Abstract
Thermal burn injuries are still a serious public health concern in the United States, due to the initial insult and resulting comorbidities. Burned patients are increasingly susceptible to colonization by endogenous and exogenous microorganisms after having lost skin, which acts as the primary protective barrier to environmental contaminants. Furthermore, the onset of additional pathophysiologies, specifically sepsis, becomes more likely in burned patients compared to other injuries. Despite improvements in the early care of burn patients, infections, and sepsis, these pathophysiologies remain major causes of morbidity and mortality and warrant further investigation of potential therapies. Vitamin E may be one such therapy. We aimed to identify publications of studies that evaluated the effectiveness of vitamin E as it pertains to thermal burn injuries, infection, and sepsis. Several investigations ranging from in vitro bench work to clinical studies have examined the impact on, or influence of, vitamin E in vitro, in vivo, and in the clinical setting. To the benefit of subjects it has been shown that enteral or parenteral vitamin E supplementation can prevent, mitigate, and even reverse the effects of thermal burn injuries, infection, and sepsis. Therefore, a large-scale prospective observational study to assess the potential benefits of vitamin E supplementation in patients is warranted and could result in clinical care practice paradigm changes.
BURN INJURY, INFECTION, AND SEPSIS
Thermal burn injuries are still a serious public health concern in the United States. Their prevalence not only impacts mortality, but also confounds long-term morbidity and organ dysfunction. In 2019 The U.S. National Fire Protection Agency reported over 1.2 million fires; these resulted in nearly 17,000 burn injuries and over 3700 deaths, an increase of 24% from a decade ago.1 In 2016 alone, approximately 338,000 people sustained flame burn injuries in the United States, according to the National Hospital Ambulatory Medical Care Survey (NHAMCS, 2016).2 Even relatively small burns (<20% of the TBSA) can be incapacitating, disfiguring, and painful; they become increasingly traumatic with the introduction of comorbidities such as smoke inhalation, prolonged inflammation, and vascular insufficiencies, all of which can lead to chronic complications and negatively impact the overall quality of life.3,4
Burned patients are also increasingly susceptible to colonization by endogenous and exogenous microorganisms after having lost skin, which acts as the primary protective barrier to environmental contaminants. Burn injury also predisposes patients to infection by local and systemic factors due to effects on metabolic and immunologic host defenses.5,6 Furthermore, burn eschar provides an environment conducive to bacterial growth because of its protein richness, wound avascularity, and release of toxic substances.7,8
Additional systematic reviews of the prevailing research literature reveal that the prevalence of sepsis, often stemming from infection in burn patients can be greater (8%–42.5%) than trauma patients (2.4%–16.9%) and even critical care patients (19%–38%).9 Improved outcomes for severely burned patients have been attributed to medical advances in resuscitation, nutritional support, pulmonary care, burn wound care, and infection control practices.10
Despite improvements in the early care of burn patients, infections, and sepsis, these pathophysiologies remain major causes of morbidity and mortality6,11 and warrant further investigation of potential therapies.
PRODUCTION OF OXIDANTS AND FREE RADICALS
Postinjury oxidative stress, a hallmark of burn injuries, infection, and sepsis, may arise when the level free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) exceeds the host’s innate antioxidant defenses. Burns as well as smoke inhalation injuries are most commonly associated with systemic inflammatory responses and significantly increased levels of RNS and ROS.12 At the acute stages of burn injury, ROS in particular are released in large quantities during what is known as the respiratory burst response of neutrophils, endothelial cells, and other phagocytes, as they produce radicals like hydrogen peroxide (H2O2) and superoxide (O2).13 It has been established that, in response to burn injuries, inflammatory cells produce these free radicals in excess, ultimately leading to lipid peroxidation14 which compromises the cellular membrane and induces further damage.15 Patients who sustain burns with concomitant smoke inhalation injury have gross evidence of systemic and pulmonary oxidant/oxidative damage, in addition to lung injury. The degree of oxidative stress after these injuries is greatly correlated to the degree of organ dysfunction and mortality.16,17
Nitric oxide (NO) is a free radical scavenger that is intricately involved with the innate immune response,18–21 and concentrations of NO in burned patients are likely to reflect the activation of the immune system and act as a surrogate of the overall immune status of the individual. The upregulation of inducible nitric oxide synthase (iNOS) during systemic inflammatory response syndrome (SIRS) post burn has been observed, along with the accumulation of NO byproducts in blood and tissue.18,21,22 Studies measuring NO byproducts in the ovine model of burn and smoke inhalation have also found that serum NO is increased at least 3-fold postinjury compared to uninjured control animals.23,24 iNOS and NO have an important role in the changes in both systemic and pulmonary blood flow and in the microvascular permeability that occurs in concomitant burn and smoke inhalation injury.23–25 Following the combined injuries, large concentrations of O2 and NO combine to produce peroxynitrite (ONOO−),26,27 which is a powerful oxidant and nitrosating and nitrating agent.28 It is particularly detrimental because it can readily trigger DNA single-strand breakage and induce significant damage to lipids and proteins.29 Without interventions that counter the effects oxidative radicals such as ONOO−, burned patients are likely to experience degraded increased infections, delays to wound healing, and prolonged ICU needs among other complications of severe oxidative stress.
VITAMIN E HISTORY, STRUCTURE, AND FUNCTION
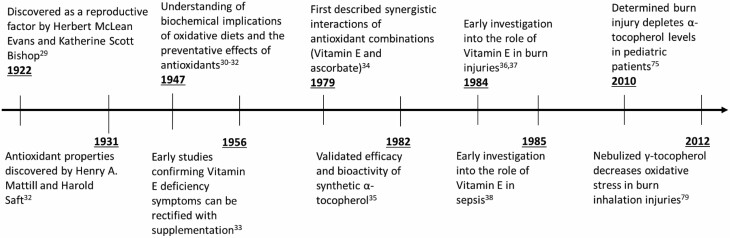
In 1922, Evans and Bishop discovered vitamin E in the form of a micronutrient that was essential for reproduction in rats.30 It was rediscovered between several groups in the late 1940s31–33 and placed under the classification of a cellular antioxidant component because it was shown to effect lipid peroxidation, which precedes diseases such as atherosclerosis.34 Vitamin E subsequently proved effective at preventing lipid peroxidation, providing electron stabilization, mitigating myopathies, and reducing RNS as well as ROS in atherosclerosis as well as burn pathophysiology.35–40 A timeline of milestones in the discoveries and utilization of vitamin E can be found in Figure 1.
Figure 1.
Timeline ranging from the year of vitamin E discovery to investigative studies on vitamin E’s role in burn injuries and sepsis.
The term “vitamin E” encompasses a group of potent, lipid-soluble chain-breaking antioxidants, and includes four tocopherols (α-, β-, γ-, and δ-tocopherol) and four tocotrienols (α-, β-, γ-, and δ-tocotrienols). Vitamin E’s chain-breaking capability allows it to react directly with free radicals by transforming them to more stable, nonradical products. Of the vitamin E antioxidants, α-tocopherol has the highest biological activity in humans and will comprise the largest focus in this review. Alpha-tocopherol is an endogenous lipid-soluble chain breaking antioxidant and is documented as protecting polyunsaturated fatty acids found in cell membrane phospholipids from the effects of free radicals by donating its hydrogen atom.41 Once introduced, α- as well as γ-tocopherols are absorbed into the small intestine and transported in large part due to the cytosolic alpha-tocopherol transfer protein (α-TTP) for storage in the human liver, brain, placenta, or delivered to tissues in need of antioxidant functions.42,43 This transfer protein maintains several advantageous physiological roles with regard to vitamin E transport and metabolism. Most notable is that it allows for near complete absorption of the most biologically active tocopherol, α-tocopherol, while also supporting vitamin E metabolism or excretion, as the most vitamin E dense organ, the liver, does not will not accumulate toxic levels of vitamin E.42,44
The molecular functions specifically fulfilled by tocopherols have yet to be fully elucidated and described in burns, infection, or sepsis. It has been seen that oxidative stress significantly reduces tocopherol concentrations in burned patients,45,46 and that it may be remedied with supplementation.47 In addition, it is possible that these compounds also have broader systemic effects. Early epidemiological studies have reported that high vitamin E intake is correlated with a reduced risk of cardiovascular diseases,48,49 fatty liver disease,50,51 and can attenuate diabetic neuropathy,52,53 whereas intake of other dietary antioxidants (such as vitamin C) have led to differing results.49,54,55 It remains to be elucidated whether these effects are strictly a product of antioxidant function but it appears at the very least that antioxidant roles in anti-inflammatory and antioxidant physiology can attenuate the resultant oxidative species.56
VITAMIN E POTENTIAL ROLE IN BURN INJURY, INFECTION, AND SEPSIS
To date, it has been noted that supplemental antioxidant vitamins added to enteral feeding solutions are well absorbed and correlated with improved antioxidant defenses, as determined in a number of in vivo and clinical studies.57–60Mito-Vit-E, a triphenyl phosphonium (TPP)-conjugated form of vitamin E, specifically protects mitochondria from oxidative stress as a result of the TPP cation accumulating within the negatively charged mitochondrial inner membrane,61 and burn injuries treated with vitamin E promote quicker reduction of exudates, pain, and bacterial load based on clinical observations, compared to untreated wounds.4 It has also been reported to attenuate lipopolysaccharide (LPS) endotoxin-induced activation of alveolar macrophages in vitro in response to and was attributed in large part to decreased TNF production and increased prostaglandin E2 production.62 Additionally, vitamin E has displayed significant involvement in mitigating and treating septic shock.63–65 This review will delve further into the previously published investigations that may further elucidate the role of vitamin E in burn injuries, infections, and sepsis; to gain a better understanding of the mechanisms involved and the potential impact vitamin E may have as a therapeutic for these pathophysiologies.
METHODS
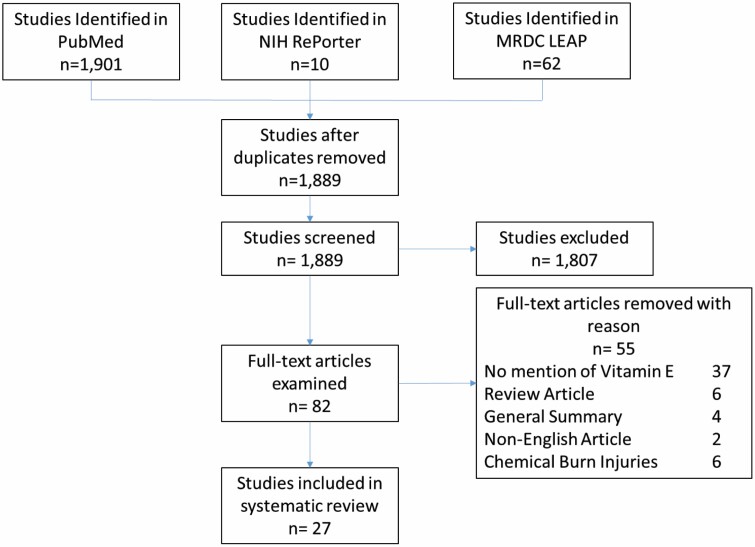
Three online databases: PubMed, NIH RePorter, and the U.S. Army Medical Research and Development Command (MRDC) Library Electronic Access Portal (LEAP) were searched for the keywords “Vitamin E,” “Tocopherol,” “Burn,” “Infection,” and “Sepsis.” Searches were limited to the year 2000 to the current date. The last search was performed in January 2022. Searches were filtered for free text on clinical studies, comparative studies, or reviews, all in English. In vivo animal studies and clinical studies focused on thermal contact injuries and inhalation injuries. Chemical burns were excluded. Relevant references cited from those articles were also examined (Figure 2).
Figure 2.
Flow chart of study selection process.
RESULTS
Studies that met the criteria for discussion of the scientific methods and results totaled 27. Ten burn studies (2 in vitro, 5 in vivo animal, 3 human), 6 infection studies (2 in vivo animal, 4 human), and 11 sepsis studies (2 in vitro, 4 in vivo, 5 human) were admitted. Some articles cited contained pertinent information but exceeded that stated window for consideration (2010–2022) and were therefore referenced but excluded discussion of data.
Vitamin E in Burn Injury
Ten studies related to thermal burn injuries and vitamin E cleared the established guidelines. Results included in vitro studies in the form of protection from induced heat shock and chemically induced oxidative damage, in in vivo large animal models of burn injury, as well as in clinical investigations of pediatric and adult burned patients.
The phenomenon of heat-shock response in cells, a cellular protective mechanism activated by different stressors such as high temperature or infections, was described as early as the 1960s66 and has since become a precursor to more complex models of understanding the cellular response to thermal burn injuries.67 Consequently, in vitro studies introducing interventions to the cellular heat-shock response are strongly correlated with the physiologic response to burn injuries.68 Inducing heat-shock also allows for the evaluation of prophylactic as well as postinjury therapies. To determine the damage preventative qualities of vitamin E before cellular heat-shock response, Butt et al69 isolated healthy fibroblasts from patient biopsies at the Jinnah hospital in Lahore, Pakistan. Dermal fibroblasts were pretreated with 100 µM vitamin E for 24 hours at 37°C, followed by induced heat shock for 10 minutes at 51°C in fresh serum free medium. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-i-tetrazolium bromide and lactate dehydrogenase assays observing cell metabolic activity and cell plasma membrane damage, respectively; results were compared to experimental groups of fibroblasts with or without pretreatment and/or induced heat shock. Vitamin E preconditioning significantly (P < .05) improved cellular viability in the vitamin E pretreated group (123.92 ± 10.63%) versus all experimental groups (110.56 ± 3.85% pretreated heat injured group; 50.8 ± 2.21% heat injured group). Butt concluded that Vitamin E preconditioning did in fact shield the cells from the damaging effects of heat-shock injury in vitro. Additional experimentation suggested the antioxidant content in culture positively affected the PI3K/Akt pathway, leading to the activation of survival or proliferation associated genes and down regulation of apoptotic genes.70,71
In a similar cytoprotective in vitro experiment, Bonferoni et al72 encapsulated α-tocopherol using chitosan oleate, an amphiphilic chitosan salt, to physically stabilize and deliver the resulting nano-emulsion to separate primary keratinocyte and primary fibroblast cultures in vitro. Cells were induced with a range of H2O2 concentrations (0.5–2.5 mM), mimicking burn-induced oxidative damage. Assays measured cytotoxicity and proliferative potential of the cultures compared to unloaded chitosan oleate and untreated controls. In keratinocytes, the α-tocopherol treated cells displayed significantly (P < .05) greater proliferation compared to all groups at 24 hours and remained significantly greater than controls after 7 days, although not-statistically different than unloaded chitosan oleate treatments alone after 7 days. α-Tocopherol loaded treatments in the range of 0.25 to 10 µM increased fibroblast viability compared to untreated controls. Fibroblasts with induced oxidative damage via H2O2 appeared to be protected by the antioxidant activity of α-tocopherol, at delivered concentrations between 5 and 10 µM.
The role of vitamin E has also been studied in the acute ovine model of burn and smoke inhalation injury, which is a highly translatable model.24,46,73 Traber et al46 tracked the depletion of plasma α-tocopherol in the ovine model of burn and smoke inhalation, which used 48 inspired breaths of cotton smoke under deep anesthesia, along with a 40% TBSA third-degree flame burn injury. Deuterated vitamin E was administered prior to injury to accurately measure depletion. Traber noted significant depletions of α-tocopherol as disappearances in the plasma were 1.5 times greater and half-lives were 8 hours shorter in injured sheep compared to sham animals after 48 hours (P < .05), supporting the hypothesis that burn and inhalation injury causes lipid peroxidation that depletes vitamin E.46 Even after 75 hours, α-tocopherol levels showed no signs of recovering back to baseline concentrations.
This depletion was found to extend to prolonged metabolic imbalances in human subjects as well.74 In a clinical study of eight pediatric patients with 25% TBSA third-degree burns, Herndon et al measured both plasma and adipose α-tocopherol levels; plasma concentrations are indicative of short-term vitamin E status, while, adipose concentrations indicative of long-term vitamin E status and are a more accurate measurement of antioxidant capacity.75 Sourced tissue was obtained as early as 2 hours postinjury peripherally to the wounded area. α-Tocopherol levels were within normal ranges (199 ± 40 nmol/g adipose tissue) within the first week of injury; however, adipose α-tocopherol levels dropped significantly at 2 and 3 weeks (133 ± 13 nmol/g adipose tissue and 109 ± 8 nmol/g adipose tissue, respectively), suggesting a decrease of 6.1 ± 0.6 nmol/g adipose tissue per day over the first month. This drastic decrease in vitamin E normally takes years of depletion. The hypermetabolic state resulting from burn injuries in both pediatric and adult patients metabolism may limit the availability of essential nutrients like vitamin E acutely and chronically.76,77 Such depletion warrants further investigation into the ability to replenish nutrients and rectify the metabolic derangement.
Yamamoto et al78 nebulized the chronic ovine model of burn and smoke inhalation injury with solution of approximately 33.3% vitamin E (32.0% γ-tocopherol, 1.3% α-tocopherol) and 66.6% ethanol. Control animals received 33.3% sterile water and 66.6% ethanol. Animals received a 20% flame burn with 24 breaths of cotton smoke. Animals received their respective treatments from 3 to 48 hours postinjury and were observed up to 3 weeks. Tocopherol levels in the lung were confirmed to be significantly (P < .01) increased compared to control animals (38.5 ± 16.8 nmol/g vs 0.39 ± 0.46) with decreases in the lipid peroxidation marker, malondialdehyde (MDA), in the vitamin E-treated group (data not provided). The ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) provides a measure of the oxygen in the blood relative to the oxygen concentration that is breathed, and was increased significantly in vitamin E-treated animals compared to controls, suggesting improved pulmonary oxygen transfer and delivery. Significant clinical measurements between groups included pulmonary shunt fraction (percentage of nonoxygenated blood pumped through the heart), peak and pause pressures (pulmonary resistance and compliance, respectively), lung bloodless wet-to-dry weight ratios (index of lung water accumulation), and bronchiolar obstruction (fraction of initial expiration relative to vital capacity) were all significantly improved in the vitamin E-treated group (P < .05).
A prospective double-blind placebo controlled pilot study by Barbosa et al59 tested the impact of vitamin E (1.35 times upper intake level), vitamin C (1.5 times upper intake level), and zinc (2.0 times upper intake level) supplementation on burned children (mean age: 54.2 ± 48.9 months; mean % burn TBSA: approx. 16%, N = 32). Antioxidant requirements were calculated by the Curreri equation79 and The new U.S. Dietary Reference Intakes for vitamins C and E.80 Antioxidant supplements were administered after meals via syringes from the second day of admittance to the hospital; administration was divided into three doses per day at regular time points and maintained for 7 days. The effects of the individual antioxidants could not be determined since all treated subjects received combined mixtures of the three micronutrients. Serum levels of vitamin E, but not vitamin C or zinc, began to resolve themselves regardless of vitamin E supplementation. The lack of change in vitamin C levels, at least, was thought to reflect its possible preferential consumption to neutralize oxyradicals, or to perform other naturally occurring antioxidant functions postinjury. As a result, the vitamin-supplemented group (n = 17) did still display a markedly significant increase in vitamin E, compared to the nonsupplemented group (n = 15). Barbosa surmised that these differences may arise from a physiologic preference or requisite depletion of vitamin C or zinc needed to neutralize the generation of oxyradicals. Malondialdehyde, which is one of the final products of polyunsaturated fatty acid peroxidation and a marker of oxidatve stress,81 was significantly decreased in treated patients (P < .05) compared to controls. Antioxidant supplementation also reduced the time to healing (wound re-epithelialization) from 7.5 ± 1 days in untreated patients to 5.3 ± 1 days in treated pediatric patients. Similar decreases in vitamin E immediately after burn injury, followed by a rapid recovery of levels upon supplementation, were seen by Mingjian nearly two decades earlier82 in 35 severely burned patients (mean age: 31.8 years; mean % burn TBSA: approx. 37.4%; male = 27, female = 8). Similarly, lipid peroxide levels acted counter to vitamin E levels, increasing as vitamin E decreased and vice versa.
Al-Kaisy et al tested the combination therapy of topical povidone-iodine ointment with systemic administration of vitamins E and C on 38 thermally injured patients of different age groups (1–60 years), sex (male = 18, female = 20), occupation, and burn TBSA ranging from 10% to 80% as estimated by The Rule of Nines.83 Injured patients were managed by surgeons according to hospital policy regimens and were treated with topical povidone-iodine ointment, another documented antioxidant product,80 in addition to drugs prescribed by the hospital drug policy. Alternatively, patients were treated with topical povidone-iodine ointment and oral vitamin E (400 mg) and vitamin C (500 mg), each once daily, inclusive of drugs prescribed by the hospital drug policy.84 In addition, 12 healthy subjects (5 males and 7 females) of the same age range as that of patients, served as controls. Unfortunately, patient attrition during the study prevented comparisons to the standard hospital policy regimen. Malondialdehyde levels were significantly (P < .05) decreased as early as 2 days and as late as 4 days for both the povidone-iodine treated (57% and 74%, respectively) and the povidone-iodine and vitamin-supplemented groups (96% and 142%, respectively) compared to injured patients receiving standard care. Povidone-iodine, and oral vitamins E and C significantly (P < 0.05) reduced the healing time by 2 days (22.7%), compared to the group treated with topical povidone-iodine ointment only.
Systemically administered vitamin E provides several benefits, as burn injuries are known to cause systemic as well as local complications specifically associated with ROS.85 Research has also proven that the local administration of vitamin E on burn injuries provides significant benefits as well. Periera et al86 investigated the topical application of polymer films loaded with vitamin E and aloe vera, on human subjects, albeit on a limited sample size of healthy humans (two males and three females between 26 and 37 years). Films were comprised of polyvinyl alcohol (PVA, 400 Da), hyaluronate, sorbitol, polyethylene oxide (PEO, 1000 kDa), sodium alginate, aloe vera, and vitamin E (3.55 ± 0.10% wt/wt) in the form of alpha-tocopherol acetate. Periera validated the delivery of vitamin E by applying the films to undamaged skin, tape stripping the skin, and subsequently quantifying the deposited vitamin E via HPLC. Vitamin E was found in the deepest portion of the stratum corneum at near milligram concentrations (101.65 ± 2.93% recovery), suggesting significant bioavailability may be achieved if applied to damaged and accessible burned skin.
Vitamin E in Burn Comorbidities
Apart from the acute damage resulting from burn injury and in conjunction with systemic inflammatory and oxidative effects, unburned tissue, specifically skeletal muscle and bone, are also affected by severe burn injury. Necrotic regions aside, tissue peripheral to the burn injury is characterized by a zone of hypoperfusion and decreased metabolic rate, followed by the initiation of reperfusion coupled with increased inflammatory cytokines, adrenergic stress, and increased glucocorticoid levels. The cytokine and glucocorticoid imbalance in particular promote energy dependent processes leading to a hypermetabolic state, ultimately contributing to skeletal muscle wasting and bone resorption due to caloric requirements and prolonged oxidative stress.
It has been found that urinary cortisol levels increase 3- to 10-fold postburn87 and can remain elevated for at least 3 months after injury.88,89 Exogenous glucocorticoids can similarly increase whole body muscle protein breakdown in humans as much as 5% to 20%.90 Hypercortisolemia can lead to both central and peripheral insulin resistance in both adipose tissue and skeletal muscle.91,92 Since both insulin resistance and hypercortisolemia cause muscle proteolysis,93,94 cortisol may directly and indirectly contribute to muscle wasting and bone resorption in response to burns.
The initial effect on bone have been characterized in a sheep model of burn injury as an acute increase in urinary excretion of the C-telopeptide of type I collagen, a biomarker of bone resorption, oberserved as early as day 1 postburn.95 Within the first 5 days there is histologic evidence of increased bone resorption87 along with increased apoptosis of osteoblasts.96
Experimentally, vitamin E supplementation has shown the ability to alleviate stress on skeletal muscle caused by increases in glucocorticoids. In vitamin E deficiency, membrane deformation and anemia have been observed due to oxidative damage to red blood cells.97 De Andrade Belo et al98 investigated if diets deficient in vitamin E negatively affected endogenous plasma cortisol levels in pacus fish submitted to normal (5 kg/m3) or elevated and stress inducing (20 kg/m3) stocking densities. Dietary supplementation of 12.6, 58.2, or 310.4 mg of vitamin E/kg dry diet was provided for a period up to 20 weeks under these stocking conditions. The one diet considered vitamin E deficient (12.6 mg/kg) was the only group resulting in a negative correlation between vitamin E supplementation and increased cortisol levels throughout the study (p2 = −0.31691), the effect of increased dietary vitamin E was attributed to decreased lipid peroxidation and oxidative stress in the 58.2 and 310.4 mg of vitamin E/kg groups.
Early work by Ohtsuka et al99 investigated the effects of vitamin E on oxidative stress in rat skeletal muscle induced by subcutaneous injections of corticosterone. Male Sprague-Dawley rats were fed a basal diet or a diet supplemented with 5000 mg dl-α-tocopherol acetate/kg for 10 days. Subcutaneous injections of corticosterone (0, 25, or 100 mg/kg body weight/d) were administered over the final 4 of those 10 days. For rats receiving the basal diet, corticosterone increased muscle loss as indicated by weights of the extensor digitorum longus (EDL) and gastrocnemius (GAST) muscles, with greater muscle loss as the corticosterone concentration increased (93.5 ± 3.6, 83.1 ± 7.5, 69.6 ± 5.6 mg EDL; 1.17 ± 0.09, 0.97 ± 0.13, 0.80 ± 0.04 g GAST). Rats with diets supplemented with vitamin E showed reduced muscle loss in the 25 and 100 mg groups; this was the case for both the EDL and GAST muscles (93.6 ± 6.5, 89.4 ± 5.1, 76.6 ± 7.2 mg EDL; 1.18 ± 0.11, 1.09 ± 0.08, 0.88 ± 0.09 g GAST). In addition, protein carbonyl content of the gastrocnemius muscle as well as lipid peroxide concentrations in the gastrocnemius and blood plasma were used as an index of oxidative damage. Protein carbonyl content was significantly (P < .001) reduced in the 100 mg vitamin E diet group, compared to the basal diet groups (1.82 ± 0.36 vs 2.70 ± 0.38 µmol/g protein), respectively. Similarly, lipid peroxide was significantly reduced in the gastrocnemius muscle compared to basal die controls (0.260 ± 0.234 vs 1.188 ± 0.501 nmol MDA/g protein), although, there were no significant difference to be found in the plasma.99
The expression of connexin43 and connexin45 hemichannels have been proposed to play a critical role in the mechanism underlying myofiber atrophy induced by the synthetic glucocorticoid, dexamethasone. Balboa et al100 treated 8-week-old C57BL6 wild-type (WT) male mice with 10 mg/kg dexamethasone for 7 days. A subset of these mice received diets supplemented with 2000 IU/kg dl-α-tocopherol acetate). The dl-α-tocopherol acetate was found to rapidly inhibit the activity of connexin43 and connexin45 hemichannels in freshly isolated myofibers of treated mice. Immunohistochemistry also showed that Atrogin-1, a protein degradation marker, was significantly reduced (P < .05) in dl-α-tocopherol acetate diet supplemented mice compared to those without supplementation (actual values not provided).
Work by Wong et al evaluated palm vitamin E (PVE) as a potential agent to prevent bone loss.101 Twelve-week-old male Wistar rats were given standard rat chow or a high-carbohydrate high-fat (HCHF) diet to induce metabolic syndrome,102 HCHF fed rats were then either treated with tocopherol-stripped corn oil as a vehicle control group, 60 mg/kg, or 100 mg/kg PVE. At the end of the study, rats were evaluated for bone density. Wong noted increased osteoblast surface, osteoid surface, bone volume, and trabecular thickness, as well as decreases eroded surface. The improvements in bone characteristics were suggested to be correlated with decreased leptin levels in the 60 mg/kg and 100 mg/kg PVE supplemented rats (2050.00 ± 246.92 and 530.36 ± 74.14 pg/ml, respectively) compared to the HCHF fed rats (3425.55 ± 377.31 pg/ml). Leptin also acts as a potent inhibitor for bone formation through the central nervous system.47 Norazlina et al103 induced osteoporosis in female Sparague-Dawley rats via ovariectomy, prior to feed treatments. Rats were fed standard feed, palm vitamin E at 30 mg/kg rat weight, palm vitamin E at 60 mg/kg rat weight, or α-tocopherol at 30 mg/kg rat weight, for 10 months postprocedure. Bone mineral density was lower in ovariectomied rats compared to intact rats and Norazlina noted there were significant (P < .05) increases in bone calcium content in the femoral and vertebral bones in intact rats supplemented with palm vitamin E at 60 mg/kg rat weight and 30 mg/kg rat weight α-tocopherol (173.9 ± 15.6, 182.3 ± 10.3 mg Femoral; 47.2 ± 2.0, 50.3 ± 1.7 mg Lumbar) compared to rats supplemented with 30 mg/kg palm vitamin E (134 ± 4.1 Femoral; 40.8 ± 1.5 mg Lumbar).103 It should be noted that the metabolic syndrome model and osteoporosis model employed by Wong and Norzalina, respectively, display distinct differences than that of a true burn injury. They do, however, carry similarities such as metabolic syndrome leading to hyperglycemia, similar to burn injuries,104,105 or a lack of antioxidant estrogen following postovariectomy leading to increased lipid peroxidation and free radical formation.106,107
Vitamin E in Infection
Seven studies looked at the role of vitamin E with regard to wound and systemic infection after thermal injuries. The body’s immune system is protective against outside bacterial, fungal, parasitic, and viral invaders, thus preventing infection which can ultimately lead to sepsis and death. Because of this, vitamin E is found in immune cells at a higher concentration compared to other blood cells, and is one of the most effective nutrients to influence immune function.108 Vitamin E reacts with peroxyl radicals and prevents oxidation of polyunsaturated fatty acids that are present in the immune cell membranes and further damage to the cell.109 Therefore, not only is vitamin E important in modulating immune function in burn infections, but it has a role in all infectious and immune etiologies.
Numerous in vivo studies have demonstrated that vitamin E is effective in reducing infection in respiratory and parasitic infections. In a study by Tantcheva et al, male ICR mice were administered doses of vitamin E (50 mg/kg) and/or vitamin C (80 mg/kg) once-daily 3 days before infection with H3N2 influenza virus.110 Results showed that lipid peroxidation levels measured in blood plasma were restored by vitamin E treatment when compared to levels increased by influenza infection. This reduction was more prominent with combination treatment with vitamin C. Parasitic infections have also been investigated in response to vitamin E treatment, as presented by De Wolf et al.111 Twenty worm-free lambs were supplemented with vitamin E with either 5.3 IU/kg/d (minimum daily requirement) or 10 IU/kg/d (requirement for optimal immune function) for 12 weeks and infected with 10,000 Haemonchus contortus (H. contortus) third stage larvae 5 weeks after vitamin E treatment.111 No differences were observed serum vitamin E, IL-4 and IFN-γ concentration, however lower reduction in packed cell volume (PCV) was observed in lambs receiving 10 IU/kg (P < .02) compared to lambs receiving 5.3 IU/kg. In addition, there was lower fecal egg count (P < .11) in 10 IU/kg-treated lambs (279 ± 72 eggs/g) versus lambs treated with the minimum daily requirement (484 ± 86 eggs/g). Overall, increased vitamin E supplementation lowered the worm burden by 49%.
Benefits of vitamin E for regulating infection have also been demonstrated in clinical human studies, including pneumonia. Shen et al studied 183 patients with stroke-associated pneumonia who were untreated or treated with low-dose (50 mg/d) or high-dose (100 mg/d) vitamin E.112 CD47 and CD55 levels, markers for neutrophil migration and activation, were measured in whole blood on the second day after admission and the day before patient discharge. Although CD55 levels did not differ between groups, CD47 levels after administration of vitamin E compared with patients receiving no treatment, with the highest levels seen in patients treated with high-dose vitamin E. The hospitalization time was also shorter with patients treated with vitamin E, leading to better clinical outcome. Vitamin E has also been shown to lower pneumonia rates in smoking adults. In a study by Hemila et al,113 male smokers aged 50 to 69 years old was administered 50 mg of vitamin E per day for 5 to 8 years, and the incidence of hospital-treated, community-acquired pneumonia was measured at the end of the study.113 Among 2216 patients who smoked 5 to 19 cigarettes a day, the incidence of pneumonia was reduced by 69% in patients receiving vitamin E and prevented pneumonia in 12.9% of patients receiving vitamin E. Among 5253 patients who smoked more than 20 cigarettes a day, the incidence of pneumonia in patients taking vitamin E supplements was lowered by 14%, indicating vitamin E may lower infection rates. In two different studies, smokers who are known to have an increase in oxidative stress had better infectious outcomes if treated with vitamin E. Generally, three factors effect burn injury mortality: age, TBSA, and inhalation injury. Of the three, inhalation injury is the strongest predictor of mortality; 114 therefore, assumptions can be made from the smoking population that vitamin E supplementation could potentially have a positive effect on decrease pneumonia in the burn patient population. Given that pneumonia is not uncommon in intubated burn patients and the mortality is high, any impact to improve outcomes is important further investigate.
Vitamin E in Sepsis
Sepsis, the body’s extreme response to infection associated with inflammation and oxidative stress, has been associated with lower vitamin E levels, leading to tissue damage and eventual multi-organ system failure.61,115–117 Initiated by the intravascular activation of host’s immune response to infection, the level of circulating pro-inflammatory cytokines increases, stimulating the pro-coagulatory state, and leading to the production of ROS, sepsis, and septic shock.118 Lowered vitamin E levels associated with sepsis has been known to cause accelerated apoptosis and tissue damage and higher mortality rate in numerous clinical studies.115,116,119 Administration of vitamin E or α-tocopherol to clinically septic patients and in vivo models has been shown to lower levels of tissue damage and organ failure with reduced oxidative stress.120–122
Ng et al studied the effects of α-tocopherol and its derivatives on lipopolysaccharide (LPS)-induced inflammatory responses in mouse peritoneal macrophages in vitro to determine whether treatment of LPS-induced macrophages decreased nitrite concentration in the cells, an indicator of nitric oxide (NO) and oxidative stress.123 Levels of pro-inflammatory cytokines including TNF-α and IL-6 were also measured by ELISA. Briefly, the cells were incubated with 1 µg/ml of LPS with varying concentrations of tocotrienol-rich fraction, α-tocopherol, and α-tocopheryl acetate. NO and PGE2 (prostaglandin E2, elevated in septic patients) levels increased 6- and 200-fold, respectively, when treated with LPS, but co-treatment with LPS and vitamin E derivatives reduced NO and PGE2 levels by up to 79.6% and 99.3%, respectively. Vitamin E and its derivatives also increased anti-inflammatory activity by reduction in TNF-α, IFN-γ, IL-1β, ad IL-6 compared to samples treated only with LPS.
In a similar study, Minter et al also tested three forms of vitamin E on human umbilical vein endothelial cells (HUVECs) treated with LPS to mimic sepsis.61 LPS exposure increased the concentration of free radicals (oxidative stress) significantly when compared to the vehicle control (P = .022). On the other hand, when the cells were treated with the three forms of vitamin E, radical production was reduced (P = .023). In addition, the metabolic activity of the HUVECs was also significantly reduced in LPS-only treated HUVECs compared to untreated cells (P < .0001) and cells treated with the vitamin E derivatives (P < .05), with MitoVitE, the mitochondria targeted form of vitamin E, downregulated the genes involved in the toll-like receptor (TLR) pathway and leading to an anti-inflammatory reaction. These in vitro models can be effective in understanding the foundation of the initial responses to vitamin E in sepsis, but these models are not always representative of what happens in a 3D environment especially in vivo.
Numerous in vivo models have been used to study the effect of vitamin E on sepsis. Similar to humans, a decrease in vitamin E in a porcine experimental sepsis model led to increased oxidative injury and lipid peroxidation.65 Atli et al induced sepsis in rats by cecal ligation and perforation (CLP) and studied the effect of selenium treatment in conjunction with vitamin E injection or selenium and vitamin E alone on the changes in lung tissue damage.120 Rats were administered selenium at 100 µg/d for 2 days and consequently at 40 µg/d for 5 days prior to CLP. If vitamin E was administered, it was given intramuscularly at 250 mg/kg/d for 7 days prior to CLP. Results showed that lung tissue damage was reduced in rats treated with selenium and/or vitamin E when compared to untreated rats through a blind pathological examination of lung histological tissue. Levels of blood oxygenation was also increased with treatment, with decreased levels of CO2. The combination of selenium and vitamin E treatment resulted in the optimal reduction in lipid peroxidation, increase in blood oxygenation, and overall reduction in tissue damage.
In a study by Godbout et al, 3-month-old mice were injected with vegetable oil containing α-tocopherol for 3 days and then injected with LPS on the fourth day to induce sepsis to determine the effects of α-tocopherol.121 Whole brain homogenates were assayed for IL-6 production, lipid peroxidation, and α-tocopherol concentration. Results showed the LPS-induced IL-6 production in the brain whereas it was almost undetectable in saline-treated mice. IL-6 production in LPS-treated mice peaked at 4 hours and remained elevated up to 24 hours compared to the control saline-treated group. When mice were treated with α-tocopherol prior to LPS injection significantly reduced (P < .001) IL-6 concentration in the brain by 77% when compared to the LPS-injected mice. IL-6 in plasma also significantly decreased (P < .02) by 25% with α-tocopherol treatment. Lipid peroxidation, an indicator of oxidative damage in cellular membranes, in the brain increased significantly (P < .02) almost 2-fold with LPS treatment compared to the saline-treated group. Similar to decrease in IL-6, treatment with α-tocopherol prior to LPS treatment decreased lipid peroxidation in the brain by 62% (P = .07), indicating that treatment was able to reduce oxidative damage. In a similar study by Lowes et al, rats were injected with LPS and peptidoglycan (PepG) to induce sepsis.124 Following injection of LPS/PepG, rats were injected with saline (control) or MitoE, a chromanol moiety of vitamin E attached to a triphenyl phosphonioum cation. This MitoE acts within the mitochondria and delivers tocopherol. Liver damage was assessed by measuring plamsa alanine amino transferase (ALT) and aspartate amino transferase (AST) activity, whereas renal function was by measuring plasma creatinine concentration. In order to measure mitochondrial respiration, mitochondria were isolated from the liver and a Clark-type oxygen electrode and oxygen consumption was used to measure the number of ATP molecules made they pass the respiratory chain to oxygen (ATP:O). Finally, oxidative damage was assessed by measuring plasma lipid hydroperoxides and liver protein carbonyl levels. 25% of rats only given LPS/PepG died under anesthesia before the end of the experiment, whereas no rats died in treated groups. ATP:O ratios in LPS/PepG treated rats were lower in the liver mitochondria than the saline or uninfected control, whereas MitoE-treated mice had similar ATP:O ratios to the saline-treated group. Liver damage (ALT and AST activity) and renal damage (creatinine level) were elevated in LPS/PepG treated rats compared to saline-treated rats, with lowered ALT and AST activity and creatinine levels in rats treated with MitoE when compared LPS/PepG treated rats. Oxidative damage (plasma lipid hydroperxoide) was also observed in LPS/PepG treated mice compared to untreated control, with reduced levels observed when treated with MitoE. These studies demonstrate that administration of vitamin E and its derivatives can reduce oxidative and tissue damage, inflammation, and mitochondrial dysfunction in in vivo models.
Vitamin E/α-tocopherol has also been investigated in the clinical setting. In a randomized, prospective study conducted by Nathens et al, 595 patients, 91% victims of trauma, were administered α-tocopherol or ascorbic acid every 8 hours (301 patients) or received the standard care to serve as the control group (294 patients).122 Lower percentage of patients developed pneumonia and acute respiratory distress syndrome (ARDS) when they received α-tocopherol compared to patients receiving the standard care (15.0%). In addition, treated patients had a lowered alveolar inflammatory response with decreased alveolar white blood cell protein concentration by 53.2% and TNF-α, IL-1β, and IL-6 concentrations compared to patients receiving standard care. Acute renal failure incidence was not significantly difference between patients receiving α-tocopherol treatment and standard care. This study concluded when administered early, vitamin E with ascorbic acid reduced the rate of organ failure and ICU hospitalization time.
Aisa-Alvarez et al studied the effect of numerous antioxidant treatments on septic shock patients with multiple organ failure.64 Ninety-seven patients were either treated with vitamin C, vitamin E, n-acetylcysteine, melatonin, or no treatment. The treatments were administered orally or through nasogastric tube for 5 days in conjunction with the standard therapy for each patient. Organ dysfunction was evaluated by assigning a SOFA (sequential organ failure assessment) score (neurologic, respiratory, hemodynamic, hepatic, and hematologic) at admission and during treatment. Oxidative stress markers were also measured collected blood plasma at the beginning and 48 hours. These included total antioxidant capacity, lipid peroxidation (LPO), and carbonylation (indication of acute kidney injury) levels were also measured. Compared to the control group, the SOFA score of patients treated with vitamin E did not significantly differ on each day up to 5 days of treatment. However, LPO and carbonylation levels did decrease (P < .17 and P < .07, respectively) with vitamin E treatment suggesting a multifactorial response to vitamin E supplementation. Only vitamin C and melatonin treatment was able to decrease the SOFA score significantly, suggesting that a combination of antioxidant treatments is required for the best clinical outcome.
Limitations of Vitamin E Treatment
As discussed in previous sections, oxidative stress as a result from burn injuries can lead to poor outcomes such as multiple organ failure and possible death. Therefore, vitamin E treatment is an attractive treatment because it has been shown to reverse the effects of oxidative stress. However, there are reported studies suggesting antioxidant treatment, including vitamin E treatment, showed no clinical efficacy in sepsis-induced multiorgan failure.125,126 It has been suggested that the effectiveness of vitamin E and other antioxidant treatments depends on the individual patient’s inflammatory response and the timing and duration of administration. A study by Heyland et al enrolled 1200 patients with at least two organ failures and were supplemented with antioxidants including selenium, zinc, β-carotene, and vitamins E and C within 24 hours of admission into the ICU.126 The study concluded there were no differences in mortality or improved outcome when patients were administered with antioxidants. This may be due to possible disruption of normal signaling involved in the response to severe infection.125 ROS produced from mitochondria are responsible for the activation of lymphocytes and monocytes during the response to infection.127,128 ROS production controls the activation of TLRs for bacterial clearance may suggest the activation of the inflammasome and T cells.69,129,130 Therefore, the disruption of this mechanism of ROS by antioxidants such as vitamin E may not improve the outcome of organ associated with multiple organ failure. ROS levels can control the level of inflammatory response; therefore, the timing and the condition of the patient is critical in administering vitamin E. Immunosuppressed patients such as older individuals or individuals with comorbidities may not benefit from vitamin E administration and can be detrimental to the patient’s outcome.131 On the other hand, during periods of a cytokine storm with exaggerated inflammatory response such as ARDS, vitamin E administration may be beneficial to suppress immune response. Further studies will need to be conducted to determine whether timing and/or the immune system profile of the patient has any effects on the benefits of vitamin E administration for burn injuries and burn injury-related sepsis.
Summary
Several investigations ranging from in vitro bench work to clinical studies have examined the impact on, or influence of, vitamin E as it pertains to thermal burn injuries, infection, and sepsis. The inflammation and oxidative stress that accompany these maladies often result in poorer healing outcomes, concomitant complications, and chronic detriments to the injured individual. The systemic impact of oxidative radicals is evident based on the significant depletions of antioxidants such as vitamin E, vitamin C, and zinc. To the benefit of subjects it has been shown that enteral or parenteral vitamin E supplementation can prevent, mitigate, and even reverse the effects of thermal burn injuries, infection, and sepsis. Additionally, as discussed, direct application of vitamin E to a wound has resulted in similarly improved wound outcomes, lending credence to its versatility. Although there are some reports indicating vitamin E has no effect on improving patient outcome, there needs to be further investigation to determine the best timing and the patient profile that benefits from vitamin E administration. In total, the published works on the use of vitamin E suggest there is value in its use as a singular therapeutic or in combination with other agents to improve healing outcomes for those afflicted by the aforementioned pathophysiologies.
FUTURE DIRECTIONS
There is clear evidence of potential benefits of vitamin E supplementation to mitigate the detrimental effects after injury. Clinical research outcomes are challenging in their reproducibility given the diversity of the patient population as well as heterogeneity of care algorithms and lack of standardized clinical metrics without a defined clinical trial. A large-scale prospective observational study to assess the potential benefits of vitamin E supplementation in patients is warranted. Additionally, the down side to vitamin E supplementation is negligible and the potential benefit is dramatic. Prospective data-driven approaches to vitamin E supplementation should be strongly considered and could result in clinical care practice paradigm changes.
ACKNOWLEDGEMENTS
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, DoD, or the U.S. Government.
Conflict of interest statement. There are no conflicts of interest to declare.
Conflict of interest statement. There are no conflicts of interest to report.
Contributor Information
Marc A Thompson, US Army Institute of Surgical Research, Combat Wound Care, JBSA Ft. Sam Houston, San Antonio, Texas, USA.
Kameel Zuniga, US Army Institute of Surgical Research, Combat Wound Care, JBSA Ft. Sam Houston, San Antonio, Texas, USA.
Linda Sousse, US Army Institute of Surgical Research, Combat Wound Care, JBSA Ft. Sam Houston, San Antonio, Texas, USA.
Robert Christy, US Army Institute of Surgical Research, Combat Wound Care, JBSA Ft. Sam Houston, San Antonio, Texas, USA.
Col Jennifer Gurney, Burn Center, US Army Institute of Surgical Research, Joint Trauma System, JBSA Ft. Sam Houston, San Antonio, Texas, USA.
References
- 1. Ahrens M, Evarts B.. Fire loss in the United States during 2019. National Fire Protection Association; 2020. [Google Scholar]
- 2. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. 2016; Aavailable from: https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2016_ed_web_tables.pdf. [Google Scholar]
- 3. Prin M, Li G. Complications and in-hospital mortality in trauma patients treated in intensive care units in the United States, 2013. Inj Epidemiol 2016;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Lonardo A, De Rosa M, Graziano A, Pascone C, Lucattelli E. Effectiveness of topical α-tocopherol acetate in burn infection treatment. Ann Burns Fire Disasters 2019;32:282. [PMC free article] [PubMed] [Google Scholar]
- 5. Alexander JW. Mechanism of immunologic suppression in burn injury. J Trauma Acute Care Surg 1990;30:70–4. [DOI] [PubMed] [Google Scholar]
- 6. Heideman M, Bengtsson A. The immunologic response to thermal injury. World J Surg 1992;16:53–6. [DOI] [PubMed] [Google Scholar]
- 7. D’Abbondanza JA, Shahrokhi S. Burn infection and burn sepsis. Surg Infect 2021;22:58–64. [DOI] [PubMed] [Google Scholar]
- 8. Ladhani HA, Yowler CJ, Claridge JA. Burn wound colonization, infection, and sepsis. Surg Infect 2021;22:44–8. [DOI] [PubMed] [Google Scholar]
- 9. Mann EA, Baun MM, Meininger JC, Wade CE. Comparison of mortality associated with sepsis in the burn, trauma, and general intensive care unit patient: a systematic review of the literature. Shock 2012;37:4–16. [DOI] [PubMed] [Google Scholar]
- 10. Capek KD, Sousse LE, Hundeshagen G, et al. Contemporary burn survival. J Am Coll Surg 2018;226:453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manning J. Sepsis in the burn patient. Crit Care Nurs Clin 2018;30:423–30. [DOI] [PubMed] [Google Scholar]
- 12. Traber D, Hawkins HK, Enkhbaatar P, Cox RA, Schmalstieg FC, Zwischenberger JB, Traber LD. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther 2007;20:163–6. [DOI] [PubMed] [Google Scholar]
- 13. Shimoda K, Nakazawa H, Traber MG, Traber DL, Nozaki M. Plasma and tissue vitamin E depletion in sheep with burn and smoke inhalation injury. Burns 2008;34:1137–41. [DOI] [PubMed] [Google Scholar]
- 14. Beiraghi-Toosi A, Askarian R, Haghighi FS, Safarian M, Kalantari F, Hashemy SI. Burn-induced oxidative stress and serum glutathione depletion; a cross sectional study. Emergency 2018;6:1–7. [PMC free article] [PubMed] [Google Scholar]
- 15. Bonucci J, Gragnani A, Trincado MM, Vincentin V, Correa SAA, Ferreira LM. The role of vitamin C in the gene expression of oxidative stress markers in fibroblasts from burn patients. Acta Cir Bras 2018;33:703–12. [DOI] [PubMed] [Google Scholar]
- 16. Duke JM, Rea S, Boyd JH, Randall SM, Wood FM. Mortality after burn injury in children: a 33-year population-based study. Pediatrics 2015;135:e903–10. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen TT, Cox CS, Traber DL, Gasser H, Redl H, Schlag G, Herndon DN. Free radical activity and loss of plasma antioxidants, vitamin E, and sulfhydryl groups in patients with burns: the 1993 Moyer Award. J Burn Care Res 1993;14:602–9. [DOI] [PubMed] [Google Scholar]
- 18. Assreuy J, Cunha FDQ, Barja-Fidalgo C, Tavares-Murta BM. Inflammatory and vascular alterations in sepsis: the role of nitric oxide-dependent mechanisms. Antiinflamm Antiallergy Agents Med Chem 2006;5:35–44. [Google Scholar]
- 19. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol 1996;271:C1424–37. [DOI] [PubMed] [Google Scholar]
- 20. Boscá L, Zeini M, Traves PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology 2005;208:249–58. [DOI] [PubMed] [Google Scholar]
- 21. Fortin CF, McDonald PP, Fulop T, Lesur O. Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock 2010;33:344–52. [DOI] [PubMed] [Google Scholar]
- 22. Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007;67:351–68. [DOI] [PubMed] [Google Scholar]
- 23. Soejima K, Schmalstieg FC, Traber LD, Szabo C, Salzman A, Traber DL. Role of nitric oxide in myocardial dysfunction after combined burn and smoke inhalation injury. Burns 2001;27:809–15. [DOI] [PubMed] [Google Scholar]
- 24. Sousse LE, Yamamoto Y, Enkhbaatar P, et al. Acute lung injury-induced collagen deposition is associated with elevated asymmetric dimethylarginine and arginase activity. Shock 2011;35:282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enkhbaatar P, Murakami K, Shimoda K, et al. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med 2003;167:1021–6. [DOI] [PubMed] [Google Scholar]
- 26. Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys 1992;298:446–51. [DOI] [PubMed] [Google Scholar]
- 27. Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett 1994;341:65–8. [DOI] [PubMed] [Google Scholar]
- 28. Bose KSC, Vyas P, Singh M. Plasma non-enzymatic antioxidants-vitamin C, E, beta-carotenes, reduced glutathione levels and total antioxidant activity in oral sub mucous fibrosis. Eur Rev Med Pharmacol Sci 2012;16:530–2. [PubMed] [Google Scholar]
- 29. Szabó C. Potential role of the peroxynitrite-poly (ADP-ribose) synthetase pathway in a rat model of severe hemorrhagic shock. Shock 1998;9:341–4. [DOI] [PubMed] [Google Scholar]
- 30. Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922;56:650–1. [DOI] [PubMed] [Google Scholar]
- 31. Schwarz K. Role of vitamin E, selenium, and related factors in experimental nutritional liver disease in Federation Proceedings. FASEB J 1965. [PubMed] [Google Scholar]
- 32. Mattill H. Antioxidants. Annu Rev Biochem 1947;16:177–92. [DOI] [PubMed] [Google Scholar]
- 33. Tappel A. The inhibition of hematin-catalyzed oxidations by alpha-tocopherol. Arch Biochem Biophys 1953;47:223–5. [DOI] [PubMed] [Google Scholar]
- 34. Huang H-Y, Appel LJ, Croft KD, Miller ER, Mori TA, Puddey IB. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: results of a randomized controlled trial. Am J Clin Nutr 2002;76:549–55. [DOI] [PubMed] [Google Scholar]
- 35. Esterbauer H, Dieber-Rotheneder M, Striegl G, Waeg G. Role of vitamin E in preventing the oxidation of low-density lipoprotein. Am J Clin Nutr 1991;53:314S–21S. [DOI] [PubMed] [Google Scholar]
- 36. Packer JE, Slater T, Willson R. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979;278:737–8. [DOI] [PubMed] [Google Scholar]
- 37. Machlin L, Gabriel E, Brin M. Biopotency of α-tocopherols as determined by curative myopathy bioassay in the rat. J Nutr 1982;112:1437–40. [DOI] [PubMed] [Google Scholar]
- 38. Chen Z. A study of lipid peroxide contents in tissues after burns in rats. dynamic changes of lipid peroxide and vitamin E in plasma, eschar and liver tissue. J Third Mil Med Univ 1984. [Google Scholar]
- 39. Wenan W. Relation of lipoperoxidation damage to burn. J First Mil Med Univ Z 1987;1. [Google Scholar]
- 40. Johnson L, Bowen Jr, Herrmann FW, et al. Relationship of prolonged pharmacologic serum levels of vitamin E to incidence of sepsis and necrotizing enterocolitis in infants with birth weight 1,500 grams or less. Pediatrics 1985;75:619–38. [PubMed] [Google Scholar]
- 41. Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J 1999;13:1145–55. [PubMed] [Google Scholar]
- 42. Kaempf-Rotzoll DE, Horiguchi M, Hashiguchi K, Aoki J, Tamai H, Linderkamp O, Arai H. Human placental trophoblast cells express α-tocopherol transfer protein. Placenta 2003;24:439–44. [DOI] [PubMed] [Google Scholar]
- 43. Atkinson J, Harroun T, Wassall SR, Stillwell W, Katsaras J. The location and behavior of α-tocopherol in membranes. Mol Nutr Food Res 2010;54:641–51. [DOI] [PubMed] [Google Scholar]
- 44. Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. The molecular basis of vitamin E retention: structure of human α-tocopherol transfer protein. J Mol Biol 2003;331:725–34. [DOI] [PubMed] [Google Scholar]
- 45. Rock CL, Dechert RE, Khilnani R, Parker RS, Rodriguez JL. Carotenoids and antioxidant vitamins in patients after burn injury. J Burn Care Res 1997;18:269–78. [DOI] [PubMed] [Google Scholar]
- 46. Traber MG, Shimoda K, Murakami K, Leonard SW, Enkhbaatar P, Traber LD, Traber DL. Burn and smoke inhalation injury in sheep depletes vitamin E: kinetic studies using deuterated tocopherols. Free Radic Biol Med 2007;42:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wong M, Lodge JK. A metabolomic investigation of the effects of vitamin E supplementation in humans. Nutr Metab 2012;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 1993;328:1444–9. [DOI] [PubMed] [Google Scholar]
- 49. Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett W.C. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 1993;328:1450–6. [DOI] [PubMed] [Google Scholar]
- 50. Harrison S, Torgerson S, Hayashi P. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Altern Med Rev 2004;9:100–1. [DOI] [PubMed] [Google Scholar]
- 51. Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao Y, Zhang W, Jia Q, et al. High dose vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front Physiol 2019;9:1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kottschade LA, Sloan JA, Mazurczak MA, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Support Care Cancer 2011;19:1769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson, EJ, O’Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol 2010;48:673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee S-W, Baek S-W, Kang K-K, et al. Vitamin C deficiency inhibits nonalcoholic fatty liver disease progression through impaired de novo lipogenesis. Am J Pathol 2021;191:1550–63. [DOI] [PubMed] [Google Scholar]
- 56. Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med 2007;43:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kayden HJ, Traber MG. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res 1993;34:343–58. [PubMed] [Google Scholar]
- 58. Jiang Q, Christen S, Shigenaga MK, Ames BN. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 2001;74:714–22. [DOI] [PubMed] [Google Scholar]
- 59. Barbosa E, Faintuch J, Moreira EAM, Goncalves da Silva VR, Pereima MJL, Fagundes RLM, Filho DW. Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: a randomized, double-blind, placebo-controlled pilot study. J Burn Care Res 2009;30:859–66. [DOI] [PubMed] [Google Scholar]
- 60. Preiser J-C, Van Gossum A, Berre J, Vincent JL, Carpentier Y. Enteral feeding with a solution enriched with antioxidant vitamins A, C, and E enhances the resistance to oxidative stress. Crit Care Med 2000;28:3828–32. [DOI] [PubMed] [Google Scholar]
- 61. Minter BE, Lowes DA, Webster NR, Galley HF. Differential effects of Mito VitE, alpha-tocopherol, and Trolox on oxidative stress, mitochondrial function and inflammatory signalling pathways in endothelial cells cultured under conditions mimicking sepsis. Antioxidants 2020;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mendez C, Garcia I, Maier RV. Antioxidants attenuate endotoxin-induced activation of alveolar macrophages. Surgery 1995;118:412–20. [DOI] [PubMed] [Google Scholar]
- 63. Goode HF, Cowley HC, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med 1995;23:646–51. [DOI] [PubMed] [Google Scholar]
- 64. Aisa-Alvarez, Soto ME, Guarner—Lans V, et al. Usefulness of antioxidants as adjuvant therapy for septic shock: a randomized clinical trial. Medicina 2020;56:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Basu S, Eriksson M. Vitamin E in relation to lipid peroxidation in experimental septic shock. Prostaglandins Leukot Essent Fatty Acids 2000;62:19–199. [DOI] [PubMed] [Google Scholar]
- 66. Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 1962;18:571–3. [Google Scholar]
- 67. Roncarati D, Scarlato V. Regulation of heat-shock genes in bacteria: from signal sensing to gene expression output. FEMS Microbiol Rev 2017;41:549–74. [DOI] [PubMed] [Google Scholar]
- 68. Hofmann E, Fink J, Eberl A, et al. A novel human ex vivo skin model to study early local responses to burn injuries. Sci Rep 2021;11:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Butt H, Mehmood A, Ali M, et al. Protective role of vitamin E preconditioning of human dermal fibroblasts against thermal stress in vitro. Life Sci 2017;184:1–9. [DOI] [PubMed] [Google Scholar]
- 70. Wegiel B, Bjartell A, Culig Z, Persson JL. Interleukin-6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survival. Int J Cancer 2008;122:1521–9. [DOI] [PubMed] [Google Scholar]
- 71. Lee S-G, Su Z-Z, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 2008;27:1114–21. [DOI] [PubMed] [Google Scholar]
- 72. Bonferoni MC, Riva F, Invernizzi A, et al. Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. Eur J Pharm Biopharm 2018;123:31–41. [DOI] [PubMed] [Google Scholar]
- 73. Hamahata A, Enkhbaatar P, Kraft ER, et al. gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free Radic Biol Med 2008;45:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Traber MG, Leonard SW, Traber DL, et al. α-Tocopherol adipose tissue stores are depleted after burn injury in pediatric patients. Am J Clin Nutr 2010;92:1378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arner P, Bernard S, Salehpour M, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 2011;478:110–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clark A, Imran J, Madni T, Wolf SE. Nutrition and metabolism in burn patients. Burns Trauma 2017;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mandell SP, Gibran NS. Early enteral nutrition for burn injury. Adv Wound Care 2014;3:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yamamoto Y, Enkhbaatar P, Sousse LE, et al. Nebulization with gamma-tocopherol ameliorates acute lung injury after burn and smoke inhalation in the ovine model. Shock 2012;37:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Turner WW Jr, Ireton CS, Hunt JL, Baxter CR. Predicting energy expenditures in burned patients. J Trauma 1985;25:11–6. [DOI] [PubMed] [Google Scholar]
- 80. Frei B, Traber MG. The new US dietary reference intakes for vitamins C and E. Redox Rep 2001;6:5–9. [DOI] [PubMed] [Google Scholar]
- 81. Gawel S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 2004;57:453–5. [PubMed] [Google Scholar]
- 82. Mingjian Z, Wang QF, Gao LX, Jin H, Wang ZY. Comparative observation of the changes in serum lipid peroxides influenced by the supplementation of vitamin E in burn patients and healthy controls. Burns 1992;18:19–21. [DOI] [PubMed] [Google Scholar]
- 83. Moore RA, Waheed A, Burns B.. Rule of nines. StatPearls [Internet]. StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 84. Al-Kaisy A, Sahib AS. Role of the antioxidant effect of vitamin E with vitamin C and topical povidone-iodine ointment in the treatment of burns. Ann Burns Fire Disasters 2005;18:19. [PMC free article] [PubMed] [Google Scholar]
- 85. Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG. Burns: pathophysiology of systemic complications and current management. J Burn Care Res 2017;38:e469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pereira GG, Guterres SS, Balducci AG, Colombo P, Sonvico F. Polymeric films loaded with vitamin E and aloe vera for topical application in the treatment of burn wounds. Biomed Res Int 2014;2014:641590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Klein G, Herndon DN, Goodman WG, et al. Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone 1995;17:455–60. [DOI] [PubMed] [Google Scholar]
- 88. Gauglitz GG, Herndon DN, Kulp GA, MeyerIII, WJ, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab 2009;94:1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Norbury WB, Herndon DN, Branski LK, Chinkes DL, Jeschki MG. Urinary cortisol and catecholamine excretion after burn injury in children. J Clin Endocrinol Metab 2008;93:1270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brillon D, Zheng B, Campbell RG, Matthews DE. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol Endocrinol Metab 1995;268:E501–13. [DOI] [PubMed] [Google Scholar]
- 91. Børsheim E, Herndon DN, Hawkins HK, Suman OE, Cotter M, Klein GL. Pamidronate attenuates muscle loss after pediatric burn injury. J Bone Miner Res 2014;29:1369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Song Y-H, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 2005;115:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006;147:4160–8. [DOI] [PubMed] [Google Scholar]
- 94. Church DD, Gwin JA, Wolfe RR, Pasiakos SM, Ferrando AA. Mitigation of muscle loss in stressed physiology: military relevance. Nutrients 2019;11:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Klein GL, Xie Y, Qin Y-X, Lin L, Hu M, Enkhbaatar P, Bonewald LF. Preliminary evidence of early bone resorption in a sheep model of acute burn injury: an observational study. J Bone Miner Metab 2014;32:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC. Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J Biol Chem 2011;286:44326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ambali SF, Ayo JO, Ojo SA, Esievo KAN. Vitamin E protects Wistar rats from chlorpyrifos-induced increase in erythrocyte osmotic fragility. Food Chem Toxicol 2010;48:3477–80. [DOI] [PubMed] [Google Scholar]
- 98. de Andrade Belo MA, Ruas de Moraes F, Yoshida, L, et al. Deleterious effects of low level of vitamin E and high stocking density on the hematology response of pacus, during chronic inflammatory reaction. Aquaculture 2014;422:124–8. [Google Scholar]
- 99. Ohtsuka A, Kojima H, Ohtani T, Hayashi K. Vitamin E reduces glucocorticoid-induced oxidative stress in rat skeletal muscle. J Nutr Sci Vitaminol 1998;44:779–86. [DOI] [PubMed] [Google Scholar]
- 100. Balboa E, Saavedra F, Cea LA, et al. Vitamin E blocks connexin hemichannels and prevents deleterious effects of glucocorticoid treatment on skeletal muscles. Int J Mol Sci 2020;21:4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wong SK, Chin K-Y, Suhaimi FH, Ahmad F, Ima-Nierwana S. The effects of vitamin E from Elaeis guineensis (oil palm) in a rat model of bone loss due to metabolic syndrome. Int J Environ Res Public Health 2018;15:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Moreno-Fernández S, Garces-Rimon M, Vera G, Astier J, Landrier JF, Miguel M. High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients 2018;10:1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Norazlina M, Ima-Nirwana S, Gapor MT, Khalid BA. Palm vitamin E is comparable to α-tocopherol in maintaining bone mineral density in ovariectomised female rats. Exp Clin Endocrinol Diabetes 2000;108:305–10. [DOI] [PubMed] [Google Scholar]
- 104. Lehnen AM, Rodrigues B, Irigoyen MC, De Angelis K, D’Agord Schaan B. Cardiovascular changes in animal models of metabolic syndrome. J Diabetes Res 2013;2013:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Berlanga-Acosta J, Iglesias-Marichal I, Rodriguez-Rodriguez N, Mendoza-Mari Y, Garcia-Ojalvo A, Fernandez-Mayola M, Playford RJ. Insulin resistance and mitochondrial dysfunction following severe burn injury. Peptides 2020;126:170269. [DOI] [PubMed] [Google Scholar]
- 106. Rodrigues MFC, Stotzer US, Domingos MM, et al. Effects of ovariectomy and resistance training on oxidative stress markers in the rat liver. Clinics 2013;68:1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Elkomy MM, Elsaid FG. Anti-osteoporotic effect of medical herbs and calcium supplementation on ovariectomized rats. J Basic Appl Zool 2015;72:81–8. [Google Scholar]
- 108. Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019;71:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee GY, Han SN. The role of vitamin E in immunity. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tantcheva LP, Stoeva ES, Galabov AS, Braykova AA, Savov VM, Mileva MM. Effect of vitamin E and vitamin C combination on experimental influenza virus infection. Methods Find Exp Clin Pharmacol 2003;25:259–64. [DOI] [PubMed] [Google Scholar]
- 111. De Wolf BM, Zajac AM, Hoffer KA, Sartini BL, Bowdridge S, LaRoith T, Petersson KH. The effect of vitamin E supplementation on an experimental Haemonchus contortus infection in lambs. Vet Parasitol 2014;205:140–9. [DOI] [PubMed] [Google Scholar]
- 112. Shen H, Zhan B. Effect of vitamin E on stroke-associated pneumonia. J Int Med Res 2020;48:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hemila H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging 2016;11:1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Knowlin L, Stanford L, Cairns B, Charles A. The effect of smoking status on burn inhalation injury mortality. Burns 2017;43:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Durant R, Klouche K, Delbosc S, et al. Superoxide anion overproduction in sepsis: effects of vitamin E and simvastatin. Shock 2004;22:34–9. [DOI] [PubMed] [Google Scholar]
- 116. Weber SU, Lehmann LE, Schewe J-C, et al. Low serum a-tocopherol and selenium are associated with accelerated apoptosis in severe sepsis. BioFactors 2008:107–19. [DOI] [PubMed] [Google Scholar]
- 117. Koekkoek K, van Zanten AR. Antioxidant vitamins and trace elements in critical illness. Nutr Clin Pract 2016;31:457–74. [DOI] [PubMed] [Google Scholar]
- 118. Victor VM, Espulgues JV, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets 2009;9:376–89. [DOI] [PubMed] [Google Scholar]
- 119. Dang H, Li J, Liu C, Xu F. The association between vitamin E deficiency and critically ill children with sepsis and septic shock. Front Nutr 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Atli M, Erikoglu M, Kaynak A, Esen HH, Kurban S. The effects of selenium and vitamin E on lung tissue in rats with sepsis. Clin Invest Med 2012;35:E48–54. [DOI] [PubMed] [Google Scholar]
- 121. Godbout JP, Berg BM, Kelley KW, Johnson RW. a-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunol 2004;149:101–9. [DOI] [PubMed] [Google Scholar]
- 122. Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002;6:814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ng LT, Ko HJ. Comparative effects of tocotrienol-rich fraction, a-tocopherol and a-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem 2012;134:920–5. [DOI] [PubMed] [Google Scholar]
- 124. Lowes D, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth 2013;110:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jain M, Chandel NS. Rethinking antioxidants in the intensive care unit. Am J Respir Crit Care Med 2013;188:1283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Heyland D, Muscedere J, Wischmeyer PE, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013;368:1489–97. [DOI] [PubMed] [Google Scholar]
- 127. Segal BH, Grimm MJ, Khan ANH, Han W, Blackwell TS. Regulation of innate immunity by NADPH oxidase. Free Radic Biol Med 2012;53:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pearce E, Pearce E. Metabolic pathways in immune cell activation and quiescence. Immunity 2013;38:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 2010;10:210–5. [DOI] [PubMed] [Google Scholar]
- 130. Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013;38:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 2013;13:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]