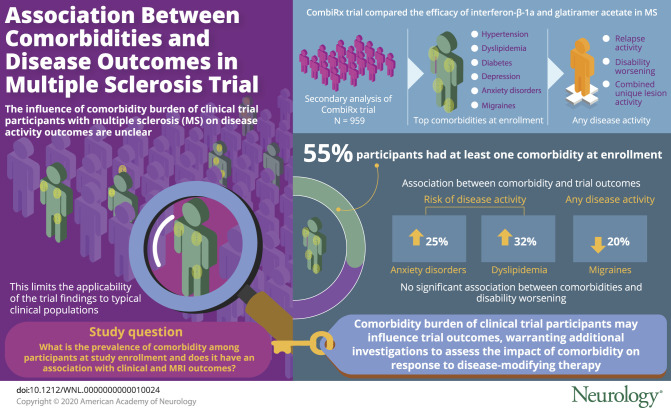
Of the 1,008 participants randomized, 959 (95.1%) were eligible for assessment of 6-month disability worsening; for this subgroup, the median length of follow-up was 3.4 years (range 0.5–6.9 years). Overall, 55.1% of participants had ≥1 comorbidity at enrollment. After adjustment, anxiety (hazard ratio [HR] 1.25, 95% confidence interval [CI] 1.01–1.55) and dyslipidemia (HR 1.32, 95% CI 1.01–1.72) were associated with an increased hazard of any disease activity, while migraine (HR 0.80, 95% CI 0.67–0.97) was associated with a decreased hazard.
Conclusions
In this large trial population with rigorously obtained outcomes, comorbid conditions were common among participants and influenced disease outcomes, including relapses. The comorbidity burden of clinical trial participants with MS may be an important factor in the outcome of clinical trials. Additional investigations of the impact of comorbidity on clinical trial outcomes and response to disease-modifying therapies are warranted.
Over the last decade, several studies have shown that comorbidity is common in multiple sclerosis (MS), even at diagnosis, and its prevalence increases with age. The most common comorbid conditions at diagnosis are depression, hypertension, anxiety, and dyslipidemia.1,2 Several studies suggest that comorbidity influences the severity of disability at diagnosis and the rate of disability worsening after diagnosis.3,4 Less is known about the influence of comorbidity on measures of disease activity such as relapse rate, and findings have conflicted,5,6 possibly due to differences in relapse measurement, frequency of assessment, and modest sample sizes.7–9 Thus, larger studies with rigorous measures of disease activity, particularly relapses, are needed.
The underrepresentation of individuals with comorbid conditions in clinical trials is a well-recognized problem that may limit the applicability of the findings to typical clinical populations.10 In MS, many trials of disease-modifying therapies have excluded potential participants with severe comorbid conditions and have not described the comorbidity status of participants who were enrolled.11 Given the association between comorbidity and commonly used clinical trial outcomes such as disability progression and relapse rates, the comorbidity profile of study participants has the potential to influence study findings in unpredictable ways in clinical trials and observational studies. The International Advisory Committee on Clinical Trials in MS recommended that “the comorbidity status of enrolled clinical trial participants…be clearly and consistently described.”11
The CombiRx clinical trial systematically collected data regarding comorbid conditions at enrollment. Using data from the CombiRx clinical trial, we aimed to determine the prevalence of comorbidity among trial participants at study enrollment and to explore the association between comorbidity and clinical (relapses, disability worsening) and MRI outcomes (combined unique active lesions).
Methods
Study design and participants
This is a post hoc analysis of data collected from the cohort of participants enrolled in the CombiRx trial. As detailed elsewhere,12 CombiRx was a double-blind, randomized, controlled clinical trial that compared the combination of interferon beta-1a (IFN) and glatiramer acetate (GA) to each of these agents as monotherapy plus placebo in treatment-naive participants; 50% of participants were assigned to combination therapy (IFN + GA). All participants were followed up for at least 3 years, but some were followed up for up to 7 years according to the study design of following up all patients until the last patient completed 3 years. The study was started in 2006 and ended in 2013.13
For this analysis, we retained the inclusion criteria for the core trial: age of 18 to 60 years, Expanded Disability Status Scale (EDSS) score of 0 to 5.5, confirmed diagnosis of relapsing-remitting MS by the Poser or McDonald criteria,14,15 and at least 2 relapses in the 3 years before enrollment (one of which could be change on MRI meeting the McDonald criteria for dissemination in time). We excluded participants who withdrew from the study before the 6-month visit because they were not eligible for assessment of EDSS worsening at 6 months.
Standard protocol approvals, registrations, and patient consents
This study was exempt from the need for research and institutional review board approval.
Study assessments
Participants underwent clinical assessments including EDSS examination at enrollment, every 3 months until month 36, and then every 6 months thereafter.12 MRIs were completed at enrollment; months 6, 12, 24, and 36; and then yearly thereafter.
Outcomes
No evidence of disease activity is increasingly proposed as a treatment target in MS and can be defined as no relapse activity, no disability worsening, and no new or gadolinium-enhancing lesions on MRI.16 Therefore, the primary outcome for this analysis was time to first evidence of disease activity (relapses, disability worsening, MRI changes) as identified with all data from enrollment through the extension phase (see below). Secondary analyses examined each of these endpoints individually: relapses (protocol defined and non–protocol defined), disability worsening, and MRI changes.
For the primary analysis, we used protocol-defined relapses, which constituted the development of new symptoms or worsening of old symptoms lasting ≥24 hours in the absence of fever, preceded by 30 days of stability and associated with changes in the EDSS score confirmed by the examining physician within 7 days of onset. The required changes in EDSS score included a ≥0.5-point increase compared to the previous visit or a ≥2-point increase in 1 functional system score (FSS) or ≥1-point change in 2 FSSs (except for bladder and cerebral FSS). Non–protocol-defined relapses met these criteria but were not confirmed within 7 days of onset and were included in secondary analyses.
Confirmed disability worsening was defined as a 1-point increase in EDSS score if the baseline EDSS score was ≤5.0 and half-point increase in the EDSS score if the baseline EDSS score was 5.5, sustained for 6 months.
Although the primary study used an MRI composite score,13 we used the combined unique lesion activity (CUA) as the MRI outcome because this most closely reflects MRI outcomes considered in defining no evidence of disease activity.16 CUA was defined as the sum of the number of new enhancing lesions, new nonenhancing T2 lesions, and enlarged nonenhancing T2 lesions observed after enrollment.
Treatment status
Treatment assignment was included as indicator variable with the combination arm (IFN + GA) as the reference group. To account for participants who stopped or changed their disease-modifying therapy particularly during the extension phase of the clinical trial, we included treatment status as a time-varying covariate (yes/no) for which “no” was defined as an interruption of therapy for at least 30 days or discontinuation of the disease-modifying therapy.
Comorbidity
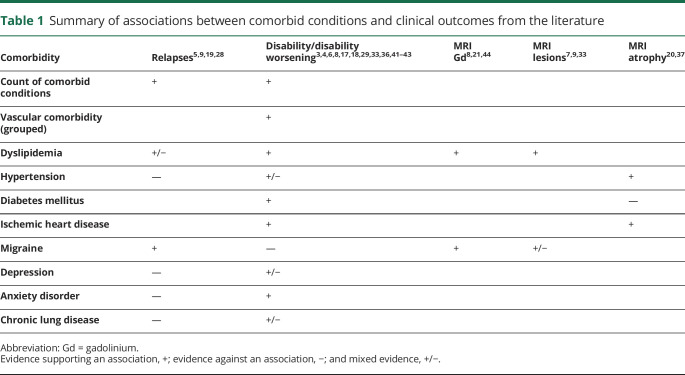
We focused on comorbid conditions reported to be relevant to MS outcomes3–7,17–21 or recommended for evaluation by International Advisory Committee on Clinical Trials in MS11 and with sufficiently high prevalence in CombiRx to be evaluable (table 1). Specifically, we considered hypertension, dyslipidemia, diabetes mellitus, depression, anxiety disorders, and migraine.
Table 1.
Summary of associations between comorbid conditions and clinical outcomes from the literature
We obtained information on comorbidity status from the medical history (enrollment) and concomitant medications (enrollment and follow-up). The medication form captured medications listed by indication for the following comorbid conditions of interest: hypertension, hyperlipidemia, diabetes mellitus, depression, and migraine. Thus, a comorbidity could be identified on the basis of the reported condition or use of a medication specific to the reported condition (e.g., diabetes mellitus could be defined having insulin recorded as a concomitant medication, even if diabetes mellitus was not recorded in the medical history). We captured incident comorbid conditions developing over the course of follow-up by reviewing reported adverse events and medications captured at follow-up. Each comorbidity was classified as present or absent (reference). For comparison with prior studies,5,19 we also summed each comorbidity to create a comorbidity count for all comorbid conditions categorized as 0 (reference), 1, 2 and ≥3; for physical comorbid conditions (including hypertension, hyperlipidemia, diabetes mellitus) categorized as 0 (reference), 1, and 2 to 3; and number of comorbid conditions treated continuously.
Covariates
Covariates included in the analysis were age at enrollment (continuous), sex (female as reference group), race (white as reference group), and body mass index (categorized as follows: underweight <18.5 kg/m2, normal [reference] 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2, and obese ≥30 kg/m2). In complementary analyses, we included the health behavior of smoking status (ever vs never [reference group] and current vs not [reference group]) as a covariate because smoking status was collected only later in the enrollment period, was not updated over the course of the study, and was missing for a subset of participants.22–24 We also examined the number of relapses in the 12 months prior to enrollment for anxiety and relapse models.
Statistical analysis
Descriptive statistics were used to summarize enrollment characteristics using mean ± SD for continuous variables and frequency (percentage) for categorical variables. Missing data were not imputed. We compared enrollment characteristics between treatment arms and number of comorbid conditions using a χ2 test for categorical characteristics, Kruskal-Wallis with pairwise comparisons adjusted using the Dwass, Steel, Critchlow-Fligner method for the EDSS, and an analysis of variance with Tukey-adjusted post hoc pairwise comparisons for continuous characteristics. The significance level for these comparisons was set at p ≤ 0.05.
We used Cox proportional hazards regression to compare time to first evidence of any disease activity for all comorbid conditions, adjusting for included covariates and treatment assignment and status. Comorbidity status was treated as time varying to account for changes in comorbidity status during follow-up. Participants were censored at the date of last follow-up or end of the trial. Hazard ratios (HRs) and 95% confidence intervals (CIs) are reported for each model. Other outcomes considered in secondary analyses were evidence of disease activity, including protocol- and non–protocol-defined relapses, and each component of evidence of disease activity separately, analyzed in order of priority as relapses, disability worsening, and CUA.
Statistical analyses used SAS version 9.4 (SAS Institute Inc, Cary, NC).
Data availability
The dataset from this study is held securely by the University of Alabama at Birmingham. Access may be granted via authorization from the Executive Committee and may be facilitated by contacting Dr. Cutter.
Results
Participants
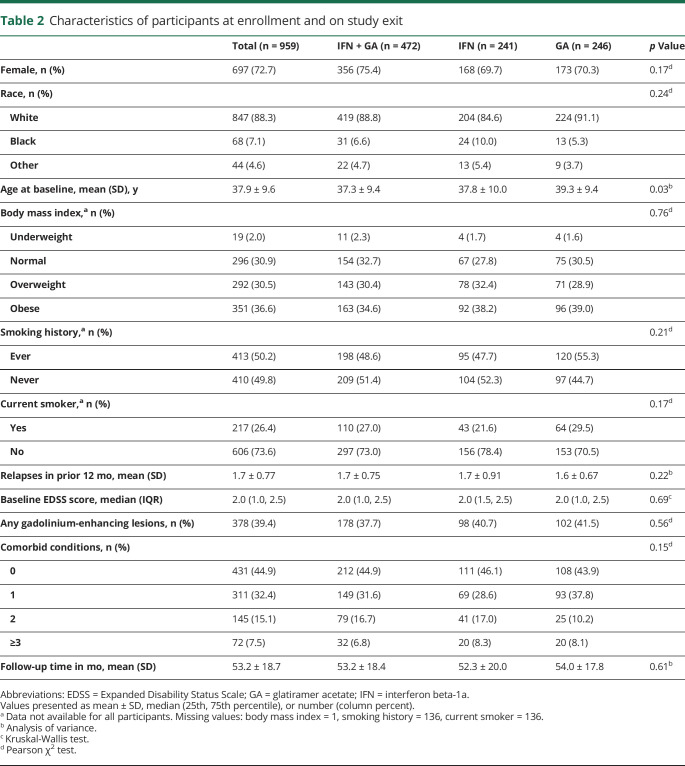
Of the 1,008 participants randomized in the CombiRx trial, 959 (95.1%) were eligible for assessment of 6-month EDSS score worsening. Enrollment characteristics were similar across treatment groups with the exception of age (table 2). On average, participants in the GA arm were 2 years older than participants in the IFN + GA arm (p = 0.027). Two-thirds (67.1%) of the participants were overweight or obese. Most participants entered the extension phase of the trial (n = 687, 71.6%), and those going into the extension phase had similar prevalence of comorbid conditions (all p > 0.05).
Table 2.
Characteristics of participants at enrollment and on study exit
Comorbidity
Overall, 55.1% of participants had at least 1 comorbidity at enrollment (table 2). The prevalence of each comorbidity and the overall count of comorbid conditions increased over the course of follow-up. The count of comorbid conditions and prevalence of individual conditions did not differ between treatment arms, at enrollment, or over the course of follow-up (all p > 0.05; table 2). The prevalence of individual conditions differed between men and women (hyperlipidemia p = 0.040, dyslipidemia p = 0.002, depression p = 0.038, migraine p < 0.001) and between those ≤50 and those >50 years of age (hyperlipidemia p < 0.001, dyslipidemia p < 0.001, depression p = 0.029).
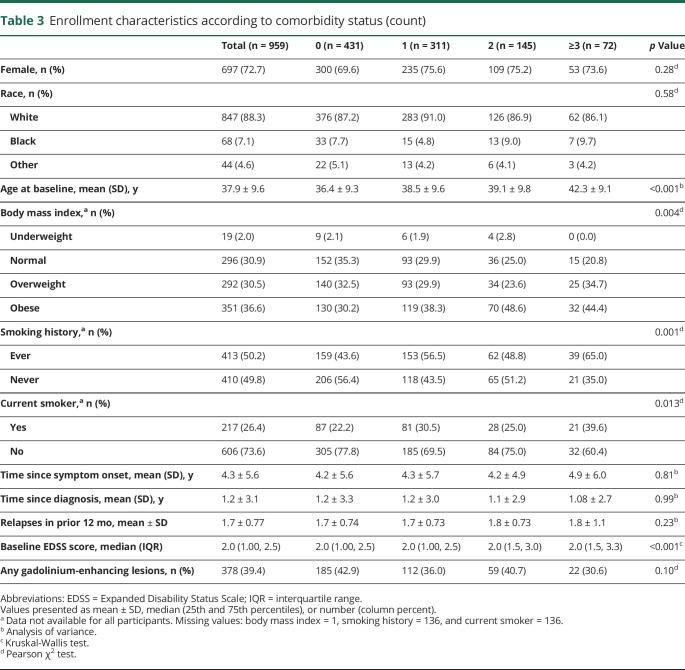
As the number of comorbid conditions increased, the mean age at enrollment increased; those with no comorbid conditions were younger compared to all other categories (0 vs 1 p = 0.015, 0 vs 2 p = 0.011, 0 vs ≥3 p < 0.0001), and those with 1 comorbidity were younger on average compared to those with ≥3 (p = 0.010) conditions (table 3). As the number of comorbid conditions increased, participants were more likely to be overweight or obese. The median disability level was lower for those with no comorbid conditions compared to those with 2 (p = 0.009) or ≥3 (p < 0.0001) comorbid conditions after adjustment for disease duration and for those with ≥3 vs 0 (p = 0.008) comorbid conditions after adjustment for age (data not shown).
Table 3.
Enrollment characteristics according to comorbidity status (count)
Comorbidity and any evidence of disease activity
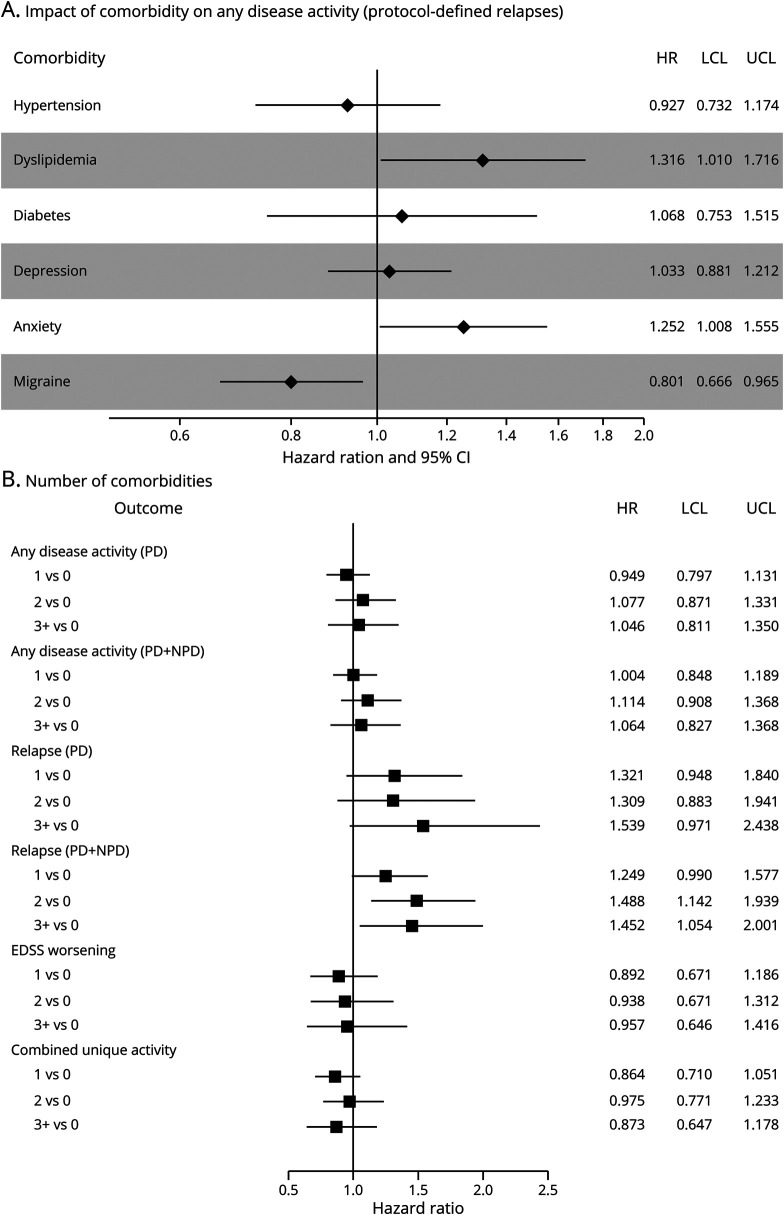
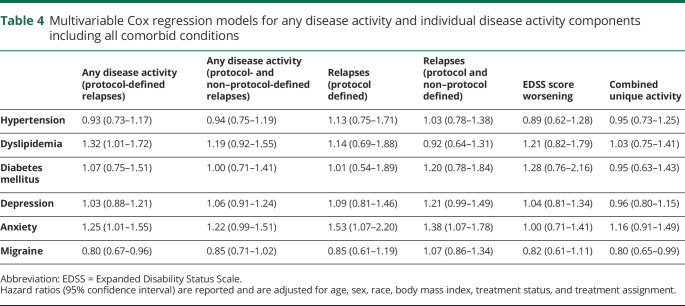
Overall, 739 (77.1%) participants had evidence of disease activity, with 280 (37.9%) of those resulting from CUA alone, 51 (6.9%) from protocol-defined relapses alone, and 68 (9.2%) from EDSS score worsening alone. The remaining 340 (46.0%) had activity through a combination of these outcomes. After adjustment for age, sex, race, body mass index, and treatment, anxiety was associated with a 25% increased hazard of any disease activity (HR 1.25, 95% CI 1.01–1.55), and dyslipidemia was associated with a 32% increased hazard of any disease activity (HR 1.32, 95% CI 1.01–1.72; figure, A and table 4). Migraine was associated with a 20% decreased hazard (HR 0.80, 95% CI 0.67–0.97; figure, A and table 4). The number of comorbid conditions was not associated with time to any disease activity (figure, B).
Figure. Association of a comorbidity accounting for (A) the presence of the other comorbid conditions and (B) number of conditions with any evidence of disease activity (PD relapses) and secondary clinical outcomes.
Adjusted hazard ratio for association of a comorbidity accounting for the presence of the other comorbidities (A) and number of conditions (B) with any evidence of disease activity (protocol-defined relapses) and secondary clinical outcomes. All models were adjusted for age, sex, race, body mass index, treatment status, treatment, and all comorbid conditions (in A only). When considering the number of conditions continuously, the results are consistent with the number of comorbidity categories. Hazard ratios (HRs) with 95% confidence intervals (CIs) are as follows: any disease activity (protocol defined [PD]) 1.03 (0.96–1.1), any disease activity (PD + non–protocol defined [NPD]) 1.03 (0.97–1.11), relapse (PD) 1.12 (0.99–1.27), relapse (PD + NPD) 1.15 (1.05–1.25), Expanded Disability Status Scale (EDSS) score worsening 1.00 (0.89–1.11), and combined unique activity 0.97 (0.89–1.05). LCL = lower confidence limit; UCL = upper confidence limit.
Table 4.
Multivariable Cox regression models for any disease activity and individual disease activity components including all comorbid conditions
Secondary analyses
Any disease activity with protocol- and non–protocol-defined relapses
When both protocol-defined and non–protocol-defined relapses were included in the definition, 786 (82.0%) participants had evidence of any disease activity. Of these participants, 98 (12.5%) had relapses alone, 203 (25.8%) had CUA alone, and 52 (6.6%) had EDSS score worsening alone. The remaining 433 (55.1%) had activity through a combination of these outcomes. The association of anxiety with any disease activity using protocol- and non–protocol-defined relapses showed an effect size (HR 1.22, 95% CI 0.99–1.51; table 4) similar to that of any disease activity using protocol-defined relapses. However, the other individual comorbid conditions were not associated with the hazard of any disease activity using protocol- and non–protocol-defined relapses, including migraine and dyslipidemia (table 4), nor was the number of comorbid conditions (figure, B).
Relapses
Anxiety was associated with an increased hazard of relapses (protocol-defined HR 1.53, 95% CI 1.07–2.20; protocol- and non–protocol-defined HR 1.38, 95% CI 1.07–1.78; table 4). To account for the possibility that prior relapses might increase anxiety, we adjusted for the number of relapses in the prior 12 months, and findings were consistent (protocol-defined HR 1.49, 95% CI 1.04–2.14; protocol- and non–protocol-defined HR 1.34, 95% CI 1.04–1.73). Depression showed an increased hazard of relapses (protocol- and non–protocol-defined HR 1.21, 95% CI 0.99–1.49). As the number of comorbid conditions increased, we observed an increased hazard of relapses (protocol and non–protocol defined) 0 vs 1, 2, and ≥3 (figure, B) after adjusting for all covariates. When we examined the number of physical comorbid conditions, those with 2 or 3 physical comorbid conditions had an increased hazard of relapses (protocol and non–protocol defined) (0 vs 2–3 HR 1.44, 95% CI 1.13–1.84).
Disability worsening
There was no significant association between any comorbidity and confirmed disability worsening after controlling for covariates (table 4 and figure, B).
Combined unique active lesions
The number of comorbid conditions was not associated with CUA. Of the individual conditions, only migraine was associated with CUA lesions (HR 0.80, 95% CI 0.65–0.99; table 4), showing a 20% decreased hazard rate of developing CUA.
Smoking
The addition of smoking status (ever/never) to the regression models did not change most of the main findings (data not shown). The effect of anxiety on any disease activity using protocol- and non–protocol-defined relapses was observed (HR 1.28, 95% CI 1.02–1.60), while some attenuation of the effect of anxiety was seen on protocol-defined relapses (HR 1.38, 95% CI 0.93–2.06). There was mild attenuation of the effect on migraine (HR 0.87, 95% CI 0.71–1.06) for any disease activity using protocol-defined relapses and CUA (HR 0.83, 95% CI 0.66–1.04). The results for current smoking status were consistent with these results.
Discussion
We investigated the association between multiple comorbid conditions with disease activity, including relapses, disability worsening, and MRI activity, among 959 CombiRx phase III clinical trial participants investigated. We found that comorbidity was common and that dyslipidemia and anxiety were associated with an increased hazard of disease activity using a stringent definition of relapses (protocol defined) over an average of 4 years of follow-up, while migraine was associated with a reduced hazard. In addition, in terms of the individual components of disease activity, we observed that anxiety was associated with an increased hazard of relapses and that a greater number of comorbid conditions (all or physical) was associated with relapses (protocol and non–protocol defined), while migraine was associated with a lower hazard of accumulating new lesions on MRI.
The CombiRx trial excluded potential participants with a history of any significant cardiac, hepatic, pulmonary, or renal disease, immune deficiency, or other medical conditions that would preclude therapy with IFN or GA. Moreover, participants were relatively young and diagnosed with MS an average of a year before enrollment. Nonetheless, we found that more than half of participants had at least 1 of the specified comorbid conditions and that the prevalence of comorbidity increased over the course of the trial. The number of comorbid conditions present was higher among participants who were overweight or obese. The prevalence of comorbidity in this trial population is consistent with the range of reported prevalence estimates for hypertension, dyslipidemia, depression, and anxiety in prevalent MS populations.25 The prevalence of diabetes mellitus was consistent with the prevalence reported at the time of MS diagnosis.1,5,17,18,20 While there are only a few reports on migraine, the prevalence was also consistent with other reports.5,21 One report saw similar increases in the prevalence of hypertension, dyslipidemia, and diabetes mellitus over 5 years of follow-up.20 The increase in the prevalence of comorbidity did not differ across treatment arms, but our sample size and duration of follow-up were likely insufficient to detect effects of IFN or GA on the risk of comorbidity. A large study based on administrative data from British Columbia, Canada, including 2,485 participants found that IFN use was associated with an increased incidence of migraine (1.55, 95% CI 1.18–2.04) and depression (1.33, 95% CI 1.13–1.56) during an average of 8.0 (SD 3.8) years of long-term follow-up.26
We observed a dose-response relationship between the number of comorbid conditions and the risk of relapses. Two prior observational studies have reported similar findings. In an online study involving 2,399 respondents, the presence of ≥3 comorbid conditions was associated with increased odds of reporting a relapse in the prior 12 months (odds ratio 2.60, 95% CI 1.56–4.31).19 In a prospective study of 885 participants, participants who reported ≥3 comorbid conditions at enrollment had a higher relapse rate, according to medical records review, over the following 2 years (rate ratio 1.45, 95% CI 1.00–2.08).5
We also found that specific comorbid conditions, including anxiety disorders and dyslipidemia, were associated with a shorter time to first evidence of any disease activity and rigorously defined relapses. Prior studies of anxiety and disease activity have been limited. One small cross-sectional study (n = 37) reported that the severity of anxiety was not associated with relapse rate.27 While the etiology of the effects of anxiety on MS clinical outcomes is unknown, further studies on the impact of treatment of anxiety on disease activity are warranted. Findings for dyslipidemia and relapses have been mixed. A Tasmanian study of 141 patients found that lipid levels were not associated with time to first relapse,28 and lipid levels were not associated with relapse rate in a study of 181 individuals with clinically isolated syndrome treated with IFN. In contrast, a Canadian study found that self-reported dyslipidemia was associated with a higher relapse rate over a 2-year period.5 An Australian study found that elevated triglyceride levels were associated with an increased hazard of relapse after an incident demyelinating event.29 These studies highlight inconsistencies in the approach used to measure lipid status and the need to develop a consistent approach in future studies to fully understand the role of lipids on relapse activity. Variable use of lipid-lowering therapies, including statins, also may have contributed to differences across studies. Trials examining statins in MS have shown mixed results.30–32
Migraine was associated with a longer time to first evidence of any disease activity, which appeared to be driven by its apparently protective effect on MRI outcomes (CUA). Cross-sectional studies have suggested that migraine is associated with more lesions on MRI, while others have reported no difference in lesion number.7,9,33 Longitudinal studies that have examined the within-person effects of migraine on accumulation of lesions over time in persons with MS are lacking. Future studies should also investigate the influence of chronic migraine therapies on MRI outcomes.
At enrollment, the median disability level was higher with a greater number of comorbid conditions, but the number of comorbid conditions was not associated with disability worsening during the trial. Specific comorbid conditions we examined also were not associated with disability worsening. Findings regarding disability at enrollment are consistent with prior cross-sectional studies that have reported that an increasing number of comorbid conditions is associated with greater disability.19,34 In a cohort of 2,375 persons with MS, the proportion reporting severe disability at diagnosis increased as the number of comorbid conditions increased.34 In an online cohort of 2,399 respondents, more comorbid conditions was also associated with greater disability.19 In contrast, the findings regarding disability worsening are discordant with some prior studies in larger cohorts that have found that depression, anxiety disorders, and vascular comorbid conditions, including dyslipidemia, are associated with greater disability worsening over time.3,18,35,36 This difference may reflect several factors, including the younger age of the cohort and shorter duration of disease or differences in the ascertainment of comorbidity. Also reflecting the early disease period of these participants, the proportion of individuals with confirmed disability worsening in the CombiRx cohort was relatively low, reducing statistical power for this analysis. We examined time to first confirmed disability worsening, whereas examining events using a recurrent events framework may be more powerful.
We focused on comorbid conditions while controlling for comorbid health indicators such as obesity and smoking at enrollment. Whereas other studies have reported that these behaviors affect clinical outcomes,8,37 smoking did not alter most disease outcomes in this analysis. However, smoking status was not available for the entire clinical trial population, which may have limited our ability to detect an effect of smoking and might have induced a selection bias. Recent studies suggest that there is a time-dependent effect of smoking cessation on disability worsening.38 Similarly, we did not account for changes in body mass index over the course of the study, but this is an important area of future work given emerging findings regarding the adverse effects of elevated body mass index on brain atrophy39 and reports of reduced treatment response in obese children with MS managed with IFN and GA.40
This study has limitations. While this study provides a consistently described cohort to study, it was not designed to investigate the role of comorbid conditions on treatment. We conducted multiple analyses, and our findings warrant replication. The ascertainment of comorbid conditions was not comprehensive, which potentially underrepresented the true burden of comorbidity of study participants, lacked information on severity, and was subject to potential misclassification bias. However, we were able to systematically account for conditions that developed during the long follow-up of trial participants, and our prevalence estimates were consistent with those reported elsewhere. Future studies should also use laboratory and formal diagnostic criteria for comorbidity assessment. Last, we were unable to explore the effects of treatment of a specified comorbidity on study outcomes because medications were used in the determination of comorbidity status. This is an important area for future research.
In CombiRx, a phase III clinical trial with up to 7 years of follow-up, comorbidity was common among participants despite the exclusion of participants with severe morbidity and influenced disease outcomes, including relapses and the accumulation of new lesions on MRI. The association of a higher count of comorbid conditions with higher relapse rates suggests that this may be a simple prognostic tool for clinicians. This study also has practical implications for the care of people with MS, highlighting the need to emphasize the importance of maintaining a healthy weight and appropriate management of comorbid conditions in the management of our patients with MS. Our findings support prior recommendations to consistently describe the comorbidity status of clinical trial populations.11 Our findings also suggest that, by influencing event rates, the comorbidity burden of clinical trial participants with MS may be an important factor in the outcome of clinical trials. Additional investigations of the impact of comorbidity on clinical trial outcomes and response to disease-modifying therapies are warranted.
Glossary
- CI
confidence interval
- CUA
combined unique lesion activity
- EDSS
Expanded Disability Status Scale
- FSS
functional system score
- GA
glatiramer acetate
- HR
hazard ratio
- IFN
interferon beta-1a
- MS
multiple sclerosis
Appendix. Authors
Footnotes
Editorial, page 193
Study funding
CombiRx (NCT00211887) was funded by the NIH, National Institute of Neurologic Disorders and Stroke (phase III study: UO1NS045719, Planning Grant R21NS41986). Study agents and matched placebo were kindly provided by their manufacturers, Biogen Idec and Teva Pharmaceutical.
Disclosure
A. Salter conducts statistical editorial services for Circulation: Cardiovascular Imaging. K. Kowalec has consulted with Emerald Lake Safety Ltd (2017–2018); has received speaker honoraria from Biogen/Fraser Health MS Clinic (2018); and has received research funding from the European Research Council, government of Canada, National MS Society, and the Consortium of MS Centers. K. Fitzgerald reports no disclosures. G. Cutter serves on the following Data and Safety Monitoring Boards: AMO Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Merck, Merck/Pfizer, Opko Biologics, Neurim, Novartis, Ophazyme, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva Pharmaceuticals, National Heart, Lung, and Blood Institute (Protocol Review Committee), National Institute of Child Health and Human Development (Obstetric Pharmacology Research Unit oversight committee). He consults or participates on advisory boards for Biogen, Brainstorm Cell Therapeutics, Charleston Labs Inc, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion Pharmaceuticals, Roche, Somahlution, Teva pharmaceuticals, TG Therapeutics, and UT Houston. Dr. Cutter is employed by the University of Alabama at Birmingham and is president of Pythagoras, Inc, a private consulting company located in Birmingham, AL. R.A. Marrie receives research funding from the Canadian Institutes of Health Research, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn's and Colitis Canada, National Multiple Sclerosis Society, and Consortium of Multiple Sclerosis Centers. She is supported by the Waugh Family Chair in Multiple Sclerosis. Go to Neurology.org/N for full disclosures.
References
- 1.Marrie RA, Patten SB, Tremlett H, et al. Sex differences in comorbidity at diagnosis of multiple sclerosis: a population-based study. Neurology 2016;86:1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrie R, Horwitz R, Cutter G, et al. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler 2008;14:1091–1098. [DOI] [PubMed] [Google Scholar]
- 3.McKay KA, Tremlett H, Fisk JD, et al. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology 2018;90:e1316–e1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhou Y, van der Mei IAF, et al. Lipid-related genetic polymorphisms significantly modulate the association between lipids and disability progression in multiple sclerosis. J Neurol Neurosurg Psychiatry 2019;90:636–641. [DOI] [PubMed] [Google Scholar]
- 5.Kowalec K, McKay KA, Patten SB, et al. Comorbidity increases the risk of relapse in multiple sclerosis: a prospective study. Neurology 2017;89:2455–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tettey P, Siejka D, Simpson S Jr, et al. Frequency of comorbidities and their association with clinical disability and relapse in multiple sclerosis. Neuroepidemiology 2016;46:106–113. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock-Guttman B, Zivadinov R, Horakova D, et al. Lipid profiles are associated with lesion formation over 24 months in interferon-β treated patients following the first demyelinating event. J Neurol Neurosurg Psychiatry 2013;84:1186–1191. [DOI] [PubMed] [Google Scholar]
- 8.Weinstock-Guttman B, Zivadinov R, Mahfooz N, et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Neuroinflammation 2011;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne RW, Weinstock-Guttman B, Horakova D, et al. Apolipoproteins are associated with new MRI lesions and deep grey matter atrophy in clinically isolated syndromes. J Neurol Neurosurg Psychiatry 2014;85:859–864. [DOI] [PubMed] [Google Scholar]
- 10.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One 2012;7:e41601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrie RA, Miller A, Sormani MP, et al. The challenge of comorbidity in clinical trials for multiple sclerosis. Neurology 2016;86:1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindsey JW, Scott TF, Lynch SG, et al. The CombiRx trial of combined therapy with interferon and glatiramer acetate in relapsing remitting MS: design and baseline characteristics. Mult Scler Relat Disord 2012;1:81–86. [DOI] [PubMed] [Google Scholar]
- 13.Lublin FD, Cofield SS, Cutter GR, et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol 2013;73:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–231. [DOI] [PubMed] [Google Scholar]
- 15.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 16.Giovannoni G, Tomic D, Bright JR, et al. “No evident disease activity”: the use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 2017;23:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conway DS, Thompson NR, Cohen JA. Influence of hypertension, diabetes, hyperlipidemia, and obstructive lung disease on multiple sclerosis disease course. Mult Scler J 2017;23:277–285. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Tremlett H, Zhu F, et al. Effects of physical comorbidities on disability progression in multiple sclerosis. Neurology 2018;90:e419–e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marck CH, Neate SL, Taylor KL, et al. Prevalence of comorbidities, overweight and obesity in an international sample of people with multiple sclerosis and associations with modifiable lifestyle factors. PLoS One 2016;11:e0148573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakimovski D, Gandhi S, Paunkoski I, et al. Hypertension and heart disease are associated with development of brain atrophy in multiple sclerosis: a 5-year longitudinal study. Eur J Neurol 2019;26:87–e8. [DOI] [PubMed] [Google Scholar]
- 21.Graziano E, Hagemeier J, Weinstock-Guttman B, et al. Increased contrast enhancing lesion activity in relapsing-remitting multiple sclerosis migraine patients. NeuroImage Clin 2015;9:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen ER, Søndergaard HB, Laursen JH, et al. Smoking is associated with increased disease activity during natalizumab treatment in multiple sclerosis. Mult Scler 2019;25:1298–1305. [DOI] [PubMed] [Google Scholar]
- 23.Petersen ER, Oturai AB, Koch-Henriksen N, et al. Smoking affects the interferon beta treatment response in multiple sclerosis. Neurology 2018;90:e593–e600. [DOI] [PubMed] [Google Scholar]
- 24.Kvistad SS, Myhr K-M, Holmøy T, et al. Body mass index influence interferon-beta treatment response in multiple sclerosis. J Neuroimmunol 2015;288:92–97. [DOI] [PubMed] [Google Scholar]
- 25.Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler 2015;21:263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jong HJI, Kingwell E, Shirani A, et al. Evaluating the safety of β-interferons in MS: a series of nested case-control studies. Neurology 2017;88:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espinola-Nadurille M, Colin-Piana R, Ramirez-Bermudez J, et al. Mental disorders in Mexican patients with multiple sclerosis. J Neuropsychiatry Clin Neurosci 2010;22:63–69. [DOI] [PubMed] [Google Scholar]
- 28.Tettey P, Simpson S, Taylor B, et al. Adverse lipid profile is not associated with relapse risk in MS: results from an observational cohort study. J Neurol Sci 2014;340:230–232. [DOI] [PubMed] [Google Scholar]
- 29.Tettey P, Simpson S, Taylor B, et al. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult Scler J 2014;20:1737–1744. [DOI] [PubMed] [Google Scholar]
- 30.Lanzillo R, Orefice G, Quarantelli M, et al. Atorvastatin Combined to Interferon to Verify the Efficacy (ACTIVE) in relapsing-remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Mult Scler J 2010;16:450–454. [DOI] [PubMed] [Google Scholar]
- 31.Kamm CP, El-Koussy M, Humpert S, et al. Atorvastatin added to interferon beta for relapsing multiple sclerosis: a randomized controlled trial. J Neurol 2012;259:2401–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Togha M, Karvigh SA, Nabavi M, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Mult Scler J 2010;16:848–854. [DOI] [PubMed] [Google Scholar]
- 33.Kister I, Caminero AB, Monteith TS, et al. Migraine is comorbid with multiple sclerosis and associated with a more symptomatic MS course. J Headache Pain 2010;11:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrie RA, Horwitz R, Cutter G, et al. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 2009;72:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tettey P, Simpson S, Taylor BV, et al. Vascular comorbidities in the onset and progression of multiple sclerosis. J Neurol Sci 2014;347:23–33. [DOI] [PubMed] [Google Scholar]
- 36.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010;74:1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kappus N, Weinstock-Guttman B, Hagemeier J, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016;87:181–187. [DOI] [PubMed] [Google Scholar]
- 38.Tanasescu R, Constantinescu CS, Tench CR, et al. Smoking cessation and the reduction of disability progression in multiple sclerosis: a cohort study. Nicotine Tob Res 2018;20:589–595. [DOI] [PubMed] [Google Scholar]
- 39.Mowry EM, Azevedo CJ, McCulloch CE, et al. Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology 2018;91:e2256–e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huppke B, Ellenberger D, Hummel H, et al. Association of obesity with multiple sclerosis risk and response to first-line disease modifying drugs in children. JAMA Neurol 2019;76:1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moccia M, Lanzillo R, Palladino R, et al. The Framingham Cardiovascular Risk Score in multiple sclerosis. Eur J Neurol 2015;22:1176–1183. [DOI] [PubMed] [Google Scholar]
- 42.Koch M, Uyttenboogaart M, van Harten A, et al. Fatigue, depression and progression in multiple sclerosis. Mult Scler J 2008;14:815–822. [DOI] [PubMed] [Google Scholar]
- 43.Binzer S, McKay KA, Brenner P, et al. Disability worsening among persons with multiple sclerosis and depression. Neurology 2019;93:e2216–e2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giubilei F, Antonini G, Di Legge S, et al. Blood cholesterol and MRI activity in first clinical episode suggestive of multiple sclerosis. Acta Neurol Scand 2002;106:109–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset from this study is held securely by the University of Alabama at Birmingham. Access may be granted via authorization from the Executive Committee and may be facilitated by contacting Dr. Cutter.