Abstract
Objectives
The use of systemic glucocorticoids (SGCs) is traditionally discouraged in the treatment of PsA and psoriasis due to the risk of psoriatic flares. However, despite this recommendation, SGCs are frequently prescribed for these patients. In this study we reappraise the old paradigm that SGCs are contra-indicated in the treatment of PsA and psoriasis.
Methods
A systematic search of MEDLINE, EMBASE and the Cochrane Library databases was performed in November 2019 to identify articles on any SGC use compared with no use in the PsA and psoriasis population. Topical glucocorticoid treatment was excluded. Our two primary outcomes focused on the prescribing characteristics and the occurrence of any type of flare.
Results
Our search yielded 4922 articles, and of these 21 full-text articles were eligible for inclusion. There were 11 retro- and prospective cohorts involving a total of 4,171,307 patients. Of these, 6727 (37.82%) of the patients with PsA and 1 460 793 (35.17%) of the patients with psoriasis were treated with any type of SGC. Ten observational/interventional studies did not report an increased risk or occurrence of psoriatic flares related to SGC use.
Conclusion
Our results indicate that SGCs are frequently prescribed for PsA and psoriasis patients. The occurrence of psoriatic flares appears to be low upon SGC exposure. In patients with a clear indication for SGCs, e.g. in need of rapid anti-inflammatory therapy or bridging of therapies, the use of SGCs should be considered in view of the low risk of skin flaring. It remains of importance to weigh risks for short- and long-term SGC-related side effects in clinical decision making.
Keywords: PsA, glucocorticoids, psoriasis, symptom flare-up, therapeutics, systematic review
Rheumatology key messages.
Systemic glucocorticoids are frequently prescribed for the treatment of psoriatic arthritis and psoriasis.
There is no solid evidence that systemic glucocorticoids increase the risk of psoriatic skin flaring.
Systemic glucocorticoids should not be withheld for the treatment of PsA/psoriasis patients when indicated.
Introduction
PsA is a heterogeneous, inflammatory autoimmune disease that is characterized by asymmetrical peripheral arthritis, dactylitis, enthesitis, SpA and psoriasis of the skin and nails [1, 2]. Approximately 20–30% of all psoriasis patients will eventually develop PsA [3]. Systemic treatment with DMARDs is essential in the management of PsA in order to prevent joint damage and erosions [4]. After initiation of a DMARD, it takes up to 3 months before treatment response can be observed in ∼40% of patients [5]. To bridge this initiation phase, systemic glucocorticoid (SGC) treatment can be given for rapid anti-inflammatory effects and to relieve pain [6]. This short-term complementary treatment has also been shown to improve long-term adherence and to improve drug survival in both psoriasis and PsA [7]. Furthermore, the addition of a SGC to current DMARD therapy gives better and faster clearance of psoriatic skin lesions and prolongs the drug-free remission period [8].
Despite these advantages, the use of SGCs for the treatment of PsA/psoriasis is traditionally discouraged by recent guidelines and textbooks due to the risks of psoriatic flares, yet they do not report evidence to support this recommendation [9–12]. Several guidelines refer to one and the same case series written in 1968, in which 19 of 104 patients developed generalized pustular psoriasis (GPP) after withdrawal of SGC therapy [13].
Several national health-care insurance databases have shown SGCs to be a frequently prescribed drug in the treatment of psoriasis in routine clinical practice. In Germany, the frequency of prescriptions for SGCs exceeds the amount prescribed for MTX, fumaric acid esters or biologics [14], and in the USA, SGC prescriptions are issued to psoriasis patients by 90% of dermatologists [15, 16]. This highlights the discrepancy between prescribing behaviour and current treatment guidelines.
Mrowietz and Domm [17] made an effort in 2012 to challenge the view of SGC use in psoriasis patients by highlighting the widespread use of these drugs without an observed increase in psoriatic flares. However, 10 years later, a shift in this old paradigm has not yet occurred. A systematic assessment of the evidence for the recommendation against the use of SGCs in psoriasis and PsA is lacking.
In this systematic review we aimed to: (1) address the general prevalence of SGC prescription in the PsA/psoriasis population, and (2) assess the risk and occurrence of psoriatic flares in PsA/psoriasis patients treated with SGCs.
Methods
Search strategy
A systematic patient/problem, intervention, comparison and outcome (PICO) search of MEDLINE, EMBASE and the Cochrane Library databases was performed in November 2019. The search strategy was constructed together with a medical librarian in order to identify any papers on SGC use compared with no use in the PsA/psoriasis population. Two primary outcomes of interest were: prevalence of SGC prescriptions and any type of psoriatic flare. We defined a flare as any type of reported exacerbation of the current psoriatic skin condition [e.g. using the psoriasis area and severity index (PASI), body surface area, clinical examination by a physician, or patients self-reporting that they had experienced a flare] or a morphological shift towards another phenotype (e.g. from psoriasis vulgaris towards erythrodermic psoriasis or psoriasis pustulosa). Search-terms used were PsA, Psoriasis, and Glucocorticoids combined with AND. Papers on topical treatment were excluded by using NOT as Boolean operator. A limit was set to English, Dutch and German language, and there was no time frame. All types of study design were considered, with the exception of case reports and case series. The PICO search strategy is presented in Supplementary Data S1, available at Rheumatology online.
Study eligibility criteria
Eligibility outcome (1): studies describing the prevalence of SGC prescriptions must contain a population of unselected PsA/psoriasis patients and must report on the usage of SGCs within this population. Eligibility outcome (2): studies describing the occurrence of flares in patients 18 years and older with PsA/psoriasis starting, using or tapering SGCs. All doses and administration routes were considered, with the exception of topical treatment regimens. Studies in which patients concomitantly used conventional synthetic DMARDs (csDMARDs) or biologic DMARDs (bDMARDs) were included. Finally, for inclusion, the article was required to include a report on any type of psoriatic flare according to the definitions described above. All flares in response to start, dose maintenance, or tapering of SGCs were deemed to be of interest.
For all the articles obtained from the search strategy, the titles and abstracts were screened according to the inclusion criteria. If there was uncertainty about the article fulfilling the criteria, it was included for full-text screening, and eligibility was discussed with J.v.L./P.W. until consensus was reached. The references listed in all the included articles were screened to check for missing papers of interest.
Data extraction and quality assessment
Three data extraction tables were created for each research question. Table 1 describes the general prevalence of SGC prescribing in the PsA/psoriasis population. Table 2 describes interventional studies reporting the risk of flares associated with SGC use in PsA/psoriasis patients compared with patients not using SGCs. Table 3 describes observational and interventional studies of PsA/psoriasis patients all using SGCs and the occurrence of flares in these populations. Publication types were clustered in order to give a clear distribution. Information on the aim of the study, number of patients, diagnosis, baseline demographics, SGC treatment regimen, and co-medication were extracted. Important outcomes were the number of patients who developed a psoriatic flare and a description of the flare according to the article.
Table 1.
General prevalence of SGC prescriptions in the PsA/psoriasis population
| Study |
Study characteristics |
Medication | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st author (year) | Publication type | Diagnosis | n | Follow-up duration | Gender (female, %) | Type of steroid | Route of administration | Prescribing physician | Description of SGC prescriptions | Other |
| Al-Dabagh 2014 | Retrospective cohort | Psoriasis | 32 375 | 1989–2010 | – | Prednisone, methylprednisolone, dexamethasone | PO, s.c. | 93% by dermatologist; remainder by primary care physician | SGCs were prescribed at 650 000 visits; (3.1% of 21 020 000 psoriasis visits) | In 50% of cases, SGCs were prescribed alone; in 45% of the cases topical regimens were prescribed. |
| Armstrong 2017 | Retrospective cohort | Psoriasis | 1 700 000 | 2007–2012 | – | Prednisone | PO | – | 11.2% patients received prednisone as a first-line treatment | The number of patients treated with SGCs decreased in later lines of therapy. |
| Augustin 2011 | Retrospective cohort | Psoriasis/PsA | 26 338 psoriasis; 2319 PsA | 2003–2007 | 42 | Betamethasone, cloprednol, cortisone, deflazacort, dexamethasone, fluocortolone, hydrocortisone, meprednisone, methylprednisolone, prednisolone, prednisone and triamcinolone | PO | 1191 prescriptions by general physician; 811 by internists; 259 by dermatologists | 8.15% of psoriasis patients and 5.39% of PsA patients |
|
| Dubreuil 2014 | Retrospective cohort | Psoriasis/PsA | 59 281 psoriasis; 4196 PsA | 1986–2010 | 51 psoriasis; 50 PsA | Glucocorticoid use (NOS) | PO | General physician | 4.3% of psoriasis patients and 8.2% of PsA patients | 25% of psoriasis and 35.2% of PsA patients received topical treatment |
| Eun 2017 | Retrospective cohort | Psoriasis | 2 321 194 | 2010–2014 | 38 | Methylprednisolone, prednisolone, dexamethasone, betamethasone, triamcinolone, other | PO, s.c. | General physician (93.9%), tertiary hospitals (2.2%), general hospitals (3.3%), small-sized hospitals (0.6%) | 26.4% of psoriasis patients got a SGC prescription. | |
| Grassi 1997 | Prospective cohort | PsA | 180 | 1990–1992 | 58 | Methylprednisolone, deflazacort, prednisone, betamethasone, dexamethasone, others | PO | – | 24.4% of PsA patients were taking SGCs. | 72.7% were simultaneously treated with a DMARD and 88.6% with a NSAID |
| Kavanaugh 2018 | Prospective cohort | Psoriasis/PsA | 7775 psoriasis; 1719 PsA; 4315 self-reported PsA | 2007–2015 | 43 psoriasis; 49 PsA | Glucocorticoid use (NOS) | – | – |
|
|
| Lee 2016 | Retrospective cohort | Psoriasis | 6072 | 2001–2011 | 46 | Glucocorticoid use (NOS) | – | – | 20.27% of all patients currently use SGCs; 9.47% have used SGCs in the past. |
|
| Madland 2005 | Retrospective cohort | PsA | 634 | 1999–2002 | 47 | Prednisolone | PO, IA | Outpatient clinics | 7.9% of PsA patients use oral SGCs; 40% of PsA patients had an IA injection. | 40% currently use a DMARD and 1.8% use a biologic. |
| Rice 2018 | Retrospective cohort | PsA | 3932 | 2006–2015 | – | Glucocorticoid use (NOS) | PO | Inpatient/outpatient clinics; general physician | All of the included PsA patients used SGCs. | 26.9% used SGCs for >60 days; the remainder used SGCs intermittently. |
| Sinnathurai 2018 | Prospective cohort | PsA | 490 | 2003–2015 | 59 | Prednisone, prednisolone | PO | – | 25.7% of PsA patients use SGCs. | 61% use MTX; 18.6% use LEF; 15.3% use SSZ; 4.1% use HCQ; 64.1% use a biologic. |
SGC: systemic glucocorticoid; NOS: not otherwise specified; PO: oral; –: not specified.
Table 2.
Papers directly comparing PsA and psoriasis patients using or not using SGCs in RCTs
| Study |
Baseline characteristics |
Treatment and assessment of psoriatic flare or morphological shift description |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st author (year) | Publication type | Aim of study | Dx | n | Age (Y, range) | SGC [dose, RoA] | Treatment duration | Co-medication | Reason for initiation of SGC | Skin flare or morphological shift during or after tapering of SGC | Percentage of flares |
| Carubbi 2016 | RCT | Comparing efficacy and safety between SGC and TNF IA treatment | PsA | 41 | 42.95 (31–68) | Triamcinolone (40 mg/month, IA), SGC (NOS) | 3 months | Stable dose of anti-TNF in combination with one or more DMARDS | Refractory arthritis | No adverse events were reported during the 52-wk follow-up | 0% |
| Gupta 2007 | Open-label-RCT | Comparing efficacy and safety of MTX + betamethasone or MTX only | Psoriasis | 40 |
|
betamethasone [3 mg/wk, PO] | Until complete clearance of lesions: 27.13 days (24.74–29.52) | 15 mg MTX PO | Psoriasis | No flares after discontinuation (91.78 days in remission) | 0% |
Dx: diagnosis; Y: year; RoA: route of administration; RCT: randomized controlled trial; SGC: systemic glucocorticoid; NOS: not otherwise specified; PO; oral.
Table 3.
Papers describing PsA and psoriasis patients all being treated with SGCs and the occurrence of flares in this population
| Study |
Baseline characteristics |
Treatment and assessment of psoriatic flare or morphological shift description |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st author (year) | Publication type | Aim of study | Dx | n | Age (Y, range) | SGC [dose, RoA] | Treatment duration | Co-medication | Reason for initiation of SGC | Skin flare or morphological shift during or after tapering of SGC | Occurrence of flares |
| Babino 2016 | Retrospective cohort | Efficacy and safety of combination therapy with ETN | PsA/Psoriasis | 37 | 59.43 (42–83) | Prednisone [25 mg/d, PO] | 7 weeks (4–10 weeks) | ETN 25 mg 2/wk or 50 mg 1/wk, MTX | Cutaneous and/or articular inefficacy of ETN monotherapy | Safety profile was assessed: no skin flares | 0% |
| Gregoire 2021 | Retrospective cohort | Assess amount of any type of psoriasis flare associated with SGC use | Psoriasis | 516 | 61.3 (SD 17.1) | Dexamethasone, fludrocortisone, hydrocortisone, methylprednisolone, prednisone [–, PO, injectable] | 18.2 weeks (SD 64.2) | MTX (30), CSA(12), adalimumab (7), ETN (5), infliximab (3), ustekinumab (2) | – | 16 flares: 15 mild plaque worsening, 1 erythrodermic | 1.42% (95% CI, 0.72%, 2.44%) |
| Ganeva 2007 | Prospective cohort | Assess adverse drug reactions of SGCd | Psoriasis | 1041 (6 psoriasis) | 48.9 (±18.9) | Methylprednisolone [–, PO], SGC NOS | Weeks – 4 years | NSAID; ACEi | Most frequently for autoimmune bullous dermatoses | None of the psoriatic exacerbation that led to hospitalization could be attributed to SGCs. | 0% |
| Brody 1966 | Single-arm trial | First study assessing efficacy and safety of triamcinolone treatment | Psoriasis | 23 | 39.8 | Triamcinolone [13 mg/2–3 weeks, i.m.] | Minimum 4 injections – maximum 50 injections over 3 years | Norethynodrel, chlordiazepoxide | Psoriasis | No flares or other adverse events were reported. | 0% |
| Cohen 1959 | Single-arm trial | Comparing efficacy and safety of different types of SGC IL and PO | Psoriasis | 25 | 44.92 (21–71) | Triamcinolone [16 mg/d]; methylprednisolone [20 mg/d]; prednisolone [30 mg/d]; hydrocortisone [PO, IL] | 4 months (1–7) | None | Psoriasis | No adverse events; up to 200 days in remission | 0% |
| Haroon 2018 | Single-arm trial | Comparing efficacy of i.m. triamcinolone on inflammatory back pain | Ax-PsA | 40 (15 PsA/15 AS/10 control) | 37.5 | Triamcinolone [80 mg once, IA] | Once | 60% of patients used DMARDs | Active PsA | No flares during follow-up period | 0% |
| Coates 2016 | RCT | Subanalyses of TICOPA trial assessing the occurrence of SGC-induced flares | PsA | 206 | 45.5 (36–55) | Methylprednisolone 40 mg [5–120 mg, IA]; 120 mg [40–160 mg, i.m.] | Single/multiple administration | 126 patients on DMARDs (90 MTX only, 30 combination) | Not specifically mentioned/inefficacy of current treatment | No adverse events reported, no significant change in PASI: patients did not report experiencing a flare. 10 patients had a PASI increase of ≥2 | 0% |
| Saviola 2007 | Open-label RCT | Comparing efficacy and safety of deflazacort/methylprednisolone | PsA/RA | 21 (7 PsA) | 60 (33–73) | Methylprednisolone [4 mg/d]; deflazacort [7.5 mg/d] [PO] | 1 year | MTX, CSA | Active PsA/RA | No flares during follow-up period | 0% |
Dx: diagnosis; Y: year; RoA: route of administration; SGC: systemic glucocorticoid; ETN: etanercept; PO; oral; NOS: not otherwise specified; ACEi: ACE-inhibitor; IL: intralesional; ax-PsA: axial psoriatic arthritis; RCT: randomized controlled trial; PASI: Psoriasis Area and Severity Index.
The methodological quality and risk of bias was assessed using the Agency for Healthcare Research and Quality (AHRQ) methodology checklist for cross-sectional and prevalence studies [18]. This manuscript was drafted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Pooling of data
It was not possible to pool the quantitative data due to large heterogeneity in terms of the type of SGC, administration route, dosage, treatment duration, and psoriatic flare definition.
Results
Study characteristics
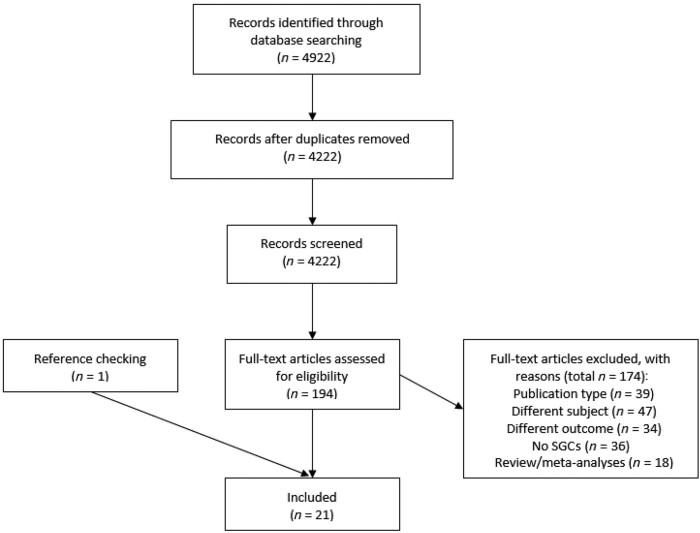
The systematic literature search resulted in a total of 4222 unique articles after duplicate removal. Of these, 194 full-text articles were screened for eligibility, after which 21 articles were found to fulfil the selection criteria Fig. 1.
Fig. 1.
Prisma flow diagram of included articles
SGCs: systemic glucocorticoids
In general, male:female distribution was equal. The type of SGC, treatment duration, dosage, indication for prescription, and use of co-medication was heterogeneous for all included papers. Methylprednisolone (5–160 mg/d, with oral or IA/i.m./intralesional administration) was prescribed most frequently, followed by triamcinolone (13–80 mg/d, with oral or IA/i.m./intralesional administration). Less frequently, patients were treated with prednisone, prednisolone, dexamethasone, fludrocortisone, hydrocortisone, betamethasone and deflazacort. (Tables 2 and 3 show details on all SGCs used.) Reporting of other possible risk factors that could contribute or lead to a psoriatic flare was lacking.
Assessment of SGC prescription prevalence for PsA and psoriasis patients
Eleven retro- and prospective cohort studies were included. Data were derived from National Health Care Insurance databases or online registries specifically designed to collect demographic data regarding PsA/psoriasis. The geographic origin of the data in the articles encompassed the USA, Germany, the UK, Korea, Australia, Norway, Italy and Taiwan. Sample sizes ranged between 180 and 2 321 194 patients, and the time period predominantly ranged from 2000 onwards. Two cohorts provided information from the period 1986–2010.
In summary, a total of 4 153 520 psoriasis and 17 787 PsA patients were analysed. A substantial proportion of patients have been treated with any type of SGC over the course of their disease: 1 460 793 psoriasis (35.17%) and 6727 PsA (37.83%) patients. Detailed study characteristics are shown in Table 1.
Detailed descriptions of PsA/psoriasis populations treated with SGCs
Al-Dabagh et. al reported that SGCs were prescribed during 650 000 (3.1%) of the 21 020 000 total psoriasis visits, which was comparable with MTX, prescribed at 3.5% of all visits. When psoriasis was the sole diagnosis and no other comorbidities were present, 50% of all these prescriptions were SGC monotherapy. No other systemic treatment was added for the prevention of skin flares. Of the SGC prescriptions, 93% were prescribed by dermatologists [15]. In Germany, SGCs were the most frequently prescribed systemic drug in psoriasis patients (2774 of 34 728 patients, 7.98%), followed by MTX (853 of 34 728 patients, 2.46%). When correcting for potential comorbidities, such as PsA or other steroid-requiring comorbidities, 64% of all these prescriptions were made for the diagnosis of psoriasis only [14]. In psoriasis patients naïve for either systemic drugs or biologics, prednisone was prescribed for 75% of 254 000 patients, predominantly by primary care physicians as the first line of treatment. This frequency gradually decreased in later lines of therapy, when DMARD or biologic therapy become more prominent [16]. In a Korean study, 612 248 of 2 321 194 psoriasis patients (26.4%) were treated with SGCs in outpatient clinics. Patients who visited their primary care physician were more likely to be treated with SGCs then patients who visited tertiary hospitals [odds ratio (OR) 11.5, 95% CI (11.26, 11.72)] [19].
A similar high frequency of SGC prescriptions was seen among PsA patients. In an international longitudinal registry, 566 of 1719 PsA patients (33%) reported using SGCs at the time of enrolment [20]. Twenty-five percent of (126 of 490) PsA patients reported using SGCs in a voluntary Australian registry [21]. In a cohort of 3932 PsA patients, 26.9% received continuous treatment with SGCs, while 73.1% received intermittent treatment with SGCs [22]. In Norway, the proportion of patients treated with oral SGCs was lower (49 of 634 PsA patients, 7.9%), while the administration of IA steroid injections remained high (247 of 634 PsA patients, 40%) [2, 23].
The risk of a SGC-induced flare: two RCTs comparing patients using SGCs with those not using SGCs
Studies in which randomization occurs have a higher level of evidence. Only two RCTs were found directly comparing PsA/psoriasis patients using and not using SGCs. Both studies show no increased risk for psoriatic flaring associated with SGC exposure. One study assessed the safety and efficacy of IA injections with triamcinolone 40 mg or a TNFα inhibitor in 41 PsA patients with mono-arthritis. Patients received a triamcinolone injection once a month for 3 consecutive months and were followed for 52 weeks to assess joint flaring. All patients used concomitant DMARD or biologic therapy, and 63.4% of patients used oral SGCs at the time of intervention. No flares were reported in either group during or after SGC treatment [24]. In order to achieve faster clearance of psoriatic lesions and prolong the remission period, Gupta et al. conducted an open-label RCT including 40 patients, where one arm received weekly doses of 15 mg MTX and 3 mg betamethasone orally and the other MTX only until complete clearance of psoriatic lesions. After clearance of the lesions, treatment was ceased, and remission was monitored every 4 weeks until lesions started to reappear or new lesions formed. However, no flares were reported. Combination therapy was significantly better for both outcomes [8]. Table 2 shows all of the details of both RCTs.
Observational and interventional studies describing flare occurrence in SGC-exposed PsA or psoriasis patients
Eight observational and interventional studies were used to explore the occurrence of SGC-related flares in PsA/psoriasis patients. Table 3 shows relevant details of the observational and interventional studies clustered by publication type. Two papers focused primarily on the research question: are patients exposed to SGC at greater risk of developing psoriatic flares. One recently published retrospective cohort included 516 patients using SGCs, with a median dose of 40 mg for a mean duration of 18.2 weeks. They identified a total of 16 psoriatic flares (1.42%) during or within 3 months of SGC exposure. Fifteen patients experienced mild worsening of plaque psoriasis, and one patient developed erythrodermic psoriasis. Six of these patients concomitantly took other medications known to induce psoriatic flares, (β-blockers, HCQ and quinacrine). The overall conclusion was that the frequency of flaring due to SGC exposure is low [25]. The other study involved a retrospective subanalysis of a RCT in which 206 PsA patients were allowed to receive IA/i.m. steroids as part of a tight control treatment regimen. A total of 161 episodes of SGC use in 101 patients were documented: 50 IA injections, with a median dose of 40 mg methylprednisolone and 111 i.m. injections with a median dose of 120 mg. A flare, defined as an increase in PASI score of ≥2, was seen in 10 patients. Overall, there was no significant PASI increase, and none of these patients self-reported experiencing an exacerbation of their skin symptoms during follow-up visits [26].
One retrospective cohort assessed the efficacy and safety of etanercept combination therapy: 4 out of 37 patients on etanercept were concomitantly treated with prednisone 25 mg/day for a mean duration of 7 weeks due to cutaneous inefficacy and/or articular inefficacy. PASI scores were monitored and no flares were reported [7]. In a prospective study aimed at identifying adverse drug reactions that led to hospitalization, none of the psoriasis vulgaris exacerbations could be attributed to SGC use [27]. In two single-arm trials, 23 and 25 psoriasis patients, respectively, received s.c. triamcinolone injections every 2–3 weeks until remission occurred or oral SGCs for an average of 4 months to determine the efficacy and safety. The longest treatment duration was 3 years. None of the participants experienced skin flaring during or after treatment [28, 29]. In an open-label controlled trial, 15 PsA patients with inflammatory axial involvement received a single dose of i.m. triamcinolone 80 mg to study improvement in inflammatory back pain. Sixty per cent of patients concomitantly used DMARDs, and no side effects were reported after a follow-up of 4 weeks [30]. In an open-label RCT study investigating the clinical efficacy and effects on bone metabolism of deflazacort or methylprednisolone, 7 PsA patients were enrolled. Patients were treated for 6 months with either deflazacort or methylprednisolone daily, after which cross-over took place to the other treatment arm. There were no flares reported during the follow-up period of the study [31].
The occurrence of psoriatic flares as reported over all studies ranged from 0 to 1.42%. This suggests that the risk of developing a flare after or during SGC exposure appears low.
Critical review of Ryan and Baker’s case series on SGC-induced generalized pustular psoriasis
Guidelines discouraging SGC use in PsA/psoriasis mainly refer to a case series published by Ryan and Baker in 1968, in which 104 patients who presented with generalized pustular psoriasis (GPP) were assessed [13]. Cases were collected from records of the authors’ hospital (n = 24) and via questionnaires filled in by 43 dermatologists (n = 80), posing a high classification bias, because no standardized dermatological diagnostic criteria for GPP existed. Furthermore, the correctness of the completed questionnaires depended on each physician’s memory regarding his or her own case notes. In 19 of 104 patients who developed GPP, the suspected trigger was SGC use, as the exacerbation developed within a few days to weeks after tapering or withdrawal of the SGC. Another 10 patients were treated with SGCs because of already rapidly deteriorating psoriasis, which eventually progressed to GPP. These cases might have developed into pustular psoriasis spontaneously, independent of SGC use. Six other patients had not received SGCs before developing pustules, indicating that SGCs might not have been the cause of flares. Other possible causes for the development of GPP described were pregnancy, hypocalcaemia, infection, or topical use of potent CSs, or they may have been idiopathic [13]. To sum up, the paper by Ryan and Baker has a high risk of bias, and the recommendation originating from this paper is based on poor evidence and should not have been reiterated over the years without critically appraising the origin.
Risk of bias
Two studies were of high quality, most were moderate, and four were of low quality. Typically, low-quality studies did not systematically report on their study protocol, nor did they elaborate on their attempt to control confounding. Moderate- to high-quality papers provided inclusion and exclusion criteria and the source of the data, elaborately reported potential confounding, and presented their primary outcomes clearly. One high-quality study applied blinding. A description of missing data was lacking in most articles. Assessment of the risk of bias of the studies is summarized in Table 4.
Table 4.
Risk of bias
| ARHQ Methodology Checklist | Al-Dabagh et al. 2014 | Armstrong et al. 2017 | Augustin et al. 2011 | Babino et al. 2016 | Brody, 1966 | Carubbi et al. 2016 | Coates, 2016 | Cohen, 1959 | Dubreuil et al. 2014 | Eun et al. 2017 | Ganeva et al. 2007 | Grassi, 1998 | Gregoire, 2021 | Gupta and Gupta, 2007 | Haroon et al. 2018 | Kavanaugh et al. 2018 | Lee et al. 2016 | Madland et al. 2005 | Rice et al. 2018 | Saviola et al. 2007 | Sinnathurai et al. 2018 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Define source of information (survey, record review). | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 2. List inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications. | + | + | + | + | – | + | + | – | + | + | + | – | + | + | + | + | + | + | + | + | + |
| 3. Indicate time period used for identifying patients. | + | – | + | + | – | – | + | – | + | + | + | + | + | – | – | + | + | + | + | – | + |
| 4. Indicate whether or not subjects were consecutive, if not population based. | + | – | + | + | – | + | + | – | + | + | + | – | + | – | + | + | + | + | + | – | + |
| 5. Indicate if evaluators of subjective components of study were masked to other aspects of the status of the participants. | NA | NA | NA | – | – | + | – | + | NA | NA | – | NA | – | – | – | NA | NA | NA | NA | – | NA |
| 6. Describe any assessments undertaken for quality assurance purposes (e.g. test/retest of primary outcome measurements). | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 7. Explain any patient exclusions from analysis. | + | – | + | + | – | + | – | – | + | + | + | – | + | + | – | – | + | – | + | + | – |
| 8. Describe how confounding was assessed and/or controlled. | + | – | + | + | – | + | + | – | + | + | + | – | + | – | + | – | + | – | – | – | – |
| 9. If applicable, explain how missing data were handled in the analysis. | NA | NA | NA | – | – | + | – | – | + | NA | – | – | – | – | + | – | – | – | NA | – | – |
| 10. Summarize patient response rates and completeness of data collection. | + | + | + | + | + | + | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 11. Clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained. | NA | NA | NA | NA | – | + | + | – | + | NA | – | NA | – | – | – | NA | NA | NA | NA | + | NA |
| Total score | 7 | 3 | 7 | 7 | 2 | 9 | 7 | 2 | 9 | 7 | 7 | 3 | 7 | 4 | 6 | 5 | 7 | 5 | 6 | 5 | 5 |
The quality of included articles was assessed using the Agency for Healthcare Research and Quality (AHRQ) methodology checklist for cross-sectional and prevalence studies. Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; high quality = 8–11. Yes = +; No = –; Not applicable/Not specified = NA.
Discussion
In this systematic review, we reappraised the old paradigm that the use of SGCs in PsA/psoriasis patients increases the occurrence of, or risk of developing, psoriatic flares. We found that SGCs are frequently used for the treatment of PsA/psoriasis in disregard of current treatment guidelines. Importantly, data describing the use of SGCs in this way mostly arise from relatively recent papers published between 2001 and 2015. By the extent of SGC usage, one would assume that the reported prevalence of SGC-related psoriatic flares would be much higher. Clinically, this is not the case. Evidence supporting advocation against the use of SGCs for psoriasis and PsA patients is mostly derived from case reports or case series [32–47]. In general, these publication types have a high risk of bias and provide low-quality evidence. Therefore, we feel that the original recommendation against the use of SGCs in this population is based on insufficient evidence.
After the publication of the case series by Ryan and Baker [13], the negative view of the use of SGCs in PsA/psoriasis patients was uncritically accepted. It is, however, important to mention that their paper has some important methodological limitations that influence the interpretation of the study results. The use of SGCs in psoriasis, and to some extent in PsA, is now traditionally discouraged and seen as malpractice. Several treatment guidelines for psoriasis and PsA directly refer to Ryan and Baker or to independent case reports/series as substantiation for the advice not to use SGCs [51–54]. Interestingly, the EULAR PsA treatment guidelines reiterate the recommendation that the use of SGCs might lead to psoriatic flaring, but mention that this recommendation is not substantiated by any evidence [12]. The GRAPPA PsA treatment guidelines indiscriminately highlight the risk of flaring without providing a direct source or critically reviewing this recommendation [9, 10]. It seems that, over the years, the recommendation to avoid SGCs for PsA/psoriasis patients has become generally accepted, and no effort has been made to critically reappraise this.
Only two papers report on flares associated with SGC use. In the paper of Coates et al. [26], in which a consensus definition of a psoriasis flare is lacking, a flare was defined as an increase in PASI of ≥2. Even though the PASI has reliable interobserver reproducibility [48, 49], it is not accurate for assessing mild psoriasis, and thus PASI might not be the best tool for monitoring flares [50]. Interestingly, all 10 patients with a PASI increase of ≥2 did not report experiencing a flare during follow-up visits. There seems to be a discordance between this clinical definition of a flare and the perception of the patients themselves. The other retrospective cohort specifically identified psoriasis patients exposed to SGCs and found that 1.42% experienced a mild worsening of their plaque psoriasis, while one patient developed erythrodermic psoriasis. An explanation for the low incidence of psoriatic flares could be that clinicians proactively take precautions to prevent flaring, for instance, by initiating combination therapy with topical or systemic DMARD therapy, and by tapering SGCs very gradually instead of by acute withdrawal.
SGCs are essential drugs that can rapidly reduce local or systemic inflammation in inflammatory diseases [55]. SGCs, whether given i.m., IA or orally, can be very beneficial in the early initiation phase of DMARD therapy in PsA or psoriasis to improve quality of life and reduce physical disability. Furthermore, SGCs have an anti-inflammatory effect by reducing pain, swelling and stiffness, and they induce immunosuppression that can ultimately prevent permanent joint damage [43, 56]. The combination of MTX and adjunctive SGCs has been shown to enhance faster psoriatic skin lesion clearance and to increase the drug-free remission period [8]. Drug survival is improved in etanercept-treated psoriasis and PsA patients who experience a loss of efficacy when temporarily co-treated with a SGC [7]. In addition to these benefits, it is generally known that SGCs also have the potential to cause adverse events, such as osteoporosis and -necrosis, infections, diabetes, cardiovascular disease, and suppression of the hypothalamic–pituitary–adrenal axis, especially when used for long-term treatment. However, when used thoughtfully, these adverse events are partially avoidable [57]. As SGCs pose multiple substantial beneficial effects, it would be undesirable to exclude them from the therapeutic armamentarium for PsA and psoriasis patients.
A limitation of this review is that the SGC prescription prevalence data is derived from insurance databases and that PsA/psoriasis patients were selected based on International Classification of Diseases codes. One cannot be certain that the SGCs prescribed at that time were solely meant for the treatment of PsA/psoriasis, or whether patients adhered to treatment. Even by filtering out patients with comorbid International Classification of Diseases codes that could explain SGC prescription (e.g. various rheumatologic conditions, urticaria, Crohn’s disease, COPD and asthma), there is no certainty for what indication the SGCs were really prescribed. Since the use of SGCs is traditionally discouraged for PsA/psoriasis, well-conducted RCTs are scarce, providing us with heterogeneous data in terms of SGC use, making it difficult to construct an evidence-based treatment recommendation. Finally, even though the search has been performed with a medical librarian, we cannot exclude the possibility that relevant articles have been missed.
This is the first systematic review questioning the old paradigm from a rheumatological and dermatological perspective. Prospective studies are needed to assess the real risk of flaring and to re-establish treatment guidelines discouraging SGC use. Considering how frequently SGC are being prescribed, the occurrence of psoriatic flares appears low and is only related to mild skin flaring, so we feel that SGC should not be withheld for the treatment of PsA/psoriasis patients when necessary. In patients with a clear indication for SGC use, e.g. those in need of rapid anti-inflammatory therapy or bridging of therapies, SGC should be considered in view of the low risk of skin flaring. It remains of importance to weigh the risks of short- and long-term SGC-related side effects in clinical decision making and possibly to treat patients in combination with a DMARD, biologic or topical treatment.
Supplementary Material
Acknowledgements
The reviewers are thanked for critically reading the manuscript and suggesting substantial improvements.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: D.M.W.B. serves as a consultant and/or speaker for AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Sanofi Genzyme, and UCB. J.M.v.L. has received honoraria from Abbvie, Boehringer Ingelheim, Celltrion, Galapagos, Magenta, Roche, and Sanofi Genzyme and research grants from Astra Zeneca, MSD, Roche, and Thermofisher.
The other authors have no conflict of interest to declare.
Contributor Information
Nanette L A Vincken, Department of Rheumatology & Clinical Immunology, University Medical Center Utrecht, Utrecht.
Deepak M W Balak, Department of Dermatology, LangeLand Ziekenhuis, Zoetermeer.
André C Knulst, Department of Dermatology & Allergology, University Medical Centre Utrecht, Utrecht, The Netherlands.
Paco M J Welsing, Department of Rheumatology & Clinical Immunology, University Medical Center Utrecht, Utrecht.
Jacob M van Laar, Department of Rheumatology & Clinical Immunology, University Medical Center Utrecht, Utrecht.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. Moll JM, Wright V.. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55–78. [DOI] [PubMed] [Google Scholar]
- 3. Mease PJ, Gladman DD, Papp KA. et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013;69:729–35. [DOI] [PubMed] [Google Scholar]
- 4. Haroon M, Gallagher P, FitzGerald O.. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015;74:1045–50. [DOI] [PubMed] [Google Scholar]
- 5. FitzGerald O, Ritchlin C.. Opportunities and challenges in the treatment of psoriatic arthritis. Best Pract Res Clin Rheumatol 2018;32:440–52. [DOI] [PubMed] [Google Scholar]
- 6. Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 7. Babino G, Giunta A, Ruzzetti M. et al. Combination therapy with etanercept in psoriasis: retrospective analysis of efficacy and safety outcomes from real-life practice. J Int Med Res 2016;44:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta R, Gupta S.. Methotrexate-betamethasone weekly oral pulse in psoriasis. J Dermatolog Treat 2007;18:291–4. [DOI] [PubMed] [Google Scholar]
- 9. Coates LC, Kavanaugh A, Mease PJ. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 10. Ritchlin CT, Kavanaugh A, Gladman DD. et al. ; Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Treatment recommendations for psoriatic arthritis. Ann Rheum Dis 2009;68:1387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gelfand JM, Armstrong AW, Bell S. et al. National Psoriasis Foundation COVID-19 Task Force Guidance for Management of Psoriatic Disease During the Pandemic: version 1. J Am Acad Dermatol 2020;83:1704–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gossec L, Smolen JS, Ramiro S. et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 13. Ryan TJ, Baker H.. Systemic corticosteroids and folic acid antagonists in the treatment of generalized pustular psoriasis. Evaluation and prognosis based on the study of 104 cases. Br J Dermatol 1969;81:134–45. [DOI] [PubMed] [Google Scholar]
- 14. Augustin M, Schafer I, Reich K, Glaeske G, Radtke M.. Systemic treatment with corticosteroids in psoriasis—health care provision far beyond the S3-guidelines. J Dtsch Dermatol Gesell 2011;9:833–8. [DOI] [PubMed] [Google Scholar]
- 15. Al-Dabagh A, Al-Dabagh R, Davis SA. et al. Systemic corticosteroids are frequently prescribed for psoriasis. J Cutan Med Surg 2014;18:195–9. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong AW, Koning JW, Rowse S. et al. Initiation, switching, and cessation of psoriasis treatments among patients with moderate to severe psoriasis in the United States. Clin Drug Investig 2017;37:493–501. [DOI] [PubMed] [Google Scholar]
- 17. Mrowietz U, Domm S.. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol 2013;27:1022–5. [DOI] [PubMed] [Google Scholar]
- 18. Rostom A, Dube C, Cranney A. et al. Celiac disease. Evid Rep Technol Assess (Summ) 2004;(104):1–6. [PMC free article] [PubMed] [Google Scholar]
- 19. Eun SJ, Jang S, Lee JY, Do YK, Jo SJ.. High rate of systemic corticosteroid prescription among outpatient visits for psoriasis: a population-based epidemiological study using the Korean National Health Insurance database. J Dermatol 2017;44:1027–32. [DOI] [PubMed] [Google Scholar]
- 20. Kavanaugh A, Papp K, Gottlieb AB. et al. Demography, baseline disease characteristics, and treatment history of psoriasis patients with self-reported psoriatic arthritis enrolled in the PSOLAR registry. BMC Rheumatol 2018;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinnathurai P, Buchbinder R, Hill C, Lassere M, March L.. Comorbidity in psoriatic arthritis and rheumatoid arthritis. Int Med J 2018;48:1360–8. [DOI] [PubMed] [Google Scholar]
- 22. Rice JB, White AG, Johnson M. et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin 2018;34:1519–27. [DOI] [PubMed] [Google Scholar]
- 23. Madland TM, Apalset EM, Johannessen AE, Rossebö B, Brun JG.. Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J Rheumatol 2005;32:1918–22. [PubMed] [Google Scholar]
- 24. Carubbi F, Zugaro L, Cipriani P. et al. Safety and efficacy of intra-articular anti-tumor necrosis factor α agents compared to corticosteroids in a treat-to-target strategy in patients with inflammatory arthritis and monoarthritis flare. Int J Immunopathol Pharmacol 2016;29:252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gregoire ARF, DeRuyter BK, Stratman EJ.. Psoriasis flares following systemic glucocorticoid exposure in patients with a history of psoriasis. JAMA Dermatol 2021;157:198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coates LC, Helliwell PS.. Psoriasis flare with corticosteroid use in psoriatic arthritis. Br J Dermatol 2016;174:219–21. [DOI] [PubMed] [Google Scholar]
- 27. Ganeva M, Gancheva T, Lazarova R, Tzvetanova Y, Hristakieva E.. A prospective study of adverse drug reactions in a dermatology department. Methods Find Exp Clin Pharmacol 2007;29:107–12. [DOI] [PubMed] [Google Scholar]
- 28. Brody SI. Parenteral triamcinolone in the systemic treatment of psoriasis. Mil Med 1966;131:619–26. [PubMed] [Google Scholar]
- 29. Cohen HJ, Baer RL.. Triamcinolone and methyl prednisolone in psoriasis. Comparison of their intralesional and systemic effects. J Invest Dermatol 1960;34:271–5. [DOI] [PubMed] [Google Scholar]
- 30. Haroon M, Ahmad M, Baig MN. et al. Inflammatory back pain in psoriatic arthritis is significantly more responsive to corticosteroids compared to back pain in ankylosing spondylitis: a prospective, open-labelled, controlled pilot study. Arthritis Res Ther 2018;20:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saviola G, Abdi. Ali L, Shams ES. et al. Compared clinical efficacy and bone metabolic effects of low-dose deflazacort and methyl prednisolone in male inflammatory arthropathies: a 12-month open randomized pilot study. Rheumatology 2007;46:994–8. [DOI] [PubMed] [Google Scholar]
- 32. Akoglu H, Dede F, Akoglu G, Gonul II, Odabas AR.. Membranoproliferative glomerulonephritis associated with psoriasis vulgaris. Ren Fail 2009;31:858–61. [DOI] [PubMed] [Google Scholar]
- 33. Annapureddy N, Agarwal SK, Ammakkanavar N. et al. Tumor necrosis factor inhibitor-induced serositis. Am J Ther 2014;21:e166–e8. [DOI] [PubMed] [Google Scholar]
- 34. Arcilla J, Joe D, Kim J. et al. Erythrodermic psoriasis treated with apremilast. Dermatol Rep 2016;8:6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonciani D, Bonciolini V, Antiga E. et al. A case of rupioid psoriasis exacerbated by systemic glucocorticosteroids. Int J Dermatol 2015;54:e100–2. [DOI] [PubMed] [Google Scholar]
- 36. Brenner M, Molin S, Ruebsam K. et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol 2009;161:964–6. [DOI] [PubMed] [Google Scholar]
- 37. Brodell RT, Williams L.. A corticosteroid-induced flare of psoriasis: how to control or, better yet, avoid. Postgraduate Med 1999;106:31–2. [DOI] [PubMed] [Google Scholar]
- 38. Edmonds EV, Morris SD, Short K, Bewley SJ, Eady RA.. Pustular psoriasis of pregnancy treated with ciclosporin and high-dose prednisolone. Clin Exp Dermatol 2005;30:709–10. [DOI] [PubMed] [Google Scholar]
- 39. Elston GE, Charles-Holmes R, Carr RA.. Precipitation of generalized pustular psoriasis by prednisolone. Clin Exp Dermatol 2006;31:133–4. [DOI] [PubMed] [Google Scholar]
- 40. Flynn A, Burke N, Byrne B. et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstetr Med 2016;9:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujimoto N, Matsuo S, Satoh T.. Psoriasis vulgaris in a patient with pemphigus vulgaris on corticosteroid therapy. J Dtsch Dermatol Ges 2018;16:606–8. [DOI] [PubMed] [Google Scholar]
- 42. Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Ind J Dermatol Venereol Leprol 2009;75:638. [DOI] [PubMed] [Google Scholar]
- 43. Mahajan VK, Sharma AL, Chauhan PS, Mehta KS, Sharma NL.. Early treatment with addition of low dose prednisolone to methotrexate improves therapeutic outcome in severe psoriatic arthritis. Indian J Dermatol 2013;58:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Westphal DC, De Souza PP, Chirano CA. et al. Generalized pustular psoriasis induced by systemic steroid dose reduction. Anais Brasileiros de Dermatologia 2016;91:664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boyd AS, Menter A.. Erythrodermic psoriasis. Precipitating factors, course, and prognosis in 50 patients. J Am Acad Dermatol 1989;21: 985–91. [PubMed] [Google Scholar]
- 46. Heinrich M, Cook E, Roach J. et al. Erythrodermic psoriasis secondary to systemic corticosteroids. Proc (Bayl Univ Med Cent) 2020;33:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choon SE, Lai NM, Mohammad NA. et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: ANALYSIS of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol 2014;53:676–84. [DOI] [PubMed] [Google Scholar]
- 48. Faria JR, Aarao AR, Jimenez LM, Silva OH, Avelleira JC.. Inter-rater concordance study of the PASI (Psoriasis Area and Severity Index). An Bras Dermatol 2010;85:625–9. [DOI] [PubMed] [Google Scholar]
- 49. Cabrera S, Chinniah N, Lock N, Cains GD, Woods J.. Inter-observer reliability of the PASI in a clinical setting. Australas J Dermatol 2015;56:100–2. [DOI] [PubMed] [Google Scholar]
- 50. Elmets CA, Korman NJ, Prater EF. et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol 2021;84:432–70. [DOI] [PubMed] [Google Scholar]
- 51. Ash Z, Gaujoux-Viala C, Gossec L. et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. [DOI] [PubMed] [Google Scholar]
- 52. Nas K, Kilic E, Cevik R. et al. Management of psoriatic arthritis: Turkish League Against Rheumatism (TLAR) expert opinions. Arch Rheumatol 2018;33:108–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurizky PS, Ferreira C de C, Nogueira LS, Mota LM.. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol 2015;90:367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nash P, Clegg DO.. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Ann Rheum Dis 2005;64(Suppl 2):ii74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hardy RS, Raza K, Cooper MS.. Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat Rev Rheumatol 2020;16:133–44. [DOI] [PubMed] [Google Scholar]
- 56. Hoes JN, Jacobs JW, Buttgereit F, Bijlsma JW.. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat Rev Rheumatol 2010;6:693–702. [DOI] [PubMed] [Google Scholar]
- 57. van der Goes MC, Jacobs JW, Bijlsma JW.. The value of glucocorticoid co-therapy in different rheumatic diseases—positive and adverse effects. Arthritis Res Ther 2014;16(Suppl 2):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.