Abstract
Background & Aims:
Helicobacter pylori induces immune tolerance and is associated with a lower risk for immune-mediated disorders, such as autoimmune and inflammatory bowel diseases (IBD). We aimed to determine the effects of treatment for H pylori infection on the incidence of autoimmune disease and IBD.
Methods:
We collected data from the National Health Insurance Research Database in Taiwan on patients younger than 18 years old without a prior diagnosis of autoimmune disease or IBD. Patients with peptic ulcer disease (PUD) with treatment of H pylori infection (PUD+HPRx), PUD without H pylori treatment (PUD–HPRx), a urinary tract infection (UTI) treated with cephalosporin, or without PUD (controls) were matched for age, sex, insurance, and Charlson’s comorbidity index score.
Results:
Of the 1 million patients we collected data from in 2005, we included 79,181 patients in the study. We compared the effects of treatment for H pylori infection on the risk of autoimmunity or IBD and found that PUD+HPRx has the highest adjusted hazard risk (aHR) for autoimmunity or IBD (aHR, 2.36), compared to PUD–HPRx (aHR, 1.91) or UTI (aHRs, 1.71) (P<.001). The increased risk of autoimmune disease was not completely accounted for by antibiotic therapy alone, because PUD+HPRx had a higher aHR than UTI (P<.001). A small but significant increase in mortality was observed in the PUD+HPRx cohort (aHR, 1.11; P=.001).
Conclusion:
In an analysis of data from the National Health Insurance Research Database in Taiwan, we found that treatment for H pylori infection is associated with a significant increase in the risk for autoimmune disease, including IBD.
Keywords: eradication, autoimmunity, immune response, bacteria
INTRODUCTION
Nearly half of the world’s population is colonized by Helicobacter pylori. Approximately 10–15% of the infected individuals will go on to develop gastroduodenal ulcers, and <3% will develop gastric cancer1. Universal vaccination has been proposed2, and yet the negative impact of eradicating H pylori in the 85–90% of otherwise asymptomatic individuals has not been evaluated. Its existence in the stomach of Otzi the Iceman’s 5000-year-old mummified remains indicates their coexistence since the beginning of human civilization3. This has led to the emerging idea of a Human-H pylori symbiosis. There is growing evidence that H pylori is a protective factor against chronic immune-mediated disorders such as asthma4–6, rheumatoid arthritis7, and inflammatory bowel disease (IBD)8–11. There is little epidemiological evidence, however, to suggest that treatment of H pylori is associated with increased incidence of immune-mediated disorders such as autoimmune diseases (AD) and IBD.
It has been hypothesized that this protection includes the induction of intestinal IL-10 and IL-18-mediated regulatory T cell (Treg) responses 12, 13. The ability of H pylori to persist in humans has been attributed to several bacterial factors that dampen host immune responses. These include VacA, CagA, H pylori LPS, and other unidentified secreted factors14–16. Rad et al. recently demonstrated that H pylori-infected individuals expressed higher levels of Foxp3, a regular T cell marker, and that the depletion of Tregs resulted in a higher degree of gastric inflammation and reduced bacterial colonization17. In fact, it has previously been shown that peripheral memory T cells are less proliferative in H pylori-infected individuals compared to uninfected controls18. In that same study, it was found that the inhibitory effect was due to the presence of suppressive T cells in infected individuals. This raises the possibility of circulating regulatory T cells in H pylori-infected host which may play a protective role against AD and IBD.
Several regions around the world are reporting a rising incidence of immune-mediated and allergic diseases19. Coincidentally, this occurs during the period of post-H pylori discovery and there is growing concern that increased testing and treatment of H pylori may have contributed to these rising incidences. While a number of host factors have been implicated including obesity, westernization of diet, changes in the hygiene and gut microbiota20, it is important to better understand the relationship between H pylori treatment and the risk of developing immune-mediated disorders in order to better inform health care providers, authors of practice guidelines, and global health policymakers on the potential negative impact of global H pylori eradication.
One of the challenges of conducting a study looking at the impact of H pylori treatment on the risks of developing AD and IBD is the relatively low prevalence of AD in regions with high rates of H pylori infection and the requirement of a large sample size to draw meaningful conclusions. In this study, we utilized the National Health Insurance Research (NHIR) Database of Taiwan, where the prevalence of H pylori approaches 80% 21, in order to determine the impact of H pylori therapy on the risk of developing AD and IBD.
MATERIALS AND METHODS
Database
This retrospective cohort study was performed using the NHIR database which was established in 1996 by the Bureau of National Health Insurance of the Department of Health and covered 99% of the 23 million residents of Taiwan and is contracted with 97% of Taiwanese hospitals and clinics. The NHIR database includes data for inpatient and ambulatory expenditures as well as orders and prescriptions dispensed at contracted pharmacies. A longitudinal database was created using a random sample of 1 million subjects in the NHIR year 2005 database and the reimbursement data from January 1, 2000 to December 31, 2010 of the eligible study subjects were included for analysis. The rationale for creating this longitudinal database is to allow a 5-year window in both directions to analyze the long-term impact of H pylori therapy on AD including IBD.
Study design
We first selected study subjects >18 years of age with or without peptic ulcer disease (PUD) identified by the International Classification of Disease (ICD)-9 codes: 531 (gastric ulcer), 532 (duodenal ulcer), and 533 (nonspecific peptic ulcer). The algorithm to identify cases from ICD-9 codes was largely similar to that used by Bernstein et al. 22 and Kappelman et al. 23; subjects with three or more outpatient ICD-9 codes were included for further analysis. Four cohorts were created: 1) subjects without PUD (Non-PUD), 2) subjects without PUD but were treated with first-generation cephalosporin antibiotics for urinary tract infection (UTI), 3) subjects with PUD without anti-H pylori treatment (PUD-HPRx), and 4) PUD subjects with H pylori treatment (PUD+HPRx). Subjects were considered to have received H pylori treatment if they were given a course of either triple or quadruple therapy for more than 7 days. H pylori testing results for diagnosis or treatment eradication are not available in the NHRI database. Subjects in the PUD-HPRx, UTI, or Non-PUD groups were matched to PUD+HPRx using propensity methods by age, gender, insurance range, and Charlson’s comorbidity index score (CCIS). Subjects selected in one group will be excluded from the database and will not be selected in the other groups. There were apparent differences between the cohorts on age, insurance range, and Charlson’s comorbidity index scores after matching largely due to fewer subjects in the UTI group.
Study sample
These autoimmune diseases (AD) were defined by diagnosis of ICD-9 code of Lupus Erythematosus (ICD-9: 710.0), Systemic Sclerosis (ICD-9:710.1), Rheumatoid Arthritis (ICD-9: 714.30 – 714.33), Polymyositis (ICD-9:710.4), Dermatomyositis (ICD-9:710.3), Vasculitis (ICD-9: 446.0, 446.2, 446.4, 446.5, 443.1, 446.7, 446.1), Pemphigus (ICD-9: 694.4), Sicca Syndrome (ICD9: 710.2), Crohn’s Disease (ICD-9: 555.x), and Chronic Ulcerative Colitis (ICD-9: 556.x). A diagnosis of UTI is identified by ICD-9 codes of 590.10–590.11, 590.80, 590.81, 590.9, 590.3, 590.00, 590.01, 590.2, 595.0–595.9, and 599.0. Only ambulatory patients who received cephalosporin were included in the UTI cohort. It is worth noting that AD including IBD are considered “Catastrophic Illnesses” which required additional review by NHRI clinical staff and approval was based on clinical data including clinical labs, radiological reports, and medication history. The person-years of follow-up were estimated from the index date plus 2-year lag time to the date of diagnosis of AD including IBD. We excluded 41,457 subjects with AD diagnosis within 2 years of receiving H pylori therapy (index date) to allow a 2-year lag time (e.g., patients treated with H pylori therapy after 2008 were excluded from the analysis). We also excluded the initial 2 years of follow-up from Non-PUD controls to avoid survival time bias. The index date is defined as the date when PUD or UTI was diagnosed and 2002 for controls. Subjects with incomplete demographical data (e.g., loss to follow-up, or withdrawal from the insurance system) (n=17,064) were also excluded from the study (Figure 1). The follow-up period begins after H pylori therapy (index date) plus 2-year lag time and ends on December 31, 2010. There are small differences in follow-up time between the groups with the shortest follow-up time in the PUD+HPRx group consistent with a higher rate of AD and IBD in that group.
Figure 1.
Study design flow chart.
Statistical Analysis
We analyzed the distribution of risk factors for the four study cohorts (PUD+HPRx, PUD-HPRx, UTI, and Non-PUD) by ANOVA test, chi-squared test or Fisher’s exact test. Cox proportional hazards regression analyses were performed to determine the crude and adjusted hazard risk (aHR) after adjustment for age, gender, comorbidities, CCIS score, and NSAIDs or antiplatelet agent (Table 2, Model i). A competing risk model was also performed to determine the risk of AD incidence (Table 2, Model ii) and mortality (Table 3). Kaplan–Meier curves estimated the probability of AD onset or mortality and the log-rank test analyzed the differences between groups. Statistical analyses were performed using SAS 9.3 software (SAS Institute, Inc., Cary, NC). Statistical significance was set at p< 0.05.
Table 2.
The adjusted hazard risk (aHR) of immune-mediated disorders, subtypes of autoimmune diseases among Non-PUD, UTI, and PUD population.
| Model i | Model ii | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| No. cases | Per 1,000 PY | (%) | aHR | (95%CI) | p value | aHR | (95%CI) | p value | |
| Autoimmune Disorders | |||||||||
| Non-PUD cohort | 614 | 4.638 | (2.6) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 728 | 7.453 | (3.7) | 1.71 | (1.54 – 1.91) | <0.001 | 1.07 | (1.00 – 1.15) | 0.028 |
| PUD-HPRx cohort | 1154 | 9.092 | (4.7) | 1.91 | (1.73 – 2.11) | <0.001 | 1.14 | (1.08 – 1.21) | <0.001 |
| PUD+HPRx cohort | 1399 | 11.299 | (5.6) | 2.36 | (2.14 – 2.59) | <0.001 | 1.34 | (1.27 – 1.42) | <0.001 |
| Lupus Erythematosus | |||||||||
| Non-PUD cohort | 9 | 0.067 | (0.0) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 24 | 0.238 | (0.1) | 3.99 | (1.85 – 8.63) | <0.001 | 0.81 | (0.74 – 0.88) | <0.001 |
| PUD-HPRx cohort | 29 | 0.220 | (0.1) | 3.24 | (1.53 – 6.86) | 0.002 | 0.83 | (0.77 – 0.90) | <0.001 |
| PUD+HPRx cohort | 39 | 0.299 | (0.2) | 4.41 | (2.12 – 9.14) | <0.001 | 0.95 | (0.88 – 1.02) | 0.164 |
| Systemic Sclerosis | |||||||||
| Non-PUD cohort | 3 | 0.022 | (0.0) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 4 | 0.040 | (0.0) | 1.92 | (0.42 – 8.69) | 0.395 | 0.79 | (0.72 – 0.87) | <0.001 |
| PUD-HPRx cohort | 5 | 0.038 | (0.0) | 1.54 | (0.36 – 6.52) | 0.554 | 0.82 | (0.76 – 0.88) | <0.001 |
| PUD+HPRx cohort | 3 | 0.023 | (0.0) | 0.92 | (0.18 – 4.67) | 0.927 | 0.93 | (0.86 – 1) | 0.051 |
| Rheumatoid Arthritis | |||||||||
| Non-PUD cohort | 153 | 1.138 | (0.7) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 191 | 1.907 | (1.0) | 2.05 | (1.65 – 2.54) | <0.001 | 0.92 | (0.84 – 0.99) | 0.044 |
| PUD-HPRx cohort | 348 | 2.664 | (1.4) | 2.29 | (1.89 – 2.77) | <0.001 | 0.96 | (0.90 – 1.03) | 0.298 |
| PUD+HPRx cohort | 368 | 2.859 | (1.5) | 2.44 | (2.01 – 2.95) | <0.001 | 1.06 | (0.99 – 1.14) | 0.054 |
| Polymyositis | |||||||||
| Non-PUD cohort | 6 | 0.044 | (0.0) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 10 | 0.099 | (0.0) | 2.55 | (0.92 – 7.08) | 0.072 | 2.55 | (0.92 – 7.08) | 0.072 |
| PUD-HPRx cohort | 10 | 0.076 | (0.0) | 1.74 | (0.63 – 4.81) | 0.283 | 1.74 | (0.63 – 4.81) | 0.283 |
| PUD+HPRx cohort | 12 | 0.092 | (0.0) | 2.16 | (0.80 – 5.81) | 0.125 | 2.16 | (0.80 – 5.81) | 0.125 |
| Dermatomyositis | |||||||||
| Non-PUD cohort | 7 | 0.052 | (0.0) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 6 | 0.059 | (0.0) | 1.33 | (0.44 – 3.98) | 0.611 | 0.79 | (0.72 – 0.86) | <0.001 |
| PUD-HPRx cohort | 14 | 0.106 | (0.1) | 2.05 | (0.82 – 5.10) | 0.122 | 0.82 | (0.76 – 0.89) | <0.001 |
| PUD+HPRx cohort | 12 | 0.092 | (0.0) | 1.79 | (0.70 – 4.58) | 0.222 | 0.93 | (0.86 – 1.00) | 0.064 |
| Vasculitis | |||||||||
| Non-PUD cohort | 7 | 0.052 | (0.0) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 10 | 0.099 | (0.1) | 1.91 | (0.72 – 5.07) | 0.189 | 0.79 | (0.73 – 0.87) | <0.001 |
| PUD-HPRx cohort | 16 | 0.121 | (0.1) | 2.13 | (0.87 – 5.20) | 0.097 | 0.83 | (0.77 – 0.89) | <0.001 |
| PUD+HPRx cohort | 24 | 0.184 | (0.1) | 3.10 | (1.32 – 7.27) | 0.009 | 0.94 | (0.87 – 1.01) | 0.099 |
| Pemphigus | |||||||||
| Non-PUD cohort | 5 | 0.037 | (0.0) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 5 | 0.049 | (0.0) | 1.71 | (0.48 – 5.98) | 0.401 | 0.79 | (0.72 – 0.87) | <0.001 |
| PUD-HPRx cohort | 5 | 0.038 | (0.0) | 0.93 | (0.26 – 3.28) | 0.918 | 0.82 | (0.76 – 0.88) | <0.001 |
| PUD+HPRx cohort | 3 | 0.023 | (0.0) | 0.57 | (0.13 – 2.44) | 0.448 | 0.92 | (0.86 – 0.99) | 0.046 |
| Sicca Syndrome | |||||||||
| Non-PUD cohort | 123 | 0.913 | (0.5) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 227 | 2.268 | (1.1) | 2.60 | (2.09 – 3.25) | <0.001 | 0.94 | (0.86 – 1.02) | 0.137 |
| PUD-HPRx cohort | 315 | 2.407 | (1.4) | 2.56 | (2.08 – 3.16) | <0.001 | 0.95 | (0.88 – 1.02) | 0.163 |
| PUD+HPRx cohort | 385 | 2.989 | (1.5) | 3.15 | (2.57 – 3.87) | <0.001 | 1.09 | (1.02 – 1.17) | 0.007 |
| Inflammatory Bowel Disease | |||||||||
| Non-PUD cohort | 317 | 2.371 | (1.3) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 289 | 2.900 | (1.5) | 1.22 | (1.04 – 1.44) | 0.012 | 0.87 | (0.80 – 0.94) | 0.001 |
| PUD-HPRx cohort | 476 | 3.662 | (1.9) | 1.52 | (1.31 – 1.75) | <0.001 | 0.94 | (0.88 – 1.00) | 0.091 |
| PUD+HPRx cohort | 666 | 5.235 | (2.7) | 2.15 | (1.88 – 2.46) | <0.001 | 1.13 | (1.06 – 1.21) | <0.001 |
| Crohn’s Disease | |||||||||
| Non-PUD cohort | 291 | 2.175 | (1.2) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 278 | 2.787 | (1.4) | 1.28 | (1.08 – 1.51) | 0.003 | 1.28 | (1.08 – 1.51) | 0.003 |
| PUD-HPRx cohort | 452 | 3.474 | (1.8) | 1.57 | (1.35 – 1.82) | <0.001 | 1.57 | (1.35 – 1.82) | <0.001 |
| PUD+HPRx cohort | 618 | 4.848 | (2.5) | 2.17 | (1.88 – 2.49) | <0.001 | 2.17 | (1.88 – 2.49) | <0.001 |
| Chronic Ulcerative Colitis | |||||||||
| Non-PUD cohort | 28 | 0.207 | (0.1) | Ref. | --- | Ref. | --- | ||
| UTI cohort | 12 | 0.119 | (0.1) | 0.62 | (0.31 – 1.23) | 0.175 | 0.78 | (0.72 – 0.86) | <0.001 |
| PUD-HPRx cohort | 27 | 0.204 | (0.1) | 0.98 | (0.58 – 1.68) | 0.965 | 0.82 | (0.76 – 0.89) | <0.001 |
| PUD+HPRx cohort | 53 | 0.407 | (0.2) | 1.97 | (1.24 – 3.13) | 0.004 | 0.94 | (0.88 – 1.01) | 0.143 |
Adjusted for age, gender, insurance range, comorbidities, CCIS score, and medication.
Non-PUD cohort: comparison cohort
PUD-HPRx cohort: non-H pylori treatment cohort
PUD+HPRx cohort: H pylori treatment cohort
Model i: Cox proportional hazards regression
Model ii: competing risk analysis
Table 3.
Mortality among Non-PUD population, UTI population and PUD population (N=79,181).
| No. Death | Cases/1,000 Person-years | aHR | (95%CI) | p value | |
|---|---|---|---|---|---|
| Non-PUD cohort | 2062 | 11.631 | Ref. | ||
| UTI cohort | 1068 | 8.023 | 0.92 | (0.85 – 0.99) | 0.036 |
| PUD-HPRx cohort | 1894 | 10.865 | 0.93 | (0.88 – 0.99) | 0.047 |
| PUD+HPRx cohort | 2170 | 12.577 | 1.11 | (1.04 – 1.18) | 0.001 |
Adjusted for age, gender, insurance range, comorbidities, CCIS score, and medication.
RESULTS
Higher cumulative hazard risk of AD and IBD in PUD+HPRx individuals compared to PUD-HPRx, UTI, or Non-PUD subjects.
To gain further insight into the risk of developing AD including IBD by treating H pylori, we compared the new incidence of AD and IBD patients with PUD diagnosed by EGD who received H pylori therapy (PUD+HPRx) or never received H pylori therapy (PUD-HPRx) with patients without PUD (Non-PUD) or UTI treated with cephalosporin (adjusted for age, gender, insurance, CCIS, and medication) (Figure 1). The patient characteristics are shown in Table 1. The average age was 49.45±16.6 with 51.6% male in Non-PUD controls, 46.52±16.9 with 51.0% male in UTI group, 49.49±16.7 with 51.7% male in PUD-HPRx group, and 49.54±16.6 with 51.7% male in PUD+HPRx group.
Table 1.
Demographic prevalence of Non-PUD, UTI, and PUD population (N=79,181).
| Non-PUD population | PUD population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| * Non-PUD cohort (n=21,051) | UTI cohort (n=16,028) | ** PUD-HPRx cohort (n=21,051) | *** PUD+HPRx cohort (n=21,051) | p value | |||||
| Age | |||||||||
| <40 | 6727 | (32.0) | 6356 | (39.7) | 6723 | (31.9) | 6723 | (31.9) | <0.001 |
| 40–59 | 8476 | (40.3) | 6137 | (38.3) | 8361 | (39.7) | 8361 | (39.7) | |
| ≧60 | 5848 | (27.8) | 3535 | (22.1) | 5967 | (28.3) | 5967 | (28.3) | |
| Mean±SD | 49.45 | (16.6) | 46.52 | (16.9) | 49.49 | (16.7) | 49.54 | (16.6) | <0.001 |
| Gender | |||||||||
| Female | 10189 | (48.4) | 7846 | (49.0) | 10170 | (48.3) | 10170 | (48.3) | 0.582 |
| Male | 10862 | (51.6) | 8182 | (51.0) | 10881 | (51.7) | 10881 | (51.7) | |
| Follow up (year) | 6.29 | (2.19) | 6.09 | (2.26) | 6.03 | (2.35) | 5.88 | (2.48) | <0.001 |
| Insurance range | |||||||||
| <15,000 | 8541 | (40.6) | 6583 | (41.1) | 8676 | (41.2) | 8423 | (40.0) | <0.001 |
| 15,000–29,999 | 6618 | (31.4) | 5761 | (35.9) | 7401 | (35.2) | 7848 | (37.3) | |
| ≧29,999 | 5892 | (28.0) | 3684 | (23.0) | 4974 | (23.6) | 4780 | (22.7) | |
| Comorbidities | |||||||||
| Diabetes | 3023 | (14.4) | 2189 | (13.7) | 3222 | (15.3) | 3496 | (16.6) | <0.001 |
| Hypertension | 5562 | (26.4) | 3896 | (24.3) | 6590 | (31.3) | 6642 | (31.6) | <0.001 |
| Hyperlipidemia | 3688 | (17.5) | 2656 | (16.6) | 4839 | (23.0) | 5091 | (24.2) | <0.001 |
| Myocardial infraction | 1045 | (5.0) | 410 | (2.6) | 826 | (3.9) | 888 | (4.2) | <0.001 |
| Congestive heart failure | 2147 | (10.2) | 1350 | (8.4) | 2589 | (12.3) | 2772 | (13.2) | <0.001 |
| Peripheral vascular disease | 1167 | (5.5) | 750 | (4.7) | 1425 | (6.8) | 1571 | (7.5) | <0.001 |
| Cerebral vascular disease | 4990 | (23.7) | 2966 | (18.5) | 5216 | (24.8) | 5261 | (25.0) | <0.001 |
| Dementia | 1104 | (5.2) | 821 | (5.1) | 1290 | (6.1) | 1299 | (6.2) | <0.001 |
| Chronic kidney disease | 4063 | (19.3) | 2568 | (16.0) | 4099 | (19.5) | 4408 | (20.9) | <0.001 |
| Cancer | 3993 | (19.0) | 2405 | (15.0) | 3903 | (18.5) | 3994 | (19.0) | <0.001 |
| Charlson’s index score | |||||||||
| ≦2 | 7822 | (37.2) | 7878 | (49.2) | 7823 | (37.2) | 7823 | (37.2) | <0.001 |
| 3 | 3175 | (15.1) | 2598 | (16.2) | 3165 | (15.0) | 3165 | (15.0) | <0.001 |
| ≧ 4 | 10054 | (47.8) | 5552 | (34.6) | 10063 | (47.8) | 10063 | (47.8) | <0.001 |
| Mean±SD | 3.79 | (3.0) | 3.28 | (2.8) | 4.18 | (3.1) | 4.36 | (3.3) | <0.001 |
| Medication | |||||||||
| NSAIDs | 1293 | (6.1) | 1407 | (8.8) | 2623 | (12.5) | 3375 | (16.0) | <0.001 |
| Antiplatelet agent | 639 | (3.0) | 426 | (2.7) | 796 | (3.8) | 949 | (4.5) | <0.001 |
| Warfarin | 70 | (0.3) | 38 | (0.2) | 56 | (0.3) | 57 | (0.3) | 0.340 |
Non-PUD cohort: comparison cohort
PUD-HPRx cohort: non-H pylori treatment cohort
PUD+HPRx cohort: H pylori treatment cohort
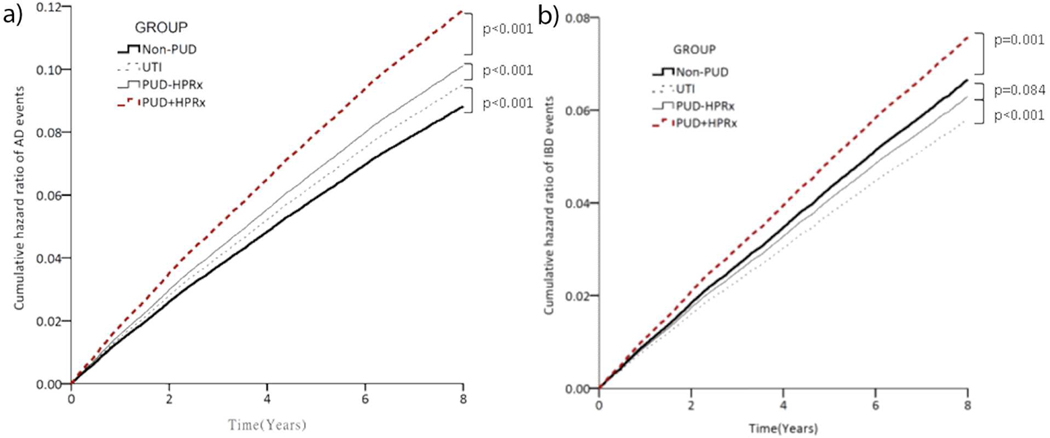
Next, after multiple regression adjusted for age, gender, comorbidities, CCIS score, and medications, we found that the risk of AD including IBD was significantly higher for patients who received H pylori eradication therapy compared to those who did not receive the therapy (p<0.001, Figures 2a and 2b). Specifically, patients who received eradication therapy had higher risk of developing Lupus Erythematosus (aHR=4.41, 95% CI [2.12, 9.14], p<0.001), Rheumatoid Arthritis (aHR=2.44, 95% CI [2.01, 2.95], p<0.001), Vasculitis (aHR=3.10, 95% CI [1.32, 7.27], p=0.009), Sicca Syndrome (aHR= 3.15, 95% CI [2.57, 3.87], p<0.001), and IBD (aHR=2.15, 95% CI [1.88, 2.46], p<0.001) (Table 2, Model i). The incidence rates of AD and IBD were also significantly higher in PUD+HPRx cohort compared to PUD-HPRx, UTI, or Non=PUD control cohort (AD=11.299 vs. 9.902, 7.453, or 4.638 per 1,000 person-years; IBD=5.235 vs 3.662, 2.900, or 2.371 per 1,000 person-years, respectively, p<0.05). We also performed additional analyses using the competing risk model to adjust for competing risk of death (Table 2, Model ii). Similar results were observed compared to the Cox proportional hazards regression analysis (Table 2, Model i) in that PUD+HPRx cohorts had a higher aHR compared to Non-PUD controls (AD=1.34, 95% CI [1.27, 1.42]; IBD=1.13, 95% CI [1.06, 1.21], p<0.001). We did find that the UTI group had a lower aHR compared to Non-PUD controls using the competing risk model (UTI=0.87, 95% CI [0.80, 0.94], p=0.001) indicating antibiotic treatment might be associated with a lower risk of IBD.
Figure 2. Higher adjusted hazard risk of AD and IBD in PUD+HPRx individuals compared to PUD-HPRx, UTI, or Non-PUD control subjects.
Kaplan–Meier curves estimated the probability of new-onset AD (a) and IBD (b) events, and the log-rank test was used for statistical analysis. A competing risk model was used to adjust for competing risk of death.
Lower cumulative survival rate in PUD+HPRx individuals compared to PUD-HPRx, UTI, and Non-PUD control subjects.
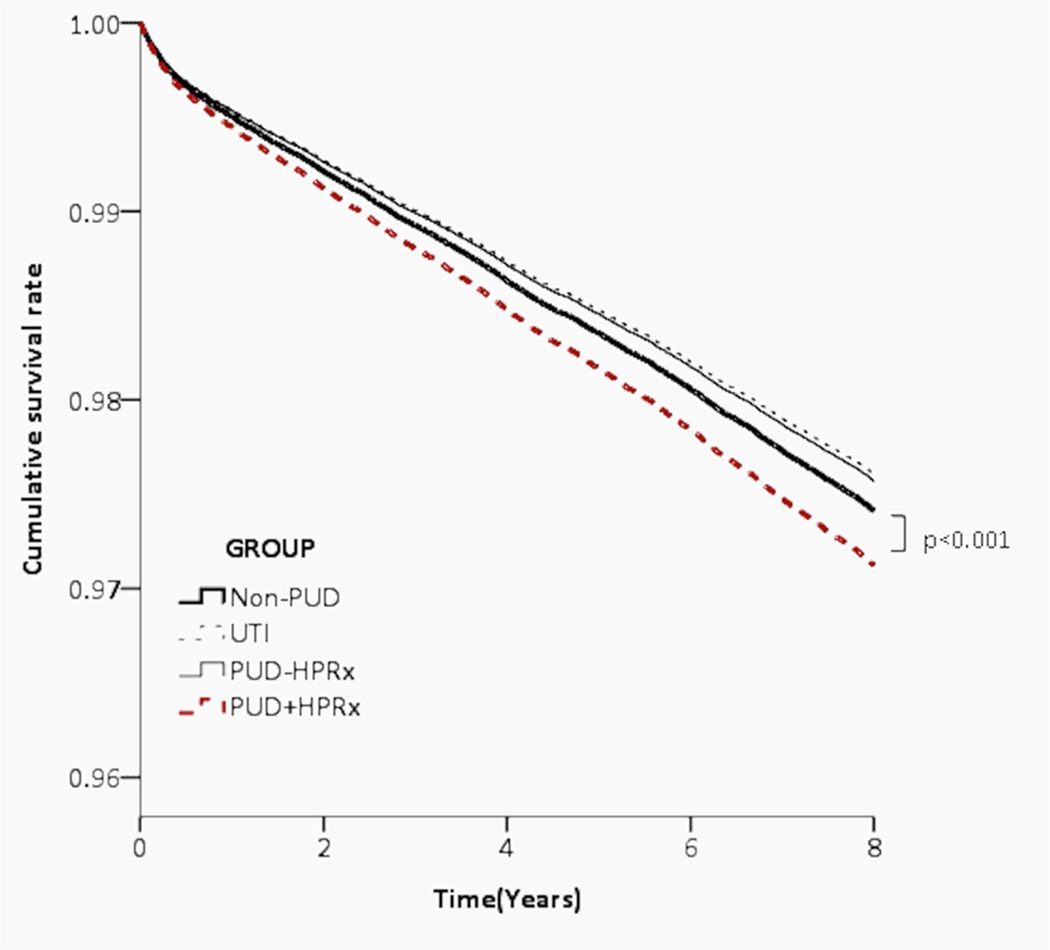
During the follow-up period, we estimated the survival rate of PUD+HPRx cohort compared to PUD-HPRx, UTI, Non-PUD control groups. We performed multiple regression to adjust for age, gender, insurance range, comorbidities, CCIS score, and medications and competing risk analysis to adjust for competing risk of death. We observed a small but significantly lower survival rate in patients who have ever received H pylori therapy (aHR= 1.11, 95% CI [1.04, 1.18], p=0.001) compared to the other three cohorts (Figure 3, Table 3).
Figure 3. Lower cumulative survival rate in PUD+HPRx individuals compared to PUD-HPRx, UTI, or Non-PUD control subjects.
Kaplan–Meier curves estimated the probability of survival rate, and the log-rank test was used for statistical analysis. A competing risk model was used to adjust for competing risk of death.
DISCUSSION
We found that PUD patients receiving H pylori treatment have a significant increase in the risk of developing AD and IBD compared to those never received H pylori treatment. The effect of antibiotic therapy on the risk of AD and IBD was assessed by adding a UTI cohort for comparison and the results showed that risk remained the highest in PUD+HPRx which supports the hypothesis that H pylori eradication may increase the risk of AD including IBD. There is also an increased risk of AD seen with UTI treatment using just one category of medication suggesting there may be an antibiotic effect which is consistent with published literature24. However, using the competing risk model to adjust for competing risk of death, we found that UTI treatment with antibiotics was associated with a lower risk of IBD when compared to Non-PUD control group, an observation that was consistent with an observation made by Ng SC et al. in Asia-Pacific CD cohorts25. There is a small increase in mortality of those individuals that received H pylori treatment but the reason is unknown as the database does not include the cause of death. A similar finding of increased mortality with H pylori therapy was reported by Ma JL et al. studying a large cohort of individuals treated with anti-H pylori therapy in China26. Our data support the current practice of checking H pylori only in symptomatic patients as H pylori treatment is associated with an increased risk of AD including IBD particular in H pylori endemic regions.
The World Health Organization classifies H pylori as a type I carcinogen based on prior studies showing that infected individuals are at increased risk of developing gastric cancer27, 28, however, the exact H pylori carcinogenic mechanisms remain unclear. Given that the majority of infected individuals will not develop H pylori-associated complications1 and the emerging evidence that H pylori infection may provide protection against chronic immune-mediated disorders, physicians are caught in a conundrum how to manage asymptomatic patients that were incidentally found to have H pylori infection. Further research is needed to propel us toward precision medicine to better identify and treat only patients with high-risk profiles for H pylori-associated complications.
Recent epidemiological data have all shown an exponentially rising incidence of IBD in Asia Pacific regions29. Our study revealed a small but significant increase in patients who have received H pylori therapy suggesting that other factors may have a greater impact on the increasing incidence of IBD in these regions. Ng SC et al. have recently shown several risk factors associated with IBD in Asia25. These include breastfeeding for less than 12 months, Westernized diet, no childhood pets, and living in urbanized cities. Another possible explanation is that the host immunomodulatory effect of H pylori infection occurs early in life and eradication of H pylori in adults only has a modest impact on their risk of immune-mediated disorders. Arnold IC et al. demonstrated in a mouse model of allergic airway disease that H pylori’s protection against the development of the disease was most robust in mice infected neonatally and was abrogated by antibiotic eradication of H pylori30. Thus, the exponentially rising incidence of IBD in Asia may be a result of increasing H pylori eradication in the prior generation.
An obvious strength of this study is the large sample size that made testing our main hypothesis feasible. However, several limitations must be noted. First, there are general limitations of claims based data, such as data entry errors and underreporting of diagnoses. Second, since the database lacks H pylori testing results, the following assumptions were made: 1) most patients diagnosed with PUD in Taiwan would be tested for H pylori, and no treatment assumed no H pylori infection; and 2) since the prevalence of H pylori infection in those over 30 years of age is 54.7% in Taiwan31, most of the patients without PUD are assumed to be H pylori-infected but not treated. Third, although we matched the study cohorts by age, gender, comorbidities, CCIS score, and medications, a generally acceptable method, it is not possible to fully account and correct for other potential confounders (e.g., smoking status, or diet) thus preventing causal inference from the observed associations. Fourth, there were apparent differences between the cohorts on age, insurance range, and Charlson’s comorbidity index scores after matching largely due to the subsequent inclusion of the UTI group which has a fewer number of cases. This is a potential confounding factor when interpreting the results comparing the risk of AD and IBD in the UTI group. Lastly, it is possible that our controls may have been treated before 2000 but this is unlikely to explain the significant increase in the risk of AD and IBD in the PUD+HPRx group. Further research using a large database with confirmed H pylori eradication is needed to verify our findings.
In the era of precision medicine, there is a need for further research to understand how to differentiate beneficial H pylori strains versus pathogenic strains and define the critical host factors which provide the “second hit” necessary for the development of gastroduodenal ulcers and malignancies. Meanwhile, the current recommendation of testing and treating only symptomatic patients or patients with a strong family history of gastric cancer32 and not asymptomatic individuals should still be followed as treatment may increase the risk of AD and IBD. Our finding also demonstrates the importance of our resident gut microbes in modulating one’s susceptibility to systemic immune-mediated disorders and the judicious use of antibiotics should continue to be emphasized in medical training to potentially limit the rapid rise of AD and IBD incidence.
What You Need to Know.
Background:
We aimed to determine the effects of treatment for H pylori infection on the incidence of autoimmune diseases and inflammatory bowel diseases.
Findings:
We collected data from the National Health Insurance Research Database in Taiwan and found that peptic ulcer disease with treatment of H pylori infection had the highest adjusted hazard risk for autoimmune and inflammatory bowel diseases (almost a 2.4-fold increase in risk), compared to peptic ulcer disease without treatment of H pylori infection or urinary tract infections treated with cephalosporin.
Implications for patient care:
Treatment for H pylori infection is associated with a significant increase in the risk for autoimmune and inflammatory bowels diseases.
GRANT SUPPORT:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01 DK087708-01 (J.Y.K.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS:
- aHR
Adjusted hazard risk
- AD
Autoimmune disease
- CCIS
Charlson’s comorbidity index score
- IBD
Inflammatory bowel disease
- ICD
International Classification of Disease
- NHIR
National Health Insurance Research
- NSAIDs
Nonsteroidal anti-inflammatory agents
- PUD
Patients with peptic ulcer disease
- PUD+HPRx
Patients with peptic ulcer disease and H pylori treatment
- PUD-HPRx
Patients with peptic ulcer disease and without H pylori treatment
- UTI
Patients with urinary tract infection and with antibiotic treatment
- Treg
Regulatory T cell
Footnotes
DISCLOSURES:
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 1991;302:1302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Giudice G, Covacci A, Telford JL, et al. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol 2001;19:523–63. [DOI] [PubMed] [Google Scholar]
- 3.Maixner F, Krause-Kyora B, Turaev D, et al. The 5300-year-old Helicobacter pylori genome of the Iceman. Science 2016;351:162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reibman J, Marmor M, Filner J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One 2008;3:e4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis 2008;198:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 2007;167:821–7. [DOI] [PubMed] [Google Scholar]
- 7.Radic M. Role of Helicobacter pylori infection in autoimmune systemic rheumatic diseases. World J Gastroenterol 2014;20:12839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.el-Omar E, Penman I, Cruikshank G, et al. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulphasalazine. Gut 1994;35:1385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halme L, Rautelin H, Leidenius M, et al. Inverse correlation between Helicobacter pylori infection and inflammatory bowel disease. J Clin Pathol 1996;49:65–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parente F, Molteni P, Bollani S, et al. Prevalence of Helicobacter pylori infection and related upper gastrointestinal lesions in patients with inflammatory bowel diseases. A cross-sectional study with matching. Scand J Gastroenterol 1997;32:1140–6. [DOI] [PubMed] [Google Scholar]
- 11.Pearce CB, Duncan HD, Timmis L, et al. Assessment of the prevalence of infection with Helicobacter pylori in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2000;12:439–43. [DOI] [PubMed] [Google Scholar]
- 12.Higgins PD, Johnson LA, Luther J, et al. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis 2011;17:1398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch KN, Hartung ML, Urban S, et al. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J Clin Invest 2015;125:3297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao JY, Rathinavelu S, Eaton KA, et al. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am J Physiol Gastrointest Liver Physiol 2006;291:G73–81. [DOI] [PubMed] [Google Scholar]
- 15.Paziak-Domanska B, Chmiela M, Jarosinska A, et al. Potential role of CagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cell Immunol 2000;202:136–9. [DOI] [PubMed] [Google Scholar]
- 16.Gebert B, Fischer W, Haas R. The Helicobacter pylori vacuolating cytotoxin: from cellular vacuolation to immunosuppressive activities. Rev Physiol Biochem Pharmacol 2004;152:205–20. [DOI] [PubMed] [Google Scholar]
- 17.Rad R, Brenner L, Bauer S, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 2006;131:525–37. [DOI] [PubMed] [Google Scholar]
- 18.Lundgren A, Suri-Payer E, Enarsson K, et al. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H pylori in infected individuals. Infect Immun 2003;71:1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–78. [DOI] [PubMed] [Google Scholar]
- 20.Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol 2014;20:6374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CW, Chang YS, Wu SC, et al. Helicobacter pylori in gastric biopsies of Taiwanese patients with gastroduodenal diseases. Jpn J Med Sci Biol 1998;51:13–23. [DOI] [PubMed] [Google Scholar]
- 22.Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis 2007;13:451–61. [DOI] [PubMed] [Google Scholar]
- 23.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424–9. [DOI] [PubMed] [Google Scholar]
- 24.Margolis DJ, Hoffstad O, Bilker W. Association or lack of association between tetracycline class antibiotics used for acne vulgaris and lupus erythematosus. Br J Dermatol 2007;157:540–6. [DOI] [PubMed] [Google Scholar]
- 25.Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 2015;64:1063–71. [DOI] [PubMed] [Google Scholar]
- 26.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–94. [DOI] [PubMed] [Google Scholar]
- 28.Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med 2009;151:121–8. [DOI] [PubMed] [Google Scholar]
- 29.Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013;145:158–165 e2. [DOI] [PubMed] [Google Scholar]
- 30.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest 2011;121:3088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi H, Bair MJ, Wu MS, et al. Prevalence of Helicobacter pylori infection in high-school students on Lanyu Island, Taiwan: risk factor analysis and effect on growth. J Formos Med Assoc 2009;108:929–36. [DOI] [PubMed] [Google Scholar]
- 32.Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212–239. [DOI] [PubMed] [Google Scholar]