Abstract
Objective
The aim of these analyses was to investigate the rate of decline in forced vital capacity (FVC) in patients with SSc-associated interstitial lung disease (SSc-ILD) with and without cough or dyspnoea in the SENSCIS trial.
Methods
Patients in the SENSCIS trial were randomized to receive nintedanib or placebo. Subgroups with and without cough or dyspnoea at baseline were defined by responses to the St George’s Respiratory Questionnaire.
Results
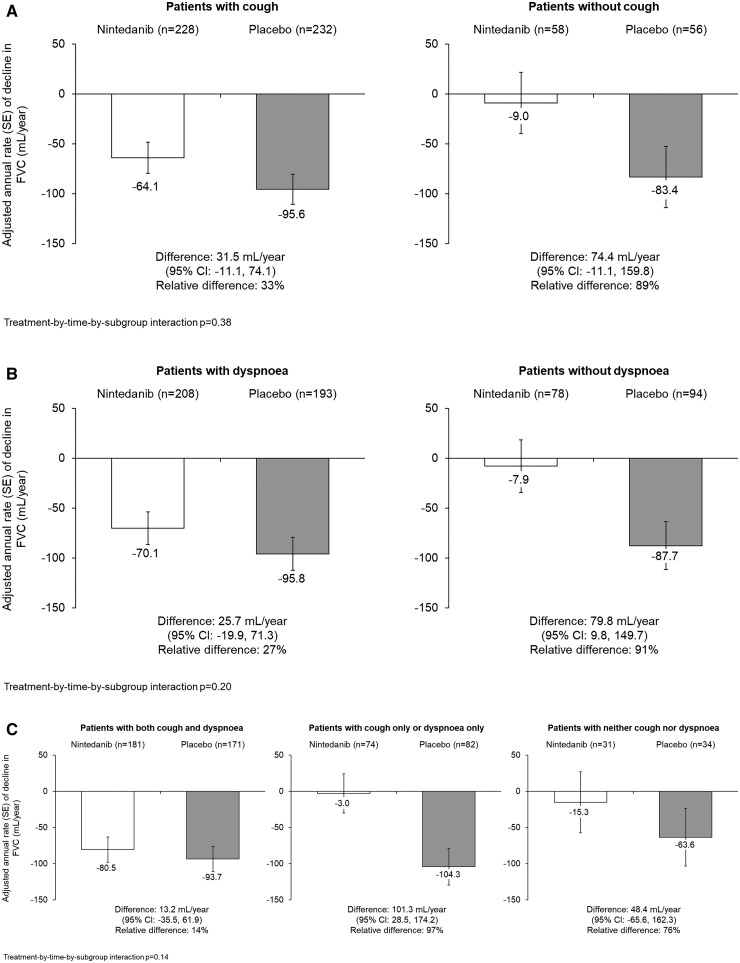
At baseline, 114/575 patients (19.8%) did not have cough and 172/574 patients (30.0%) did not have dyspnoea. In the placebo group, the rate of FVC decline over 52 weeks was similar in patients with and without cough (−95.6 and −83.4 mL/year, respectively) or dyspnoea (−95.8 and −87.7 mL/year, respectively). The effect of nintedanib vs placebo on reducing the rate of FVC decline was numerically more pronounced in patients without than with cough [difference: 74.4 (95% CI −11.1, 159.8) vs 31.5 (−11.1, 74.1)] and without than with dyspnoea [79.8 (9.8, 149.7) vs 25.7 (−19.9, 71.3)], but interaction P-values did not indicate heterogeneity in the treatment effect between these subgroups (P = 0.38 and P = 0.20, respectively).
Conclusion
In the placebo group of the SENSCIS trial, the rate of FVC decline was similar irrespective of the presence of cough or dyspnoea at baseline. The effect of nintedanib on reducing the rate of FVC decline was numerically more pronounced in patients without than with cough or dyspnoea at baseline, but no statistically significant heterogeneity was observed between the subgroups.
Trial registration
ClinicalTrials.gov, https://clinicaltrials.gov, NCT02597933.
Keywords: nintedanib, connective tissue diseases, vital capacity, systemic scleroderma
Rheumatology key messages.
The SENSCIS trial enrolled patients with systemic sclerosis–associated interstitial lung disease regardless of respiratory symptoms.
The rate of forced vital capacity (FVC) decline was similar irrespective of cough or dyspnoea.
The effect of nintedanib on FVC decline was similar irrespective of cough or dyspnoea.
Introduction
SSc is a rare and heterogeneous autoimmune disease characterized by immune dysregulation, microvascular damage and progressive fibrosis of the skin and internal organs [1]. Interstitial lung disease (ILD) is the leading cause of death in patients with SSc [2]. Dyspnoea and cough are common in patients with SSc-ILD [3–7] and have a negative impact on patients’ quality of life [4, 8–10]. Cough in patients with SSc-ILD may be due to the SSc-ILD itself [4–6], to gastroesophageal reflux disease (GERD) [11, 12], to other comorbidities [13], or to medication use [14]. Similarly, dyspnoea is a common manifestation of ILD, but may also occur due to comorbidities such as pulmonary hypertension or cardiac involvement [15, 16].
The severity and timing of onset of cough and dyspnoea in patients with SSc-ILD are variable. Many patients do not report symptoms even when ILD is evident on high-resolution CT (HRCT) and their lung function is impaired [7, 17–19]. This may be due in part to patients with SSc-ILD reducing activities that require exertion, or to limitations in the questionnaires used to assess respiratory symptoms.
The SENSCIS trial enrolled patients with and without respiratory symptoms, in contrast to Scleroderma Lung Studies I and II, which only enrolled patients with respiratory symptoms. In the SENSCIS trial, the rate of decline in forced vital capacity (FVC) (mL/year) over 52 weeks was reduced by 44% in patients randomized to nintedanib compared with placebo [20]. Furthermore, nintedanib reduced the risk of an absolute decline in FVC of ≥10% predicted or death over 52 weeks [21].
While there is some evidence that the presence of cough or dyspnoea may be associated with the progression of ILDs [22–25], the relationships between cough and dyspnoea and the course of SSc-ILD have not been established. The purpose of the present analyses was to assess the rate of FVC decline, and the effect of nintedanib on the rate of FVC decline, in patients with and without cough and dyspnoea at baseline in the SENSCIS trial.
Material and methods
Trial design
The design of the SENSCIS trial (NCT02597933) has been published, together with the trial protocol [20]. Briefly, eligible patients had SSc with onset of first non-RP symptom ≤7 years before screening, extent of fibrotic ILD ≥10% on HRCT (based on assessment of the whole lung), FVC ≥40% predicted, and diffusion capacity of the lung for carbon monoxide (DLCO) 30–89% predicted. Patients on prednisone ≤10 mg/day or equivalent and/or stable therapy with mycophenolate or MTX for ≥6 months were allowed to participate. Patients were randomized 1:1 to receive nintedanib or placebo stratified by the presence of anti-topo I antibody (ATA). Patients remained on blinded treatment until the last patient had reached week 52 but for ≤100 weeks. The trial was carried out in compliance with the principles of the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice of the International Conference on Harmonization. The trial was performed at 194 sites in 32 countries and was approved by an independent ethics committee or institutional review board at every site. The sites are listed in the primary manuscript on the trial results [20]. Patients provided written informed consent before trial entry.
Analyses
In posthoc analyses, we analysed the efficacy and safety of nintedanib in subgroups of patients with and without cough at baseline and with and without dyspnoea at baseline based on patients’ answers to the questions in the St George’s Respiratory Questionnaire (SGRQ) [26]. Patients who ticked boxes for ‘most days a week’, ‘several days a week’ or ‘a few days a month’ (rather than ‘only with chest infection’ or ‘not at all’) in response to the question ‘Over the last month, I have coughed …’ were considered to have cough. Patients who ticked these boxes in response to the question ‘Over the last month, I have had shortness of breath ….’ were considered to have dyspnoea.
We assessed the following outcomes in the nintedanib and placebo groups in subgroups with and without cough and with and without dyspnoea: rate of decline in FVC (mL/year) over 52 weeks; proportion of patients with absolute decline in FVC >5% predicted and >10% predicted at week 52; proportion of patients with relative decline in FVC >5% predicted and >10% predicted at week 52; time to absolute decline in FVC ≥10% predicted or death over 52 weeks; absolute change from baseline in SGRQ total score at week 52; proportion of patients with an absolute increase (worsening) in SGRQ total score of ≥4 points at week 52. An increase in SGRQ total score of ≥4 points has been suggested to represent a meaningful change in patients with idiopathic pulmonary fibrosis [27]. We also assessed the rate of decline in FVC over 52 weeks in subgroups with both cough and dyspnoea, either cough only or dyspnoea only, and neither cough nor dyspnoea at baseline.
The rate of decline in FVC (mL/year) over 52 weeks was analysed using a random coefficient regression model (with random slopes and intercepts) with fixed categorical effects of ATA status and sex, fixed continuous effects of baseline FVC (mL), age, and height and including baseline-by-time, treatment-by-subgroup and treatment-by-subgroup-by-time interaction terms. The analysis was based on all measurements taken over 52 weeks, including those from patients who discontinued trial medication, in patients who had a baseline and ≥1 post-baseline FVC measurement. The proportions of patients with absolute/relative declines in FVC >5% or >10% predicted and with an increase in SGRQ total score of ≥4 points at week 52 were compared between subgroups using a logistic regression model including terms for treatment, ATA status, subgroup and treatment-by-subgroup interaction. Odds ratios were estimated for the effect of treatment within each subgroup. Missing values were imputed using a worst-value-carried-forward approach.
The time to absolute decline in FVC ≥10% predicted or death over 52 weeks was analysed using a Cox proportional hazards model stratified by ATA status with terms for treatment, subgroup and treatment-by-subgroup interaction. Absolute change from baseline in SGRQ total score at week 52 was based on a mixed model for repeated measures (MMRM), with fixed categorical effects of ATA status, treatment-by-subgroup-by-visit interaction and a fixed continuous covariate of baseline SGRQ total score-by-visit interaction. For all analyses, an interaction test was applied to assess potential heterogeneity in the effect of nintedanib between the subgroups, with no adjustment for multiple testing. Adverse events were coded according to preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA) and are presented descriptively.
Results
Patients
Of 575 patients with information on cough and 574 patients with information on dyspnoea, 114 patients (19.8%) did not have cough and 172 patients (30.0%) did not have dyspnoea at baseline. Of 574 patients with information on both symptoms, 353 patients (61.5%) had both cough and dyspnoea, 156 patients (27.2%) had cough only or dyspnoea only, and 65 patients (11.3%) had neither cough nor dyspnoea. Of the patients with cough, 353 (76.6%) reported cough most or several days a week and 108 (23.4%) reported cough a few days a month. Of the patients who reported dyspnoea, 283 (70.4%) reported dyspnoea most or several days a week and 119 (29.6%) reported dyspnoea a few days a month.
At baseline, in patients with and without cough, respectively, the mean (s.d.) extent of fibrotic ILD on HRCT was 37.2 (21.3)% and 30.7 (20.3)%, the mean (s.d.) FVC was 71.5 (16.1) and 76.7 (18.3)% predicted, the mean (s.d.) high-sensitivity CRP was 5.6 (9.7) and 9.6 (29.2) mg/l, 66.6% and 46.5% had GERD, 80.3% and 77.2% were taking drugs for gastric acid–related disorders, 50.1% and 49.1% were taking CSs, 48.4% and 49.1% were taking mycophenolate, and 8.7% and 6.1% were taking angiotensin-converting enzyme (ACE) inhibitors (Table 1). Among the patients who reported cough on their baseline SGRQ, 10.0% in the nintedanib group and 9.1% in the placebo group had cough noted as a ‘baseline medical condition’ by the investigator in the case report form completed at screening; among patients who did not report cough on their baseline SGRQ, these proportions were 5.2% and 1.8%, respectively.
Table 1.
Baseline characteristics in subgroups by cough at baseline
|
Patients with cough
a
|
Patients without cough
a
|
|||
|---|---|---|---|---|
| Nintedanib (n = 229) | Placebo (n = 232) | Nintedanib (n = 58) | Placebo (n = 56) | |
| Age (years) | 54.8 (11.8) | 53.5 (12.8) | 53.8 (11.9) | 52.6 (11.8) |
| Female | 172 (75.1) | 166 (71.6) | 49 (84.5) | 46 (82.1) |
| Years since onset of first non-RP symptom | 3.5 (1.6) | 3.5 (1.8) | 3.5 (1.6) | 3.5 (1.8) |
| dcSSc | 120 (52.4) | 122 (52.6) | 32 (55.2) | 24 (42.9) |
| ATA positive | 138 (60.3) | 142 (61.2) | 35 (60.3) | 35 (62.5) |
| High-sensitivity CRPb | 5.3 (8.9) | 5.9 (10.6) | 8.4 (19.5) | 10.9 (36.9) |
| Extent of fibrotic ILD on HRCTc | 38.5 (21.8) | 36.0 (20.7) | 29.7 (20.1) | 31.9 (20.7) |
| FVC (mL) | 2443 (701) | 2535 (839) | 2511 (867) | 2565 (714) |
| FVC % predicted | 71.3 (15.8) | 71.8 (16.4) | 77.0 (19.6) | 76.4 (17.1) |
| DLCO % predictedd,e | 51.8 (14.7) | 53.1 (15.2) | 57.5 (15.6) | 53.5 (14.9) |
| SpO2 (%)f | 97.6 (1.8) | 97.5 (2.6) | 97.7 (2.2) | 97.6 (2.4) |
| mRSSg | 11.6 (9.4) | 10.7 (8.7) | 10.1 (8.3) | 11.6 (9.3) |
| SGRQ total scoref | 43.9 (18.8) | 42.3 (20.3) | 28.0 (20.7) | 27.1 (19.4) |
| Phlegmh | 154 (67.2) | 152 (65.5) | 7 (12.1) | 13 (23.2) |
| Asthmai | 12 (5.2) | 8 (3.4) | 2 (3.4) | 0 |
| Gastroesophageal reflux diseasej | 158 (69.0) | 149 (64.2) | 27 (46.6) | 26 (46.4) |
| Drugs for gastric acid–related disordersk | 183 (79.9) | 187 (80.6) | 45 (77.6) | 43 (76.8) |
| Immunosuppressantsk | 198 (86.5) | 195 (84.1) | 54 (93.1) | 47 (83.9) |
| CSsk | 124 (54.1) | 107 (46.1) | 28 (48.3) | 28 (50.0) |
| Inhaled CSsl | 34 (14.8) | 30 (12.9) | 5 (8.6) | 4 (7.1) |
| Mycophenolate (mofetil or sodium) | 109 (47.6) | 114 (49.1) | 30 (51.7) | 26 (46.4) |
| ACE inhibitorsm | 22 (9.6) | 18 (7.8) | 3 (5.2) | 4 (7.1) |
| Cough reported by investigator at screeningn | 23 (10.0) | 21 (9.1) | 3 (5.2) | 1 (1.8) |
Data are n (%) or mean (s.d.).
Information on cough at baseline was missing for 1 patient in the nintedanib group.
Normal range: ≤4.99 mg/L; data missing for 45 patients.
Assessed in whole lung to nearest 5% by central review. Pure (non-fibrotic) ground-glass opacity was not included.
Corrected for haemoglobin.
Data missing for 7 patients.
Data missing for 10 patients.
Data missing for 2 patients.
Patients who ticked boxes for ‘most days a week’, ‘several days a week’ or ‘a few days a month’ (rather than ‘only with chest infection’ or ‘not at all’) to the question ‘Over the last month, I have brought up phlegm …’ were considered to have phlegm at baseline.
Based on preferred term in the Medical Dictionary for Regulatory Activities (MedDRA).
Oesophageal involvement (dysphagia, reflux) reported at screening.
Customized drug grouping.
WHO Anatomical Therapeutic Chemical (ATC) codes for ‘adrenergics, inhalants’ and ‘other drugs for obstructive airway diseases, inhalants’ within the customized drug grouping ‘corticosteroids’.
mWHO ATC codes for ‘ACE inhibitors, plain’ and ‘ACE inhibitors, combinations’ within the customized drug grouping ‘antihypertensives’.
Based on MedDRA preferred terms ‘cough’, ‘productive cough’ and ‘upper−airway cough syndrome’. ACE, angiotensin-converting enzyme; ATA, anti-topo 1 antibody; DLCO, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; HRCT, high-resolution CT; ILD, interstitial lung disease; mRSS, modified Rodnan skin score; SGRQ, St George’s Respiratory questionnaire; SpO2, oxygen saturation.
At baseline, in patients with and without dyspnoea, respectively, the mean (s.d.) extent of fibrotic ILD on HRCT was 37.7 (21.7)% and 31.6 (19.4)%, the mean (s.d.) FVC was 71.0 (16.3)% and 76.5 (16.8)% predicted, the mean (s.d.) high-sensitivity CRP was 5.8 (10.3) and 7.8 (24.3) mg/l, 68.9% and 47.7% had GERD, 82.8% and 72.1% were taking drugs for gastric acid–related disorders, 53.5% and 41.9% were taking CSs, and 50.0% and 44.8% of patients were taking mycophenolate (Table 2). Among the patients who reported dyspnoea on their baseline SGRQ, 13.4% in the nintedanib group and 7.3% in the placebo group had dyspnoea noted as a ‘baseline medical condition’ by the investigator in the case report form completed at screening; among those who did not report dyspnoea on their baseline SGRQ, these proportions were 5.1% and 3.2%, respectively.
Table 2.
Baseline characteristics in subgroups by dyspnoea at baseline
|
Patients with dyspnoea
a
|
Patients without dyspnoea
a
|
|||
|---|---|---|---|---|
| Nintedanib (n = 209) | Placebo (n = 193) | Nintedanib (n = 78) | Placebo (n = 94) | |
| Age (years) | 54.8 (12.0) | 53.6 (12.7) | 53.9 (11.2) | 53.0 (12.5) |
| Female | 159 (76.1) | 137 (71.0) | 62 (79.5) | 74 (78.7) |
| Years since onset of first non-RP symptom | 3.4 (1.6) | 3.5 (1.7) | 3.6 (1.7) | 3.4 (1.9) |
| dcSSc | 113 (54.1) | 95 (49.2) | 39 (50.0) | 50 (53.2) |
| ATA positive | 132 (63.2) | 113 (58.5) | 41 (52.6) | 63 (67.0) |
| High-sensitivity CRPb | 5.5 (9.4) | 6.0 (11.2) | 6.8 (16.8) | 8.6 (29.4) |
| Extent of fibrotic ILD on HRCTc | 38.7 (21.8) | 36.6 (21.6) | 31.3 (20.8) | 31.9 (18.3) |
| FVC (mL) | 2395 (672) | 2531 (829) | 2623 (871) | 2570 (790) |
| FVC % predicted | 70.6 (16.1) | 71.4 (16.6) | 77.4 (17.5) | 75.6 (16.1) |
| DLCO % predictedd,e | 50.9 (14.7) | 50.9 (14.2) | 58.4 (14.8) | 58.2 (15.7) |
| SpO2 (%)f | 97.6 (1.8) | 97.6 (2.4) | 97.6 (2.1) | 97.3 (2.7) |
| mRSSg | 11.3 (9.3) | 10.5 (9.0) | 11.4 (8.9) | 11.7 (8.6) |
| SGRQ total scoref | 47.8 (16.9) | 45.8 (18.7) | 21.6 (15.4) | 25.9 (18.8) |
| Asthmah | 12 (5.7) | 7 (3.6) | 2 (2.6) | 1 (1.1) |
| Gastroesophageal reflux diseasei | 147 (70.3) | 130 (67.4) | 38 (48.7) | 44 (46.8) |
| Drugs for gastric acid–related disordersj | 174 (83.3) | 159 (82.4) | 54 (69.2) | 70 (74.5) |
| Immunosuppressantsj | 185 (88.5) | 167 (86.5) | 67 (85.9) | 74 (78.7) |
| CSsj | 121 (57.9) | 94 (48.7) | 31 (39.7) | 41 (43.6) |
| Inhaled CSsk | 36 (17.2) | 28 (14.5) | 3 (3.8) | 6 (6.4) |
| Mycophenolate (mofetil or sodium) | 102 (48.8) | 99 (51.3) | 37 (47.4) | 40 (42.6) |
| ACE inhibitorsl | 17 (8.1) | 11 (5.7) | 8 (10.3) | 11 (11.7) |
| Dyspnoea reported by investigator at screeningm | 28 (13.4) | 14 (7.3) | 4 (5.1) | 3 (3.2) |
Data are n (%) or mean (s.d.).
Information on dyspnoea at baseline was missing for 1 patient each in the nintedanib and placebo groups.
Normal range: ≤4.99 mg/L; data missing for 45 patients.
Assessed in whole lung to nearest 5% by central review. Pure (non-fibrotic) ground-glass opacity was not included.
Corrected for haemoglobin.
Data missing for 7 patients.
Data missing for 10 patients.
Data missing for 2 patients.
Based on preferred term in the Medical Dictionary for Regulatory Activities (MedDRA).
Oesophageal involvement (dysphagia, reflux) reported at screening.
Customized drug grouping.
WHO Anatomical Therapeutic Chemical (ATC) codes for ‘adrenergics, inhalants’ and ‘other drugs for obstructive airway diseases, inhalants’ within the customized drug grouping ‘corticosteroids’.
WHO ATC codes for ‘ACE inhibitors, plain’ and ‘ACE inhibitors, combinations’ within the customized drug grouping ‘antihypertensives’.
Based on MedDRA preferred terms ‘dyspnoea’ and ‘dyspnoea exertional’.
ACE, angiotensin-converting enzyme; ATA, anti-topo 1 antibody; DLCO, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; HRCT, high-resolution CT; ILD, interstitial lung disease; mRSS, modified Rodnan skin score; SGRQ, St George’s Respiratory questionnaire; SpO2, oxygen saturation.
Outcomes in subgroups by cough at baseline
In the placebo group, the rate of decline in FVC (mL/year) over 52 weeks was similar in patients with and without cough at baseline (Fig. 1A). The effect of nintedanib vs placebo on reducing the rate of decline in FVC (mL/year) was numerically more pronounced in patients without than with cough, but the exploratory interaction P-value did not indicate heterogeneity in the treatment effect between these subgroups (P = 0.38) (Fig. 1A). The proportions of patients with absolute/relative declines in FVC >5% or >10% predicted were similar or lower in patients treated with nintedanib than placebo in patients with and without cough at baseline, with no heterogeneity detected between the subgroups (Table 3). Similarly, no heterogeneity was detected between these subgroups in the time to an absolute decline in FVC ≥10% predicted or death (P = 0.33) (Table 3).
Fig. 1.
Rate of decline in FVC (mL/year) over 52 weeks in subgroups by respiratory symptoms at baseline in the SENSCIS trial
Rate of decline in FVC (mL/year) over 52 weeks in subgroups by (A) cough at baseline, (B) dyspnoea at baseline, and (C) both cough and dyspnoea, cough only or dyspnoea only, or neither cough nor dyspnoea. FVC, forced vital capacity.
Table 3.
Outcomes in subgroups by cough at baseline
| Patients with cough |
Patients without cough |
|||
|---|---|---|---|---|
| Nintedanib (n = 228) |
Placebo
(n = 232) |
Nintedanib
(n = 58) |
Placebo
(n = 56) |
|
| Rate of decline in FVC (mL/year) over 52 weeks, adjusted rate (s.e.) | −64.1 (15.6) | −95.6 (15.1) | −9.0 (30.7) | −83.4 (30.8) |
| Difference (95% CI) | 31.5 (−11.1, 74.1) | 74.4 (−11.1, 159.8) | ||
| Treatment-by-time-by-subgroup interaction | P = 0.38 | |||
| Relative decline in FVC (mL) >5% at week 52, n (%) | 80 (35.1) | 100 (43.1) | 15 (25.9) | 25 (44.6) |
| Odds ratio (95% CI) | 0.71 (0.49, 1.04) | 0.43 (0.20, 0.95) | ||
| Treatment-by-subgroup interaction | P = 0.26 | |||
| Relative decline in FVC (mL) >10% at week 52, n (%) | 41 (18.0) | 42 (18.1) | 7 (12.1) | 10 (17.9) |
| Odds ratio (95% CI) | 0.99 (0.62, 1.60) | 0.63 (0.22, 1.81) | ||
| Treatment-by-subgroup interaction | P = 0.44 | |||
| Absolute decline in FVC >5% predicted at week 52, n (%) | 49 (21.5) | 65 (28.0) | 10 (17.2) | 17 (30.4) |
| Odds ratio (95% CI) | 0.70 (0.46, 1.08) | 0.48 (0.20, 1.17) | ||
| Treatment-by-subgroup interaction | P = 0.45 | |||
| Absolute decline in FVC >10% predicted at week 52, n (%) | 17 (7.5) | 19 (8.2) | 3 (5.2) | 5 (8.9) |
| Odds ratio (95% CI) | 0.90 (0.46, 1.78) | 0.55 (0.13, 2.44) | ||
| Treatment-by-subgroup interaction | P = 0.56 | |||
| Absolute decline in FVC ≥10% predicted or death at week 52, n (%) | 34 (14.8)a | 49 (21.1) | 6 (10.3) | 13 (23.2) |
| Hazard ratio (95% CI)b | 0.71 (0.46, 1.10) | 0.41 (0.16, 1.09) | ||
| Treatment-by-subgroup interaction | P = 0.33 | |||
| Absolute change from baseline in SGRQ total score at week 52, adjusted mean (s.e.)c | 0.2 (1.0) | −1.2 (1.0) | 3.1 (2.0) | 0.5 (2.1) |
| Difference (95% CI) | 1.4 (−1.3, 4.1) | 2.6 (−3.0, 8.1) | ||
| Treatment-by-time-by-subgroup interaction | P = 0.72 | |||
| Absolute increase from baseline in SGRQ total score of ≥4 points at week 52, n (%)d | 87 (39.9) | 72 (31.9) | 24 (44.4) | 21 (40.4) |
| Odds ratio (95% CI) | 1.42 (0.96, 2.09) | 1.18 (0.54, 2.55) | ||
| Treatment-by-subgroup interaction | 0.68 | |||
Analysed in 229 patients in the nintedanib group with cough at baseline.
Time-to-event analysis.
Analysed in 226 patients in the nintedanib group and 230 patients in the placebo group with cough at baseline, and 56 patients in the nintedanib group and 53 patients in the placebo group without cough at baseline.
Analysed in 218 patients in the nintedanib group and 226 patients in the placebo group with cough at baseline, and 54 patients in the nintedanib group and 52 patients in the placebo group without cough at baseline. FVC, forced vital capacity; SGRQ, St George’s Respiratory Questionnaire.
Changes in SGRQ total score at week 52 were small in both the nintedanib and placebo groups in both subgroups by cough at baseline. No heterogeneity was detected in the effect of nintedanib on the absolute change in SGRQ total score between the subgroups by cough (Table 3). The proportion of patients with an increase (worsening) in SGRQ total score of ≥4 points at week 52 was similar in patients treated with nintedanib and placebo both in patients with and without cough, with no heterogeneity detected between the subgroups (Table 3).
The adverse event profile of nintedanib was similar in the subgroups by cough (Supplementary Table S1, available at Rheumatology online). The proportions of patients with adverse events that led to discontinuation of trial medication in the nintedanib and placebo groups, respectively, were 15.7% and 6.5% in patients with cough at baseline and 17.2% and 17.9% in those without cough at baseline.
Outcomes in subgroups by dyspnoea at baseline
In the placebo group, the rate of decline in FVC (mL/year) was similar in patients with and without dyspnoea at baseline (Fig. 1B). The effect of nintedanib vs placebo on reducing the rate of decline in FVC was numerically more pronounced in patients without than with dyspnoea, but the exploratory interaction P-value did not indicate heterogeneity in the treatment effect between these subgroups (P = 0.20) (Fig. 1B). The proportions of patients with absolute/relative declines in FVC >5% or >10% predicted were similar or lower in patients treated with nintedanib than placebo in patients with and without dyspnoea at baseline, with no heterogeneity detected between the subgroups (Table 4). Similarly, no heterogeneity was detected between these subgroups in the time to an absolute decline in FVC ≥10% predicted or death (P = 0.81) (Table 4).
Table 4.
Outcomes in subgroups by dyspnoea at baseline
| Patients with dyspnoea |
Patients without dyspnoea |
|||
|---|---|---|---|---|
| Nintedanib (n = 208) | Placebo (n = 193) | Nintedanib (n = 78) | Placebo (n = 94) | |
| Rate of decline in FVC (mL/year) over 52 weeks, adjusted mean (s.e.) | −70.1 (16.3) | −95.8 (16.5) | −7.9 (26.4) | −87.7 (23.9) |
| Difference (95% CI) | 25.7 (−19.9, 71.3) | 79.8 (9.8, 149.7) | ||
| Treatment-by-time-by-subgroup interaction | P = 0.20 | |||
| Relative decline in FVC (mL) >5% at week 52, n (%) | 77 (37.0) | 84 (43.5) | 18 (23.1) | 40 (42.6) |
| Odds ratio (95% CI) | 0.76 (0.51, 1.13) | 0.41 (0.21, 0.80) | ||
| Treatment-by-subgroup interaction | P = 0.13 | |||
| Relative decline in FVC (mL) >10% at week 52, n (%) | 40 (19.2) | 39 (20.2) | 8 (10.3) | 13 (13.8) |
| Odds ratio (95% CI) | 0.93 (0.57, 1.52) | 0.74 (0.29, 1.89) | ||
| Treatment-by-subgroup interaction | P = 0.67 | |||
| Absolute decline in FVC >5% predicted at week 52, n (%) | 48 (23.1) | 58 (30.1) | 11 (14.1) | 24 (25.5) |
| Odds ratio (95% CI) | 0.69 (0.44, 1.07) | 0.50 (0.23, 1.10) | ||
| Treatment-by-subgroup interaction | P = 0.49 | |||
| Absolute decline in FVC >10% predicted at week 52, n (%) | 18 (8.7) | 17 (8.8) | 2 (2.6) | 7 (7.4) |
| Odds ratio (95% CI) | 0.99 (0.49, 1.99) | 0.32 (0.06, 1.57) | ||
| Treatment-by-subgroup interaction | P = 0.20 | |||
| Absolute decline in FVC ≥10% predicted or death at week 52, n (%) | 33 (15.8)a | 47 (24.4) | 7 (9.0) | 15 (16.0) |
| Hazard ratio (95% CI)b | 0.63 (0.40, 0.99) | 0.48 (0.19, 1.20) | ||
| Treatment-by-subgroup interaction | P = 0.81 | |||
| Absolute change from baseline in SGRQ total score at week 52, adjusted mean (s.e.)c | 0.0 (1.1) | −0.7 (1.1) | 2.9 (1.8) | −1.3 (1.6) |
| Difference (95% CI) | 0.7 (−2.2, 3.6) | 4.3 (−0.2, 8.8) | ||
| Treatment-by-time-by-subgroup interaction | P = 0.19 | |||
| Absolute increase from baseline in SGRQ total score of ≥4 points at week 52, n (%)d | 77 (39.1) | 63 (33.7) | 34 (45.3) | 30 (33.3) |
| Odds ratio (95% CI) | 1.27 (0.84, 1.93) | 1.63 (0.86, 3.07) | ||
| Treatment-by-subgroup interaction | P = 0.52 | |||
Analysed in 209 patients in the nintedanib group with dyspnoea at baseline.
Time-to-event analysis.
Analysed in 206 patients in the nintedanib group and 190 patients in the placebo group with dyspnoea at baseline, and 76 patients in the nintedanib group and 92 patients in the placebo group without dyspnoea at baseline.
Analysed in 197 patients in the nintedanib group and 187 patients in the placebo group with dyspnoea at baseline, and 75 patients in the nintedanib group and 90 patients in the placebo group without dyspnoea at baseline. FVC, forced vital capacity; SGRQ, St George’s Respiratory Questionnaire.
Changes in SGRQ total score at week 52 were small in both the nintedanib and placebo groups in both subgroups by dyspnoea at baseline. No heterogeneity was detected in the effect of nintedanib vs placebo on the absolute change in SGRQ total score between the subgroups (Table 4). The proportion of patients with an increase (worsening) in SGRQ total score of ≥4 points at week 52 was similar in patients treated with nintedanib and placebo both in patients with and without dyspnoea at baseline, with no heterogeneity detected between the subgroups (Table 4).
The adverse event profile of nintedanib was similar in the subgroups by dyspnoea (Supplementary Table S2, available at Rheumatology online). The proportions of patients with adverse events that led to discontinuation of trial medication in the nintedanib and placebo groups, respectively, were 17.7% and 7.8% in patients with dyspnoea at baseline and 11.5% and 10.6% in those without dyspnoea at baseline.
Outcomes in subgroups by cough and dyspnoea at baseline
In the placebo group, the rate of decline in FVC was similar in patients who had both cough and dyspnoea, cough only or dyspnoea only, or neither cough nor dyspnoea (Fig. 1C). The effect of nintedanib vs placebo on reducing the rate of decline in FVC was numerically more pronounced in patients with fewer or no symptoms, but the exploratory interaction P-value did not indicate heterogeneity in the treatment effect across subgroups (P = 0.14).
Discussion
Our analyses demonstrated that patients with SSc-ILD enrolled in the SENSCIS trial who had cough or dyspnoea at baseline had a numerically greater extent of fibrotic ILD and a numerically lower FVC % predicted and DLCO % predicted at baseline than patients without these symptoms. Data from Scleroderma Lung Studies I and II, which enrolled patients with SSc-ILD and exertional dyspnoea based on Mahler’s Dyspnoea Index, indicated that the presence of cough at baseline (based on cough severity index or on responses to the questions about cough and phlegm in the SGRQ) was associated with lower DLCO, worse dyspnoea and a greater extent of fibrosis on HRCT, but not with lower FVC [5, 10]. It should be noted that in the SENSCIS trial, both the patients who did not have cough and the patients who did not have dyspnoea had a mean extent of fibrotic ILD (based on assessment of the whole lung) of over 30%. These findings highlight that respiratory symptoms may be a late presentation of SSc-ILD, at least in a subgroup of patients, and support the screening of all patients with SSc for ILD, irrespective of symptoms, as recommended by experts [28–30].
Among patients in the SENSCIS trial who reported cough or dyspnoea in response to the question in the SGRQ, only around 10% had that symptom recorded by the investigator. The reasons for this difference are unknown, but it is possible that the investigators did not ask the patients explicit questions about these symptoms at screening. This observation suggests that patient-reported-outcomes measures such as the SGRQ may support physicians in identifying patients with these symptoms in routine clinical practice.
In our analyses, no difference in the rate of loss of lung function over the following year was detected between subgroups based on the presence of cough or dyspnoea at baseline. This is consistent with an analysis of data from 535 patients with SSc-ILD in the EUSTAR database, which found no significant difference in the 5-year decline in FVC between patients with or without dyspnoea at baseline [31]. These findings highlight the importance of regular monitoring of all patients with SSc-ILD for progression, not just those with severe or symptomatic disease [28].
Compared with patients who received placebo, patients treated with nintedanib had a lower rate of decline in FVC across the subgroups. Interestingly, we observed numerically greater effects of nintedanib in the subgroups without dyspnoea and without cough, despite these subgroups having less severe disease at baseline based on the extent of fibrotic ILD on HRCT. These results suggest that the presence of cough or dyspnoea alone should not be used as an indicator of when SSc-ILD is ‘severe’ enough to warrant treatment. Experts in the field have proposed that the initiation of treatment for SSc-ILD should be based on more than one measure of disease severity (e.g. extent of fibrosis on HRCT, pulmonary function tests, symptoms) as well as on other factors, including risk factors for progression and patient preferences [28, 30, 32]. Prompt treatment of SSc-ILD may help to preserve lung function and ultimately improve outcomes.
Consistent with previous studies [5, 7], there was a high prevalence of cough (80%) and dyspnoea (70%) in the SENSCIS trial. Managing symptoms and maintaining patients’ quality of life should be part of a holistic approach to the care of patients with SSc-ILD [33]. However, alleviation of cough and dyspnoea in patients with ILD is challenging given the lack of treatments with robust evidence of efficacy [6, 34]. In Scleroderma Lung Study II, patients with SSc-ILD and exertional dyspnoea who were treated with CYC or mycophenolate for 24 months experienced significant improvements in dyspnoea (based on the Transitional Dyspnoea Index) and in SGRQ total score, but there was no placebo control [35]. In the SENSCIS trial, there was no statistically significant effect of nintedanib on change in SGRQ total score [20], and no heterogeneous effect of nintedanib on change in SGRQ total score between patients with or without cough or dyspnoea at baseline was detected.
Limitations of our analyses include that they were not powered for formal statistical testing of the individual subgroups, and the interaction P-values should be regarded as exploratory. Our analyses were not adjusted for the presence of comorbidities (such as GERD, pulmonary hypertension, or cardiac involvement) that may have an impact on respiratory symptoms or the course of FVC decline, nor for the use of comedications. The presence of cough and dyspnoea at baseline were based solely on responses to two questions in the SGRQ, which have not been validated as a means of determining the presence of cough or dyspnoea in patients with ILDs. We only investigated subgroups based on the presence of cough or dyspnoea at baseline and did not consider whether development or resolution of cough during the trial was associated with a different course of SSc-ILD.
In conclusion, in patients with SSc-ILD in the SENSCIS trial, patients with cough or dyspnoea at baseline had a numerically greater extent of fibrotic ILD and numerically lower FVC % predicted than patients without dyspnoea or cough, but patients without cough or dyspnoea also showed considerable fibrosis on HRCT and impairment in FVC. The rate of decline in FVC in the placebo group was numerically similar irrespective of the presence of cough or dyspnoea at baseline. The effect of nintedanib on reducing the rate of FVC decline was numerically more pronounced in patients without than with cough or dyspnoea at baseline, but no statistically significant heterogeneity was observed between these subgroups. These data suggest that the presence of cough or dyspnoea alone should not be used to inform when to initiate nintedanib in patients with SSc-ILD. More research is needed to understand the underlying mechanisms and potential prognostic value of cough and dyspnoea in patients with SSc-ILD.
Supplementary Material
Acknowledgement
We thank the patients who participated in the SENSCIS trial. The SENSCIS trial was funded by Boehringer Ingelheim International GmbH (BI). The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment for development of this manuscript. Writing assistance was provided by Elizabeth Ng and Wendy Morris of FleishmanHillard, London, UK, which was contracted and funded by BI. Support with statistical programming was provided by Rafal Falkowski on behalf of BI. BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Contributor Information
Elizabeth R Volkmann, Department of Medicine, Division of Rheumatology, University of California, David Geffen School of Medicine, Los Angeles, CA, USA.
Michael Kreuter, Center for Interstitial and Rare Lung Diseases, Pneumology and Respiratory Care Medicine, Thoraxklinik, University of Heidelberg, Member of the German Center for Lung Research, Heidelberg, Germany.
Anna M Hoffmann-Vold, Department of Rheumatology, Oslo University Hospital, Oslo, Norway.
Marlies Wijsenbeek, Department of Respiratory Medicine, Erasmus MC, University Medical Centre, Rotterdam, The Netherlands.
Vanessa Smith, Department of Rheumatology and Internal Medicine, Ghent University Hospital, Ghent, Belgium.
Dinesh Khanna, Department of Medicine, University of Michigan, Ann Arbor, MI, USA.
Christopher P Denton, University College London Division of Medicine, Centre for Rheumatology and Connective Tissue Diseases, London, UK.
Wim A Wuyts, Unit for Interstitial Lung Diseases, Department of Pulmonary Medicine, University Hospitals Leuven, Leuven, Belgium.
Corinna Miede, mainanalytics GmbH, Sulzbach (Taunus).
Margarida Alves, Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany.
Steven Sambevski, Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany.
Yannick Allanore, Department of Rheumatology A, Descartes University, APHP, Cochin Hospital, Paris, France.
Funding: This work was supported by Boehringer Ingelheim International GmbH (BI). The authors did not receive payment for development of this manuscript.
Disclosure statement: E.R.V. reports grants from Forbius and Kadmon and has served on advisory boards and received payment for lectures from BI. M.K. reports grants, consulting fees and payment for presentations from BI and Roche; he has a leadership or fiduciary role with the German Respiratory Society and Deutsche gesellschaft für Pneumologiex. A.M.H.-V. reports grants, consulting fees, support for attending meetings, and payment for presentations from BI; consulting fees, support for attending meetings and payment for presentations from Actelion, Medscape, Roche; consulting fees and payment for presentations from Arxx Therapeutics, Bayer, Lilly, Merck Sharp & Dohme; she has a leadership or fiduciary role with EUSTAR, the Nordic PH vision group, and the Norwegian SSc study group. M.W. reports grants, consulting fees, support for attending meetings and payment for presentations from BI and Hoffmann-la Roche; and grants from The Netherlands Organization for Health Research and Development, The Dutch Lung Foundation, The Dutch Pulmonary Fibrosis Patient Association, The Thorax Foundation, ErasmusMC and Sarcoidosis.nl; she has received consulting fees and served on a Data Safety Monitoring Board or Advisory Board with Galapagos; she has received consulting fees from Bristol-Myers Squibb, Galecto, Respivant; she has received payment for presentations from Novartis; she has served on a Data Safety Monitoring Board or Advisory Board with Savara; she is secretary of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society, a member of the board of the Netherlands Respiratory Society, a member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and Related Disorders Federation, chair of the educational committee of the European Reference Network for Rare Lung Diseases, and a member of the advisory board of the Dutch lung fibrosis and sarcoidosis patient associations. V.S. reports grants and speaker fees from Janssen-Cilag; grants from the Belgian Fund for Scientific Research in Rheumatic Diseases; research support from Research Foundation Flanders; research support, consulting fees, support for attending meetings and speaker fees from BI; payment for presentations from Accord Healthcare and UCB; support for attending meetings from Celgene; she is chair of the EULAR study group on microcirculation in rheumatic diseases and co-chair of the ACR study Group on microcirculation and the SCTC working group on capillaroscopy. D.K. reports grants and consultancy fees from Bristol-Myers Squibb; grants, consulting fees and payment for presentations from Horizon Therapeutics; grants from Pfizer; royalties or licences from the University of California Los Angeles Scleroderma Clinical Trials Consortium Gastrointestinal Tract 2.0; consulting fees and payment for presentations from AbbVie, Actelion, BI, CSL Behring, Genentech; consulting fees from Talaris and Theraly; he has a leadership or fiduciary role and owns stock in Eicos Sciences. C.P.D. reports grants and consulting fees from CSL Behring, GlaxoSmithKline, Inventiva; grants from Arxx Therapeutics and Servier; consulting fees and payment for presentations from BI and Corbus; consulting fees from AbbVie, Acceleron Pharma, Bayer, Horizon Therapeutics, Roche and Sanofi; and payment for presentations from Janssen. W.A.W. reports grants from BI, Galapagos, and Roche. C.M. is an employee of mainanalytics GmbH, Sulzbach (Taunus), Germany, which was contracted by BI to assist with these analyses. M.A. and S.S. are employees of BI. Y.A. has received consulting fees and payment for presentations from BI; consulting fees from Sanofi; and has participated in a Data Safety Monitoring Board or advisory board for BI, Chemomab, Curizon, Medsenic, Menarini, and Sanofi.
Data availability statement
See online supplementary material, available at Rheumatology online.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann-Vold AM, Fretheim H, Halse AK. et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med 2019;200:1258–66. [DOI] [PubMed] [Google Scholar]
- 3. Faisal A, Alghamdi BJ, Ciavaglia CE. et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med 2016;193:299–309. [DOI] [PubMed] [Google Scholar]
- 4. Cheng JZ, Wilcox PG, Glaspole I. et al. Cough is less common and less severe in systemic sclerosis–associated interstitial lung disease compared to other fibrotic interstitial lung diseases. Respirology 2017;22:1592–7. [DOI] [PubMed] [Google Scholar]
- 5. Tashkin DP, Volkmann ER, Tseng CH. et al. Improved cough and cough-specific quality of life in patients treated for scleroderma-related interstitial lung disease: results of Scleroderma Lung Study II. Chest 2017;151:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birring SS, Kavanagh JE, Irwin RS. et al. ; Collaborators. Treatment of interstitial lung disease associated cough: CHEST guideline and expert panel report. Chest 2018;154:904–17. [DOI] [PubMed] [Google Scholar]
- 7. Hu S, Hou Y, Wang Q. et al. Prognostic profile of systemic sclerosis: analysis of the clinical EUSTAR cohort in China. Arthritis Res Ther 2018;20:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khanna D, Clements PJ, Furst DE. et al. ; for the Scleroderma Lung Study Group. Correlation of the degree of dyspnea with health-related quality of life, functional abilities, and diffusing capacity for carbon monoxide in patients with systemic sclerosis and active alveolitis: results from the Scleroderma Lung Study. Arthritis Rheum 2005;52:592–600. [DOI] [PubMed] [Google Scholar]
- 9. Baron M, Sutton E, Hudson M. et al. The relationship of dyspnoea to function and quality of life in systemic sclerosis. Ann Rheum Dis 2008;67:644–50. [DOI] [PubMed] [Google Scholar]
- 10. Theodore AC, Tseng CH, Li N. et al. Correlation of cough with disease activity and treatment with cyclophosphamide in scleroderma interstitial lung disease: findings from the Scleroderma Lung Study. Chest 2012;142:614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kahrilas PJ, Altman KW, Chang AB. et al. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest 2016;150:1341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Q, Wallace L, Patnaik P, Alves M. et al. Disease frequency, patient characteristics, comorbidity outcomes and immunosuppressive therapy in systemic sclerosis and systemic sclerosis–associated interstitial lung disease: a US cohort study. Rheumatology (Oxford) 2021;60:1915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irwin RS, French CL, Chang AB. et al. CHEST Expert Cough Panel. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest 2018;153:196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shim J, Song W, Morice AH.. Drug-induced cough. Physiol Res 2020;69:S81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vacca A, Meune C, Gordon J. et al. ; Scleroderma Clinical Trial Consortium Cardiac Subcommittee. Cardiac arrhythmias and conduction defects in systemic sclerosis. Rheumatology (Oxford) 2014;53:1172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galiè N, Humbert M, Vachiery JL. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903–75. [DOI] [PubMed] [Google Scholar]
- 17. Steen VD, Conte C, Owens GR, Medsger TA Jr.. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994;37:1283–9. [DOI] [PubMed] [Google Scholar]
- 18. Adler S, Huscher D, Siegert E. et al. ; EUSTAR co-workers on behalf of the DeSScipher project research group within the EUSTAR network. Systemic sclerosis associated interstitial lung disease – individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group. Arthritis Res Ther 2018;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forestier A, Le Gouellec N, Béhal H. et al. Evolution of high-resolution CT-scan in systemic sclerosis–associated interstitial lung disease: description and prognosis factors. Semin Arthritis Rheum 2020;50:1406–13. [DOI] [PubMed] [Google Scholar]
- 20. Distler O, Highland KB, Gahlemann M. et al. ; SENSCIS Trial Investigators. Nintedanib for systemic sclerosis–associated interstitial lung disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 21. Maher TM, Mayes MD, Kreuter M. et al. ; SENSCIS Trial Investigators. Effect of nintedanib on lung function in patients with systemic sclerosis–associated interstitial lung disease: further analyses of a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2021;73:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishiyama O, Taniguchi H, Kondoh Y. et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J 2010;36:1067–72. [DOI] [PubMed] [Google Scholar]
- 23. du Bois RM, Weycker D, Albera C. et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:459–66. [DOI] [PubMed] [Google Scholar]
- 24. Ryerson CJ, Abbritti M, Ley B. et al. Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology 2011;16:969–75. [DOI] [PubMed] [Google Scholar]
- 25. Case AH, Hellkamp AS, Neely ML. et al. Associations between patient-reported outcomes and death or lung transplant in idiopathic pulmonary fibrosis. Data from the Idiopathic Pulmonary Fibrosis Prospective Outcomes Registry. Ann Am Thorac Soc 2020;17:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones PW, Quirk FH, Baveystock CM.. The St George’s Respiratory Questionnaire. Respir Med 1991;85:25–31. [DOI] [PubMed] [Google Scholar]
- 27. Swigris JJ, Wilson H, Esser D. et al. Psychometric properties of the St George's Respiratory Questionnaire in patients with idiopathic pulmonary fibrosis: insights from the INPULSIS trials. BMJ Open Respir Res 2018;5:e000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffmann-Vold AM, Maher TM, Philpot EE. et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol 2020;2:E71–83. [DOI] [PubMed] [Google Scholar]
- 29. Perelas A, Silver RM, Arrossi AV, Highland KB.. Systemic sclerosis–associated interstitial lung disease. Lancet Respir Med 2020;8:304–20. [DOI] [PubMed] [Google Scholar]
- 30. Roofeh D, Lescoat A, Khanna D.. Treatment for systemic sclerosis–-associated interstitial lung disease. Curr Opin Rheumatol 2021;33:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoffmann-Vold AM, Allanore Y, Alves M. et al. ; EUSTAR collaborators. Progressive interstitial lung disease in patients with systemic sclerosis–associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Volkmann ER. Natural history of systemic sclerosis–related interstitial lung disease: how to identify a progressive fibrosing phenotype. J Scleroderma Relat Disord 2020;5:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffmann-Vold AM, Allanore Y, Bendstrup E. et al. The need for a holistic approach for SSc-ILD – achievements and ambiguity in a devastating disease. Respir Res 2020;21:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parshall MB, Schwartzstein RM, Adams L. et al. ; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society Statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012;185:435–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Volkmann ER, Tashkin DP, LeClair H. et al. Treatment with mycophenolate and cyclophosphamide leads to clinically meaningful improvements in patient-reported outcomes in scleroderma lung disease: results of Scleroderma Lung Study II. ACR Open Rheumatol 2020;2:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See online supplementary material, available at Rheumatology online.