Abstract
Objectives
Nailfold videocapillaroscopy (NVC) plays a well-established role in differentiating primary from secondary RP due to SSc. However, the association of NVC with novel severe organ involvement/progression in SSc has never been evaluated in a multicentre, multinational study, which we now perform for the first time.
Methods
Follow-up data from 334 SSc patients [265 women; 18 limited SSc (lSSc)/203 lcSSc/113 dcSSc] registered between November 2008 and January 2016 by seven tertiary centres in the EUSTAR-database, were analysed. Novel severe organ involvement/progression was defined as new/progressive involvement of the peripheral vasculature, lungs, heart, skin, gastrointestinal tract, kidneys, musculoskeletal system, or death, at the 12- or 24-month follow-up. NVC images at enrolment were quantitatively and qualitatively evaluated according to the standardized definitions of the EULAR Study Group on Microcirculation in Rheumatic Diseases. Uni- and multivariable logistic regression modelling (ULR, MLR) was performed.
Results
Of the 334 included SSc patients, 257 (76.9%) developed novel overall severe organ involvement/progression. Following MLR, normal capillary density was associated with less-frequent novel overall severe organ involvement/progression [odds ratio (OR) = 0.77, P < 0.001] and novel peripheral vascular involvement (OR = 0.79, P = 0.043); microhaemorrhages were associated with less novel pulmonary hypertension (OR = 0.47, P = 0.029); and a ‘severe’ (active/late) NVC pattern was associated with novel overall severe organ involvement/progression (OR = 2.14, P = 0.002) and skin progression (OR = 1.70, P = 0.049).
Conclusions
Our results suggest that NVC may be a promising biomarker in SSc, certainly warranting further investigation. Despite the participation of tertiary centres, which follow their patients in a standardized way, we were underpowered to detect associations with infrequent severe organ involvement/progression.
Keywords: SSc, microcirculation, nailfold videocapillaroscopy, organ involvement, disease progression, EULAR Study Group on Microcirculation in Rheumatic Diseases, EUSTAR
Rheumatology key messages.
This is the first multicentre/multinational SSc study investigating associations between NVC and severe organ involvement/progression.
Additional evidence is provided for NVC’s future integration as a predictive biomarker of high-risk SSc subgroups.
Further research in longitudinal inception cohorts, with uniform, standardized and long-term follow-up is recommended.
Introduction
SSc is a rare CTD, hallmarked by a triad of microvascular damage, complex autoimmune dysfunction, and chronic progressive fibrosis of the skin and internal organs. These mechanisms synergistically lead to a heterogeneous phenotype of this orphan disease, with some patients exhibiting an indolent disease course, but others developing severe organ dysfunction, resulting in a major burden of severe morbidity and mortality [1–3]. Despite an increasing understanding of its pathogenesis, SSc remains a devastating disease with a high disease-related mortality [2, 3]. Today, pulmonary complications, such as interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH), are both prevalent and the leading causes of SSc-related mortality, with up to 60% of disease-related deaths in clinically overt SSc (i.e. lcSSc and dcSSc) [2–5]. However, during the disease course, it is not only the lungs that may be severely affected, but also several other organ systems, including the peripheral vasculature, heart, gastrointestinal tract, kidneys and musculoskeletal system [1, 6–8]. Although there is currently no definitive treatment that can stop the natural progression of the disease, patients at risk of developing life-threatening organ involvement should be identified as early as possible so that they can benefit from close monitoring and optimized organ-specific treatment [9]. The hunt for biomarkers that can identify at an early stage, preferably non-invasively, those patients who are at highest risk of developing severe organ complications remains a topic of great interest in SSc research [6, 7, 10–14].
Over the last decade, nailfold videocapillaroscopy (NVC) has often been proposed as an ideal biomarker [4, 6, 13, 15–21]. This safe and easy-to-handle instrument allows non-invasive evaluation of the peripheral microcirculation at the nailfold, using optical instruments [14, 15, 17, 22–26]. By reliably detecting structural microvascular alterations already present in the preclinical stages of SSc (i.e. the presence of a ‘scleroderma pattern’), NVC is able to distinguish primary from secondary RP due to SSc [14, 15, 17, 25]. This pivotal role of NVC in the diagnosis of the disease is reflected by its inclusion in the 2013 ACR/EULAR criteria for SSc [27]. In addition to its well-established diagnostic ability, the prognostic role of NVC has been investigated in several studies [4, 6, 11–13, 15–20, 28]. As such, two cross-sectional pilot studies independently demonstrated a predictive role of baseline qualitative NVC assessment (i.e. presence of a ‘scleroderma pattern’) for the future occurrence of peripheral vascular involvement, which was recently confirmed by both a French national study and a pan-European multicentre study [6, 10, 20, 28]. Moreover, the latter study also examined quantitative NVC parameters, with capillary density (i.e. loss of capillaries) proving to be the most promising NVC predictor of new digital ulcers [20]. Associations between serial NVC examinations and future severe organ dysfunction have also been the subject of some longitudinal monocentre studies, in which progressive capillary loss and progression to a more ‘severe’ (i.e. active or late) NVC pattern have been identified as potential biomarkers of disease progression [16, 18, 19]. Finally, two systematic reviews devoted to this subject have corroborated these findings [11, 12].
However, as the association between NVC and future severe organ involvement and/or progression has never been thoroughly evaluated in a multicentre, multinational study, we set up such a study for the first time in a joint collaboration of the European Scleroderma Trials and Research Group (EUSTAR) and the EULAR Study Group on Microcirculation in Rheumatic Diseases.
Materials and methods
Study population and design
Seven EUSTAR centres (Ghent University Hospital, Ghent, Belgium; University of Genoa, Genova, Italy; Sapienza University of Rome, Rome, Italy; University (Hospital) Zurich, Zurich, Switzerland; University Cochin Hospital, Paris, France; University Hospital of Lille, Lille, France; and Instituto Gaetano Pini, Milan, Italy) participated in this study, entitled ‘EUSTAR Clinical Project 61’ (EUSTAR CP61)/‘EULAR Study Group on Microcirculation in Rheumatic Diseases Project 9’ (Supplementary Table S1, available at Rheumatology online). The study was approved by the local Ethical Committee of each participating centre and by the EUSTAR Clinical Research Committee (EC 2008/385, Supplementary Table S1, available at Rheumatology online). The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient before registration in the EUSTAR-database.
Data were collected over a 24-month follow-up period on consecutive SSc patients (excluding overlapping syndromes), classified according to the 1980 ACR or 2013 ACR/EULAR criteria for SSc, and stratified into subsets according to LeRoy and Medsger [limited systemic sclerosis (lSSc), lcSSc and dcSSc], prospectively registered in the EUSTAR-database between November 2008 and January 2016 [27, 29, 30]. Patients eligible for this study were adult SSc patients, regardless of disease duration, in whom NVC had been performed on their first date of registration in the EUSTAR-database (considered the baseline). Data on demographics and disease-specific investigations (i.e. clinical, serological and diagnostic data) at baseline and at dates closest to the 12- and 24-month follow-up were included for analysis. A detailed report on the structure, minimum essential data and inclusion criteria of the EUSTAR-database has been previously published [8, 31].
Nailfold videocapillaroscopy
Collection and blinding of NVC images
NVC examination, using a videocapillaroscopic probe equipped with a contact lens of ×200 magnification, was performed at each participating centre upon enrolment. In particular, the nailfolds of the 2nd–5th fingers were examined bilaterally in each patient. Two consecutive fields in the middle of the nailfold extending over 1 mm and corresponding to the distal row of capillaries were captured per finger, resulting in 16 images per patient [14, 17, 25]. The investigators were asked to place a 1 mm grid on each image. Subsequently, the acquired NVC images were anonymized and sent for central reading at the Ghent University Hospital through a website specially designed for this study (www.eustarcp61.ugent.be).
Assessment of NVC images
The collected NVC images were randomly analysed by a blinded single trained assessor (K.M.), supervised by a microcirculation and capillaroscopy expert (V.S.). NVC assessment was performed in a standardized manner by applying the capillaroscopic definitions consented by the EULAR Study Group on Microcirculation in Rheumatic Diseases [17].
The quantitative assessment consisted of examining the following NVC parameters per linear millimetre, per image: ‘capillary density’ (i.e. number of distal row capillaries, considered normal if n ≥ 7), ‘capillary dimension’ (more specifically number of giant capillaries, i.e. with apical limb width >50µm), ‘capillary morphology’ [i.e. number of abnormally shaped capillaries not meeting the following definition of normal: hairpin shaped, (once or twice) crossing or tortuous shape, provided that the tip of the capillary is convex] and presence of ‘microhaemorrhages’ (i.e. red or brownish hemosiderin deposits in the pericapillary and/or periungual area) [17, 24–26].
The qualitative assessment was performed by first determining the overall capillary pattern for each patient, by categorizing the images into a ‘(non-)scleroderma pattern’, according to the standardized Fast Track Algorithm [17, 25]. Then, the ‘scleroderma patterns’ were further subcategorized according to Cutolo et al. based on the most dominant/worst pattern as ‘early’, ‘active’ or ‘late’ [17, 23]. ‘Active’ and ‘late’ NVC patterns were considered ‘severe’ NVC patterns.
Visibility of the images was scored at the image level. A capillaroscopic parameter was coded as ‘non-evaluable’ if the assessor was unable to evaluate it. If all NVC parameters in an image were evaluable, the visibility of that image was considered ‘good’. Patients with less than four ‘good’ images were considered to have ‘poor overall visibility’ and were excluded from further analysis.
Definitions of novel severe organ involvement/progression
Novel involvement
The occurrence of novel future severe organ involvement was defined as new involvement of one of the following organ systems at a future visit (i.e. at 12 or 24 months) that did not exist at enrolment, or at the prior visit: peripheral vascular system, lungs, heart, gastrointestinal tract, kidneys or musculoskeletal system. Novel peripheral vascular involvement was defined as the presentation of a new episode of a pitting scar, ischaemic digital ulcer or gangrene [18, 20]. Pulmonary involvement was subdivided into ILD and lung vascular involvement. Novel ILD was defined as the new presence of interstitial abnormalities on high-resolution CT (pulmonary fibrosis, ground-glass opacities or honeycombing) [5, 7, 32, 33]. Novel lung vascular involvement was further subdivided into novel pulmonary hypertension (PH) and novel PAH. Novel PH was defined as a new finding of systolic pulmonary arterial pressure ≥35 mmHg on transthoracic echocardiography (TTE). Novel PAH was defined as a new finding of mean pulmonary arterial pressure ≥25 mmHg combined with a pulmonary capillary wedge pressure ≤15, on right heart catheterization [34]. Novel heart involvement was established when there was new left heart failure, defined as a left ventricular ejection fraction <50%, or new pericardial effusion, on TTE [18]. Novel gastrointestinal tract involvement was defined as the new occurrence of intestinal obstruction or malabsorption syndrome. Novel renal involvement was defined as a new episode of scleroderma renal crisis [18]. Novel musculoskeletal involvement was defined as a new elevation of creatinine kinase or new onset of joint arthritis or muscle weakness. Finally, the date and causes of death were also recorded [3].
Novel progression
Progression of known ILD was defined as deterioration of lung function tests (≥10% relative decline in forced vital capacity expressed as percentage of predicted normal values (FVC%pred), or ≥5% to <10% relative decline in FVC%pred combined with ≥15% relative decline in diffusing capacity for carbon monoxide expressed as percentage of predicted normal values (DLCO%pred)) [5, 32, 35]. Progression of skin involvement was defined as worsening of the modified Rodnan skin score (mRSS) by ≥25% and ≥5 points or new presence of scleredema [18].
Statistics
Handling missing data
A detailed description of the missing data is provided in Supplementary Data S1a, available at Rheumatology online.
Statistical analysis
Uni- and multivariable logistic regression modelling (ULR and MLR) was performed to assess independent associations between the occurrence of novel severe organ involvement and/or progression (‘outcome measure’) and candidate risk factors (‘covariables’). Model selection was based on a backward elimination method, to progressively exclude those independent covariables that had only minor associations with the outcome measure when combined in one model. This method implies starting with a model that includes all candidate covariables, assessing the quality of the model using the Akaike Information Criterion (AIC), and subsequently deleting the covariable that least deteriorates the model, taking into account the complexity of the model [36]. This process was repeated until no further simplification was possible (i.e. lowest possible AIC). Nevertheless, NVC parameters were always retained in the model since they were the main subject of our research question. In order to be as inclusive as possible, and not to miss a key covariable independently associated with each organ involvement, covariables for the backward elimination MLR were selected based on data from published literature and upon consensus among the authors. Finally, the MLR was adjusted for gender, age, disease duration, SSc-related antibodies, dcSSc subset, immunomodulatory medication (i.e. MTX, MMF, CYC, rituximab, AZA and autologous stem cell transplantation) and the NVC parameter of interest. Specifically, for peripheral vascular involvement, the MLR was additionally adjusted for vasodilator medication (i.e. prostacyclin, calcium antagonists, endothelin receptor antagonists and phosphodiesterase type 5 inhibitors) and history of peripheral vascular lesions; and for PH, the MLR was additionally adjusted for vasodilator medication and dyspnoea (Supplementary Tables S2 and S3, available at Rheumatology online).
The strength of associations was summarized as odds ratios (ORs) and 95% CIs. Firth logistic regression was used in case of (quasi-)complete separation [37]. The risk of overfitting was assessed using the 1-in-10 rule of thumb (Supplementary Data S1b, available at Rheumatology online) [38].
For descriptive statistics, data for categorical variables are presented as absolute numbers and percentages, and for continuous variables as means with s.d., unless otherwise stated. Significance was defined as a P-value < 0.05. All statistical analyses were performed using IBM SPSS statistics Version 26 (IBM SPSS Inc., Chicago, Illinois, USA), extension ‘STATS FIRHTLOG’.
Results
Study population
Data from 361 consecutive SSc patients were collected. Of these, 4 patients were excluded because their NVC images were ‘non-evaluable’, and 23 others had no follow-up data available. Thus, a total of 334 SSc patients was finally retained for analysis (Table 1).
Table 1.
Baseline characteristics of the study population (n = 334)
57.9 (13.6)
| Baseline characteristics | All patients | ‘Non-scleroderma pattern’ | ‘Scleroderma pattern’ | ‘Early’ | ‘Active’ | ‘Late’ |
|---|---|---|---|---|---|---|
| (n = 334) | (n = 92) | (n = 242) | (n = 53) | (n = 170) | (n = 19) | |
| Age, mean (s.d.), years | 59.03 (12.9) | 57.4 (13.9) | 56.2 (14.2) | 57.2 (14.0) | 62.7 (10.9) | |
| Gender | ||||||
| male, n (%) | 69 (20.7) | 17 (18.5) | 52 (21.5) | 7 (13.2) | 40 (23.5) | 5 (26.3) |
| female, n (%) | 265 (79.3) | 75 (81.5) | 190 (78.5) | 46 (86.6) | 130 (76.5) | 14 (73.7) |
| Disease duration (years), median (IQR) | 5 (1–12) | 5 (2–14) | 5 (1–11) | 4 (0–9) | 5 (1–13) | 9 (2–13) |
| SSc classification criteria | ||||||
| ACR criteria, n (%) | 277 (82.9) | 76 (82.6) | 201 (83.1) | 39 (73.6) | 145 (85.3) | 17 (89.5) |
| 2013 ACR/EULAR criteria, n (%) | 302 (90.4) | 78 (84.8) | 224 (92.6) | 47 (88.7) | 158 (92.9) | 19 (100.0) |
| LeRoy subset | ||||||
| lSSc, n (%) | 18 (5.4) | 6 (6.5) | 12 (5.0) | 7 (13.2) | 5 (2.9) | 0 (0.0) |
| lcSSc, n (%) | 203 (60.8) | 51 (55.4) | 152 (62.8) | 33 (62.3) | 111 (65.3) | 8 (42.1) |
| dcSSc, n (%) | 113 (33.8) | 35 (38.0) | 78 (32.2) | 13 (24.5) | 54 (31.8) | 11 (57.9) |
| SSc-specific antibodies | 231 (69.2) | 65 (70.7) | 166 (68.6) | 33 (62.3) | 120 (70.6) | 13 (68.4) |
| ACA, n (%) | 115 (34.4) | 28 (30.4) | 87 (36.0) | 19 (35.8) | 64 (37.6) | 4 (21.1) |
| Anti-topo-1 (Scl-70) antibodies, n (%) | 99 (29.6) | 28 (30.4) | 171 (29.3) | 11 (20.8) | 52 (30.6) | 8 (42.1) |
| Anti-RNA polymerase III antibodies, n (%) | 17 (5.1) | 9 (9.8) | 8 (3.3) | 3 (5.7) | 54 (2.4) | 1 (5.3) |
| NVC parameters | ||||||
| Quantitative assessment | ||||||
| Capillary density | ||||||
| mean number of capillaries/mm, mean (s.d.) | 5.4 (1.6) | 6.4 (1.7) | 5.1 (1.3) | 6.9 (0.8) | 4.6 (0.9) | 4.0 (1.2) |
| Capillary dimension | ||||||
| number of patients with giant capillaries, n (%) | 223 (66.8) | 0 (0.0) | 223 (92.1) | 53 (100.0) | 170 (100.0) | 0 (0.0) |
| mean number of giant capillaries/mm, mean (s.d.) | 0.4 (0.5) | 0.0 (0.0) | 0.5 (0.5) | 0.4 (0.4) | 0.6 (0.5) | 0.0 (0.0) |
| Capillary morphology | ||||||
| number of patients with abnormally shaped capillaries, n (%) | 311 (93.1) | 83 (90.2) | 228 (94.2) | 51 (96.2) | 158 (92.9) | 19 (100.0) |
| mean number of abnormally shaped capillaries/mm, mean (s.d.) | 0.9 (0.7) | 0.9 (0.7) | 0.9 (0.7) | 0.7 (0.5) | 0.8 (0.7) | 1.9 (0.6) |
| Microhaemorrhages | ||||||
| number of patients with microhaemorrhages, n (%) | 197 (59.0) | 31 (33.7) | 166 (68.6) | 37 (69.8) | 121 (71.2) | 8 (42.1) |
| mean number of microhaemorrhages/mm, mean (s.d.) | 0.2 (0.2) | 0.1 (0.1) | 0.2 (0.2) | 0.2 (0.2) | 0.2 (0.2) | 0.1 (0.1) |
| Qualitative assessment | ||||||
| ‘Non-scleroderma pattern’ | 92 (27.5) | 92 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ‘Scleroderma pattern’ | 242 (72.5) | 0 (0.0) | 242 (100.0) | 53 (100.0) | 170 (100.0) | 19 (100.0) |
| Early NVC pattern | 53 (21.9) | 0 (0.0) | 53 (21.9) | 53 (100.0) | 0 (0.0) | 0 (0.0) |
| Active NVC pattern | 170 (70.2) | 0 (0.0) | 170 (70.2) | 0 (0.0) | 170 (100.0) | 0 (0.0) |
| Late NVC pattern | 19 (7.9) | 0 (0.0) | 19 (7.9) | 0 (0.0) | 0 (0.0) | 19 (100.0) |
| Severe NVC patterna | 189 (78.1) | 0 (0.0) | 189 (78.1) | 0 (0.0) | 170 (90.0) | 19 (10.0) |
| Peripheral vascular involvement | 160 (47.9) | 35 (38.0) | 125 (51.7) | 22 (41.5) | 92 (54.1) | 11 (57.9) |
| History | 129 (38.6) | 29 (31.5) | 100 (41.3) | 16 (30.2) | 76 (44.7) | 8 (42.1) |
| History of digital ulcers, n (%) | 112 (33.5) | 26 (28.3) | 86 (35.5) | 12 (22.6) | 66 (38.8) | 8 (42.1) |
| History of gangrene, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| History of pitting scars, n (%) | 28 (8.4) | 7 (7.6) | 7 (7.6) | 4 (7.5) | 17 (10.0) | 0 (0.0) |
| Active | 130 (38.9) | 27 (29.3) | 103 (42.6) | 17 (32.1) | 76 (44.7) | 10 (52.6) |
| Active digital ulcers, n (%) | 75 (22.5) | 18 (19.6) | 57 (23.6) | 11 (20.8) | 38 (22.4) | 8 (42.1) |
| Active gangrene, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Active pitting scars, n (%) | 63 (18.9) | 12 (13.0) | 51 (21.1) | 7 (13.2) | 42 (24.7) | 2 (10.5) |
| Pulmonary involvement | ||||||
| ILD, n (%) | 149 (44.6) | 43 (46.7) | 106 (43.8) | 22 (41.5) | 75 (44.1) | 9 (47.4) |
| Vascular lung involvement | ||||||
| PH, n (%) | 62 (18.6) | 16 (17.4) | 46 (19.0) | 4 (7.5) | 36 (21.2) | 6 (31.6) |
| PAH, n (%) | 2 (0.6) | 1 (1.1) | 1 (0.4) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| Skin | ||||||
| mRSS, mean (s.d.) | 8.8 (6.3) | 8.9 (6.7) | 8.7 (6.2) | 6.9 (4.7) | 8.9 (5.9) | 11.3 (10.3) |
| Scleredema, n (%) | 103 (30.8) | 23 (25.0) | 80 (33.1) | 18 (34.0) | 59 (34.7) | 3 (15.8) |
| Heart | 12 (3.6) | 4 (4.3) | 8 (3.3) | 1 (1.9) | 5 (2.9) | 2 (10.5) |
| LVEF < 50%, n (%) | 2 (0.6) | 1 (1.1) | 1 (0.4) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| Pericardial effusion, n (%) | 10 (3.0) | 3 (3.3) | 7 (2.9) | 1 (1.9) | 4 (2.4) | 2 (10.5) |
| Gastrointestinal tract | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Intestinal obstruction, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Malabsorption, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| SRC, n (%) | 2 (0.6) | 1 (1.1) | 1 (0.4) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| Musculoskeletal involvement | 68 (20.4) | 19 (20.7) | 49 (20.2) | 7 (13.2) | 35 (20.6) | 7 (36.8) |
| Synovitis, n (%) | 37 (11.1) | 13 (14.1) | 24 (9.9) | 1 (1.9) | 18 (10.6) | 5 (26.3) |
| Muscle weakness, n (%) | 27 (8.1) | 8 (8.7) | 19 (7.9) | 4 (7.5) | 14 (8.2) | 1 (5.3) |
| CK elevation, n (%) | 23 (6.9) | 7 (7.6) | 16 (6.6) | 4 (7.5) | 11 (6.5) | 1 (5.3) |
| Medication | ||||||
| Vasodilator therapy, n (%) | 232 (69.5) | 58 (63.0) | 174 (71.9) | 34 (64.2) | 126 (74.1) | 14 (73.7) |
| Prostacyclin, n (%) | 86 (25.7) | 18 (19.6) | 68 (28.1) | 10 (18.9) | 54 (31.8) | 4 (21.1) |
| Calcium antagonists, n (%) | 192 (57.5) | 49 (53.3) | 143 (59.4) | 29 (54.7) | 101 (59.4) | 13 (68.4) |
| Endothelin receptor antagonists, n (%) | 47 (14.1) | 13 (14.1) | 34 (14.0) | 4 (7.5) | 28 (16.5) | 2 (10.5) |
| Phosphodiesterase type 5 inhibitor, n (%) | 14 (4.2) | 2 (2.2) | 12 (5.0) | 1 (1.9) | 9 (5.3) | 2 (10.5) |
| Immunosuppressive therapy | 104 (31.1) | 34 (37.0) | 70 (28.9) | 15 (28.3) | 46 (27.1) | 9 (47.4) |
| MTX, n (%) | 60 (18.0) | 20 (21.7) | 40 (16.5) | 8 (15.1) | 27 (15.9) | 5 (26.3) |
| MMF, n (%) | 20 (6.0) | 7 (7.6) | 13 (5.4) | 3 (5.7) | 9 (5.3) | 1 (5.3) |
| CYC, n (%) | 4 (1.2) | 3 (3.3) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (5.3) |
| Rituximab, n (%) | 16 (4.8) | 6 (6.5) | 10 (4.1) | 1 (1.9) | 5 (2.9) | 4 (21.1) |
| AZA, n (%) | 13 (3.9) | 3 (3.3) | 10 (4.1) | 3 (5.7) | 7 (4.1) | 0 (0.0) |
| Autologous stem cell transplantation, n (%) | 2 (0.6) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 2 (1.2) | 0 (0.0) |
| Renal therapy, n (%) | 41 (12.3) | 12 (13.0) | 29 (12.0) | 4 (7.5) | 21 (12.4) | 4 (21.1) |
| ACE-inhibitor, n (%) | 41 (12.3) | 12 (13.0) | 29 (12.0) | 4 (7.5) | 21 (12.4) | 4 (21.1) |
| Dialysis, n (%) | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
‘Active’ and ‘late’ NVC patterns were considered ‘severe’ NVC patterns. ACE: angiotensin-converting enzyme; ILD: interstitial lung disease; IQR: interquartile range; lSSc: limited SSc; LVEF: left ventricular ejection fraction; mRSS: modified Rodnan skin score; NVC: nailfold videocapillaroscopy; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; SRC: scleroderma renal crisis.
Baseline NVC evaluation
Ninety-two patients (27.5%) had a ‘non-scleroderma pattern’ and 242 (72.5%) a ‘scleroderma pattern’. Of the latter, 53/242 (21.9%) were subclassified as an ‘early’, 170/242 (70.2%) as an ‘active’, and 19/242 (7.9%) as a ‘late’ pattern (Table 1).
Novel overall severe organ involvement and/or progression
During the 24-month follow-up period, 257/334 (76.9%) unique patients were diagnosed with novel overall severe organ involvement and/or progression (Table 2). Among these patients, 199/257 (77.4%) were women, 159/257 (61.6%) were classified as having lcSSc and 90/257 (35.0%) as having dcSSc. Their median (IQR) disease duration was 5 (1–12) years. The proportion of patients with a ‘scleroderma pattern’ was similar in the patients with vs those without novel overall severe organ involvement and/or progression (72.8% vs 71.4%, P = 0.818). The proportion of patients with ‘severe’ NVC patterns was significantly higher in the patients who developed novel overall severe organ involvement and/or progression than in those without (61.4% vs 42.9%, P = 0.004).
Table 2.
Occurrence of novel severe organ involvement and/or progression during follow-up
| Novel severe organ involvement and/or progressiona | Follow-up period |
||
|---|---|---|---|
| 0–12 months | 12–24 months | Entire follow-up 0–24 months | |
| (n = 320) | (n = 301) | (n = 334) | |
| Novel peripheral vascular involvement, n (%) | 65 (20.3) | 50 (16.6) | 111 (33.2) |
| Novel pulmonary involvement | |||
| Structural | |||
| New ILD, n (%) | 10 (3.1) | 9 (3.0) | 19 (5.7) |
| Progression of known ILD, n (%) | 36 (11.3) | 42 (14.0) | 67 (20.1) |
| Vascular | |||
| New PH, n (%) | 24 (7.5) | 21 (7.0) | 45 (13.5) |
| New PAH, n (%) | 1 (0.3) | 4 (1.3) | 5 (1.5) |
| Skin progression, n (%) | 75 (23.4) | 72 (23.9) | 140 (41.9) |
| Death, n (%) | 1 (0.3) | 4 (1.3) | 5 (1.5) |
| Novel heart involvement, n (%) | 9 (2.8) | 9 (3.0) | 18 (5.4) |
| Novel gastrointestinal tract involvement, n (%) | 4 (1.3) | 1 (0.3) | 5 (1.5) |
| Novel SRC, n (%) | 2 (0.6) | 3 (1.0) | 5 (1.5) |
| Novel musculoskeletal involvement, n (%) | 33 (10.3) | 29 (9.6) | 59 (17.7) |
Novel severe organ involvement and/or progression is defined as new involvement of any organ system at a future visit (i.e. 12 or 24 months) that did not exist at enrolment or at the prior visit. Of 334 unique SSc patients, 257 (76.9%) had novel severe organ involvement and/or progression. ILD: interstitial lung disease; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; SRC: scleroderma renal crisis.
Quantitative NVC assessment
Normal capillary density was associated with less likelihood of future overall development of novel severe organ involvement and/or progression in ULR (OR = 0.76, 95% CI: 0.65, 0.88, P < 0.001) (Table 3). MLR adjusted for gender and immunomodulatory medication gave similar findings (OR = 0.77, 95% CI: 0.66, 0.89, P < 0.001) (Fig. 1, Supplementary Table S2a, available at Rheumatology online). No association could be established for the other quantitative NVC parameters (Table 3).
Table 3.
Associations between NVC and novel severe organ involvement/progression during follow-up (0–24 months)—univariable logistic regression
| Novel severe organ involvement and/or progression (0–24 months) | QUANTITATIVE ASSESSMENT |
QUALITATIVE ASSESSMENT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capillary density |
Capillary dimension |
Capillary morphology |
Micro-haemorrhages |
Presence of scleroderma pattern |
Presence of ‘severe’ NVC patternsa |
|||||||
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||
| Overall future organ involvement, (n = 257) | 0.76 (0.65, 0.88) | <0.001 | 1.25 (0.77, 2.01) | 0.372 | 1.33 (0.52, 3.13) | 0.533 | 1.01 (0.63, 1.62) | 0.955 | 1.34 (0.81, 2.21) | 0.251 | 2.136 (1.33, 3.44) | 0.002 |
| Peripheral vascular, (n = 111) | 0.68 (0.57, 0.80) | <0.001 | 1.15 (0.70, 1.92) | 0.585 | 0.58 (0.22, 1.54) | 0.271 | 0.72 (0.44, 1.16) | 0.170 | 1.26 (0.74, 2.18) | 0.399 | 1.82 (1.12, 2.98) | 0.015 |
| Pulmonary | ||||||||||||
| Structural | ||||||||||||
| New ILD, (n = 19) | 0.78 (0.56, 1.06) | 0.110 | 0.41 (0.16, 1.06) | 0.065 | 2.86 (0.37, 368.71) | 0.393 | 0.92 (0.36, 2.44) | 0.865 | 0.48 (0.19, 1.27) | 0.135 | 0.65 (0.25, 1.65) | 0.355 |
| Progression of known ILD, (n = 67) | 0.85 (0.70, 1.02) | 0.084 | 0.85 (0.48, 1.53) | 0.586 | 2.75 (0.76, 17.70) | 0.136 | 0.74 (0.42, 1.30) | 0.295 | 0.84 (0.46, 1.57) | 0.578 | 0.81 (0.47, 1.43) | 0.470 |
| Vascular | ||||||||||||
| New PH, (n = 45) | 0.87 (0.70, 1.07) | 0.201 | 0.60 (0.31, 1.15) | 0.122 | 1.54 (0.42, 9.94) | 0.552 | 0.42 (0.22, 0.80) | 0.008 | 0.88 (0.45, 1.83) | 0.728 | 1.46 (0.76, 2.93) | 0.260 |
| New PAH, (n = 5) | 0.82 (0.44, 1.46) | 0.521 | 1.90 (0.28, 37.34) | 0.547 | 0.77 (0.08, 102.11) | 0.864 | 0.95 (0.16, 7.33) | 0.959 | 2.26 (0.04, 22.71) | 0.365 | 3.01 (0.44, 59.17) | 0.283 |
| Skin progression, (n = 140) | 0.97 (0.83, 1.14) | 0.729 | 1.46 (0.86, 2.47) | 0.165 | 1.00 (0.232, 2.97) | 1.000 | 1.31 (0.78, 2.20) | 0.308 | 1.63 (0.93, 2.85) | 0.087 | 1.71 (1.02, 2.87) | 0.042 |
| Death, (n = 5) | 0.82 (0.43, 1.44) | 0.501 | 0.71 (0.12, 5.48) | 0.718 | 0.28 (0.04, 5.64) | 0.327 | 0.44 (0.06, 2.66) | 0.359 | 1.50 (0.22, 29.60) | 0.707 | 6.86 (0.72, 912.33) | 0.104 |
| Heart, (n = 18) | 0.98 (0.72, 1.33) | 0.917 | 0.818 (0.31, 2.30) | 0.692 | 0.50 (0.12, 3.36) | 0.417 | 1.31 (0.49, 3.89) | 0.595 | 1.02 (0.38, 3.12) | 0.969 | 1.01 (0.39, 2.75) | 0.977 |
| Gastrointestinal tract, (n = 5) | 1.56 (0.91, 2.72) | 0.106 | 1.92 (0.28, 37.75) | 0.540 | 0.76 (0.08, 101.70) | 0.862 | 0.45 (0.07, 2.36) | 0.339 | 1.49 (0.22, 29.33) | 0.714 | 0.18 (0.01, 1.26) | 0.088 |
| SRC, (n = 5) | 0.98 (0.54, 1.69) | 0.944 | 1.94 (0.28, 38.16) | 0.533 | 0.76 (0.08, 101.29) | 0.860 | 0.90 (0.17, 5.44) | 0.894 | 1.51 (0.22, 29.71) | 0.705 | 2.29 (0.42, 23.08) | 0.355 |
| Musculoskeletal, (n = 59) | 0.98 (0.82, 1.18) | 0.866 | 0.84 (0.47, 1.53) | 0.560 | 4.50 (0.90, 81.92) | 0.072 | 0.84 (0.47, 1.51) | 0.552 | 1.02 (0.55, 1.98) | 0.948 | 1.12 (0.63, 2.02) | 0.705 |
Underlined text: Firth log used. Bold text: Statistically significant finding (P < 0.05). a‘Active’ and ‘late’ NVC patterns were considered ‘severe’ NVC patterns. ILD: interstitial lung disease; NVC: nailfold videocapillaroscopy; OR: odds ratio; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; SRC: scleroderma renal crisis.
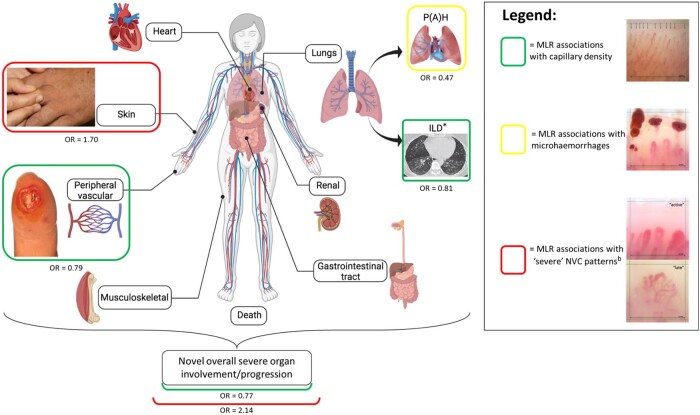
Fig. 1.
Significant associations between NVC and novel severe organ involvement/progression in SSc, after MLR.
aCross-sectional association. b‘Active’ and ‘late’ NVC patterns were considered ‘severe’ NVC patterns. ILD: interstitial lung disease; MLR: multivariable logistic regression analysis; NVC: nailfold video capillaroscopy; OR: odds ratio; P(A)H: pulmonary (arterial) hypertension.
(Image created with BioRender.com).
This figure illustrates the main study findings, as found after multivariable logistic regression analysis: normal capillary density is associated with a lower incidence of novel overall organ involvement/progression (i.e. new involvement of any organ system at a future visit [i.e. 12 or 24 months] that did not exist at enrolment or at the prior visit) and a lower incidence of novel peripheral vascular involvement; microhaemorrhages are associated with a lower incidence of novel pulmonary hypertension; and ‘severe’ NVC patterns are associated with a higher incidence of novel overall organ involvement/progression and a higher incidence of novel skin progression. Note that capillary density is cross-sectionally associated with the presence of ILD. For capillaroscopic image acquisition, a nailfold videocapillaroscope with a ×200 magnification was used. Each centre was asked to place a 1-mm grid on each image, using centre-dependent image analysis. Images were evaluated quantitatively (i.e. capillary density, capillary dimension, capillary morphology and presence of microhaemorrhages) and qualitatively [i.e. presence/absence of a scleroderma pattern (early, active or late)] according to the standardized definitions of the EULAR Study Group on Microcirculation in Rheumatic Diseases, by a single trained assessor (K.M.) who was blinded to the clinical data [17].
Qualitative NVC assessment
ULR showed that ‘severe’ NVC patterns were associated with future overall development of novel severe organ involvement and/or progression during follow-up (OR = 2.14, 95% CI: 1.33, 3.44, P = 0.002) (Table 3). MLR adjusted for gender and immunomodulatory medication gave similar findings (OR = 2.14, 95% CI: 1.33, 3.48, P = 0.002) (Fig. 1, Supplementary Table S3a, available at Rheumatology online).
Novel severe organ involvement and/or progression, by organ system (Table 2)
Quantitative NVC assessment
Normal capillary density was associated with a lower development of novel peripheral vascular involvement, both in ULR (OR = 0.68, 95% CI: 0.57, 0.80, P < 0.001) and MLR, adjusted for dcSSc and history of peripheral vascular lesions (OR = 0.79, 95% CI: 0.61, 0.99, P = 0.043) (Fig. 1, Table 3, Supplementary Table S2b, available at Rheumatology online). Microhaemorrhages were less associated with novel PH in ULR analysis (OR = 0.42, 95% CI: 0.22, 0.80, P = 0.008) and MLR, adjusted for age and dyspnoea (OR = 0.47, 95% CI: 0.23, 0.93, P = 0.029) (Fig. 1, Table 3, Supplementary Table S2c, available at Rheumatology online). No associations could be established for capillary dimension or capillary morphology (Table 3).
Qualitative NVC assessment
ULR showed that patients with ‘severe’ NVC patterns were more likely to develop peripheral vascular involvement (OR = 1.82, 95% CI: 1.12, 2.98, P = 0.015) and skin progression (OR = 1.71, 95% CI: 1.02, 2.87, P = 0.042) (Table 3). MLR adjusted for gender and dcSSc, yielded similar findings only for the latter (OR = 1.70, 95% CI: 1.00, 2.89, P = 0.049) (Fig. 1, Supplementary Table S3b and c, available at Rheumatology online).
Discussion
We evaluated for the first time in a multicentre, multinational setting (i.e. in a joint collaboration of EUSTAR and the EULAR Study Group on Microcirculation in Rheumatic Diseases), associations between NVC (using internationally agreed upon definitions) and novel severe organ involvement and/or progression in SSc [17].
Concerning quantitative NVC parameters, encouraging results were generated. For instance, fewer patients with normal capillary density were found to develop novel overall severe organ involvement/progression and peripheral vascular involvement. This observation is consistent with what has been previously demonstrated [6, 10, 18, 20]. More specifically, both the cross-sectional mono- and bi-centre pilot studies by Smith et al., the monocentre longitudinal study by Avouac et al. and the large multicentre ‘CAP’ study by Cutolo et al. have highlighted capillary density as the most robust predictor for identifying SSc patients who will develop severe disease [6, 10, 13, 20]. Moreover, capillary density is considered representative of advanced disease and has been shown to be the most (inter-rater) reliable quantitative capillaroscopic parameter [22]. Our data further point towards a promising role for this capillaroscopic parameter, supporting its future prognostic use in daily SSc practice.
Another finding was that, although the literature has shown microhaemorrhages to be good indicators of SSc disease activity, their presence in our multicentre study was associated with fewer novel PH events [39–41]. Future studies will be needed to further elucidate their role.
Examining qualitative NVC parameters, we found that patients with ‘severe’ NVC patterns had a significantly higher relative risk of developing novel overall severe .organ involvement/progression, peripheral vascular involvement, and skin progression. These data confirm the findings of previous reports, which showed that more ‘severe’ NVC patterns were predictive of severe organ dysfunction, and particularly vascular damage [6, 10, 20, 28]. Given that progressive worsening of NVC patterns represents the gradual progression of microvasculopathy in SSc, this finding is not surprising [17]. However, new to our study is that it provides robust evidence for an association between NVC and skin progression, even after MLR. This is particularly important, as skin fibrosis is not only a prominent debilitating clinical manifestation of SSc but is also of prognostic importance, as the rate and degree of progression appear to correlate with organ involvement and mortality [1, 2, 32, 42–45].
Other than for the peripheral vasculature and skin, no other significant associations were revealed between NVC and the individual organ systems, which is probably due to the small number of events in these categories.
Somewhat surprisingly, we were unable to confirm the hypothesis suggested by recent systematic reviews stating that NVC might be a promising biomarker for severe pulmonary complications [11, 12]. Against this background, an important caveat for our study is that, although a large sample size was examined, an unexpectedly low proportion of patients developed novel pulmonary involvement (1.4% PAH and 5.3% ILD) during follow-up. One would have expected an incidence of 10% and 50% for novel PAH and ILD, respectively [34, 46, 47]. Our observed low incidence could be partly explained by the already relatively long disease duration of our patients and partly by an insufficient follow-up time [46–50]. Another explanation could be that there was a flaw in pulmonary involvement reporting inherent in the data collection for the EUSTAR-database. Therefore, one can assume that the predictive power of NVC for future pulmonary involvement is underestimated in this study. Hence, to circumvent bias and investigate cross-sectional associations between NVC and pre-existing ILD at enrolment, a post hoc ULR and MLR analysis was performed. Interestingly, lower capillary density was significantly associated with ILD, confirming previous findings (Fig. 1, Supplementary Data S2, available at Rheumatology online) [11, 17].
Our study had several strengths. First, data quality was ensured by the participation of highly specialized tertiary centres accustomed to the daily care of SSc patients according to the available clinical practice guidelines [7]. Second, heterogeneity regarding NVC image analysis was avoided by having one independent trained assessor rating the images, and by following recently published consensus recommendations [17, 25]. Third, to minimize the inherent subjectivity of capillaroscopic assessment, NVC images were centrally rated by one trained assessor, who was blinded to the clinical data.
A first limitation of this study was that the patient population consisted of many patients with already clinically overt disease (as evidenced by subset, disease duration, and frequency of organ involvement at enrolment), which may have led to an underestimation of the frequency of novel severe organ involvement/progression. It is likely that studies with a larger number of patients with early disease (i.e. within 2 years of diagnosis) could avoid this bias and thus demonstrate robust associations with NVC. Second, we are well aware that the definitions used are very stringent for some categories of novel severe organ involvement and/or progression (e.g. gastrointestinal involvement); this was inherent in the design of the EUSTAR-database. Consequently, events in these groups may be infrequent, and our study may be underpowered. Although not within the aims of our study, future studies may overcome this limitation by examining associations with more frequent, and therefore less severe, SSc-related organ involvement. Third, because NVC was performed only once, at the time of enrolment, we may have overlooked associations with the microvascular alterations that usually occur during the natural course of the disease. Moreover, the statistical analysis only considered the effect of the covariables that were present at the time of enrolment, thus excluding possible changes that may have occurred during follow-up. Another limitation was the missingness of data inherent an an observational dataset, such as the EUSTAR-database, which forced us to perform several imputations (i.e. assumptions), creating a risk of bias. Finally, it should not be overlooked that NVC is currently not widely used outside academic centres in Europe, nor in most centres in the USA, thus limiting its potential feasibility as a biomarker (at this time).
This study provides additional evidence for a future shift towards the use of NVC as an optional indicator of more severe disease in SSc, with important clinical implications for its overall management. Longitudinal studies, conducted in inception cohorts, with uniform and long-term follow-up, using standardized microvascular techniques, are warranted to further elucidate and confirm the predictive role of NVC in SSc. The generation of consistent high-quality research in this area may lead to the integration of NVC as a predictive biomarker into daily SSc clinical practice, to enable timely initiation and/or escalation of treatment in specific high-risk subgroups.
Conclusion
Our results suggest that NVC may be a promising biomarker in SSc. Despite the participation of tertiary centres, which assess and follow their patients in a standardized way, we were underpowered to detect associations with infrequent severe organ involvement and/or progression.
Supplementary Material
Acknowledgements
The Ghent University Hospital is a member of the European Reference Network on Rare and Complex Connective Tissue and Musculoskeletal Diseases (ERN ReCONNET), of the Flemish Network on Rare Connective Tissue Diseases as well as the Centre of Excellence for Imaging of EULAR. The Laboratory of Experimental Rheumatology and the Academic Clinical Division of Rheumatology of the University of Genoa are members of the European Reference Network on Rare and Complex Connective Tissue and Musculoskeletal Diseases (ERN ReCONNET) as well as the Centre of Excellence for Imaging of EULAR. We would like to thank all of the collaborators in the EULAR Study Group on Microcirculation in Rheumatic Diseases. We would like to thank Melissa De Decker for her dedication in daily coordinating the logistics of the multidisciplinary care in the Ghent University Scleroderma Unit. A.V. made substantial contributions to the design of the study, acquisition of data, analysis and interpretation of data, drafting of the article, critical revision of the intellectual content, and final approval of the version to be published. M.C. contributed to ideation of the study, and made substantial contributions to the design of the study, acquisition of data, drafting of the article, critical revision of the intellectual content, and final approval of the version to be published. O.D., V.R., Y.A., C.P.D., E.H., F.I. and A.S. made substantial contributions to the design of the study, acquisition of data, critical revision of the intellectual content, and final approval of the version to be published. E.D. made substantial contributions to analysis and interpretation of data, critical revision of the intellectual content, and final approval of the version to be published. J.A., S.J., D.L., K.M., C.P. and M.V. made substantial contributions to acquisition of data, critical revision of the intellectual content, and final approval of the version to be published. A.L.H. made substantial contributions to the design of the study, critical revision of the intellectual content, and final approval of the version to be published. V.S. contributed to ideation of the study, and made substantial contributions to the design of the study, analysis and interpretation of data, drafting of the article, critical revision of the intellectual content, and final approval of the version to be published.
Funding: V.S. is a Senior Clinical Investigator of the Research Foundation—Flanders (Belgium) (FWO) [1.8.029.20N]. The FWO was not involved in the study design, collection, analysis and interpretation of data, writing of the report, nor in the decision to submit the manuscript for publication. V.S. is supported by an unrestricted educational chair on systemic sclerosis of Janssen-Cilag NV. Janssen-Cilag NV was not involved in the study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication.
Disclosure statement: O.D. has/had a consultancy relationship with and/or has received research funding from or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last 3 years: Abbvie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, Baecon, Blade, Bayer, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, Novartis, Roche, Roivant, Sanofi, Serodapharm, Topadur and UCB. O.D. has/had a patent issued: ‘mir-29 for the treatment of systemic sclerosis’ (US8247389, EP2331143). O.D. has received research grants from Kymera and Mitsubishi Tanabe. V.S. has/had received speaker fees from Boehringer-Ingelheim Pharma GmbH&Co, Janssen-Cilag NV, UCB Biopharma Sprl; consulting fees from Boehringer-Ingelheim Pharma GmbH&Co; and grant/research support from the Research Foundation—Flanders (FWO), the Belgian Fund for Scientific Research in Rheumatic Diseases (FWRO), Boehringer-Ingelheim Pharma GmbH&Co and Janssen-Cilag NV. The other authors have declared no conflicts of interest.
Contributor Information
Amber Vanhaecke, Department of Internal Medicine, Ghent University; Department of Rheumatology, Ghent University Hospital, Ghent, Belgium.
Maurizio Cutolo, Laboratory of Experimental Rheumatology and Academic Division of Clinical Rheumatology, Department of Internal Medicine, University of Genoa, IRCCS San Martino Polyclinic Hospital, Genoa, Italy.
Oliver Distler, Department of Rheumatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Valeria Riccieri, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Rome, Italy.
Yannick Allanore, Service de Rheumatology, Université de Paris, Hôpital Cochin, AP-HP CUP, Paris, France.
Christopher P Denton, Department of Rheumatology, University College London, Royal Free Hospital, London, UK.
Eric Hachulla, Institute for Translational Research in Inflammation (INFINITE), Université de Lille; INSERM; Service de Médecine Interne et Immunologie Clinique, Centre de Référence des Maladies Autoimmunes Systémiques Rares du Nord et Nord-Ouest de France (CeRAINO), CHU Lille, Lille, France.
Francesca Ingegnoli, Division of Clinical Rheumatology, ASST Pini-CTO; Department of Clinical Sciences and Community Health, Università degli Studi di Milano, Milano, Italy.
Ellen Deschepper, Biostatistics Unit, Department of Public Health and Primary Care, Ghent University, Ghent, Belgium.
Jérôme Avouac, Service de Rheumatology, Université de Paris, Hôpital Cochin, AP-HP CUP, Paris, France.
Suzana Jordan, Department of Rheumatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
David Launay, Institute for Translational Research in Inflammation (INFINITE), Université de Lille; INSERM; Service de Médecine Interne et Immunologie Clinique, Centre de Référence des Maladies Autoimmunes Systémiques Rares du Nord et Nord-Ouest de France (CeRAINO), CHU Lille, Lille, France.
Karin Melsens, Department of Internal Medicine, Ghent University; Department of Rheumatology, Ghent University Hospital, Ghent, Belgium.
Carmen Pizzorni, Laboratory of Experimental Rheumatology and Academic Division of Clinical Rheumatology, Department of Internal Medicine, University of Genoa, IRCCS San Martino Polyclinic Hospital, Genoa, Italy.
Alberto Sulli, Laboratory of Experimental Rheumatology and Academic Division of Clinical Rheumatology, Department of Internal Medicine, University of Genoa, IRCCS San Martino Polyclinic Hospital, Genoa, Italy.
Massimiliano Vasile, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Rome, Italy.
Ariane L Herrick, Division of Musculoskeletal and Dermatological Sciences, University of Manchester, Salford Royal NHS Foundation Trust; NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, UK.
Vanessa Smith, Department of Internal Medicine, Ghent University; Department of Rheumatology, Ghent University Hospital, Ghent, Belgium; Unit for Molecular Immunology and Inflammation, VIB Inflammation Research Centre (IRC), Ghent, Belgium.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Medsger TA Jr, Bombardieri S, Czirjak L. et al. Assessment of disease severity and prognosis. Clin Exp Rheumatol 2003;21(Suppl 29):S42–6. [PubMed] [Google Scholar]
- 2. Elhai M, Meune C, Boubaya M. et al. ; EUSTAR group. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. [DOI] [PubMed] [Google Scholar]
- 3. Steen VD, Medsger TA.. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007;66:940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Distler O, Assassi S, Cottin V. et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur Respir J 2020;55:1902026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goh NS, Desai SR, Veeraraghavan S. et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248–54. [DOI] [PubMed] [Google Scholar]
- 6. Smith V, Riccieri V, Pizzorni C. et al. Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. J Rheumatol 2013;40:2023–8. [DOI] [PubMed] [Google Scholar]
- 7. Smith V, Scire CA, Talarico R. et al. Systemic sclerosis: state of the art on clinical practice guidelines. RMD Open 2018;4(Suppl 1):e000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meier FM, Frommer KW, Dinser R. et al. ; EUSTAR Co-authors. Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2012;71:1355–60. [DOI] [PubMed] [Google Scholar]
- 9. Nihtyanova SI, Tang EC, Coghlan JG. et al. Improved survival in systemic sclerosis is associated with better ascertainment of internal organ disease: a retrospective cohort study. QJM 2010;103:109–15. [DOI] [PubMed] [Google Scholar]
- 10. Smith V, Decuman S, Sulli A. et al. Do worsening scleroderma capillaroscopic patterns predict future severe organ involvement? A pilot study. Ann Rheum Dis 2012;71:1636–9. [DOI] [PubMed] [Google Scholar]
- 11. Smith V, Vanhaecke A, Vandecasteele E. et al. Nailfold videocapillaroscopy in systemic sclerosis–related pulmonary arterial hypertension: a systematic literature review. J Rheumatol 2020;47:888–95. [DOI] [PubMed] [Google Scholar]
- 12. Smith V, Vanhaecke A, Guerra MG. et al. May capillaroscopy be a candidate tool in future algorithms for SSC-ILD: are we looking for the holy grail? A systematic review. Autoimmun Rev 2020;19:102619. [DOI] [PubMed] [Google Scholar]
- 13. Paxton D, Pauling JD.. Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review. Semin Arthritis Rheum 2018;48:482–94. [DOI] [PubMed] [Google Scholar]
- 14. Koenig M, Joyal F, Fritzler MJ, Roussin A. et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud‘s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum 2008;58:3902–12. [DOI] [PubMed] [Google Scholar]
- 15. Cutolo M, Smith V.. Detection of microvascular changes in systemic sclerosis and other rheumatic diseases. Nat Rev Rheumatol 2021;17:665–77. [DOI] [PubMed] [Google Scholar]
- 16. Sulli A, Pizzorni C, Smith V. et al. Timing of transition between capillaroscopic patterns in systemic sclerosis. Arthritis Rheum 2012;64:821–5. [DOI] [PubMed] [Google Scholar]
- 17. Smith V, Herrick AL, Ingegnoli F. et al. ; EULAR Study Group on Microcirculation in Rheumatic Diseases and the Scleroderma Clinical Trials Consortium Group on Capillaroscopy. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud‘s phenomenon and systemic sclerosis. Autoimmun Rev 2020;19:102458. [DOI] [PubMed] [Google Scholar]
- 18. Avouac J, Lepri G, Smith V. et al. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum 2017;47:86–94. [DOI] [PubMed] [Google Scholar]
- 19. Sulli A, Paolino S, Pizzorni C. et al. Progression of nailfold capillaroscopic patterns and correlation with organ involvement in systemic sclerosis: a 12 year study. Rheumatology (Oxford) 2020;59:1192. [DOI] [PubMed] [Google Scholar]
- 20. Cutolo M, Herrick AL, Distler O. et al. ; CAP Study Investigators. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a multicenter, prospective cohort study. Arthritis Rheum 2016;68:2527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith V, Distler O, Cutolo M.. Might nailfold capillaroscopy be a “Proxy” for lung involvement in connective tissue diseases? J Rheumatol 2019;46:1061–3. [DOI] [PubMed] [Google Scholar]
- 22. Smith V, Pizzorni C, De Keyser F. et al. Reliability of the qualitative and semiquantitative nailfold videocapillaroscopy assessment in a systemic sclerosis cohort: a two-centre study. Ann Rheum Dis 2010;69:1092–6. [DOI] [PubMed] [Google Scholar]
- 23. Cutolo M, Sulli A, Pizzorni C, Accardo S.. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 2000;27:155–60. [PubMed] [Google Scholar]
- 24. Cutolo M, Melsens K, Herrick AL. et al. ; EULAR Study Group on Microcirculation in Rheumatic Diseases. Reliability of simple capillaroscopic definitions in describing capillary morphology in rheumatic diseases. Rheumatology (Oxford) 2018;57:757–9. [DOI] [PubMed] [Google Scholar]
- 25. Smith V, Vanhaecke A, Herrick AL. et al. Fast track algorithm: how to differentiate a “scleroderma pattern” from a “non-scleroderma pattern“. Autoimmun Rev 2019;18:102394. [DOI] [PubMed] [Google Scholar]
- 26. Smith V, Beeckman S, Herrick AL. et al. ; EULAR study group on microcirculation. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology (Oxford) 2016;55:883–90. [DOI] [PubMed] [Google Scholar]
- 27. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 28. Boulon C, Aiouaz S, Blaise S. et al. Correlation between capillaroscopic classifications and severity in systemic sclerosis: results from SCLEROCAP study at inclusion. Clin Exp Rheumatol 2019;37(Suppl 119):63–8. [PubMed] [Google Scholar]
- 29. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 30. LeRoy EC, Medsger TA Jr.. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573–6. [PubMed] [Google Scholar]
- 31. Walker UA, Tyndall A, Czirjak L. et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffmann-Vold AM, Allanore Y, Alves M. et al. ; EUSTAR collaborators. Progressive interstitial lung disease in patients with systemic sclerosis–associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roofeh D, Jaafar S, Vummidi D, Khanna D.. Management of systemic sclerosis–associated interstitial lung disease. Curr Opin Rheumatol 2019;31:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galie N, Humbert M, Vachiery JL. et al. ; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 35. Khanna D, Mittoo S, Aggarwal R. et al. Connective Tissue Disease-associated Interstitial Lung Diseases (CTD-ILD) – report from OMERACT CTD-ILD Working Group. J Rheumatol 2015;42:2168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akaike H. Information theory and an extension of the maximum likelihood principle. In: 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, USSR, 1971 .Budapest, Hungary: Akadémiai Kiadó, 1973: 267–81. [Google Scholar]
- 37. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27–38. [Google Scholar]
- 38. Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004;66:411–21. [DOI] [PubMed] [Google Scholar]
- 39. Pignataro F, Maglione W, Minniti A. et al. NEMO score in nailfold videocapillaroscopy is a good tool to assess both steady state levels and overtime changes of disease activity in patients with systemic sclerosis: a comparison with the proposed composite indices for this disease status entity. Arthritis Res Ther 2019;21:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Del Papa N, Pignataro F, Maglione W. et al. High NEMO score values in nailfold videocapillaroscopy are associated with the subsequent development of ischaemic digital ulcers in patients with systemic sclerosis. Arthritis Res Ther 2020;22:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andracco R, Irace R, Zaccara E. et al. The cumulative number of micro-haemorrhages and micro-thromboses in nailfold videocapillaroscopy is a good indicator of disease activity in systemic sclerosis: a validation study of the NEMO score. Arthritis Res Ther 2017;19:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clements PJ, Hurwitz EL, Wong WK. et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum 2000;43:2445–54. [DOI] [PubMed] [Google Scholar]
- 43. Shand L, Lunt M, Nihtyanova S. et al. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum 2007;56:2422–31. [DOI] [PubMed] [Google Scholar]
- 44. Steen VD, Medsger TA Jr.. Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum 2001;44:2828–35. [DOI] [PubMed] [Google Scholar]
- 45. Wu W, Jordan S, Graf N. et al. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann Rheum Dis 2019;78:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vandecasteele E, Melsens K, Vanhaecke A. et al. Incidence, prevalence and long-term progression of Goh algorithm rated interstitial lung disease in systemic sclerosis in two independent cohorts in Flanders: a retrospective cohort study. Semin Arthritis Rheum 2021;51:969–76. [DOI] [PubMed] [Google Scholar]
- 47. Nihtyanova SI, Schreiber BE, Ong VH. et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheum 2014;66:1625–35. [DOI] [PubMed] [Google Scholar]
- 48. LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 49. Denton CP, Khanna D.. Systemic sclerosis. Lancet (London) 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 50. Rubio-Rivas M, Corbella X, Pestana-Fernandez M. et al. First clinical symptom as a prognostic factor in systemic sclerosis: results of a retrospective nationwide cohort study. Clin Rheumatol 2018;37:999–1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.