Abstract
Purpose
We investigated the effect of therapeutic plasma exchange (TPE) on life-threatening COVID-19; presenting as acute respiratory distress syndrome (ARDS) plus multi-system organ failure and cytokine release syndrome (CRS).
Materials and methods
We prospectively enrolled ten consecutive adult intensive care unit (ICU) subjects [7 males; median age: 51 interquartile range (IQR): 45.1–55.9 years old] with life-threatening COVID-19 infection. All had ARDS [PaO2/FiO2 ratio: 110 (IQR): 95.5–135.5], septic shock, CRS and deteriorated within 24 h of ICU admission despite fluid resuscitation, antibiotics, hydroxychloroquine, ARDS-net and prone position mechanical ventilation. All received 5–7 TPE sessions (dosed as 1.0 to 1.5 plasma volumes).
Results
All of the following significantly normalized (p < 0.05) following the TPE completion, when compared to baseline: Sequential Organ Function Assessment score, PaO2/FiO2 ratio, levels of lymphocytes, total bilirubin, lactate dehydrogenase, ferritin, C-reactive protein and interleukin-6. No adverse effects from TPE were observed. Acute kidney injury and pulmonary embolism were observed in 10% and 20% of patients, respectively. The duration of mechanical ventilation was 9 (IQR: 7 to 12) days, the ICU length of stay was 15 (IQR: 13.2 to 19.6) days and the mortality on day-28 was 10%.
Conclusion
TPE demonstrates a potential survival benefit and low risk in life-threatening COVID-19, albeit in a small pilot study.
Keywords: Life-threatening COVID-19, Acute respiratory distress syndrome, Cytokine release syndrome, Therapeutic plasma exchange, Intensive care unit
Highlights
-
•
Life-threatening COVID-19 is defined as ARDS, sepsis, MSOF, and at least one criterion for cytokine release syndrome.
-
•
The application of plasma exchange resulted in decreased levels of inflammatory biomarkers in life-threatening COVID-19.
-
•
The application of plasma exchange resulted in improved clinical outcomes in life-threatening COVID-19.
-
•
Plasma exchange demonstrates a potential survival benefit, and low complication risk in life-threatening COVID-19.
1. Introduction
Coronaviruses (CoV) can cause infections ranging from the common cold to severe disorders such as the Middle East Respiratory Syndrome and the Severe Acute Respiratory Syndrome (SARS-CoV) [1,2]. In December 2019 in Wuhan city, China, a novel coronavirus was identified and subsequently named the Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) [3]. It is responsible for a pandemic that has threatened the world's health, economy and way of life. Hyperbole aside, treatments are desperately needed. A minority of patients develop fulminant (life-threatening) SARS-CoV-2 disease (COVID-19): defined by acute respiratory failure/acute respiratory distress syndrome (ARF/ARDS), septic shock and/or multi-system organ failure (MSOF) [[4], [5], [6]]. While antiviral agents, such as remdesivir, and convalescent plasma transfusion (CPT) containing a high concentration of neutralizing antibodies, show promise, there is currently no treatment supported by robust evidence [[7], [8], [9], [10], [11], [12], [13], [14], [15]]. Moreover COVID-19 antibodies titers in infected cases can vary and decrease over time [14,15]. Therapeutic plasma exchange (TPE), without protective antibodies, has been previously used with success in patients with severe sepsis, MSOF and fulminant SARS-CoV; although its benefit is unclear in severe ARDS [16,17]. This is a pilot study using TPE as rescue therapy in ten adult intensive care unit (ICU) patients with fulminant COVID-19.
2. Materials and methods
2.1. Subjects
We prospectively enrolled ten consecutive subjects with life-threatening COVID-19 who were admitted to King Saud Medical City (KSMC) and Al Imam Abdulrahman Al Feisal Hospital (AIAF) [Saudi Ministry of Health (MOH), Cluster One Health Care Facilities, Riyadh, Kingdom of Saudi Arabia] between March 22 and May 11, 2020. We primarily evaluated 28 day mortality, and the safety of TPE in life-threatening COVID-19. Secondary outcomes were: improvement in Sequential Organ Function Assessment (SOFA) score [18], changes in inflammation markers, days on mechanical ventilation and ICU length of stay. Inclusion criteria were: 1) Age ≥ 18 years old; 2) Intubation and ICU admission; and 3) Life-threatening COVID-19 [[4], [5], [6],12,13,15,[19], [20], [21], [22]].defined as: i) ARDS (according to the Berlin criteria) [23,24], ii) Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥ 20 upon ICU admission [25], iii) Presence of severe sepsis/septic shock, and/or multi-system organ failure (MSOF) [26,27], and one or more criteria for defining cytokine release syndrome (CRS). The criteria for CRS are fully outlined in Table 1 [12,13,19,20,28,29]. SARS-CoV-2 infection was confirmed by Real-Time-Polymerase-Chain-Reaction (RT-PCR) assays using QuantiNova Probe RT-PCR kit (Qiagen) in a Light-Cycler 480 real-time PCR system (Roche, Basel, Switzerland) [[30], [31], [32], [33], [34], [35]]. Exclusion criteria were: 1) previous allergic reaction to plasma exchange or its ingredients (i.e., sodium citrate) and 2) two consecutive negative RT-PCR tests for SARS-CoV-2 taken at least 24 h apart. The study was conducted according to the principles of the Declaration of Helsinki and approved by our Institutional Review Board [36]. Written informed consent was obtained from patients or legal representatives. Therapeutic Plasma Exchange TPE was initiated using the Spectra Optia ™ Apheresis System equipped with the Depuro D2000 Adsorption Cartridge (Terumo BCT Inc., USA) [37]. Spectra Optia ™ Apheresis System operates with acid-citrate dextrose anticoagulant (ACDA) as per Kidney Disease Improving Global Outcomes (KDIGO) 2019 guidelines [38]. TPE was utilized with an adsorption cartridge containing activated uncoated coconut shell (carbon granules) charcoal (100 g), and the nonionic resins Amberlite XAD-7HP and Amberchrom GC300C [37]. These adsorption materials could remove significant proportions of interferon-gamma, interleukins −3, −10, −1B, −6, −8, and tumor necrosis factor-alpha [[39], [40], [41], [42], [43], [44], [45], [46], [47]]. A dose of 1.5 plasma volumes was used for the first dose then one plasma volume daily for five to seven doses per clinical case. Plasma was replaced with albumin 5% or fresh frozen plasma in patients with coagulopathy (prothrombin time > 37 s; international normalized ratio > 3; activated partial thromboplastin time > 100 or fibrinogen level < 100 mg/d) [48]. TPE sessions were performed daily over four hours and laboratory markers were measured daily [[4], [5], [6],12,13,37,38]. To evaluate the effect of TPE in removing inflammatory mediators known to be increased in COVID-19 patients [[4], [5], [6],12,13,28,29], we measured serum levels of C-reactive protein (CRP), d-dimers, LDH, ferritin and IL-6 prior to the initiation of TPE and after the last TPE session. CRP was defined as elevated if it was >5.0 mg/l and IL-6 if >7.0 pg/ml [49].
Table 1.
Criteria for defining CRS.
| One or more of the following criteria should be present* |
| C-reactive protein >100 or > 50 mg/l but doubled in the past 48 h |
| lymphocyte count < 0.6 × 109/l |
| Serum Interleukin-6 (IL-6) ≥ 3× upper normal limit |
| Ferritin > 300 μg/l (or surrogate) with doubling within 24 h |
| Ferritin > 600 μg/l at presentation and LDH >250 U/l |
| Elevated D-dimer (> 1 μg/ml) |
Abbreviations: CRS = cytokine release syndrome, LDH = lactate dehydrogenase; * We defined as low risk for developing CRS the presence of one criterion, moderate risk the presence of two to three criteria and high risk the presence of more than three criteria.
2.2. Statistical analysis
Continuous variables were expressed as medians with interquartile ranges (IQR) and categorical variables were expressed as absolute numbers and proportions. We utilized the Wilcoxon signed rank test for non-parametric data to compare parameters before and after TPE. All tests were two-tailed and considered significant when the p value was <0.05. Statistical analysis was performed using SPSS, version 23.0.
3. Results
3.1. Patients and therapeutic interventions
Out of one hundred and sixty-five consecutive patients who were admitted to the ICU between March 22 and May 11, 2020, eighteen COVID-19 patients were eligible for the study. Out of these eighteen cases, five patients expired upon ICU admission and could not be recruited. For three patients the legal representatives did not provide their informed consent for participation in the study. Finally, ten consented patients were enrolled in this pilot study (please, refer to the flow diagram Fig. S1 suppl. of the Appendix). All enrolled patients had ARDS and septic shock, within 24 h of ICU admission, and all had more than three risk factors for CRS (Table 1). All received empiric treatment (see below) for COVID-19 along with standard ICU support [[4], [5], [6],12,13,15,[19], [20], [21], [22], [23], [24], [25], [26], [27]]. All deteriorated within 24 h of ICU admission despite recruitment maneuvers, low tidal volume and prone position mechanical ventilation resulting in a PaO2/FiO2 ratio of 110 (IQR: 95.5 to 135.5) and increasing norepinephrine requirements [norepinephrine (1.9 μg/kg/min; IQR: 1.8 to 2 μg/kg/min)]. After the administration of vasopressin (infusion rate: 0.07 units/min; IQR: 0.05 to 0.09 units/min) the dose of norepinephrine was reduced to 0.9 μg/kg/min (IQR: 0.7 to 1 μg/kg/min). Co-interventions included, lung recruitment, prone positioning, and empiric hydroxychloroquine (400 mg twice daily on day 1, followed by 200 mg twice daily on days 2–5) [[4], [5], [6],9,10,50,51], broad spectrum antibiotics, intravenous hydrocortisone (200 mg daily for 3 days) [26,27], and prophylactic anticoagulation (enoxaparin 40 mg subcutaneously once daily and intermittent pneumatic compression) in all patients [[52], [53], [54], [55], [56], [57], [58]]. Plasma exchange was administered as rescue therapy to all patients and extracorporeal membrane oxygenation (ECMO) was added in one patient who exhibited a PaO2/FiO2 ratio < 80 for ≥6 h (case number 8, Table 2). Baseline features are presented in Table 2 (also, please, refer to Table S1 suppl. of the Appendix). Median patient age was 51 (IQR: 45.1 to 55.9) years old and body mass index (BMI) 24.9 (IQR: 20.5 to 29.8) kg/m2. Time of symptom onset to ICU admission was 6.5 (IQR: 3.5 to 7.4) days. All cases were enrolled approximately 24 h post ICU admission. Their most common symptoms were: cough (10/10 cases), fever (8/10 cases), and dyspnea (5/10 cases); while less common symptoms included sputum production (4/10 cases), vomiting and nausea (4/10 cases), diarrhea, altered level of consciousness and anosmia (2/10 cases). Six patients had underlying chronic diseases: diabetes mellitus (60%), essential hypertension (50%) and cardiovascular disease (10%). Pulmonary embolism (PE) was ruled out in 8/10 cases and confirmed in 2/10 following contrast chest computed tomography (CT). The two positives (patients 8 and 9, Table 2) exhibited subsegmental PE without hemodynamic compromise and received therapeutic anticoagulation. Baseline point-of-care cardiac ultrasonography showed no significant cardiac dysfunction in any of our ten patients; while, their cardiac enzymes were within normal limits. Acute kidney injury, which was defined per the “risk,” “injury,” and “failure” (RIFLE) criteria [59], was observed in 10% of cases (Table S1 suppl., Appendix). In all cases, chest (CT) scans, performed upon ICU admission, showed bilateral ground-glass opacities and variable pulmonary parenchymal consolidations consistent with COVID-19 pneumonia (Fig. 1 and Fig. S2 suppl., Fig. S3 suppl. of the Appendix) [[60], [61], [62]]. Five patients had multi-lobe involvement and two had chronic lung parenchymal changes. Effects of TPE All TPE patients had an increase in their PaO2/FiO2 ratio above 250 following the 3rd or later treatment. Radiologic findings showed variable degrees of improvement after the completion of five or more TPE sessions (Fig. 1 and Fig. S2 , Fig. S3 of the Appendix). Nine of ten patients were successfully liberated from mechanical ventilation and extubated, survived and were discharged from the hospital to home isolation after 20 days (IQR: 17.6 to 22.6), and without complications. A comparison of parameters in COVID-19 patients prior to and after TPE is outlined in Table 3.
Table 2.
Baseline features of the TPE patients (n = 10).
| Patient no. | Sex |
Age |
Classification of disease severity | Days of symptom onset to ICU admission | Number of TPE sessions | Clustering infection* | Main symptoms prior to hospital admission | Comorbidities |
|---|---|---|---|---|---|---|---|---|
| Male/Female | Years | |||||||
| 1 | Male | 45 | Life-threatening | 5 | 5 | Yes | Fever, cough, dyspnea, sputum production | None |
| 2 | Female | 51 | Life-threatening | 6 | 5 | No | Fever, cough, dyspnea, nausea, vomiting | None |
| 3 | Male | 38 | Life-threatening | 7 | 5 | No | Cough, dyspnea, chest pain, anosmia | None |
| 4 | Female | 52 | Life-threatening | 2 | 5 | Yes | Cough, nausea, vomiting, altered level of consciousness | Diabetes |
| 5 | Male | 61 | Life-threatening | 3 | 5 | No | Fever, cough, dyspnea, sputum production | Hypertension, diabetes |
| 6 | Female | 48 | Life-threatening | 2 | 5 | No | Fever, cough, dyspnea, chest pain, diarrhea | Hypertension, diabetes |
| 7 | Male | 55 | Life-threatening | 8 | 7 | No | Fever, cough, sputum production, anosmia | Hypertension, diabetes |
| 8 | Male | 58 | Life-threatening | 7 | 7 | No | Fever, cough, nausea, vomiting, diarrhea | Hypertension, diabetes |
| 9 | Male | 65 | Life-threatening | 7 | 6 | No | Fever, cough, nausea, vomiting, altered level of consciousness | Hypertension, diabetes, cardio- vascular disease |
| 10 | Male | 51 | Life-threatening | 7 | 6 | No | Fever, cough, sputum production, chest pain | None |
Abbreviations: TPE = therapeutic plasma exchange; ICU = intensive care unit. *clustering infection means that these patients were infected in an area where several other cases were discovered at the same time (cluster).
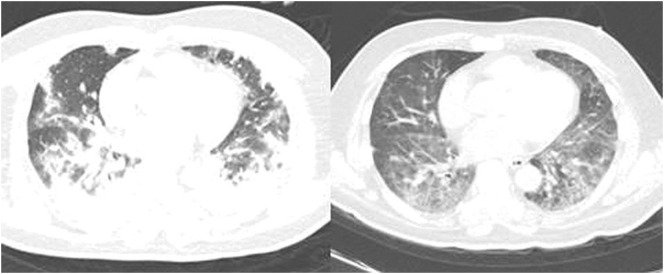
Fig. 1.
Contrast chest computed tomography scan in a life-threatening COVID-19 patient (case 3; Table 2) showing diffuse bilateral mixed ground glass opacities and consolidation with airbronchogram associated with septum thickening and mild right pleural effusion prior to plasma exchange (left panel); and after five plasma exchange sessions showing gradual improvement (right panel).
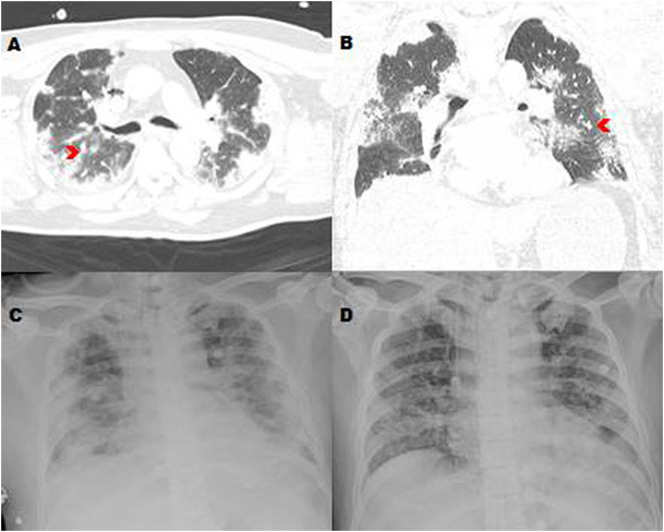
Fig. S2.
Contrast chest computed tomography (CT) scan (A, B) showing bilateral mixed ground glass opacities (red chevrons) in a TPE patient (case 1; Table 2) with COVID-19; his chest X-ray on admission showing acute respiratory distress-like picture (C) and gradual improvement after five plasma exchange treatments (D).
Fig. S3.
Contrast chest CT scan showing bilateral ground glass opacities in a TPE patient (case 10; Table 2) with COVID-19 (left panel) and gradual improvement after six TPE sessions (right panel).
Table 3.
Comparison of parameters in patients (n = 10) before and after TPE.
| Parameters | Before TPE | After 5 to 7 TPE sessions |
|---|---|---|
| Sequential Organ Function Assessment score | 11 (8.9 to 11.5) | 2 (1.4 to 3.6) * |
| PaO2/FiO2 ratio | 110 (95.5 to 135.5) | 340 (310.5 to 370.6) * |
| Lymphocyte count (109/l, normal range 1.1 to 3.2) | 0.6 (0.45 to 0.8) | 1.15 (0.8 to 1.4) * |
| C-reactive protein (mg/, normal range 10 to 5) | 71.3 (51.3 to 89.7) | 13.2 (7.2 to 26.4) * |
| Total bilirubin (μmol/l, normal range 0 to 26) | 28.2 (17.7 to 33.4) | 11.6 (8.2 to 15.8) * |
| Alanine aminotransferase (U/l, normal range 9 to 50) | 66.5 (52.3 to 91.2) | 42.5 (22.7 to 53.6) * |
| Aspartate aminotransferase (U/l, normal range 15 to 40) | 45.9 (39.2 to 78.3) | 33.2 (30.9 to 41.6) * |
| Creatinine (mg/dL, normal range 0.6 to 1.2) | 1.2 (0.9 to 1.4) | 1.1 (0.8 to 1.2) |
| Serum lactate (mmol/l, normal range 1.0 to 2.5) | 5.5 (3.4 to 8.9) | 1.7 (1.2 to 2.6) * |
| Lactate dehydrogenase (U/l, normal range 100 to 190) | 576.5 (378.4 to 673.4) | 199.5 (156.1 to 232.3) * |
| Ferritin (ng/ml, normal range 23–336) | 1233 (799 to 1758) | 290 (201 to 322) * |
| D-dimers (mcg/ml, normal values <1) | 7.4 (4.9 to 11.7) | 0.9 (0.7 to 1.2) * |
| Interleukin-6 (pg/ml, normal range 1–7) | 159.5 (88.9 to 182.3) | 31.2 (15.4 to 49.8) * |
Abbreviations: TPE = therapeutic plasma exchange; PaO2/FiO2 = partial arterial pressure of oxygen to fractional inspired concentration of oxygen; values are medians with interquartile range; the median time between the baseline data and the data after 5–7 TPE sessions was 5 days; * p values <0.05 were statistically significant by Wilcoxon signed rank test for non-parametric data. The value for creatinine did not reach statistical significance (p = 0.56).
All patients were weaned off vasopressors following TPE completion. There was a significant improvement of all laboratory parameters related to the CRS, the PaO2/FiO2 ratio and SOFA scores (all p < 0.05; Table 3). The duration of mechanical ventilation was 9 (IQR: 7 to 12) days, the ICU length of stay was 15 (IQR: 13.2 to 19.6) days, the hospital discharge rate was 90% and the mortality on day-28 was 10% (Table S1 . suppl. Appendix). One patient had a secondary infection from a Gram negative bacterium (case 7). The expired patient (case 8, Table 2) developed cardiac arrest after completing all TPE sessions, and while undergoing weaning trials. No patient had any severe coagulopathy apart from elevated D-dimer levels (Table 3). No arrhythmias and/or other cardiac events that might have been linked to hydroxychloroquine were identified [[63], [64], [65], [66]]. No adverse effects (i.e., transfusion/allergic reactions, line complications) from the TPE were observed in this case-series. Screening for other viral infections and systemic disorders was negative. SARSCoV-2 RNA, assayed by RT-PCR, became negative in all discharged cases after 18 (16 to 20) days.
4. Discussion
The use of TPE, with a variety of adsorption cartridges, has been the subject of cohort studies and clinical trials [[39], [40], [41], [42], [43], [44], [45], [46], [47]]. The putative benefit is assumed to come from reducing inflammatory cytokines such as IL-6 and endotoxins [27,28,[39], [40], [41], [42], [43], [44], [45], [46], [47],67,68]. The objective of our small pilot study was to determine if using TPE in critically ill COVID-19 patients could ameliorate inflammatory mediators and potentially rescue extremely sick patients. In brief, we demonstrated that the use TPE for life-threatening COVID-19 infection appears safe and feasible, and may be associated with improved survival.
When compared to other studies [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]], our cohort, of COVID-19 patients who received TPE had acceptable and better survival, hospital discharge rates, days on mechanical ventilation and ICU length of stay compared to other clinical studies [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. CRS could be a unique feature of life-threatening COVID-19, as previously described [27,28,[67], [68], [69]]. Elevated levels of inflammation markers and lymphopenia are early predictors of severe COVID-19 and death [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. In all of our TPE patients, serum C-RP, d-dimers, ferritin, LDH and IL-6 were markedly increased; hence all patients had more than three risk factors for CRS (Table 1). We demonstrated that all of these acute inflammatory markers decreased after five to seven TPE sessions. However, it is important to acknowledge that these markers might have decreased over time in patients not treated with TPE. It is believed that IL-6 is central to CRS, and accordingly, a new monoclonal antibody against IL-6 (TCZ) has been used in trials for treatment of COVID-19 with CRS [[67], [68], [69]]. The larger of these pilot studies recruited 15 COVID-19 patients [69]. The authors observed baseline IL-6 levels comparable to ours; however, their reported outcome was variable with a dramatic increase of IL-6 (> 2000 pg/ml) in four patients that died.
The potential survival benefit observed in our pilot study is comparable to recent trials in which CPT was used for severe COVID-19 [12,13]. CPT shows promise but is complex and time consuming and may be associated with a higher transmission risk of pathogens compared to classic TPE. Another concern with CPT, but not TPE, is the possibility of antibody-dependent infection enhancement. This could suppress innate immunity and thereby allow intracellular viral growth [12,13,70]. Thus, TPE might be a rational and less expensive rescue therapy for life-threatening COVID-19 compared to CPT, especially if natural immunity does not occur [71].
In this pilot study, no adverse effects of TPE were observed. The patient who expired (case 8, Table 2) developed cardiac arrest after completing all TPE sessions, and while undergoing weaning trials. This is unusual, unexpected and insufficiently explained, although he had underlying cardiovascular disease. The event occurred more than two days after his last TPE session; thus could not be linked directly to therapy. Although no myocardial injury was documented, we cannot exclude the possibility of sudden cardiac arrest due to the development of arrhythmias related to the virus per se and/or the administration of hydroxychloroquine as previously reported [[74], [75], [76], [77], [78]]. Notably, in the revised version of Saudi MOH guidelines for COVID-19 ICU management, which was released after the completion of this study, hydroxychloroquine is no longer included as an optional empiric therapy [20]. Hence, if it was the initiating event then at least we are not using the medication in the ICU anymore. It is also noteworthy that our PE observed prevalence was 20% and that there is an increased incidence of microthrombi and PE in serious COVID-19 [[52], [53], [54], [55], [56], [57], [58]]. The suggested pathophysiological mechanism of microthrombosis and vascular dysfunction associated with COVID-19 may differ from macrophage activation syndrome with disseminated intravascular coagulation (DIC) [72,73]. Importantly, in this case-series, we confirmed CRS rather than just DIC [27,28,[67], [68], [69]]. Study limitations This pilot study has limitations which confines its generalizability. Apart from TPE, patients received other empiric medications and supportive interventions despite uncertainty as to their effectiveness. As these were not controlled for, we are uncertain of their effects on inflammatory mediator levels and survival. All patients received 200 mg of hydrocortisone (40 mg prednisone equivalent), and hence steroids might have affected the immune response or viral clearance. Also, the median initial dose of norepinephrine was higher than in septic shock trials; however, we believe this reflected how sick patients were and was part of our justification for rescue TPE. While our study did not set out to compare TPE to CPT, the time from onset of symptoms to initiation of TPE was shorter compared to that reported in CPT trials (6.5 vs. 16.5 days) [12,13]. Notably, the natural course of SARS-CoV-2's viremia is not well established. Hence, it is difficult to know the relationship between viral RNA reduction and TPE, or the optimal TPE regime. Instead, we intuited that the sooner that TPE was initiated for life-threatening COVID-19, the better for our patients. Conceivably, patients could have recovered without TPE. Resource limitations restricted us from measuring a full panel of cytokines but those measured are comparable to published literature [[12], [13], [14],[39], [40], [41], [42], [43], [44], [45], [46], [47],[67], [68], [69], [70]]. Our patient sample was too small to draw definitive conclusions and/or perform meaningful subgroup analysis. Despite the aforementioned limitations, our preliminary work suggests that TPE is associated with discrete improvement in the biochemical markers of CRS, ARDS, septic shock and MSOF related to fulminant COVID-19. TPE also appeared to be low risk despite the high illness severity of our patients. Larger randomized control trials are required to confirm or refute our findings and we are working towards that end.
Summary of baseline characteristics and outcome measures of TPE patients.
Flow diagram of consecutive intensive care unit (ICU) admissions and COVID-19 patients being enrolled to receive therapeutic plasma exchange.
Declarations authors' contributions
All authors contributed to data acquisition, analysis, and interpretation. All authors reviewed and approved the final version.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of King Saud Medical City, Riyadh, Kingdom of Saudi Arabia [H-01-R-053, IORG0010374#, serial number: H1-R-20-00]. Written informed consent was obtained by all eligible patients or their legal representatives.
Funding
No financial support was received for this preliminary study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to thank Mr. Daood Saied Ahmad Asad for his invaluable technical support during plasma exchange sessions and Ms. Zahra Alfrdan for her secretarial support; we thank all the members of the COVID-19 Crisis Management Team at King Saud Medical City and at Al Imam Abdulrahman Al Feisal Hospital: Ms. Huda Ahmad Mhawish (Head of ICU nursing staff), Mr. Basel Hamid Almuabbadi, Ms. Bobby Rose Marasigan, Ms. Karen Joyce Calamba, Ms. Mary Bay Obra, Ms. Ashley Diane Cabrales, Ms. Faranadz Fazar, Mr. Anil Kumar, Ms. Katrina Baguisa, Ms. Anitha Vargese and Mr. Ayman Alsalmi (Head of Respiratory Therapists). Finally, we acknowledge all health-care workers involved in the diagnosis and treatment of COVID-19 patients in Riyadh, KSA.
References
- 1.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update. 11 June 2014. [Google Scholar]
- 2.CDC Middle East Respiratory Syndrome (MERS) 25th June, 2014. [Google Scholar]
- 3.Lu R., Zhao X., Li J., et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. (Jan 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. China Medical Treatment Expert Group for Covid-19. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. (Feb 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. (11 March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 6 April. [DOI] [PMC free article] [PubMed]
- 7.Grein J., Ohmagari N., Shin D., et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020; Apr 10;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicenter trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020:1–3. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautret P., Lagier J.-C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B., Wang Y., Wen D., et al. A trial of Lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020; Apr 6;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen C., Wang Z., Zhao F., et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020; 27 March;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao R., Li M., Song H., et al. Early detection of SARS-CoV-2 antibodies in COVID-19 patients as a serologic marker of infection. Clin Infect Dis. 2020; May 1 doi: 10.1093/cid/ciaa523. ciaa523. [DOI] [Google Scholar]
- 15.Deng Y., Liu W., Liu K., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J. 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel P., Nandwani V., Vanchiere J., et al. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A--an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011;12(2):e87–e89. doi: 10.1097/PCC.0b013e3181e2a569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaup H., Stahl K., Schmidt B.M.W., et al. Early therapeutic plasma exchange in septic shock: a prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care. 2018;22:285. doi: 10.1186/s13054-018-2220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour C.W., Liu V.X., Iwashyna T.J. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016; Feb 23;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saudi Ministry of Health Coronavirus Diseases 19 (COVID-19) guidelines. March 2020; version 1.2. https://covid19.moh.gov.sa
- 20.Saudi Ministry of Health Coronavirus Diseases 19 (COVID-19) guidelines. (revised version 1.7) May 25th, 2020. https://covid19.moh.gov.sa
- 21.World Health Organization, Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: Interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infectionwhen-novel-coronavirus-(ncov)-infection-is-suspected.
- 22.https://covid19.cdc.gov.sa
- 23.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;23(315(8)):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson N.D., Fan E., Camporota L., et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 25.Salluh J.I. Soares M. ICU severity of illness scores: APACHE, SAPS and MPM. Curr Opin Crit Care. 2014;20(5):557–565. doi: 10.1097/MCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes A., Evans L.E., Alhazzani W., Campaign Surviving Sepsis, et al. International Guidelines for Management of Sepsis and Septic Shock. Intensive Care Med 2017. 2016;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 27.Levy M.M., Evans L.E., Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 28.Azkur A.K., Akdis M., Azkur D., et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020; May 12;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 30.Nalla A.K., Casto A.M., Huang M.W., et al. Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J Clin Microbiol. 2020; April 8;58(6) doi: 10.1128/JCM.00557-20. e00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020; April 1;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020; March 11;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R., Han H., Liu F., et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan J.F., Yip C.C., To KK, et al. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol. 2020; Apr 23;58(5) doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. (pii2000045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Medical Association World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 37.D2000 Cartridge Operation Manual for Use of D2000 with the Terumo Spectra Optia™ Apheresis System; for use in the U.S. under FDA EUA200148: Authorization for Emergency Use in patients with COVID-19 admitted to the ICU with confirmed or imminent respiratory failure. https://www.fda.gov/media/136837/download:
- 38.Wang A.Y., Akizawa T., Bavanandan S., et al. 2017 kidney disease: improving global outcomes (KDIGO) chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update implementation: Asia summit conference report. Kidney Int Rep. 2019;4(11):1523–1537. doi: 10.1016/j.ekir.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellinger R.P., Bagshaw S.M., Antonelli M., EUPHRATES Trial Investigators, et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018;320(14):1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein D.J., Foster D., Walker P.M., Bagshaw S.M., Mekonnen H., Antonelli M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018;44(12):2205–2212. doi: 10.1007/s00134-018-5463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piccinni P., Dan M., Barbacini S., et al. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006;32(1):80–86. doi: 10.1007/s00134-005-2815-x. [DOI] [PubMed] [Google Scholar]
- 42.Bellomo R., Tetta C., Ronco C. Coupled plasma filtration adsorption. Intensive Care Med. 2003;29(8):1222–1228. doi: 10.1007/s00134-003-1796-x. [DOI] [PubMed] [Google Scholar]
- 43.Cole L., Bellomo R., Hart G., Journois D., Davenport P., Tipping P., et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002;30(1):100–106. doi: 10.1097/00003246-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Daga Ruiz D., Fonseca San Miguel F., González de Molina F.J., et al. Plasmapheresis and other extracorporeal filtration techniques in critical patients. Med Intensiva. 2017;41(3):174–187. doi: 10.1016/j.medin.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Hardersen R., Enebakk T., Christiansen D., et al. Comparison of cytokine changes in three different lipoprotein apheresis systems in an ex vivo whole blood model. J Clin Apher. 2020;35(2):104–116. doi: 10.1002/jca.21765. [DOI] [PubMed] [Google Scholar]
- 46.Kronbichler A., Brezina B., Quintana L.F., Jayne D.R. Efficacy of plasma exchange and immunoadsorption in systemic lupus erythematosus and antiphospholipid syndrome: a systematic review. Autoimmun Rev. 2016;15(1):38–49. doi: 10.1016/j.autrev.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Sloan S.R., Andrzejewski C., Jr., Aqui N.A., Kiss J.E., Krause P.J., Park Y.A. Role of therapeutic apheresis in infectious and inflammatory diseases: current knowledge and unanswered questions. J Clin Apher. 2015;30(5):259–264. doi: 10.1002/jca.21370. [DOI] [PubMed] [Google Scholar]
- 48.Pai M., Moffat K.A., Plumhoff E., Hayward C.P.M. Critical values in the coagulation laboratory: results of a survey of the north American specialized coagulation laboratory association. Am J Clin Pathol. 2011;136(6):836–841. doi: 10.1309/AJCP8O8GIPPPNUSH. [DOI] [PubMed] [Google Scholar]
- 49.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meo S.A., Klonoff D.C., Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 51.Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369 doi: 10.1136/bmj.m1432. (m 1432) [DOI] [PubMed] [Google Scholar]
- 52.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. (S0049384820301201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thachil Jecko, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Ning, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. 2020; May 6 doi: 10.7326/M20-2003. (M20-2003) [DOI] [PubMed] [Google Scholar]
- 56.Menter T., Haslbauer J.D., Nienhold R., et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020; May 4 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hékimian G., Lebreton G., Bréchot N., et al. Severe pulmonary embolism in COVID-19 patients: a call for increased awareness. Crit Care. 2020;24:274. doi: 10.1186/s13054-020-02931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fraissé M., Logre E., Pajot O., et al. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24:275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ostermann M., Chang R.W. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35(8):1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 60.Xu Y.H., Dong J.H., An W.M., et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARSCoV- 2. J Infect. 2020;80:394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020; Feb 12:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ai T., Yang Z., Hou H., et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020; Feb 26:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyerowitz E.A., Vannier A.G.L., Friesen M.G.N., et al. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J. 2020;34(5):6027–6037. doi: 10.1096/fj.202000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guastalegname M., Vallone A. Could chloroquine /hydroxychloroquine be harmful in Coronavirus Disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020; Mar 24;71(15):888–889. doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yazdany J., Kim A.H.J. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 2020;172(11):754–755. doi: 10.7326/M20-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg E.S., Dufort E.M., Udo T., et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020; May 11:e208630. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020; Mar 29:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020; Apr 10:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020; Apr 6;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halstead S.B. Dengue antibody-dependent enhancement: Knowns and unknowns. Microbiol Spectr. 2014;2(6) doi: 10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 71.Lan L., Xu D., Ye G., et al. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020; Feb 27;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Puja Mehta, McAuley Daniel F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGonagle Dennis, et al. Why the immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia are distinct from macrophage activation syndrome with disseminated Intravascular coagulation. Lancet Rheum. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [Google Scholar]
- 74.Liu P.P., Blet A., Smyth D., Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 2020; Apr 15;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 75.Jankelson L., Karam G., Becker M.L., Chinitz L.A., Tsai M.C. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: A systematic review. Heart Rhythm. 2020; 11 doi: 10.1016/j.hrthm.2020.05.008. (S1547-5271(20)30431-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuck K.H. Arrhythmias and sudden cardiac death in the COVID-19 pandemic. Herz. 2020;45(4):325–326. doi: 10.1007/s00059-020-04924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giudicessi J.R., Roden D.M., Wilde A.A.M., Ackerman M.J. Genetic susceptibility for COVID-19-associated sudden cardiac death in African Americans. Heart Rhythm. 2020;S1547-5271(20) doi: 10.1016/j.hrthm.2020.04.045. (30419–7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saleh M., Gabriels J., Chang D., et al. The Effect of Chloroquine, Hydroxychloroquine and Azithromycin on the Corrected QT Interval in Patients with SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol. 2020; Apr 29;13(6):e008662. doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of baseline characteristics and outcome measures of TPE patients.
Flow diagram of consecutive intensive care unit (ICU) admissions and COVID-19 patients being enrolled to receive therapeutic plasma exchange.