Abstract
Background
Equations for estimated glomerular filtration rate (eGFR) based on serum creatinine include terms for sex/gender. For transgender and gender-diverse (TGD) youth, gender-affirming hormone (GAH) treatment may affect serum creatinine and in turn eGFR.
Methods
TGD youth were recruited for this prospective, longitudinal, observational study prior to starting GAH treatment. Data collected as part of routine clinical care were abstracted from the medical record.
Results
For participants designated male at birth (DMAB, N = 92), serum creatinine decreased within 6 months of estradiol treatment (mean ± SD 0.83 ± 0.12 mg/dL to 0.76 ± 0.12 mg/dL, p < 0.001); for participants designated female at birth (DFAB, n = 194), serum creatinine increased within 6 months of testosterone treatment (0.68 ± 0.10 mg/dL to 0.79 ± 0.11 mg/dL, p < 0.001). Participants DFAB treated with testosterone had serum creatinine similar to that of participants DMAB at baseline, whereas even after estradiol treatment, serum creatinine in participants DMAB remained higher than that of participants DFAB at baseline. Compared to reference groups drawn from the National Health and Nutritional Examination Survey, serum creatinine after 12 months of GAH was more similar when compared by gender identity than by designated sex.
Conclusion
GAH treatment leads to changes in serum creatinine within 6 months of treatment. Clinicians should consider a patient’s hormonal exposure when estimating kidney function via eGFR and use other methods to estimate GFR if eGFR based on serum creatinine is concerning.
Keywords: Sex steroids, Transgender, Glomerular filtration rate, Serum creatinine, Sex, Gender
Introduction
Gender-affirming care for transgender and gender-diverse (TGD) youth may include treatment with gender-affirming hormones (GAH) [1]. Gender-affirming care has been associated with decreased lifetime suicidal ideation and favorable mental health outcomes [2–4]. In addition to inducing secondary sex characteristics concordant with the individual’s gender identity, GAH can alter multiple laboratory and anthropometric features including hemoglobin, cholesterol, and body composition [5–10]. Understanding these changes, especially when they affect clinical decision making, is important for the clinical care of TGD youth.
One example of a significant clinical laboratory measurement that may change during GAH treatment is serum creatinine and its use in estimating glomerular filtration rate (GFR). Assessment of GFR is an important clinical tool for screening and diagnosis of kidney disease and injury, dosing medications appropriately, assessing safety of radiological contrast, and assessing need for dialysis and eligibility for kidney transplantation. Given the challenges and cost of measurement of glomerular filtration rate via an ideal filtration marker such as inulin, clinicians commonly use equations for estimated GFR (eGFR) using serum creatinine [11]. However, serum creatinine is influenced by multiple factors in addition to GFR, including muscle mass and diet; these factors contribute to differences in serum creatinine between men and women, among racial and ethnic groups, and across age [12, 13].
Commonly used equations for eGFR have attempted to compensate for these differences in serum creatinine by adjusting for race and sex/gender using empirically derived coefficients [14]. These race and sex/gender-based coefficients act as surrogates for multiple other factors that impact GFR including measurable factors (e.g., sex-steroid concentrations, body size, and muscle mass) and factors that are more difficult to measure (e.g., genetic factors and diet) [15]. The underlying factors captured by the race and sex/gender coefficients in eGFR equations and precisely how these factors impact GFR are incompletely understood.
The appropriateness of incorporating race in eGFR equations has recently been called into question [16, 17]. The use of race as an imprecise proxy for physiological differences due to genetic ancestry can contribute to health disparities by introducing other social and external factors associated with race such as differences in access to medical care, minority stress, and socioeconomic status [15, 18].
Similarly, because adjustment for sex/gender in eGFR equations may capture multiple factors including sex-chromosome composition, sex-specific hormones, as well as societal and environmental factors, this adjustment, likewise, may not accurately reflect these factors for all patients. For TGD patients in particular, clinicians have encountered difficulty determining which sex/gender-based equation is most appropriate [19]. For example, Whitley et al. reported a case of a transgender man with chronic kidney disease who experienced delays in both transplant eligibility and dialysis initiation because of confusion surrounding whether to use the creatinine-based eGFR equation for men or for women [20]. Additional knowledge regarding the impact of GAH on serum creatinine will aid in clinical decision making for TGD individuals.
Here, we report the changes in serum creatinine during GAH treatment in a cohort of TGD youth and compare these changes to a comparison group of adolescents drawn from the National Health and Nutrition Examination Survey (NHANES). In addition, we report the impact of these changes on eGFR as determined by equations without race adjustment: the Chronic Kidney Disease Epidemiology Collaboration 2021 (CKD-EPI 2021) and the Chronic Kidney Disease in Children Under 25 (CKiDU25) equations [21].
Methods
Participants were recruited prior to initiating GAH treatment at one of four study sites (e.g., Boston Children’s Hospital, Benioff Children’s Hospital, Lurie Children’s Hospital, and Children’s Hospital Los Angeles) as part of the Trans Youth Care—United States study. A full description of the study protocol has been published for reference [22]. Informed consent was obtained from all participants prior to study entry. The research protocol was approved by the Institutional Review Boards at all study sites. Laboratory, medication, and anthropometric data were collected as part of routine clinical care and abstracted from the medical record. Creatinine measurements were performed at the clinical laboratories of study sites and at outside facilities.
In order to take into account age-related changes in creatinine measurements, standardized serum creatinine levels (SCr/Q) were calculated by dividing serum creatinine levels by Q values calculated as follows [23]:
In addition, serum creatinine of study participants was compared to creatinine measurements performed as part of NHANES 2017–2018 [24]. NHANES creatinine measurements from participants between the ages of 12 and 22 years who were not pregnant at the time of measurement were used as reference data to generate an algorithm to calculate Z-scores by age and sex.
Estimates of GFR for study participants were made via 1) the CKiDU25 equation [21]:
where height is in units of meters, serum creatinine (SCr) is in units of mg/dL, and k is calculated as follows:
For males:
For females:
and 2) the CKD-EPI 2021 equation [17]:
in which age is in units of years, κ = 0.7 for the female equation and 0.9 for the male equation, α = −0.302 for the male equation and −0.241 for the female equation. Additional eGFR calculations utilizing the bedside Schwartz, FAS, and EKFC equations are available in supplementary materials (Supplemental Table 1) [23, 25, 26]. Longitudinal analysis was completed via mixed-effects modeling. All analyses were performed using race and age as covariates. A p value <0.05 was considered significant. Stata Statistical Software: Release 16 (College Station, TX) was used for analyses.
Results
The analysis included 286 participants, 92 (32%) designated male at birth (DMAB) and 194 (68%) designated female at birth (DFAB) (Supplemental Fig. 1, Table 1). The majority of participants identified as white (64% of participants DMAB and 57% of participants DFAB).
Table 1.
Baseline Characteristics of Participants
| Designated male at birth (n = 92) | Designated female at birth (n = 194) | |
|---|---|---|
| Age, median (interquartile range), y* | 17.3 (16.2, 18.6) | 16.2 (15.1, 17.5) |
| Affirmed Gender, n (%)* | ||
| Male | 0 (0%) | 78 (40%) |
| Female | 36 (39%) | 0 (0%) |
| Transgender Female (male-to-female) | 51 (55%) | 0 (0%) |
| Transgender Male (female-to-male) | 0 (0%) | 103 (53%) |
| Gender Fluid | 0 (0%) | 2 (1%) |
| Gender Queer | 1 (1%) | 1 (0.5%) |
| Non-binary | 4 (4%) | 10 (5%) |
| Race/Ethnicity, n (%) | ||
| White | 59 (64%) | 111 (57%) |
| Black or African American | 2 (2%) | 5 (3%) |
| Multi-Race | 11 (12%) | 20 (10%) |
| Asian, Native Hawaiian, or Pacific Islander | 2 (2%) | 8 (4%) |
| Hispanic / Latino, Non-White | 16 (16%) | 31 (16%) |
| Hispanic / Latino, White | 2 (2%) | 19 (10%) |
| Other | 1 (1%) | 0 (0%) |
| Tanner Stage at Baseline Visit, n (%) * | ||
| III | 3 (4%) | 1 (0.5%) |
| IV | 10 (12%) | 17 (9%) |
| V | 73 (85%) | 164 (90%) |
| Height at Baseline Visit, mean (SD), cm* | 173.4 (7.6) | 163.1 (6.7) |
| Weight at Baseline Visit, median (interquartile range), kg* | 67.2 (59.4, 80.5) | 61.9 (54.7, 73.2) |
| Body Mass Index at Baseline Visit, median (interquartile range), kg/m2* | 22.2 (20.1, 25.8) | 23.5 (20.6, 28.1) |
| Estrogen Type, n (%)** | ||
| Oral | 77 (84%) | - |
| Transdermal | 12 (13%) | - |
| Intramuscular | 3 (3%) | - |
| Estradiol Dose, median (interquartile range)*** | ||
| Oral administration, mg/day | 4 (2–4) | |
| Transdermal administration, mg/day | 0.5 (0.25–0.1) | |
| Intramuscular, mg/week | 15 (10–15) | |
| Testosterone Type, n (%)** | ||
| Subcutaneous | - | 189 (97%) |
| Transdermal gel | - | 5 (3%) |
| Testosterone Dose, median (interquartile range)*** | ||
| Subcutaneous administration, mg/week | 40 (26–50) | |
| Transdermal administration, mg/day | 40.5 (25–50) | |
| Using spironolactone, n (%) * | 58 (63%) | 1 (0.5%) |
p < 0.05 for difference between designated sex
Anticipated formulation at time of baseline visit
Calculated over 24-month study period
Serum creatinine measurements during GAH in TGD youth
Both participants DMAB and participants DFAB had significant changes in serum creatinine by 6 months of GAH treatment. Participants DMAB treated with estradiol had a decrease in serum creatinine of 0.07 ± 0.14 mg/dL over 6 months of treatment, from 0.83 ± 0.12 mg/dL to 0.76 ± 0.12 mg/dL, p <0.001 (Table 2 and Fig. 1). There were no further changes in creatinine in participants DMAB following the 6-month visit. Participants DFAB treated with testosterone had an initial increase in serum creatinine of 0.11 ± 0.1 mg/dL over the first 6 months of treatment (from 0.68 ± 0.10 mg/dL to 0.79 ± 0.11 mg/dL, p < 0.001) and an additional increase between 6 and 12 months, for a total increase of 0.14 ± 0.11 mg/dL over the first year (from 0.79 ± 0.11 mg/dL to 0.82 ± 0.12 mg/dL, p = 0.03). There were no additional increases in serum creatinine beyond the first year of treatment.
Table 2.
Creatinine and change in estimated GFR during gender-affirming hormone treatment
| Baseline | 6 months | 12 months | 18 months | 24 months | |
|---|---|---|---|---|---|
| Designated male at birth treated with estradiol (n = 92) | |||||
| n | 75 | 54 | 51 | 35 | 29 |
| Serum Creatinine, mean ± SD, mg/dL | 0.83 ± 0.12 | 0.76 ± 0.12* | 0.74 ± 0.11 | 0.75 ± 0.11 | 0.75 ± 0.12 |
| Male SCr/Q, mean ± SD | 1.04 ± 0.15 | 0.93 ± 0.15* | 0.90 ± 0.14 | 0.90 ± 0.14 | 0.89 ± 0.13 |
| Female SCr/Q, mean ± SD | 1.24 ± 0.17 | 1.12 ± 0.18* | 1.09 ± 0.16 | 1.10 ± 0.16 | 1.10 ± 0.16 |
| Baseline | Change from baseline | Change from 6 months | Change from 12 months | Change from 18 months | |
| Male CKiDU25, median (IQR), mL/min/1.73 m2 | 104.3 (93.9, 113.5) | 10.3 (2.2,22.9) | 2.3 (−6.3, 9.1) | 0.7 (−6.8, 11.8) | 3.9 (−12.3,8.7) |
| Female CKiDU25, median (IQR), mL/min/1.73 m2 | 85.5 (77.7,93.9) | 7.6 (1.8,18.0) | 1.9 (−7.0, 7.4) | 0.3 (−5.6,9.6) | 1.1 (−10.0,7.1) |
| Male CKD-EPI 2021, median (IQR), mL/min/1.73 m2 | 131.2 (126.4,134.1) | 2.7 (−0.4,6.9) | −0.1 (−2.8,2.4) | −0.4 (−2.5,3.4) | −0.4 (−3.9,2.4) |
| Female CKD-EPI 2021, median (IQR), mL/min/1.73 m2 | 107.2 (94.9,117.3) | 6.6 (−0.3,12.6) | −0.1 (−4.3,5.8) | −0.4 (−3.3,4.4) | −0.3 (−5.1,5.3) |
| Designated female at birth treated with testosterone (n = 194) | |||||
| n | 150 | 116 | 93 | 77 | 83 |
| Serum Creatinine, mean ± SD, mg/dL | 0.68 ± 0.10 | 0.79 ± 0.11* | 0.82 ± 0.12† | 0.81 ± 0.12 | 0.82 ± 0.14 |
| Male SCr/Q, mean ± SD | 0.88 ± 0.14 | 1.00 ± 0.16* | 1.03 ± 0.14 | 0.99 ± 0.15 | 1.00 ± 0.17 |
| Female SCr/Q, mean ± SD | 1.04 ± 0.16 | 1.20 ± 0.18* | 1.24 ± 0.16 | 1.20 ± 0.18 | 1.21 ± 0.20 |
| Baseline | Change from baseline | Change from 6 months | Change from 12 months | Change from 18 months | |
| Male CKiDU25, median (IQR), mL/min/1.73 m2 | 113.5 (102.8,126.3) | −13.5 (−22.4,−7.2) | −2.2 (−7.3,4.8) | 2.9 (−5.1,8.5) | 0.2 (−8.2,8.1) |
| Female CKiDU25, median (IQR), mL/min/1.73 m2 | 96.0 (87.0,106.9) | −12.4 (−19.7,−6.5) | −2.4 (−6.1,3.4) | 1.9 (−5.2,6.5) | −1.4 (−6.7,6.2) |
| Male CKD-EPI 2021, median (IQR), mL/min/1.73 m2 | 139.8 (135.1,144.8) | −7.1 (−10.3,−3.8) | −2.0 (−6.0,0.8) | 0.1 (−4.3,3.9) | −1.9 (−5.9,2.5) |
| Female CKD-EPI 2021, median (IQR), mL/min/1.73 m2 | 130.9 (121.1,134.7) | −12.2 (−22.9,−5.4) | −3.3 (−9.9,2.9) | 1.2 (−6.6,8.3) | −3.5 (−9.5,6.7) |
p< 0.05 for change from baseline measurement
p <0.05 for change from 6-month measurement
p values were calculated utilizing a mixed-effects model with age and race as covariates
Fig. 1.
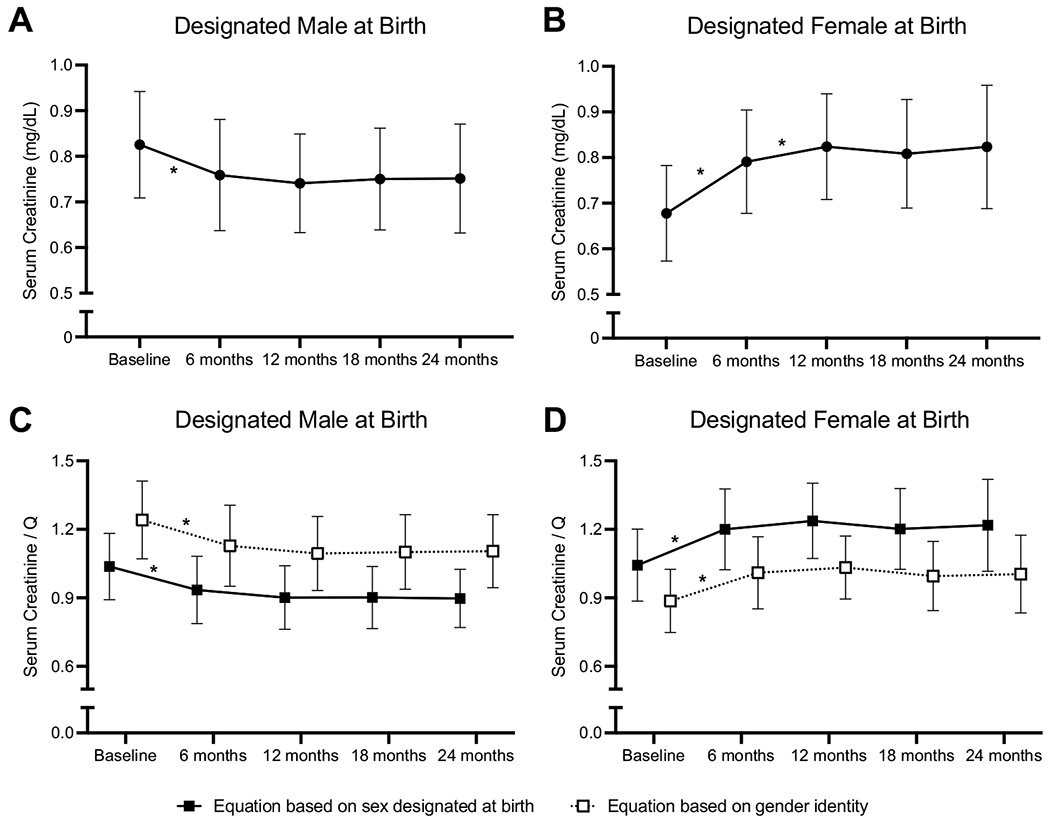
Change in serum creatinine (A and B) and standardized creatinine (SCr/Q; C and D) in participants designated male at birth (DMAB) treated with estradiol (A and C) and in participants designated female at birth (DFAB) treated with testosterone (B and D) during gender-affirming hormone treatment. SCr/Q was calculated using the Q equations corresponding to the participants’ sex designated at birth (closed squares and solid line) and corresponding to the participants’ gender identity (open squares and dashed lines). Symbols indicate means, and lines represent standard deviations. An asterisk indicates a significant change from the previous timepoint (p < 0.05). Creatinine decreased significantly in participants DMAB and increased significantly in participants DFAB from baseline to 6 months (p < 0.001 for both). Serum creatinine continued to increase from 6 to 12 months in participants DFAB (p < 0.001). Standardized creatinine increased from 0 to 6 months in both participants DMAB and DFAB using either equation (p < 0.001 for all comparisons) and did not increase further
After 12 months of testosterone treatment, participants DFAB had serum creatinine similar to the serum creatinine of DMAB participants at baseline (0.82 ± 0.12 mg/dL vs. 0.83 ± 0.12 mg/dL). Although the serum creatinine in participants DMAB decreased with estradiol treatment, it did not decrease to the level of participants DFAB at baseline (0.76 ± 0.12 mg/dL vs. 0.68 ± 0.10 mg/dL, p = 0.0003).
Serum creatinine increases with age across adolescence. To determine if the changes that we observed in serum creatinine during GAH in TGD youth were simply due to increasing age, SCr/Q scores were calculated and compared across GAH therapy (Table 2 and Fig. 1). SCr/Q values decreased in participants DMAB during the first 6 months of estradiol therapy when compared using the male (1.0 ± 0.15 to 0.9 ± 0.15, p < 0.001) and female (1.3 ± 0.17 to 1.1 ± 0.18, p< 0.001) formulas. In participants DFAB, SCr/Q values increased in the first 6 months of testosterone treatment by both the male (0.9 ± 0.14 to 1.0 ± 0.16, p < 0.001) and female formulas (1.0 ± 0.16 to 1.2 ± 0.18, p < 0.001). There were no further changes in SCr/Q after the 6-month visit in either designated sex group.
As an additional method of standardizing by age, we used serum creatinine measurements drawn from NHANES participants as reference data (Supplemental Table 2) to allow us to convert serum creatinine measurements from study participants to Z-scores for age and sex, with a Z-score of 0 indicating that a measurement was equal to the mean NHANES measurement for matched age and specified sex.
Compared to female NHANES participants, participants DFAB had higher mean serum creatinine at baseline (with a mean Z-score significantly greater than 0: + 0.3 ± 1.0, p = 0.002). After 12 months of testosterone treatment, this difference was even more pronounced (Z-score for female reference + 1.4 ± 1.0, p < 0.0001 compared to baseline), demonstrating that the increase in serum creatinine was beyond that expected solely based on increasing age (Supplemental Table 3 and Supplemental Fig. 3). Moreover, after 12 months of treatment, participants DFAB had serum creatinine that was more similar to that of male NHANES participants (Z-score −0.3 ± 0.8) than that of female NHANES participants.
Participants DMAB had mean serum creatinine at baseline that was not significantly different from the reference group of male NHANES participants (Z-score −0.2 ± 0.9, p = 0.06, Supplemental Table 3 and Supplemental Fig. 3). Similar to participants DFAB, after 12 months of estradiol treatment the difference between mean serum creatinine measurements and a reference group of male adolescents was more pronounced (Z-score for male reference −1.1 ± 0.9, p < 0.0001 compared to baseline). Likewise, after 12 months of estradiol participants DMAB had serum creatinine measurements that were closer to the mean for female NHANES participants (Z-score −0.6 ± 1.0) than male NHANES participants. Thus, in both participants DFAB and those DMAB the changes in serum creatinine during GAH were not due to increased age alone.
In participants DFAB, the increase in serum creatinine during the study period was positively associated with the increase in serum testosterone (R2 = 0.11, p = 0.003, Supplemental Fig. 2). In participants DMAB, the association between the changes in serum creatinine and in serum testosterone was not statistically significant (R2 = 0.14, p = 0.051, Supplemental Fig. 2).
Impact of spironolactone use on serum creatinine
There were 58 participants (63%) DMAB who were concurrently treated with spironolactone in addition to estradiol, with spironolactone started before, at the same time, or shortly after estradiol was started. There was no difference in baseline serum creatinine or SCr/Q levels between those taking and those not taking spironolactone (serum creatinine 0.83 ± 0.11 mg/dL vs. 0.79 ± 0.16 mg/dL, p = 0.2; SCr/Q 1.0 ± 0.02 vs. 1.0 ± 0.04, p = 0.9). Spironolactone was associated with differences in the rate of decrease in creatinine with estradiol treatment; those not taking spironolactone had more of a decrease than those taking spironolactone, resulting in significant differences in serum creatinine and SCr/Q measured via the male equation at the 6-month follow-up visit (no spironolactone: serum creatinine 0.67 ± 0.07 mg/dL and SCr/Q 0.83 ± 0.03, spironolactone: serum creatinine 0.78 ± 0.12 mg/dL and SCr/Q 0.95 ± 0.02, p = 0.011 for serum creatinine and p = 0.02 for SCr/Q).
Change in body mass index during GAH in TGD youth
Because serum creatinine is affected by muscle mass [12], we analyzed changes in body mass index (BMI) during GAH to roughly estimate changes in body composition. For participants DFAB, BMI increased in the first 6 months of testosterone treatment (25.1 ± 6.5 kg/m2 to 25.8 ± 6.2 kg/m2, p < 0.0001), with no further changes from 6 to 12 months (Supplemental Table 4). Participants DMAB experienced changes in BMI later in their course of estradiol treatment, with no significant change in BMI from 0 to 6 months (23.9 ± 6.4 kg/m2 to 24.0 ± 5.8 kg/m2, p = 0.6), then a significant increase in BMI from 6 to 12 months (24.0 ± 5.8 kg/m2 to 25.2 ± 7.2 kg/m2, p = 0.0001).
Estimated GFR during GAH in TGD youth
eGFR calculated via the CKiDU25 equation, which accounts for age-related creatinine changes, was inversely associated with changes in creatinine: rising in participants DMAB treated with estradiol by 10.3 (2.2, 22.9) mL/min/1.73 m2 using the male equation and by 7.6 (1.8,18.0) mL/min/1.73 m2 using the female equation over the first 6 months of treatment. For participants DFAB treated with testosterone, eGFR calculated via the CKiDU25 equation fell over 6 months using both the female (−13.5 (−22.4,−7.2) mL/min/1.73 m2) and male equations (−12.4 (−19.7,−6.5) mL/min/1.73 m2).
We also calculated eGFR via the CKD-EPI 2021 equation using both the male and female equations for all participants. For participants DMAB, estradiol-induced decreases in serum creatinine led to an increase in eGFR after 6 months calculated via both the CKD-EPI 2021 equation for males (2.7 (−0.4, 6.9) mL/min/1.73 m2) and the equation for females (6.6 (−0.3,12.6) mL/min/1.73 m2). For participants DFAB, testosterone-induced increases in serum creatinine led to a decrease in eGFR after 6 months by both the male equation (−7.1 (−10.3,−3.8) mL/min/1.73 m2) and the female equation (−12.2 (−22.9,−5.4) mL/min/1.73 m2) (Table 2 and Fig. 2). Changes in eGFR were similar using the bedside Schwartz, FAS, and EKFC equations (Supplemental Table 1).
Fig. 2.
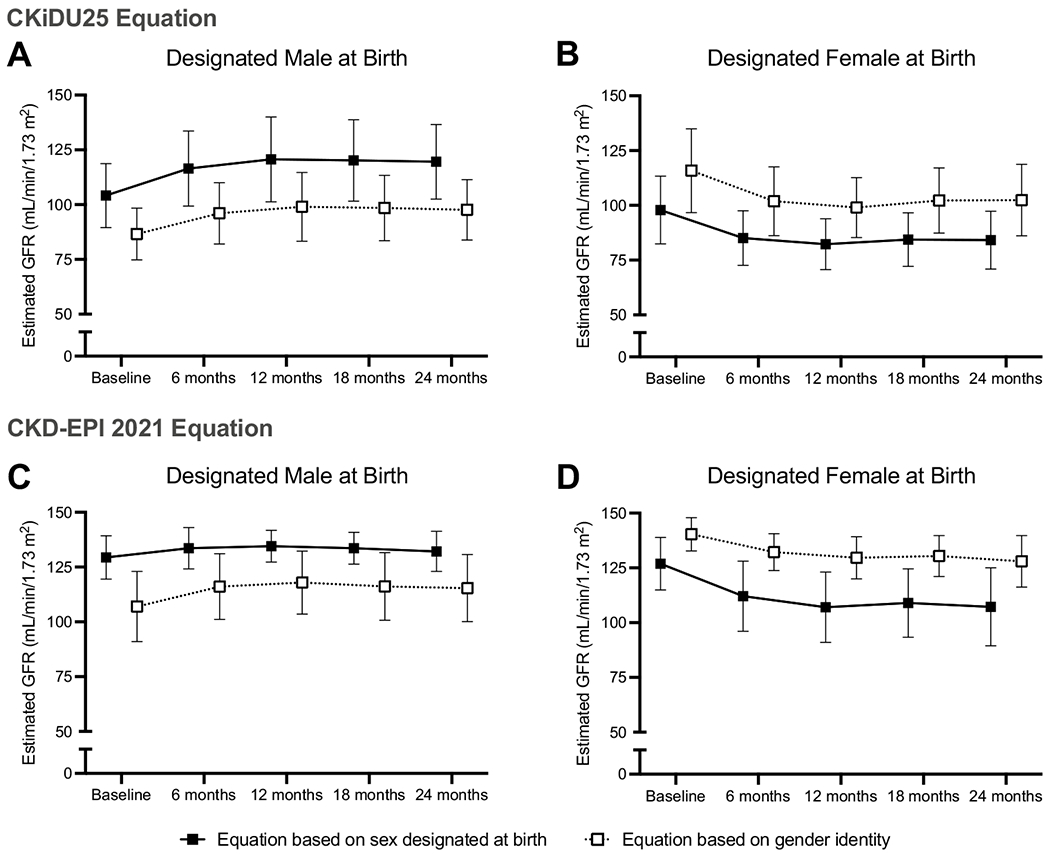
Change in estimated GFR by the CKiDU25 equation (A and B) and CKD-EPI 2021 equation (C and D) during gender-affirming hormone treatment in participants designated male at birth (DMAB) treated with estradiol (A and C) and in participants designated female at birth (DFAB) treated with testosterone (B and D). Estimated GFR (eGFR) was calculated using equations corresponding to the participants’ sex designated at birth (closed squares and solid line) and corresponding to the participants’ gender identity (open squares and dashed lines). Symbols indicate means, and lines indicate standard deviations
Prevalence of chronic kidney disease by eGFR
Although there were no changes in mean serum creatinine for the entire cohort beyond 12 months of GAH, two DFAB participants treated with testosterone demonstrated significant changes between the 12- and 24-month visits and had an eGFR concerning for chronic kidney disease via the definition of <75 mL/min/1.73 m2 proposed by Pottel et al. when the female CKD-EPI 2021 equation was used [12, 27, 28]. One participant had an eGFR of 60.8 mL/min/1.73 m2 at the 18-month follow-up visit, which improved by the 24-month visit (female Eq. 74.1 mL/min/1.73 m2; male Eq. 99.0 mL/min/1.73 m2). The other participant had an eGFR of 69.1 mL/min/1.73 m2 by the female equation at the 24-month visit after having eGFR > 75 mL/min/1.73 m2 at all other study visits. Notably, both individuals had higher eGFR measurements when the male equation was used (81.3 mL/min/1.73 m2 and 92.3 mL/min/1.73 m2, respectively).
Discussion
We observed significant changes in serum creatinine and corresponding eGFR within 6 months of GAH treatment in TGD youth. In participants DFAB, testosterone therapy caused serum creatinine to increase to concentrations similar to the baseline measurements for participants DMAB. Participants DMAB experienced a decrease in serum creatinine during estradiol treatment, but at 12 months of treatment, the mean serum creatinine in this group remained above that of participants DFAB at baseline. When compared to a reference group of adolescents, serum creatinine in TGD youth treated with GAH was more similar when compared by gender than when compared by designated sex.
Serum creatinine and eGFR begin to diverge during puberty, with adolescents undergoing a testosterone-driven puberty displaying an increasingly higher eGFR than adolescents undergoing an estradiol-driven puberty [11]. The change in serum creatinine during puberty and during GAH in TGD youth is likely influenced by changes in body size and composition [29]. GAH treatment in TGD youth leads to changes in muscle mass; within 1 year of GAH treatment, transmasculine adolescents DFAB treated with testosterone have higher lean mass than cisgender female adolescents, and transfeminine adolescents DMAB treated with estradiol have lower lean mass than cisgender male adolescents [8]. These changes in body composition likely underlie some if not all of the changes in serum creatinine with GAH. We found that changes in BMI in transmasculine individuals mirrored changes in serum creatinine, though we did not find a significant change in BMI in transfeminine individuals after the first 6 months of GAH. This is most likely because BMI does not fully reflect body composition, though it may also raise the possibility that GAH can affect serum creatinine through factors independent of body composition.
Indeed, in addition to the impact of changes in body composition, GAH treatment may have a direct effect on kidney function. In one study, female rats given exogenous testosterone for 4 months had increased kidney size with a larger glomerular area when compared to the female control group [30]. To our knowledge, there have been no human studies directly examining renal morphology or measuring GFR during sex-steroid exposure.
Lastly, other factors that were not measured in this study such as diet could impact serum creatinine and GFR. Additional medications including those utilized routinely in gender-affirming care can also have an impact on expected creatinine change. For example, spironolactone, which is routinely used in the care of transfeminine youth for its antiandrogen properties, attenuates the decrease in serum creatinine with estradiol treatment. Our results show that TGD youth can compensate for the slight volume contraction and decreased renal blood flow caused by spironolactone, as evidenced by the lack of a significant difference in baseline creatinine between participants taking and not taking spironolactone. It is possible that this compensation masks the effect of estradiol on serum creatinine. It is also possible that there is an interaction between the effects of estradiol and spironolactone on kidney function.
In the CKD-EPI 2021 equation, the sex-based covariate is an empirical proxy for one or more factors that affect GFR including some that are measurable (e.g., muscle mass) and some more difficult to measure (e.g., diet) [14, 31]. Additional studies directly measuring both body composition (e.g., via dual-energy X-ray absorptiometry) as well as GFR in TGD individuals would clarify the effect of sex steroids both on GFR itself and on creatinine and eGFR.
In the absence of such studies of serum creatinine and GFR changes in diverse TGD individuals, the existing creatinine-based eGFR equations will undoubtedly continue to be used for clinical decisions that require approximation of GFR. Some researchers have suggested using the eGFR equation associated with the patient’s gender identity for TGD individuals who have received GAH for at least 1 year [10]. Our data show that serum creatinine of TGD youth receiving GAH is more similar to that of reference population when matched by gender identity than when matched by sex designated at birth, and thus GFR equations corresponding to gender identity may be better at estimating GFR than equations corresponding to sex designated at birth. However, after 2 years of GAH therapy, transfeminine youth continued to have serum creatinine levels slightly above the mean for female adolescents, and transmasculine youth had serum creatinine levels slightly below the mean for male adolescents. Thus, using estimating eGFR equations corresponding to a patient’s gender identity may also not be a perfect approach, though studies involving direct measurement of GFR are needed to confirm this possibility.
In the meantime, clinicians concerned about kidney function in TGD youth should consider measuring GFR directly, or if such measurement is not possible, using other endogenous markers. For example, cystatin C is not affected by lean muscle mass, unlike serum creatinine, and equations for eGFR utilizing cystatin C have been recommended as confirmatory tests for eGFR in individuals with low or borderline low eGFR by equations based on serum creatinine [32, 33]. Recent efforts to produce eGFR equations without a race adjustment found that equations utilizing both creatinine and cystatin C are more accurate at predicting eGFR than equations with creatinine alone [17]. It is plausible that given its independence from changes in body composition, cystatin C may be a more reliable marker of kidney function in transgender individuals receiving GAH, but formal studies of cystatin C in transgender individuals are needed to confirm this possibility.
Direct measurement of GFR and analysis of body composition were not performed as part of this study, presenting significant limitations to the study. Additionally, information regarding the method and precision of serum creatinine measurements was not collected. Lack of racial diversity in the cohort is an additional limitation. As our cohort consisted of healthy adolescents, our findings may not be applicable to patients with chronic kidney disease. Gender-affirming therapy serves to both increase the administered sex hormone level as well as decrease endogenous sex hormones; in this study, we could not ascertain if the decrease in endogenous sex steroids, the increase in the administered sex-steroid levels, or both were responsible for changes in serum creatinine in TGD youth.
Until future research efforts come to fruition, for patients who have been taking GAH for at least 6 months, we recommend calculating eGFR based on both the patient’s sex designated at birth and their gender identity. If eGFR is found to be low by either equation, we recommend using an eGFR equation based on cystatin C (though such equations also need validation in TGD cohorts) and/or direct measurement of GFR [27].
Supplementary Material
Funding
This work was supported by National Institutes of Health (R01 HD082554) and the Doris Duke Charitable Foundation (Grant 2019119 to KM).
Abbreviations
- TGD
Transgender/gender diverse
- GAH
Gender-affirming hormones
- DMAB
Designated Male at Birth
- DFAB
Designated Female at Birth
- BMI
Body Mass Index
- GFR
Glomerular Filtration Rate
- eGFR
Estimated Glomerular Filtration Rate
- CKD
Chronic Kidney Disease
- NHANES
National Health and Nutrition Examination Survey
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00467-022-05445-0.
Conflict of Interest The authors have no relevant financial conflicts of interest to disclose.
References
- 1.Hembree WC, Cohen-Kettenis PT, Gooren LJ, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GGR (2017) Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 102:3869–3903 [DOI] [PubMed] [Google Scholar]
- 2.Turban JL, King D, Carswell JM, Keuroghlian AS (2020) Pubertal Suppression for Transgender Youth and Risk of Suicidal Ideation. Pediatrics 145:e20191725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher AD, Castellini G, Ristori J, Casale H et al. (2016) Cross-sex hormone treatment and psychobiological changes in transsexual persons: Two-year follow-up data. J Clin Endocrinol Metab 101:4260–4269 [DOI] [PubMed] [Google Scholar]
- 4.de Vries ALC, McGuire JK, Steensma TD, Wagenaar ECF, Doreleijers TAH, Cohen-Kettenis PT (2014) Young Adult Psychological Outcome After Puberty Suppression and Gender Reassignment. Pediatrics 134:696–704 [DOI] [PubMed] [Google Scholar]
- 5.Chew D, Anderson J, Williams K, May T, Pang K (2018) Hormonal Treatment in Young People With Gender Dysphoria: A Systematic Review. Pediatrics 141:e20173742. [DOI] [PubMed] [Google Scholar]
- 6.Olson-Kennedy J, Okonta V, Clark LF, Belzer M (2018) Physiologic Response to Gender-Affirming Hormones Among Transgender Youth. J Adolesc Heal 62:397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millington K, Finlayson C, Olson-Kennedy J, Garofalo R, Rosenthal SM, Chan Y-M (2021) Association of High-Density Lipoprotein Cholesterol with Sex Steroid Treatment in Transgender and Gender-Diverse Youth. JAMA Pediatr 175:520–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nokoff NJ, Scarbro SL, Moreau KL, Zeitler P, Nadeau KJ, Juarez-Colunga E, Kelsey MM (2020) Body Composition and Markers of Cardiometabolic Health in Transgender Youth Compared With Cisgender Youth. J Clin Endocrinol Metab 105:704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarin J, Pine-Twaddell E, Trotman G, Stevens J, Conard LA, Tefera E, Gomez-Lobo V (2017) Cross-Sex Hormones and Metabolic Parameters in Adolescents With Gender Dysphoria. Pediatrics 139:e20163173. [DOI] [PubMed] [Google Scholar]
- 10.Cheung AS, Lim HY, Cook T, Zwickl S, Ginger A, Chiang C, Zajac JD (2020) Approach to Interpreting Common Laboratory Pathology Tests in Transgender Individuals. J Clin Endocrinol Metab 106:893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mian AN, Schwartz GJ (2017) Measurement and Estimation of Glomerular Filtration Rate in Children. Adv Chronic Kidney Dis 24:348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing Kidney Function — Measured and Estimated Glomerular Filtration Rate. N Engl J Med 354:2473–2483 [DOI] [PubMed] [Google Scholar]
- 13.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP (2008) Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol 3:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diao JA, Inker LA, Levey AS, Tighiouart H, Powe NR, Manrai AK (2021) In Search of a Better Equation — Performance and Equity in Estimates of Kidney Function. N Engl J Med 384:396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonham VL, Green ED, Pérez-Stable EJ (2018) Examining How Race, Ethnicity, and Ancestry Data Are Used in Biomedical Research. JAMA 320:1533–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA, Eneanya ND, Coresh J, Tighiouart H et al. (2021) New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med 385:1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado C, Baweja M, Burrows NR, Crews DC et al. (2021) Reassessing the Inclusion of Race in Diagnosing Kidney Diseases: An Interim Report from the NKF-ASN Task Force. J Am Soc Nephrol 32:1305–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collister D, Saad N, Christie E, Ahmed S (2021) Providing Care for Transgender Persons With Kidney Disease: A Narrative Review. Can J Kidney Heal Dis. 10.1177/2054358120985379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitley CT, Greene DN (2017) Transgender man being evaluated for a kidney transplant. Clin Chem 63:1680–1683 [DOI] [PubMed] [Google Scholar]
- 21.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2021) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson-Kennedy J, Chan Y-M, Garofalo R, Spack N, Chen D, Clark L, Ehrensaft D, Hidalgo M, Tishelman A, Rosenthal S (2019) The Impact of Early Medical Treatment in Transgender Youth: The Trans Youth Care Study (Preprint). JMIR Res Protoc. 10.2196/14434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pottel H, Björk J, Courbebaisse M, Couzi L et al. (2021) Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate. Ann Intern Med 174:183–191 [DOI] [PubMed] [Google Scholar]
- 24.(2017) National Health and Nutrition Examination Survey Laboratory Protocol. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville, MD [Google Scholar]
- 25.Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V, Selistre L, Mariat C, Martens F, Delanaye P (2016) An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 31(5):798—806. 10.1093/ndt/gfv454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staples A, Leblond R, Watkins S, Wong C, Brandt J (2010) Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 25:2321–2326 [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation (2002) K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. [Google Scholar]
- 28.Pottel H, Hoste L, Delanaye P (2015) Abnormal glomerular filtration rate in children, adolescents and young adults starts below 75 mL/min/1.73 m2. Pediatr Nephrol 30:821–828 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Haycock GB, Edelmann CMJ, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263 [PubMed] [Google Scholar]
- 30.Lichtenecker DCK, Argeri R, de Moura Castro CH, Dias-da-Silva MR, Gomes GN (2021) Cross-Sex Testosterone Therapy Modifies the Renal Morphology and Function in Female Rats and Might Underlie Increased Systolic Pressure. Clin Exp Pharmacol Physiol 48:978–986 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Inker LA, Coresh J (2014) GFR estimation: From physiology to public health. Am J Kidney Dis 63:820–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH et al. (2012) Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N Engl J Med 367:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinge E, Lindergård B, Nilsson-Ehle P, Grubb A (1999) Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59:587–592 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


