Abstract
In an effort to implicate immune responses to specific Borrelia burgdorferi proteins that may have a role in chronic Lyme arthritis, we studied the natural history of the antibody response to B. burgdorferi in serial serum samples from 25 patients monitored throughout the course of Lyme disease. In these patients, the immunoglobulin G (IgM) and IgG antibody responses to 10 recombinant B. burgdorferi proteins, determined during early infection, early arthritis, and maximal arthritis, were correlated with the severity and duration of maximal arthritis. The earliest responses were usually to outer surface protein C (OspC), P35, P37, and P41; reactivity with OspE, OspF, P39, and P93 often developed weeks later; and months to years later, 64% of patients had responses to OspA and OspB. During early infection and early arthritis, the levels of IgG antibody to P35 correlated inversely with the subsequent severity or duration of maximal arthritis. In contrast, during periods of maximal arthritis, the levels of IgG antibody to OspA and OspB, especially to a C-terminal epitope of OspA, correlated directly with the severity and duration of arthritis. Thus, the higher the IgG antibody response to P35 earlier in the infection, the milder and briefer the subsequent arthritis, whereas during maximal arthritis, the higher the IgG response to OspA and OspB, the more severe and prolonged the arthritis.
Lyme disease, which is caused by the tick-borne spirochete Borrelia burgdorferi, usually begins with localized infection of the skin, erythema migrans, followed days to weeks later by dissemination of the spirochete to multiple sites, including joints (28, 40). Weeks to months later, brief attacks of arthritis or arthralgia, lasting days or weeks, often occur in a few large joints. Months later, more prolonged episodes of arthritis, lasting months, may develop, and about 10% of patients with arthritis have continuous joint inflammation for 1 year or longer, a condition which we have termed chronic Lyme arthritis (44). The synovial histology in these patients is similar to that seen in other forms of chronic inflammatory arthritis, including rheumatoid arthritis (22, 41).
B. burgdorferi contains at least 30 immunogenic proteins (2, 9, 11, 12, 27, 33, 39) with as many as 10 cell membrane or outer membrane proteins, including outer surface lipoproteins (Osp)A through -F (3–6, 19, 21, 25, 30, 31). The spirochete expresses different proteins at different times in its life cycle, and this may be critical in the spirochete’s homing to and survival in various tissues (1, 10, 14, 37). We have been interested in the expression of B. burgdorferi in the joints of patients with Lyme arthritis and in immune responses that influence this phase of the illness. In two previous studies, we used a unique set of serial serum samples from untreated patients monitored throughout the course of Lyme disease in the late 1970s prior to the use of antibiotic therapy for this illness (23, 24). Only with this set of serum samples is it possible to determine how the antibody responses to B. burgdorferi develop and change during the various stages of the illness.
In the initial study, 11 of the 15 patients (73%) monitored throughout the illness developed strong immunoglobulin G (IgG) responses to OspA and OspB near the beginning of prolonged episodes of arthritis, from 7 months to 5 years after disease onset (23). Moreover, the combination of the HLA-DR4 specificity and OspA or OspB reactivity was associated with chronic arthritis and lack of response to antibiotic therapy. However, other recombinant proteins were not yet available to test the specificity of these associations.
In the second study (24), OspA epitope mapping was done in 10 patients monitored throughout the illness. In these patients, an early IgM response was often found to epitopes throughout the protein. Of the 10 patients, 7 who developed arthritis of moderate or prolonged duration did not have IgG responses to OspA early in the illness, but they had strong responses to this protein near the beginning of prolonged arthritis. In contrast, two of the three patients who had only brief attacks of arthritis had weak IgG responses to OspA early in the illness and during periods of arthritis. Thus, patients who had difficulty with IgG isotype switching to OspA early in the illness seemed more likely to develop prolonged arthritis. However, the number of patients tested was too small for meaningful statistical comparisons.
Our goal in this study was to address two major questions raised by the previous studies. First, in an effort to compare initial responses according to the severity and duration of subsequent arthritis, we increased the sample size to include all 25 untreated patients in whom serial serum samples in our archival collection were available at appropriate time points. Second, to determine the specificity of the OspA and OspB associations with prolonged arthritis, we tested the serum samples for reactivity with 10 recombinant spirochetal proteins that are now available. The ultimate purpose of these studies was to implicate immune responses to specific B. burgdorferi proteins that may have a role in the pathogenesis of chronic Lyme arthritis.
MATERIALS AND METHODS
Patients.
During the late 1970s, all patients were monitored by one of us in the Lyme disease clinic at the Yale University School of Medicine. Clinical data were recorded in patients’ charts, and blood samples from each visit were stored at −70°C. At the time of this study, serial serum samples were available from 25 untreated patients during early infection when erythema migrans was present, during early episodes of arthritis or arthralgia, and during periods of maximal arthritis. All 25 patients met Centers for Disease Control and Prevention (CDC) criteria for the diagnosis of Lyme disease (7): they had erythema migrans followed by oligoarticular arthritis with positive IgG responses to B. burgdorferi as interpreted by the CDC/ASTPHLD criteria (8). Their ages ranged from 3 to 59 years (median, 32 years); 13 were male and 12 were female. Joint involvement in these individuals was representative of the range of severity and duration of Lyme arthritis (44).
Before determining antibody responses, we determined from patients’ charts the severity and duration of maximal arthritis. The period of maximal arthritis was defined as the most prolonged episode of continuous joint swelling. In all 25 patients, knees were the joints affected at that time. Therefore, the severity of arthritis was based on the volume of knee effusions, which had been estimated at each visit as follows: 1 to 10 ml, score of 1; 10 to 30 ml, score of 2; 30 to 50 ml, score of 3; and >50 ml, score of 4. In many instances, joint effusions were aspirated; therefore, the size of the effusion was known with certainty.
Recombinant B. burgdorferi antigens.
Full-length, unlipidated OspA (amino acids [aa] 16 to 273), the fragments OspA1 (aa 16 to 108), OspA2 (aa 105 to 201), and OspA3 (aa 168 to 273), and full-length, unlipidated OspB and OspC were generated as recombinant fusion proteins with Escherichia coli maltose binding protein (MBP) as previously described (23). The plasmid vectors pTRH44 and pTRH46, containing OspA and OspB from B. burgdorferi B31, respectively, were kindly provided by Alan Barbour (21), whereas the DNA used for the OspC construct was obtained from B. burgdorferi 297 (19). Restriction fragments containing these gene segments were inserted at the 3′ end of the E. coli malE gene, which encodes MBP. During the logarithmic growth phase of E. coli, protein production was induced, and the bacteria were lysed by passage through a French pressure cell. The supernatant was then passed over a cross-linked amylose column by using buffer containing maltose, and the MBP fusion proteins were eluted. Purified unlipidated OspE, OspF, P35, and P37 were generated at the Yale University School of Medicine by using similar techniques. E. coli carrying the appropriate plasmids encoding these B. burgdorferi proteins and the fusion partner glutathione transferase were lysed by sonication, and the lysates were passed over a glutathione column to elute the purified recombinant proteins (13, 25). P39, P41, and P93 were a kind gift from John M. Robinson, Abbott Laboratories, Abbott Park, Ill. (33).
ELISA.
The serum samples, which had been stored at −70°C, were tested by enzyme-linked immunosorbent assay (ELISA) for IgM and IgG antibodies to various recombinant B. burgdorferi antigens, using modifications of previously described methods (24). Ninety-six-well Immulon plates (Dynatech Inc., Kensington, Md.) were coated with each of the antigens at a concentration of 1 μg/well. These concentrations were shown to be in antigen excess by using checkerboard dilutions of each recombinant antigen and an appropriate strongly positive patient serum sample. After incubation overnight at 4°C, the plates were washed with 0.05% phosphate-buffered saline–Tween 20 and incubated with 5% nonfat dried milk in phosphate-buffered saline–Tween 20 (milk buffer) for 45 min at 37°C. After washing, 200 μl of patient serum samples (1:50 dilution, except 1:200 for P35 and P37) were plated in duplicate and incubated for 45 min at 37°C. After washing again, the plates were incubated with alkaline phosphatase-conjugated, goat anti-human IgG (1:750) or IgM (1:500) in milk buffer (Tago). The substrate was freshly prepared p-nitrophenyl phosphate. The plates were read at 405 nm when the lowest dilution of the positive control sample, which was included on each plate, reached 1.0. The cutoff for a positive value was defined as 3 standard deviations above the mean optic density of seven negative control samples which were also included on the same plate. The negative control samples were obtained from healthy individuals with no prior history of Lyme disease. Samples from the same patient were always tested together on the same plate.
Statistics.
For each of the three time points, the IgM or IgG absorbance value for each recombinant protein was correlated with the severity and duration of maximal arthritis, using the Spearman rank correlation test. The P values are two tailed. Because testing was done with 10 borrelial proteins, a P value of ≤0.005 rather than ≤0.05 (a Bonferroni correction) was considered statistically significant.
RESULTS
Natural history of the antibody responses to B. burgdorferi during the course of Lyme disease.
When erythema migrans was present (2 weeks to 3 months after disease onset), 60 to 80% of the 25 patients had IgM or IgG antibody responses to OspC, P35, P37, and P41; the most common early IgG response was to P35 (Table 1). The percentage of patients with reactivity with each of these proteins remained high during early, brief attacks of arthritis or arthralgia (2 to 12 months after disease onset) and during the most prolonged period of arthritis (7 months to 4.5 years after disease onset). Although many patients had IgM responses with OspE early in the illness, IgM reactivity with OspF, P39, or P93 was less common at that time. The number of patients with IgG responses to OspE, OspF, P39, and P93 increased at each subsequent time point. Only three to five patients (12 to 20%) had weak IgM or IgG reactivity with OspA or OspB early in the illness, and no additional patients developed responses to these proteins during early periods of arthritis or arthralgia. Instead, 16 of the 25 patients (60%), including all 5 with chronic arthritis, had IgG responses to OspA and OspB during periods of maximal arthritis. Thus, the only new responses that sometimes developed during the period of maximal arthritis were to OspA and OspB; reactivity with the other spirochetal proteins developed prior to that time.
TABLE 1.
Number of Lyme arthritis patients with positive responses to B. burgdorferi antigens
| Time of responsea | Antigen | No. (%) of patients with positive response
|
|||||
|---|---|---|---|---|---|---|---|
| IgM
|
IgG
|
||||||
| EMb | Early arthralgia/arthritis | Maximal arthritis | EM | Early arthralgia/arthritis | Maximal arthritis | ||
| Early | OspC | 20 (80) | 10 (40) | 4 (16) | 17 (68) | 21 (84) | 20 (80) |
| P35 | 8 (35) | 9 (36) | 4 (16) | 21 (84) | 20 (83) | 23 (92) | |
| P37 | 17 (68) | 12 (48) | 7 (28) | 14 (56) | 16 (67) | 20 (80) | |
| P41 | 17 (68) | 10 (40) | 6 (24) | 19 (76) | 19 (79) | 24 (96) | |
| Weeks later | OspE | 17 (74) | 13 (54) | 13 (48) | 1 (4) | 10 (40) | 18 (72) |
| OspF | 8 (35) | 11 (46) | 8 (35) | 6 (25) | 14 (58) | 20 (80) | |
| P39 | 12 (48) | 9 (36) | 6 (24) | 9 (36) | 18 (72) | 25 (100) | |
| P93 | 8 (32) | 7 (28) | 5 (20) | 4 (16) | 14 (56) | 22 (88) | |
| Months to years later | OspA | 4 (16) | 0 (0) | 8 (35) | 5 (20) | 3 (12) | 16 (64) |
| OspB | 3 (12) | 0 (0) | 7 (28) | 3 (12) | 6 (24) | 16 (64) | |
Based on the timing of the IgG responses of the majority of patients.
EM, erythema migrans.
The mean levels of IgM antibody to each of the spirochetal proteins tested, except for OspA and OspB, were highest early in the infection and declined at each subsequent time point (Fig. 1A). The mean IgG antibody levels to OspC, OspE, OspF, P39, P41, and P93 were low initially and increased at each subsequent time point; the mean IgG antibody responses to P35 and P37 remained at a moderate level at each time point, and the mean IgG antibody levels to OspA and OspB did not increase until the last time point during prolonged periods of arthritis (Fig. 1B). Reactivity was usually stronger with OspA than OspB. As noted previously (24), the responses to OspA were directed primarily against epitopes in the N-terminal (A1) and C-terminal (A3) thirds of OspA (data not shown).
FIG. 1.
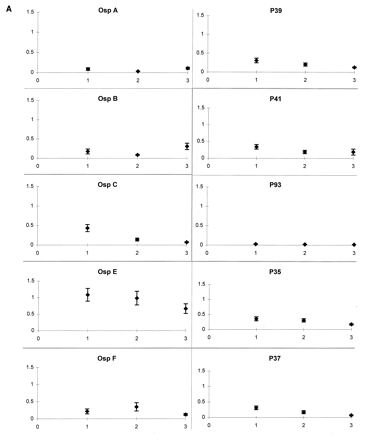
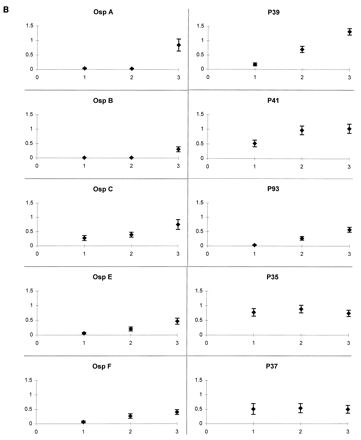
IgM (A) and IgG (B) responses to B. burgdorferi antigens over the course of Lyme disease. Data points representing the mean absorbance (optical density) ± the standard error of the mean of serum samples from all 25 patients are plotted at three time points: (i) when erythema migrans was present, 1 to 12 weeks from disease onset; (ii) during early, brief attacks of early arthritis or arthralgia, 2 to 12 months after disease onset; and (iii) during periods of maximal arthritis, 7 months to 4.5 years after disease onset. Except for OspA and OspB, the mean levels of IgM antibody were highest early in the infection and declined thereafter. The mean IgG antibody levels to OspC, OspE, OspF, P39, P41, and P93 were low initially and increased at each subsequent time point; the mean IgG antibody responses to P35 and P37 remained at a moderate level at each time point, and the mean levels of IgG antibody to OspA and OspB did not increase until the last time point, during periods of maximal arthritis.
Correlation of antibody responses with duration and severity of arthritis.
Early in the illness, the levels of IgG antibody to P35, the most common early response, correlated inversely with the subsequent duration of arthritis (r = −0.54, P = 0.005) (Table 2). Similarly, patients who had higher levels of antibody to OspC or P41 early in the illness tended to have milder and briefer arthritis. However, these trends were not significant at the 0.005 level, a Bonferroni correction for the 10 borrelial antigens tested in this analysis. Several weeks to months later during early, brief attacks of arthritis or arthralgia, IgG reactivity with P35 again correlated inversely with the amount of joint swelling during subsequent, prolonged attacks of arthritis (r = −0.67, P = 0.0004). In addition, during early arthritis, higher levels of antibody to OspE tended to correlate with less subsequent joint swelling. Thus, the greater the early responses to P35, and to a lesser degree to OspC, OspE, and P41, the milder and briefer the subsequent arthritis.
TABLE 2.
Correlation of the severity and duration of the most prolonged period of arthritis with IgG antibody responses to spirochetal proteins during early infection, early arthritis, and maximal arthritis
| Antigen | Antibody response during early infection correlated with maximal arthritis
|
Antibody response during early arthritis correlated with maximal arthritis
|
Antibody response during maximal arthritis correlated with maximal arthritis
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Swelling
|
Duration
|
Swelling
|
Duration
|
Swelling
|
Duration
|
|||||||
| Coefa | P valueb | Coef | P value | Coef | P value | Coef | P value | Coef | P value | Coef | P value | |
| OspA | −0.16 | 0.44 | −0.09 | 0.67 | 0.02 | 0.93 | 0.11 | 0.59 | 0.66 | 0.0003 | 0.48 | 0.01 |
| OspA1 | −0.08 | 0.72 | 0.08 | 0.7 | 0.05 | 0.81 | 0.16 | 0.44 | 0.56 | 0.005 | 0.45 | 0.02 |
| OspA2 | −0.16 | 0.45 | −0.04 | 0.83 | 0.04 | 0.85 | 0.03 | 0.88 | 0.01 | 0.97 | 0.19 | 0.37 |
| OspA3 | −0.17 | 0.43 | 0.06 | 0.78 | −0.12 | 0.56 | 0.04 | 0.83 | 0.63 | 0.0007 | 0.56 | 0.005 |
| OspB | −0.16 | 0.45 | 0.15 | 0.48 | −0.16 | 0.44 | 0 | 0.99 | 0.64 | 0.0005 | 0.48 | 0.02 |
| OspC | −0.43 | 0.03 | −0.30 | 0.15 | −0.20 | 0.34 | −0.21 | 0.32 | 0.25 | 0.22 | 0.19 | 0.37 |
| OspE | −0.19 | 0.37 | 0 | 0.98 | −0.53 | 0.009 | −0.24 | 0.27 | −0.30 | 0.15 | −0.07 | 0.75 |
| OspF | −0.13 | 0.54 | 0.29 | 0.16 | −0.31 | 0.16 | −0.04 | 0.84 | −0.30 | 0.15 | 0.04 | 0.86 |
| P35 | −0.24 | 0.24 | −0.54 | 0.005 | −0.67 | 0.0004 | −0.54 | 0.07 | −0.33 | 0.10 | −0.54 | 0.006 |
| P37 | −0.05 | 0.80 | −0.25 | 0.23 | −0.37 | 0.07 | −0.21 | 0.33 | −0.18 | 0.40 | −0.35 | 0.09 |
| P39 | −0.29 | 0.16 | −0.30 | 0.14 | −0.40 | 0.05 | −0.3 | 0.15 | −0.02 | 0.93 | 0.14 | 0.5 |
| P41 | −0.25 | 0.22 | −0.44 | 0.03 | −0.40 | 0.05 | −0.25 | 0.24 | 0.15 | 0.47 | 0.03 | 0.87 |
| P93 | −0.12 | 0.58 | −0.08 | 0.72 | −0.18 | 0.40 | −0.01 | 0.97 | 0.32 | 0.11 | 0.2 | 0.33 |
Coef, correlation coefficient.
P values of <0.005 (a Bonferroni correction for the 10 B. burgdorferi antigens tested) are shown in bold.
Months to years later, during periods of maximal arthritis, there was a strong direct correlation between the degree of joint swelling and the strength of the IgG responses to OspA (r = 0.66, P = 0.0003) and OspB (r = 0.64, P = 0.0005), and the levels of IgG antibody to the C-terminal fragment of OspA correlated directly with both the swelling (r = 0.63, P = 0.0007) and duration of arthritis (r = 0.56, P = 0.005) (Table 2). Thus, the higher the levels of antibody to OspA and OspB, especially to a C-terminal epitope of OspA, the more severe and prolonged the arthritis.
At each of the three time points, there were no statistically significant associations between the levels of IgM antibody to any spirochetal protein and the severity or duration of arthritis (data not shown).
Antibody responses in representative patients.
To further illustrate these correlations, Fig. 2 shows the antibody responses to OspA, OspB, and P35 for four representative patients. At the mild end of the spectrum, patient A had only one short attack of arthritis, and patient B had only short episodes of arthralgia. During erythema migrans or during early periods of arthritis or arthralgia, both of these patients had high levels of IgG antibody to P35. Neither patient had responses to OspA or OspB at any time in the illness. In contrast, patients C and D had chronic Lyme arthritis with severe and prolonged episodes of knee swelling lasting more than 1 year. Both had the HLA-DR4 specificity. Both patients had only moderate or low levels of IgG antibody to P35 throughout the illness, but both developed marked IgG responses to OspA and OspB more than 1 year after disease onset during periods of maximal arthritis with marked knee swelling. In both, the response was stronger to OspA than OspB.
FIG. 2.
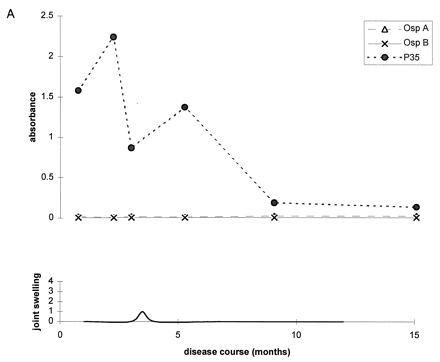
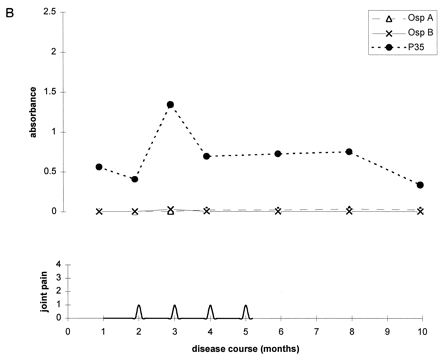
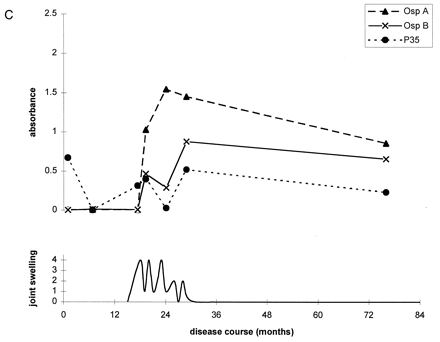
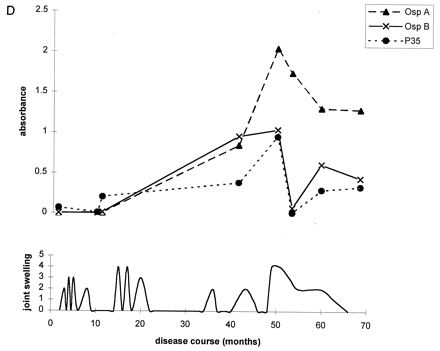
Antibody responses to OspA, OspB, and P35 in four representative patients at opposite ends of the spectrum of Lyme arthritis. (A) Patient with one short attack of arthritis; (B) patient with arthralgias of short duration; (C and D) patients with severe and prolonged chronic Lyme arthritis. Patients A and B had high levels of IgG antibody to P35 early in the infection or during early attacks of arthritis or arthralgia, and they had no responses to OspA or OspB at any time. In contrast, patients C and D had minimal or no IgG reactivity with P35 early in the infection, and they developed marked IgG responses to OspA and OspB during periods of maximal arthritis.
DISCUSSION
In this study of 25 untreated patients monitored longitudinally throughout the course of Lyme disease, common IgM and IgG responses early in the infection were to OspC, P35, P37, and P41. Within weeks, many patients also had responses to P39, P93, OspE, and, to a lesser extent, OspF. Reactivity with most of these proteins increased at each time point, suggesting that these proteins are often expressed by the spirochete throughout the infection. Although many patients had IgM reactivity with OspC early in the infection, the levels of IgG antibody to this protein remained low until periods of prolonged arthritis. Individual differences in the strength and timing of antibody responses may be due to differential expression of these proteins by the spirochete or to variations in the host immune response. Although strain variation among spirochetes may be a factor, the 25 study patients came from the Lyme, Conn., area, where this infection is thought to be caused by a single borrelial species, B. burgdorferi sensu stricto.
When patients were stratified according to the severity and duration of arthritis, higher levels of IgG antibody to P35 earlier in the infection correlated with milder and briefer arthritis. Based on cloning studies, P35 was reported to be a newly recognized spirochetal protein that is expressed only in vivo, not in culture (14). However, with the recent publication of the complete sequence of the B. burgdorferi genome (18), it became apparent that P35 contains the C-terminal 261 aa of a 354-aa protein encoded by a gene called bbk32. Most recently, Probert and Johnson reported that bbk32 encodes a differentially expressed, fibronectin binding protein (32). Thus, we would postulate that a marked antibody response to this fibronectin binding protein early in the illness reduces the number of spirochetes that reach or remain in the joints, resulting in milder arthritis of shorter duration.
In contrast with the responses to all other antigens, only a small percentage of patients had reactivity with OspA and OspB early in the infection or during early attacks of arthritis or arthralgia. Although the spirochete down-regulates expression of these two related proteins in the tick prior to transmission to the vertebrate host (17), we and other investigators have noted that patients with early infection or acute neuroborreliosis may have an ephemeral immune response to OspA, primarily of the IgM isotype (24, 35, 36), suggesting that some spirochetes may still express this protein early in the illness. However, in this study, this early response did not correlate with the severity or duration of subsequent arthritis. Instead, as we have shown previously (23, 24), IgG reactivity with these proteins developed in the majority of patients more than 1 year after disease onset in association with periods of maximal arthritis. Thus, in most patients, B. burgdorferi may not express OspA and OspB until late in the illness in joints.
This study confirms that the stronger the responses to OspA and OspB, particularly to the C-terminal epitope of OspA, the longer and more severe the arthritis. To explain this association, one must postulate either that the expression of OspA and OspB has survival value for the spirochete in the joint or that host immunity to one or both of these closely related proteins enhances the severity and duration of joint inflammation. It is surprising that expression of OspA and OspB would have survival value in joints since high levels of antibody to a C-terminal epitope of OspA kill spirochetes in the midgut of the tick (17), thereby protecting mice as well as human subjects from reinfection with B. burgdorferi (13, 34, 45). Perhaps spirochetes in joints express OspA only intermittently; OspA might be masked by other antigens, or it may undergo antigenic variation in the joint (16). In one patient, an OspA frameshift, identified from DNA in Lyme arthritis synovial fluid, resulted in an OspA that did not bind protective antibodies (15).
Alternately, autoreactive immune phenomena may be a factor in explaining severe and prolonged Lyme arthritis. The first clue to this possibility was the observation that a small percentage of patients have persistent Lyme arthritis for months or even several years after prolonged courses of antibiotic therapy (43). Although B. burgdorferi DNA can frequently be detected in the joint fluid of such patients prior to antibiotic therapy, it cannot usually be demonstrated there after treatment (29). Moreover, this outcome is associated with HLA-DR4 alleles (23, 42, 43) and with cellular as well as humoral immunity to OspA (23, 24, 26). We recently showed that in HLA-DRB1*0401-positive individuals, there is molecular mimicry between the dominant T-cell epitope of OspA and human leukocyte function-associated antigen (hLFA-1) (20). Both hLFA-1 and OspA induced T-cell reactivity in 9 of 11 patients tested with treatment-resistant Lyme arthritis but not in those with other forms of chronic inflammatory arthritis. A cross-reactive T-cell response to OspA and hLFA-1 would provide an amplification mechanism to explain more severe and prolonged arthritis in the natural infection, and it would also explain the persistence of joint inflammation after the apparent eradication of the spirochete from the joint in antibiotic-treated patients. We do not think that anti-OspA antibody is pathogenic itself; rather, we suspect that it is simply a marker for this critical T-cell response. We observed in this study a statistically significant association with the C-terminal fragment of OspA presumably because this is usually the dominant antibody epitope of OspA (24, 38). The similar but less significant association with OspB, which has 56% sequence homology with OspA (5), may result from shared antibody epitopes with OspA.
In summary, the higher the IgG antibody response to P35 earlier in the infection, the shorter the severity and duration of subsequent arthritis, whereas during the period of maximal arthritis, the higher the IgG response to OspA and OspB, especially to a C-terminal epitope of OspA, the more severe and prolonged the arthritis. These clinical correlations presumably result from both the differential expression of these proteins by the spirochete and genetically determined differences in the host immune response.
ACKNOWLEDGMENTS
We thank John M. Robinson for the recombinant P39, P41, and P93 proteins used in this study; Manchuan Chen for preparing P35, P37, OspE, and OspF, and Robin Ruthazer for assistance with the statistical analysis.
This study was supported by grant AR-20358 from the National Institutes of Health (A.C.S.), grant U5-CCU-106581 from the Centers for Disease Control and Prevention (R.A.F. and E.F.), and the Eshe Fund (A.C.S.). E.A. received support from the Lincoln National Foundation of Fort Wayne, Ind. E.F. is the recipient of the Wellcome Clinical Scientist Award in translational research.
REFERENCES
- 1.Akin D R, Bourell K W, Caimaro M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Burgdorfer W, Grunwaldt E, Steere A C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Investig. 1983;72:504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G, Tessier S L, Hayes S F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984;45:94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergstrom S, Bundoc V G, Barbour A G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 6.Brandt M E, Riley B S, Randolf J D, Norgard M V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. Case definitions for public health surveillance. Morbid Mortal Weekly Rep. 1990;39:19–21. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on the Serological Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 9.Craft J E, Fischer D K, Shimamoto G T, Steere A C. Antigens of Borrelia burgdorferi recognized during Lyme disease: appearance of a new IgM response and expansion of the IgG response late in the illness. J Clin Investig. 1986;78:934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S, Barthold S W, Giles S S, Montgomery R R, Telford S R, Fikrig E. Temporal pattern of Borrelia burgdorferi P21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 12.Fawcett P T, Rose C, Gibney K M, Chase C A, Kiehl B, Doughty R A. Detection of antibodies to the recombinant P39 protein of Borrelia burgdorferi using enzyme immunoassay and immunoblotting. J Rheum. 1993;20:734–738. [PubMed] [Google Scholar]
- 13.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 14.Fikrig E, Barthold S W, Sun W, Feng W, Telford III S R, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 15.Fikrig E, Liu B, Fu L L. An ospA frame shift, identified from DNA in Lyme arthritis synovial fluid, results in an outer surface protein A that does not bind protective antibodies. J Immunol. 1995;155:5700–5704. [PubMed] [Google Scholar]
- 16.Fikrig E, Tao H, Barthold S W, Flavell R A. Selection of variant Borrelia burgdorferi isolates from mice immunized with outer surface protein A or B. Infect Immun. 1995;63:1658–1662. doi: 10.1128/iai.63.5.1658-1662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fikrig E, Telford III S R, Barthold S W, Kantor F S, Spielman A, Flavell R A. Elimination of Borrelia burgdorferi from vector ticks feeding on Osp A-immunized mice. Proc Natl Acad Sci USA. 1992;89:5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleishmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 19.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross D W, Forsthuber T, Tary-Lehmann M, Etling C, Kouichi I, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 21.Howe T R, Mayer L W, Barbour A G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985;227:645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- 22.Johnston Y E, Duray P H, Steere A C, Kashgarian M, Buza J, Malawista S E, Askenase P W. Lyme arthritis: spirochetes found in synovial microangiopathic lesions. Am J Pathol. 1985;118:26–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Kalish R A, Leong J M, Steere A C. Association of treatment resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalish R A, Leong J M, Steere A C. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect Immun. 1995;63:2228–2235. doi: 10.1128/iai.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam T T, Nguyen T P, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengl-Janβen B, Strauss A F, Steere A C, Kamradt T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A (OspA) in patients with treatment-resistant or treatment-responsive Lyme arthritis. J Exp Med. 1994;180:2069–2078. doi: 10.1084/jem.180.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luft B J, Mudri S, Jiang W, Dattwyler R J, Gorevic P D, Fischer T, Munoz P, Dunn J J, Schubach W H. The 93-kilodalton protein of Borrelia burgdorferi: an immunodominant protoplasmic cylinder antigen. Infect Immun. 1992;60:4309–4321. doi: 10.1128/iai.60.10.4309-4321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadelman R B, Nowakowski J, Forseter G, Goldberg N S, Bittker S, Cooper D, Aguero-Rosenfeld M, Wormser G P. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med. 1996;100:502–508. doi: 10.1016/s0002-9343(95)99915-9. [DOI] [PubMed] [Google Scholar]
- 29.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid in Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 30.Norris S J, Carter C J, Howell J K, Barbour A G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padula S J, Dias F, Sampieri A, Craven R B, Ryan R W. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol. 1994;32:1733–1738. doi: 10.1128/jcm.32.7.1733-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Probert W S, Johnson B J B. Identification of a 47 kilodalton fibronectin binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1999;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 33.Robinson J M, Pilot-Matias T J, Pratt S D, Patel C B, Bevirt T S, Hunt J C. Analysis of the humoral response to the flagellin protein of Borrelia burgdorferi: cloning of regions capable of differentiating Lyme disease from syphilis. J Clin Microbiol. 1993;31:629–635. doi: 10.1128/jcm.31.3.629-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoen R T, Meurice F, Brunet C M, Cretella S, Krause D S, Craft J E, Fikrig E. Safety and immunogenicity of an outer surface protein A vaccine in subjects with previous Lyme disease. J Infect Dis. 1995;172:1324–1329. doi: 10.1093/infdis/172.5.1324. [DOI] [PubMed] [Google Scholar]
- 35.Schutzer S E, Coyle P K, Krupp L B, Deng Z, Belman A L, Dattwyler R, Luft B J. Simultaneous expression of Borrelia OspA and OspC and IgM response in cerebrospinal fluid in early neurologic Lyme disease. J Clin Investig. 1997;100:763–767. doi: 10.1172/JCI119589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schutzer S E, Coyle P K, Dunn J J, Luft B J, Brunner M. Early and specific antibody response to OspA in Lyme disease. J Clin Investig. 1994;94:454–457. doi: 10.1172/JCI117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sears J E, Fikrig E, Nakagawa T Y, Deponte K, Marcantonio N, Kantor F S, Flavell R A. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991;147:1995–2000. [PubMed] [Google Scholar]
- 39.Simpson W J, Schrumpf M E, Schwan T G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steere A C, Bartenhagen N H, Craft J E, Hutchinson G J, Newman J H, Rahn D W, Sigal L H, Spieler P H, Stenn K S, Malawista S E. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 41.Steere A C, Duray P H, Butcher E C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis: comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31:487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 42.Steere A C, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 43.Steere A C, Levin R E, Molloy P J, Kalish R A, Abraham III J H, Liu N Y, Schmid C H. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–888. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 44.Steere A C, Schoen R T, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 45.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination for Lyme disease with recombinant Borrelia burgdorferi outer-surface protein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]


