Abstract
Hypertrophic scars are a common negative outcome of deep partial-thickness (DPT) burn wounds resulting in increased dermal thickness, wound area contracture, and inflammation of the affected area. The red Duroc and Yorkshire porcine breeds are common large animal models for studying dermal wounds due to their structural similarities to human skin; however, the porcine transcriptomic profiles of dermal burn wounds and healing process are not well known. In response, a longitudinal transcriptomic comparative study was conducted comparing red Duroc and Yorkshire superficial and DPT burn wounds to their respective control uninjured tissue. Using next-generation RNA sequencing, total RNAs were isolated from burn wound tissue harvested on 0, 3, 7, 15, 30, and 60 days postburn, and mRNA-seq and gene expression read counts were generated. Significant differentially expressed genes relative to uninjured tissue were defined, and active biological processes were determined using gene set enrichment analyses. Additionally, collagen deposition, α-smooth muscle actin (SMA) protein concentration, epidermal and dermal thickness measurements, and wound area changes in response to burn injury were characterized. Overall, the red Duroc pigs, in response to both burn wound types, elicited a more robust and prolonged inflammatory immune response, fibroblast migration, and proliferation, as well as heightened levels of extracellular matrix modulation relative to respective burn types in the Yorkshire pigs. Collectively, the red Duroc DPT burn wounds produce a greater degree of hypertrophic scar-like response compared with Yorkshire DPT burn wounds. These findings will facilitate future porcine burn studies down-selecting treatment targets and determining the effects of novel therapeutic strategies.
Based on the extent of the injury, burn wounds are grouped into superficial (first degree), partial- (second degree), and full-thickness burns (third degree).1, 2 Superficial burns, which affect the epidermal2 and some of the underlying uppermost dermal layers, normally heal in 14 days or less, leaving no or minimal scarring of the affected area. By contrast, in full-thickness burns, the injury extends into the entire dermis down to subcutaneous tissue. The standard of care for full-thickness burns is early excision of dead skin and tissue and autologous skin grafting to close the wounds for reduced scarring.1 Partial-thickness (second degree) burns include superficial partial- and deep partial-thickness (DPT) burns. In superficial partial-thickness burns, the injury involves the epidermis and the upper dermal layers, whereas in DPT burns, tissue damages are extended into the reticular layer of dermis, affecting approximately 75% of the dermis.1 And as much as half or more DPT wounds develop severe hypertrophic scars (HSs), scar contractures, and loss of function and aesthetics of the affected area.3 Human HSs form thick, raised, and firm itchy dermal lesions that can persist for years .4, 5 Treatments to prevent HS formation after burn injury is one of the unmet challenges.6 Additionally, current treatments in reversing HSs are also minimally effective.7 Therefore, hypertrophic scarring is a major concern in DPT burn injuries, and effective prevention and treatment strategies are needed.
Unlike full-thickness burns, DPT burn wounds retain some dermal elements including keratinocyte stem cells and fibroblasts that provide regenerative capacity. However, exuberant activation of these cells and prolonged inflammation of slowly healing wounds can drive HS formation.8, 9 The severity of fibrotic scarring is characterized by excessive production and deposition of extracellular matrix (ECM) proteins (in particular, collagens), fibroblast hyper-proliferation and resistance to apoptosis, profuse differentiation of fibroblasts into myofibroblasts, and a decrease in collagen degradation.10 These cellular events are largely driven by the transforming growth factor (TGF)-β pathway, which is a potent stimulator of fibrosis. TGF-β receptor activation results in translocation of the SMAD2–SMAD3 transcription factor complex into the nucleus to promote expression of ECM genes such as COL1A1, COL3A1, matrix metalloproteinase (MMP)-3, MMP-9, and tissue inhibitor of metalloproteinases (TIMPs), as well as about 60 other ECM-related genes.11TGF-β also activates a non-SMAD pathway that includes signaling through p38 mitogen-activated protein (MAP) kinase, Jun N-terminal kinase (JNK), and Ras-extracellular signal-regulated kinase (Erk), triggering inflammation in response to stress and other stimulatory signals.12 Additionally, despite the importance of angiogenesis for wound repair, excessive angiogenesis promotes pro-fibrotic environments in the wound bed, and its reduction is linked to improved healing.13
The lack of efficacious treatments to prevent or mitigate the onset of HS compels the need to understand the biological responses to DPT burns to uncover potential treatment targets at molecular levels using clinically relevant animal models of burn wounds.14 Due to the structural similarity of porcine and human skin, a number of porcine burn models using Yorkshire and red Duroc breeds15 have been developed to study the healing and scarring of burn wounds. Although literature suggests that the red Duroc produces more human-like HS than Yorkshire, little research has directly compared the two breeds with respect to molecular events during scaring in response to DPT burns.16–19 For these reasons, the current study performed a direct comparison of superficial burns affecting the epidermis layer and DPT burns which affect the epidermis and lower portion of the dermis in Yorkshire and red Duroc pigs. Transcriptomic profiling of mRNA transcripts obtained from different time points postburn allowed an unbiased assessment of differentially expressed genes (DEGs) (normalized to uninjured tissue) of activated pathways and biomarkers associated with each burn wound type longitudinally in these two breeds.
During each postoperative (burn) day (POD) studied, red Duroc DPT burn wounds demonstrated a higher number of DEGs relative to Yorkshire. Gene set enrichment analysis (GSEA) of the DPT burn wounds in red Duroc pigs demonstrated a longer duration of inflammation and ECM modulation compared with Yorkshire. Both porcine breeds demonstrated a decline in collagen levels starting at POD 7 in their DPT burn wounds. A significant increase in α-SMA protein levels in the DPT burn wounds was detected at PODs 15 and 30 for the red Duroc and Yorkshire, respectively. Additionally, a noticeable increase in wound area contraction and a significant increase in both epidermal and dermal thickness were observed in the red Duroc DPT burn wounds. By contrast, compared with DPT burn wounds, differences in numbers of DEGs in superficial burn wounds between these two breeds are less for each of the studied POD, and inflammatory response and ECM modulations are less profound relative to their respective DPT burns. Overall, our longitudinal transcriptomics study revealed that the porcine breeds respond differently to burn injury with red Duroc demonstrating a more robust and prolonged inflammatory response to burn and a propensity to form HS-like characteristics compared with Yorkshire which exhibited outwardly healed characteristics.
METHOD
Animal Study Statement
The study on animals was carried out in compliance with the ARRIVE guidelines.
Animal Ethics Statement
The research was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. The United States Army Institute of Surgical Research (USAISR) Institutional Animal Care and Use Committee (IACUC) approved all research conducted in this study (Animal Protocol A-17-018). The USAISR facility where this research was conducted is fully accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC).
Burn Wound Porcine Model
Twelve superficial and 12 DPT position-matched 1.25″-diameter burn wounds were created on the mid-region of the dorsum of two female Yorkshire and two female red Duroc pigs, each weighing 45 to 55 kg (Supplementary Figure S1). The total wound area including controls was less than 10% TBSA. The TBSA calculation was based on the Meeh’s formula (A = 10 × W2, 3), A = area in cm2, 10 is a constant, and W = body weight in grams).
Prior to wounding, the pigs were anesthetized, and the placement of burn wounds and controls was traced with a skin marker. Burn wounds were created with a burn device designed by our group consisting of dual 1.25″ copper cylinders attached to a heat-insulated Teflon handle to allow safe handling by the operator. The burn device is electronically controlled by a J-type thermocouple embedded inside the copper cylinder. Superficial and DPT burn wounds were created by applying the dual copper cylinders to the skin at 100°C with a pressure of 378.819 g/cm2 for 2 and 13 seconds, respectively, in Yorkshire pigs and 5 and 36 seconds, respectively, in red Duroc pigs. Due to differences in average dermal thicknesses of Yorkshire and red Duroc pigs (2.16 and 3.69 mm, respectively), the creation of superficial and DPT burn depths in red Duroc pigs requires longer burn device contact times than equivalent burn wounds in the Yorkshire pigs. Superficial and DPT burn depth was determined by assessing the degree of apoptosis20–22 as measured by terminal deoxynucleotidyl transferase 2´-Deoxyuridine, 5´-Triphosphate (dUTP) nick-end labeling (TUNEL) stains of tissue cross-sections (Supplementary Table S1).
Following the creation of burn wounds and excision of tissue on designated days, the wounds were dressed with Xeroform (Covidien, Mansfield, MA, USA), cotton gauze, Ioban (3M Health Care, St. Paul, MN, USA), and a fabric vest (DeRoyal, Powell, TN, USA).
Superficial, and DPT wounds, and control uninjured tissues were position-matched along the midline on the mid-region of the pig’s dorsum to allow direct comparisons of burn types and controls (Supplementary Figure S2). Superficial and DPT burns and control tissues were collected after the burn on PODs 0, 3, 7, 15, 30, and 60. From each pig, for each POD, one 12-mm-diameter and one 14-mm-diameter biopsies were collected from the center of each wound type from the skin to the entire dermis layer (Supplementary Table S1).
The 12-mm-diameter circular biopsy was utilized for collagen deposition assays, and the 14-mm-diameter circular biopsy was sectioned in half utilized for mRNA sequencing and Real-time PCR (RT-PCR), and histology (Supplementary Table S1). Tissue samples isolated for collagen were placed in cryotubes (Thermo Scientific, Waltham, MA), frozen in liquid nitrogen, and stored at −80°C. Tissue samples isolated for total RNA sequencing were placed in cryotubes suspended in RNAlater (Thermo Scientific, Waltham, MA) and stored at 4°C.
Wound Contraction
Following the induction of burn wounds, the burned and control uninjured tissues were outlined with a tattoo on POD 3 to allow for wound size measurements using a SilhouetteStar wound assessment camera (Aranz Medical, Christchurch, New Zealand). Due to the animal’s natural growth, the wound area was affected by the burn type and the physical growth of the animal. To account for the natural animal growth, a 2.25-cm diameter growth control was tattooed proximal to each burned and control uninjured skin. Growth controls were used to normalize the area. Wound area contracture was calculated according to Supplementary Figure S3. The wound area ratio was determined, and the percentage relative to POD 3 was calculated. Assay was performed in technical duplicates. Data plot and statistical analysis were performed using GraphPad Prism 8.4.3.471 (GraphPad Software, Inc. San Diego, CA). Comparisons were performed using two-way analysis of variance (ANOVA) with Tukey’s correction for multiple comparison and an α set to 0.05. Data were graphed as the mean ± SD.
Collagen Concentration Assay
Total collagen concentration was determined with a total collagen assay kit (QuickZyme Biosciences, The Netherlands). Frozen tissue samples were crushed with a Bessman Tissue Pulverizer (Spectrum, Inc., Rancho Dominguez, CA) and then stored in a pre-weighed screw cap microtube (Sarstedt, Nümbrecht, Germany). The tissue samples were lyophilized with a VirTis Advantage Plus EL-85 Freeze Dryer (Thermo Scientific, Waltham, MA.). The dry weight of the tissue samples was recorded and used to normalize the tissue weight with 6 M HCl. The tissue samples were hydrolyzed with 6M HCl incubated at 95°C for 20 hours. The hydrolyzed samples were diluted to 4 M HCl with water, and collagen concentration was determined based on the standard curve generated from the collagen standard included in the kit. Data plot and statistical analysis were performed using GraphPad Prism 8.4.3.471 GraphPad Software, Inc. San Diego, CA). Assay was performed in technical triplicates. Comparisons were performed using two-way ANOVA with Tukey’s correction for multiple comparison and an α set to 0.05. Data were graphed as the mean ± SD.
Histology
Dermal tissue was collected with a 14-mm cutaneous punch (Delasco, Council Bluffs, IA). Cutaneous samples collected included the epidermis, dermis, and hypodermis layers. Dermal tissue samples were fixed in 10% buffered formalin in phosphate-buffered saline (PBS) (Fisher Diagnostics, Kalamazoo, MI) for at least 1 week and then processed and embedded in paraffin wax. About 4- to 5-μm thin sections of the embedded tissue samples were stained with hematoxylin-eosin, Masson’s trichrome, and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL).
Immunohistochemical α-SMA Protein Quantification
Burn wound tissue α-SMA protein expression was quantified as previously described by Wang et al.23 Immunohistochemistry colorimetric α-SMA-stained tissue sections were scanned with an Aperio Versa 200 pathology slide scanner, and α-SMA protein expression within burn wound tissue sections was quantified using a pixel positive algorithm (ImageScope V12.3.3.5048). α-SMA protein expression is scored based on the number and colorimetric intensity of the positive stain containing pixels. α-SMA protein expression index is expressed as ×108 positively scored pixels per area (mm2). Assay was performed in technical triplicates. Data plot and statistical analysis were performed using GraphPad Prism 8.4.3 (471) (GraphPad Software, Inc. San Diego, CA). Comparisons were performed using two-way ANOVA with Tukey’s correction for multiple comparison and an α set to 0.05. Data were graphed as the mean ± SD.
TUNEL Staining
Detection of apoptotic cells in histological cross-sections of dermal wound tissue was accomplished with terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) using a Click-iT Plus TUNEL Assay kit (Life Technologies, Carlsbad, CA). As previously described by Brandenburg et al,20 briefly, histological tissue sections were deparaffinized, rehydrated, and incubated in 4% paraformaldehyde for 15 minutes. Tissue samples were permeabilized with Proteinase K treatment and then washed. The tissue sample cross-sections were treated with terminal deoxynucleotidyl transferase (TdT) reaction buffer incorporating a bio-orthogonal alkyne moiety modified dUTP at the 3′-OH ends of fragmented DNA. Fluorescent labeling of fragmented DNA in apoptotic cells was accomplished by treating the tissue cross-sections with the Click-iT Plus TUNEL reaction cocktail which includes an Alexa Fluor 594 azide and an alkyne. General nuclei were stained with DAPI. Visualization of apoptotic cells was accomplished with a ×10 objective on a Leica Aperio Versa 200 (Leica Biosystems, Inc., Buffalo Grove, IL) slide scanner with Ex/Em wavelengths of 590/615 nm, while the cellular nuclei were visualized using Ex/Em wavelengths of 358/461 nm.
Dermal Thickness Measurement
Cross-sections of burn wound tissue samples were stained with hematoxylin–eosin then visualized and imaged with a ×10 objective on a Leica Aperio Versa 200 (Leica Biosystems, Inc., Buffalo Grove, IL). Image analysis and dermal thickness measurements were conducted using the Leica ImageScope V12 software. For each burn wound tissue cross-section, using the software, the epidermis–dermis border and the dermis–hypodermis border were traced and then the software measured the distance between the two traced borders. Assay was performed in technical triplicates. Data plot and statistical analysis were performed using GraphPad Prism 8.4.3 (471) (GraphPad Software, Inc. San Diego, CA). Comparisons were performed using two-way ANOVA with Tukey’s correction for multiple comparison and an α set to 0.05. Data were graphed as the mean ± SD.
Total RNA Extraction
To obtain total RNA, each biopsy sample was transferred to 2 ml TRIzol (Life Technologies) and homogenized using the T25 ULTRATURRAX at 20,000 rpm (IKA, Germany). RNA was extracted with 400 µl chloroform. The RNA was purified using an RNEasy Mini-Kit (Qiagen, MD) with a QIAcube extraction robot (Qiagen, MD). Genomic DNA was removed by treatment with DNAse I (Qiagen), and the absence of DNA contamination was confirmed using a minus-reverse transcriptase control demonstrating a Cycle threshold (Ct) value 10 cycles higher than the reverse transcribed samples. The RNA was quantified by Qubit (Thermo Fisher Scientific, Waltham, MA) and checked for purity (Fragment Analyzer, Agilent Technologies, Santa Clara, CA).
Quantitative RT-PCR
Quantitative RT-PCR was performed as described by Karna et al.24 Briefly, quantitative real-time PCR was performed using the SYBR green master mix (Bio-Rad) with specified primers (Supplementary Table S2) and was analyzed using the StepOne System (Applied Biosystems). Expression of mRNA in wound tissue was determined by the 2−ΔΔCt method, and normalized by metal regulatory transcription factor 1 as the housekeeping gene. These RT-qPCR results validated the RNA-Seq data with the selected genes (Supplementary Figures S4 and S5). Assay was performed in technical triplicates.
Next-Generation Sequencing (mRNAseq)
Total RNA extracted from biopsies collected from the middle dorsum region of four animals with two for each breed represented days 0, 3, 7, 15, 30, and 60 and was submitted to the Genome Sequencing Facility of UT Health (San Antonio, TX, USA) for RNA sequencing. Briefly, the quality of total RNA was assessed by RNA quality number (RQN) using the Agilent Fragment analyzer. Following the manufacturer’s instructions, total RNA (~500 ng) with RQN > 7.0 was used for RNA-seq library preparation with the TruSeq stranded mRNA-seq sample prep kit (Illumina, CA). After, RNA-seq libraries were subjected to quantification process, pooled for cBot amplification, and a subsequent 50-bp single-read sequencing run was performed with Illumina HiSeq 3000 platform. After the sequencing run, demultiplexing with Bcl2fastq2 was employed to generate the fastq file for each sample. An average of 30 million reads was obtained for this set of samples. For more information about sequenced samples, refer to Supplementary Table S3. Sequenced reads were submitted to the NCBI Sequence Read Archive (SRA) and are accessible under BioProject PRJNA706694.
Gene Expression Data Analysis
Short sequence reads (50 bp) from sequencing facility were aligned using TopHat2 aligner25 to the pig genome (Ensembl Sus scrofa 11.1 genome build). After the alignment, all known transcripts (GTF file obtained from Ensembl in Dec 2017) were summarized by using HTSeq26 for gene expression measurement in unit of read counts and then converted to fragment per kilobase of transcript per million transcripts mapped (FPKM). The DESeq algorithm27 was used to estimate the differential expression in read counts and their statistical significance for all samples from different burn degree and regions to their corresponding control groups. Significant DEGs with FDR (adjusted P-value using Benjamini–Hochberg method for multiple-test correction) below .05 (or 5%) and fold change greater than 2 were selected as significant DEGs. Principal component analysis (PCA) was performed on top variable genes (with averaged normalized expression level [size factor normalization by DESeq] > 1 and std. > 0.5 across time points) to visualize the trajectory of global expressional changes following burn wounds. Functional assessment of these DEGs was performed using fast GSEA and TopGO package in R/Bioconductor (https://bioconductor.org/). Heatmap, Volcano plot, k-means, and/or hierarchical clustering algorithms were performed using custom codes implemented in R (https://www.R-project.org) or MATLAB (Mathworks, Inc, MA). K-means algorithm was performed on DEGs collected from any group-wise comparisons of each porcine model. The RPKM data were z-transformed, and DEGs were clustered based on their expression patterns across time points. Here, we set k at 16 to form 16 clusters. Gene set enrichment analyses were performed as described by Subramanian et al.28 Briefly, gene expression fold changes (FCs) were determined for each burn wound type and time point relative to the control uninjured tissue using the DESeq algorithm. All genes were ranked by log2 FC, and enrichment scores for each gene set were calculated as Kolmogorov–Smirnov statistic based on the ranked genes implemented in Fast GSEA (Bioconductor Package).29 Normalized enrichment scores (NES) increase when genes belonging to the gene set are enriched to the higher ranks (positive) or lower ranks (negative), and NES near 0 when targeted gene set is randomly present on the ranked list.
RESULTS
Duroc DPT Burn Wounds Displayed More DEGs at Each POD
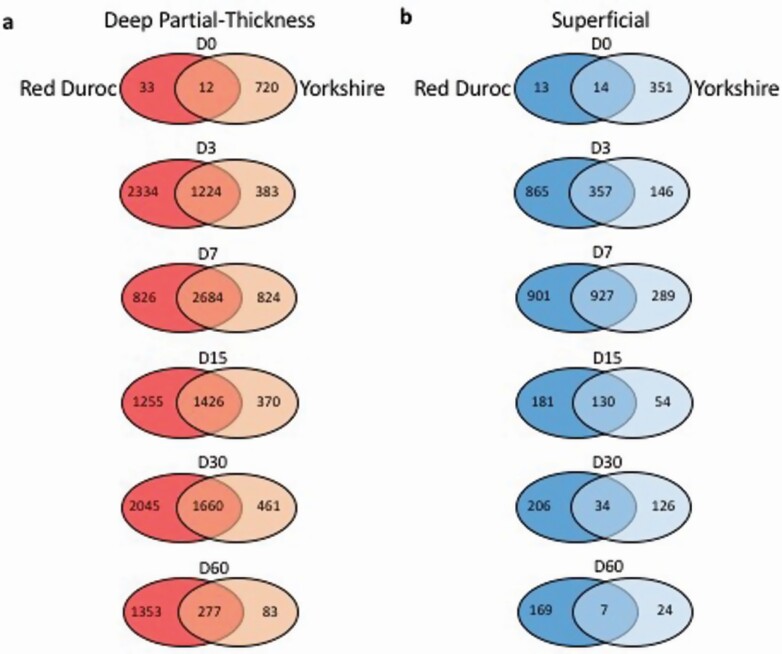
DEGs were determined by comparing the transcriptome of the burn wound to control uninjured tissue for each POD. In the red Duroc DPT burn wounds, the number of DEGs increased and peaked at POD 3 and POD 30 with 3558 and 3705 identified DEGs, respectively, and remained at 1630 DEGs at POD 60. In contrast, the Yorkshire DPT burn wounds exhibited 2121 and 360 DEGs at PODs 30 and 60, respectively (Figure 1a). The number of shared DEGs in the DPT burn wounds between both breeds peaked at POD 7 with 2684 identified DEGs and declined to 277 DEGs by POD 60. Proportions of upregulated and downregulated DEGs for each burn type, timepoint, and breed are listed in Supplementary Table S4.
Figure 1.
Differentially expressed gene (DEG) interaction analysis of red Duroc and Yorkshire porcine superficial and deep partial-thickness (DPT) burn wounds. DEGs in Red Duroc and Yorkshire porcine (a) DPT and (b) superficial burn wounds were selected based on P-value < .05 and fold change ≥ 2. The number of DEGs selected for each burn type compared with the control uninjured tissue was counted and illustrated for days 0, 3, 7, 15, 30, and 60 postburn. The Venn diagrams showed unique and shared DEGs of each burn type, compared with the control uninjured tissue.
Compared with DPT burn wounds, we observed much fewer DEGs across the superficial burn wounds in both breeds (Figure 1b). The number of DEGs peaked at POD 7 for both Yorkshire and red Duroc with 1216 and 1828 DEGs identified, respectively. Comparable to DPT burns in both breeds, the number of shared superficial DEGs peaked at POD 7 with 927 DEGs profiled and decreased to 7 DEGs at POD 60. Additionally, by POD 60, the number of identified DEGs between superficial burn wounds and control uninjured tissue in red Duroc and Yorkshire porcine breeds was far less than the DEGs profiled between DPT burns and the control uninjured tissue in the respective porcine breeds with only 176 and 31 DEGs identified, respectively (Figure 1b).
Functional Comparative Analysis of Porcine Burn Wound Transcriptome
To gain insight into the active and inactive molecular pathways during our course of study for each burn wound type and porcine breed, gene set enrichment analyses (GSEA) were applied to our profiled DEGs, using Gene Ontology (GO) dataset.30 The identified enriched pathways were arranged by NES with positive NESs indicating genes that delegate a particular biological function as active pathways.28 Conversely, gene sets with negative NESs indicate the biological pathway to be inactive or downregulated. The top 10 scored gene sets of DPT and superficial burns of both porcine breeds demonstrate upregulation of processes related to inflammation and immune responses such as leukocyte chemotaxis, migration, adhesion, and activation until approximately at POD 15 in DPT and at POD 7 in superficial burn wounds, in both porcine breeds (Figure 2a–e; Supplementary Figure S6). Compared with superficial burn wounds, prolonged inflammation was evident in DPT burn wounds for both porcine breeds. This observation was supported by the reduced migration, chemotaxis, and activation of leukocytes in superficial burn wounds in both breeds (Figure 2a–e; Supplementary Figure S6). In relation to ECM modulation, DEGs of both Yorkshire and red Duroc burn wound samples were enriched in pathways associated with collagen fibril organization (GO:0030199) (Figure 2f; Supplementary Figure S6). This function was ranked in the top 10 upregulated gene sets for the red Duroc superficial burn wound samples collected on PODs 15 and 30, as well as PODs 30 and 60 in the DPT burn wounds. The DEGs of DPT wounds in Yorkshire were highly enriched for pathways functioning in collagen fibril organization (GO:0030199) at POD 30 as well. Similar to red Duroc superficial burn wounds, this pathway was also identified in the top 10 active Yorkshire superficial burn wound pathways in PODs 15 and 30 (Figure 2f; Supplementary Figure S6). Interestingly, the activity of collagen fibril and extracellular structure organization was tailed off by POD 60 only for Duroc superficial burn wounds (Figure 2f and g). Furthermore, in DPT wounds, collagen fibril and extracellular structure organization started early at PODs 7 and 3, respectively, for Duroc pigs, whereas these processes started late at PODs 15 and 7, respectively, for Yorkshire breed.
Figure 2.
Gene Ontology (GO) terms for biological processes identified in red Duroc and Yorkshire porcine deep partial-thickness (DPT) and superficial burn wounds relative to control uninjured tissue. Gene set enrichment analysis of differentially expressed genes identified biological processes and graphs represent normalized enrichment scores determined for days 0, 3, 7, 15, 30, and 60 postburn. a. Inflammatory response, b. leukocyte migration, c. leukocyte chemotaxis, d. leukocyte activation, e. lymphocyte migration, f. collagen fibril organization, and g. extracellular structure organization.
Principle Component Analysis
A PCA of variably expressed genes of DPT and superficial burn wounds and control uninjured tissue in both breeds was performed to give an overview of the relative gene expression profiles (Supplementary Figures S7 and S8). The PCA of the red Duroc and Yorkshire transcriptomes captured 60.3% and 49% of the total variance, respectively. Overall, the clustering of identical PODs and burn types as well as control uninjured tissue of each Yorkshire pig were tighter compared with the red Duroc pigs, suggesting that the transcriptomes of the Yorkshire breed in this study were more similar than that of the Duroc breed. Also, the response of the Yorkshire breed to both burn types from both pigs (Y0 and Y4) was more consistent than the equivalent data plots in the red Duroc pigs. Comparing Duroc with Yorkshire pigs, PCA of DPT wounds of both breeds captured 53.1% compared with 35.2% of the total variance in the PCA of Superficial (SUP) wounds of both breeds (Supplementary Figures S9 and S10).
Collagen Deposition Decreased Over Time in Duroc DPT Burn Wounds
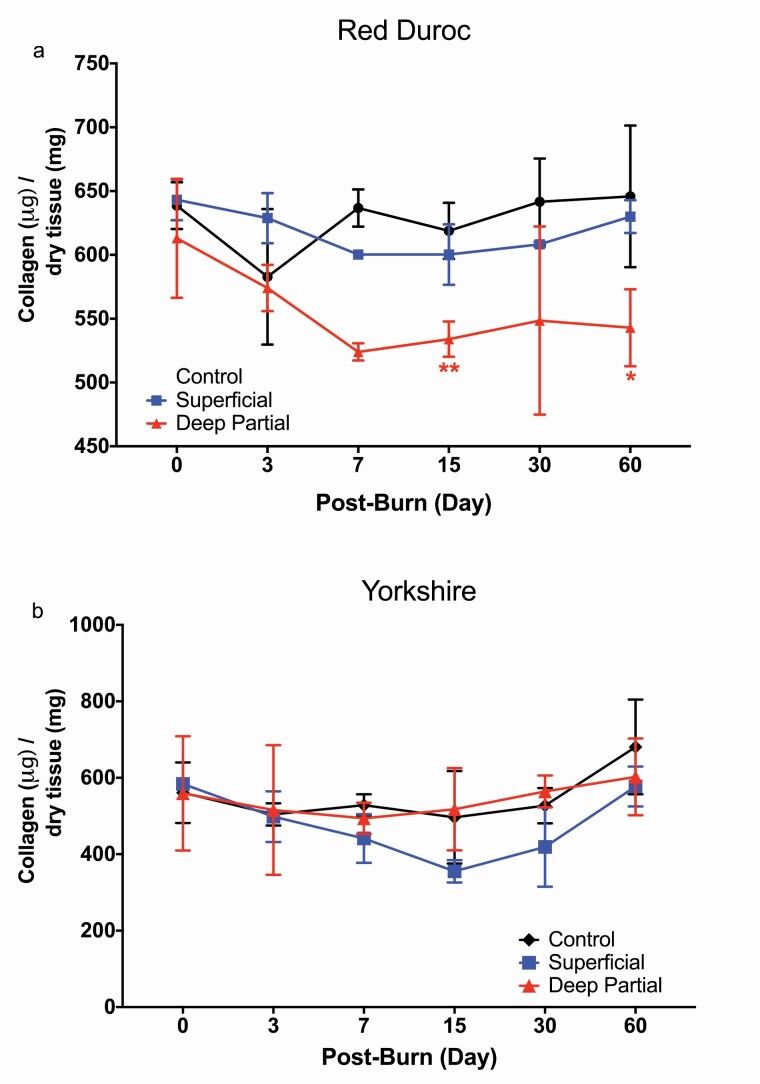
To compare collagen deposition in dermal tissue in response to burn injury, total collagen of dermal wound tissue was collected on PODs 0, 3, 7, 15, 30, and 60 and calculated as the mass of total collagen per dry weight of the dermal tissue sample (Figure 3). Total collagen deposition of DPT and superficial burn wound tissues was compared with their respective control uninjured tissue (Figure 3).
Figure 3.
Changes of red Duroc porcine collagen deposition in response to superficial and deep partial-thickness (DPT) burns. Collagen concentrations of a. Red Duroc and b. Yorkshire superficial and DPT burn wound tissue, compared with control uninjured tissue. Concentrations were expressed as micrograms of collagen to milligram of total dry tissue. Red Duroc porcine DPT burn wounds demonstrated a significant decrease in collagen concentration on days 15 and 60 postburn. Statistical significance was determined by two-way analysis of variance (ANOVA; (*P < .05; **P < .01, ***P < .001 in comparison with control uninjured tissue).
Collagen concentrations in the red Duroc DPT burn wounds significantly declined to 533.993 μg collagen/mg dry tissue by POD 15 (Figure 3a), and this decreasing trend continued to POD 60 (542.889 μg collagen/mg dry tissue) showing significantly lower collagen levels than the superficial burn wounds and the control uninjured tissue of the Duroc pigs (629.994 and 645.875 μg collagen/mg dry tissue). In contrast, no significant differences were observed in total collagen per dry weight of DPT and superficial burn wounds in the Yorkshire pigs (Figure 3b).
Dermal Thickness Increased Over Time in DPT Burn Wounds Between Porcine Breeds
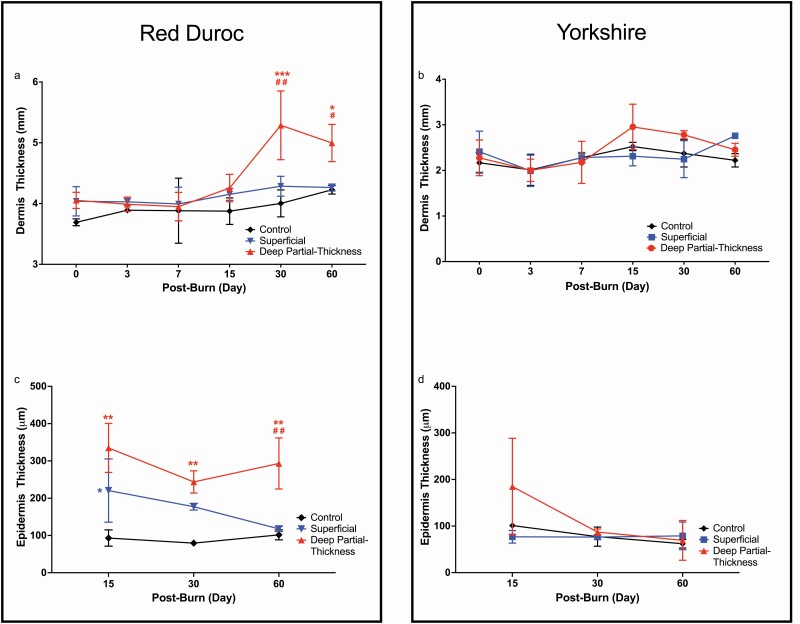
As a characteristic of HS, we measured the dermal and epidermal thickness in superficial and DPT burn wounds (Figure 4). Compared with the control uninjured tissue, the DPT burn wounds of red Duroc pigs showed significantly thicker dermis starting at POD 30 (4.00 and 5.29 mm, respectively), and although the difference in dermal thickness decreased by POD 60, the thickness remained significantly greater (Figure 4a) (4.26 and 5.00 mm, respectively). By contrast, there was no significant increase of dermal thickness in DPT burn wounds of Yorkshire pigs throughout the study duration (Figure 4b). Additionally, no significant changes were evident in the dermal thickness of SUP burn wounds relative to the control uninjured tissue in both breeds (Figure 4a and b).
Figure 4.
Changes in red Duroc porcine dermal thickness in response to deep partial-thickness (DPT) burn. Dermis thickness of superficial, DPT burn wounds, and control uninjured tissue of a. red Duroc and b. Yorkshire pigs. Epidermis thickness of superficial, DPT burn wounds, and control uninjured tissue of c. red Duroc and b. Yorkshire pigs. In response to DPT burn injury, the red Duroc pigs significantly increased in dermal thickness at day 30 postburn and remained significantly higher than control uninjured tissue and superficial burn wounds at day 60 postburn. Statistical significance was determined by two-way analysis of variance (ANOVA; *P < .05, **P < .01, ***P < .001 in comparison with control uninjured tissue; #P < .05; ##P < .01 in comparison with superficial burn wound samples).
Histological assessments of red Duroc and Yorkshire DPT burn wounds showed full epithelization at POD 15 (Supplementary Table S5). Superficial burn wounds of red Duroc pigs demonstrated significantly thicker epidermis at POD 15 relative to the control uninjured tissue (Figure 4c). The epidermal thickness of these wounds decreased to a similar thickness as the control uninjured tissue by POD 60. By contrast, compared with control uninjured tissue, the epidermal thickness of DPT wound in Duroc pigs increased significantly at POD 15 and maintained at a similar level throughout the study period (Figure 4c). The epidermal thickness of Yorkshire pigs showed no significant changes in thickness in both burn types and the control uninjured tissue (Figure 4d).
Wound Contraction Differed Between Duroc and Yorkshire Pigs
Burn wound area percentage change of each POD relative to wound area measured at POD 3 was calculated to determine the degree of wound contraction. Percent change of measured wound areas of superficial and DPT burn wounds were obtained by normalizing these wound areas to the area of growth controls tattooed around each burn wound at POD 3 (Supplementary Figure S3). Although not significant, the red Duroc DPT wound area decreased by 22% at POD 60 (Figure 5a) relative to its wound area at POD 3. Conversely, the red Duroc superficial wound area increased by 12% at POD 60 relative to POD 3; however, the increase was not significant. Comparing the red Duroc DPT burns to the superficial burns at POD 60, there were significantly more changes to the wound area percentage in DPT wounds (Figure 5a). However, Yorkshire superficial and DPT burn did not show significant differences in wound area percentage changes (Figure 5b).
Figure 5.
Red Duroc and Yorkshire wound area percent change. a. Red Duroc and b. Yorkshire wound area were determined relative to growth controls to account for growth of the animals and wound area percentage was determined relative to day 3 wound area. (#P < .05 in comparison with superficial burn wound samples).
α-SMA Protein Increased in DPT Burn Wounds in Red Duroc and Yorkshire Pigs
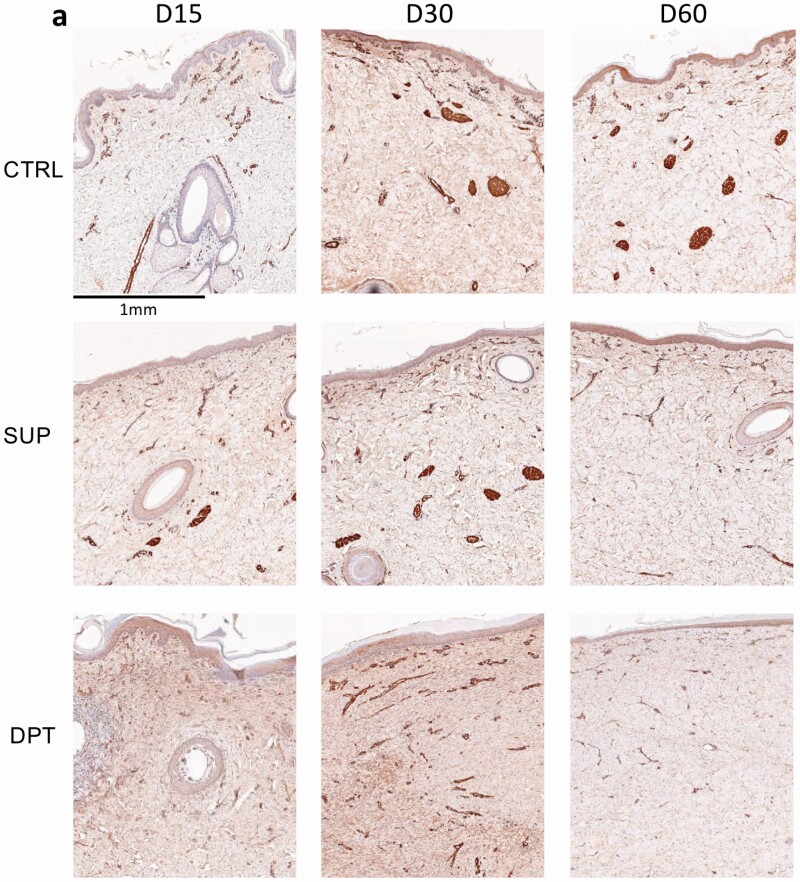
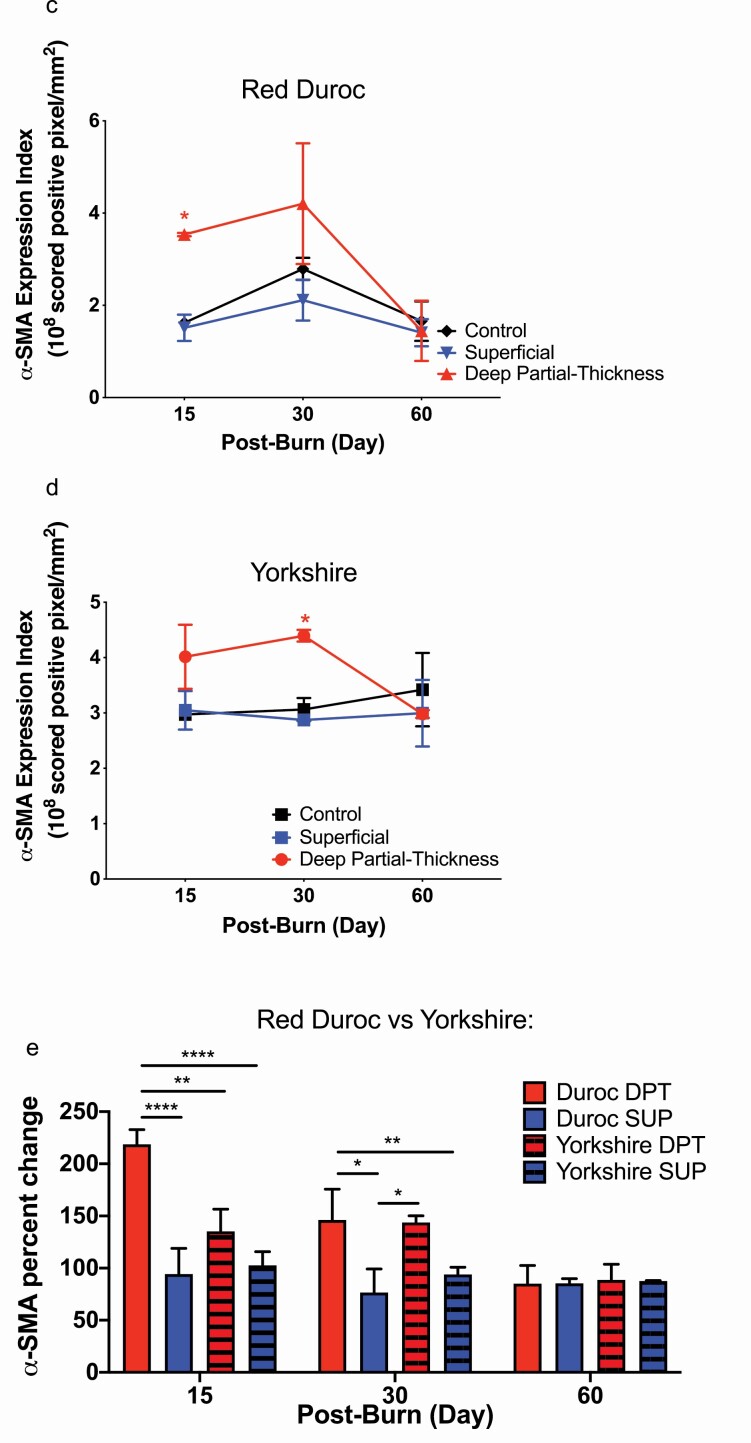
Since α-SMA protein expression associated with burn depth and wound contracture,23 porcine α-SMA protein levels in superficial and DPT burn wounds in both red Duroc and Yorkshire pigs were determined. Yorkshire and red Duroc DPT burn wound sections collected on PODs 15 and 30 demonstrated positive α-SMA staining of blood vessels and dermal fibroblasts. (Figure 6a and b). α-SMA protein levels in DPT burn wounds were significantly higher than superficial burn and control uninjured tissue at POD 15 (3.54, 1.51, and 1.62 expression index respectively P-value < .05) and noticeably even higher at POD 30 (4.21, 2.11, and 2.79 expression index, respectively) (Figure 6c) in Duroc pigs. Likewise, Yorkshire DPT burn wounds showed a higher α-SMA protein level than superficial burn and control uninjured tissue at POD 30 (4.40, 2.87, and 3.06 expression index, respectively, P-value < .05) postburn (Figure 6d). By POD 60, α-SMA protein levels in superficial and DPT burn wounds of both breeds were comparable to the control uninjured tissue (Figure 6c and d).
Figure 6.
Detection and quantification of Red Duroc and Yorkshire porcine tissue α-SMA protein expression in response to deep partial-thickness (DPT) burn relative to control uninjured tissue. Immunohistochemical staining of α-SMA protein in cross-sections of a. red Duroc and b. Yorkshire porcine DPT, superficial (SUP), and control uninjured tissue harvested on days 15, 30, and 60 postburn. Cross-section images of stained α-SMA obtained from red Duroc, Yorkshire porcine superficial, DPT, and control uninjured tissue samples harvested on postoperative (burn) days 15, 30, and 60 were analyzed with Aperio Image Scope Positive Pixel Count Algorithm. α-SMA protein expression index is expressed as ×108 positively scored pixels per area (mm2). c. α-SMA protein expression indices of Red Duroc and d. Yorkshire superficial and DPT wounds. e) α-SMA percent change (normalized to their respective control uninjured tissue) in superficial, and DPT tissue for red Duroc vs Yorkshire pigs. Statistical significance was determined by two-way analysis of variance (ANOVA) (*P < .05; **P < .01; ***P < .001).
When normalizing the α-SMA protein expression in DPT and superficial burn wounds of red Duroc and Yorkshire pigs to their respective control uninjured tissue, α-SMA protein level percentage changes in red Duroc DPT burn wounds were significantly higher than the respective Yorkshire porcine burn wounds at POD 15 (Figure 6e).
Discussion
Severe burn injury is considered one of the most debilitating injuries with risk of shock, sepsis, as well as long-term consequences of immunosuppression, hyper-metabolism, remote organ dysfunction, and hypertrophic scarring.1, 6, 8, 31–33 With advances in burn treatments, the likelihood of surviving a severe burn injury is high; however, debilitating outcomes such as HSs and wound contractures negatively impact the function, range of motion (18–45% loss), and aesthetics of the burn wound area.34 Approximately 32 to 72% of burn injuries result in HSs and wound contractures.35, 36 The frequency of burn injuries among civilian and military populations and the required specialized care drive the need to ensure that current and future prevention and treatment strategies are optimal for recovery with minimal lasting negative effects.
Porcine models, in particular the Yorkshire and the red Duroc,18, 37–39 are commonly used for studying dermal wounds due to similarity in skin or wound structure and response to burn injury with humans.40, 41 Previous work suggests that, due to inheritable genetic traits,42 these breeds respond differently to dermal burn injury with red Duroc burn wound outcomes more similar in physiology to human HS .16, 18, 43, 44
To compare transcriptional responses to superficial vs DPT burn injury over time in red Duroc vs Yorkshire breeds, porcine mRNA from burn wound biopsies was isolated and sequenced for downstream functional analysis. Verification of our RNA-Seq transcriptomic data was conducted by comparing expression profiles of select set of genes (MMP-9, MMP-3, PLAUR, CTGF, IL-6, ANGPT2, TGFB1, COL1A1, COL1A2, and COL3A1) with RT-PCR approaches (Supplementary Figures S4 and S5). Higher gene expression levels were obtained from RT-PCR for some genes compared with RNA-Seq, but the overall expression trends remained the same with similarities observed in MMP-3, CTGF, IL-6, COL1A2, and TGFB1 genes.
Overall, our longitudinal study showed an initial increase of DEGs followed by a gradual decrease, suggesting that the burn wounds were in a process of resolution over time. The POD 60 DPT burn wound DEGs identified in the Yorkshire were lower than the Duroc, suggesting earlier DPT burn wound resolution in the Yorkshire than the red Duroc. Interestingly, Duroc DPT wounds showed two distinct peaks in DEGs on POD3 and 30. Further analysis of these peaks and comparison of upregulated vs downregulated DEGs from each time point (Supplementary Table S4) identified only 83 DEGs shared between the list of upregulated genes in POD 3 and downregulated genes in POD 30. Similarly, comparing the downregulated DEGs identified in POD 3 to the upregulated DEGs in POD 30 showed only 65 shared DEGs. This relatively low number of shared DEGs among the peaks from POD 3 and 30 suggests two different groups of genes were expressed transiently, one upregulated during the early stage and the other during the late stage of wound healing, conferring unique functions during the wound resolution process.
Using GSEA method28 with profiled DEGs, we focused on the top 10 enriched GO terms and observed a robust inflammatory response, indicated by enrichment of GOs related to immune responses, in both burn types and breeds (Supplementary Figure S6). When all enriched gene sets were considered in the analysis, notable differing trends were detected in immune response function between the porcine breeds. Red Duroc breed produced a more prolonged and robust immune response toward burn injury than the Yorkshire. For example, in response to a DPT burn injury, the Yorkshire genes associated with macrophage activation (GO:0042116) were upregulated from POD 3 to at least POD 30 (Supplementary Figure S11), whereas the red Duroc macrophage activation-related genes remained upregulated until the termination of the study at POD 60. The same trend was detected in the superficial burn wounds between these breeds. Additionally, in response to superficial and DPT burn injury, upregulation of neutrophil migration (GO:1902624) was prolonged in red Duroc compared with the Yorkshire breed (Supplementary Figure S11). Furthermore, histological assessments of H&E-stained tissue sections (Supplementary Figures S12 and S13) also supported overall greater and longer duration of inflammation in the red Duroc DPT and superficial burn wounds (Supplementary Table S5) relative to Yorkshire breed.
Interestingly, in the same vein, we observed prolonged mast cell responses—mast cell-mediated immunity (GO:0002448) and regulation of mast cell activation (GO:0033006)—in the red Duroc breed, regardless of the burn types (Supplementary Figure S11). Mast cells contribute to dermal scar formation and fibrosis with HSs having a higher number of mast cells as well as increased level of mast cell activation than normotrophic scars or scar-less wounds.45 Such differences have also been reported in red Duroc and Yorkshire full-thickness excisional wound studies,46 further supporting the conclusion that the red Duroc DPT burns were more conducive to HSs than the corresponding burns in the Yorkshire.
Transcriptional differences in immune responses between these porcine breeds were also seen in gene expressions of pro-inflammatory cytokines, IL-6 and IL-1A, which were significantly higher in the red Duroc DPT and SUP burn wounds, compared with the respective Yorkshire burn wounds at POD 3 (Supplementary Figure S14). For MMPs, while the Yorkshire burn wounds showed downregulation of the matrix metallopeptidase 9 (MMP-9) gene, a gelatinase enzyme necessary for wound healing by mediating leukocyte migration,47 we detected upregulation of MMP-9 gene in red Duroc DPT and SUP burn wounds (Supplementary Figure S14). In fact, the MMP-9 gene expression peaked between POD 3 and 7 then progressively decreased in the red Duroc DPT burn wound, which is similar to reports of deep dermal excisional wounds, as published by Blackstone et al.39 The MMP-9 gene expression profile of red Duroc superficial burn wounds differed with previously reported superficial excisional wounds in terms of timeline with peak upregulation observed at POD7 with eventual downregulation by POD 60.39 In summary, duration of inflammatory and immune responses toward induction of DPT and superficial burns was more robust and prolonged in the red Duroc, compared with the Yorkshire breed. Consistent with earlier findings,9 our transcriptomic data collectively suggest that prolonged inflammation in Duroc DPT wounds could drive the increase in scarring, as supported by increased levels of α-SMA at POD 15 and wound contraction observed at POD 60 in our burn wounds.
Despite significant differences in dermal thickness and noticeable variations in dermal density, our results did not demonstrate significant increases in collagen deposition in both superficial and DPT burns in the Yorkshire or red Duroc pigs. Interestingly, the water retention ratio of red Duroc DPT burn wounds was significantly higher than the red Duroc superficial burn wounds and control uninjured tissue (data not shown). Increased water retention in HSs is contributed by accumulation of high molecular weight proteoglycans such as versican which have an affinity to water.48–50 In relation to wound healing, proteoglycans such as aggrecan, biglycan, and versican contribute to cell–cell and cell–matrix interactions, cell proliferation and migration, and ECM remodeling.51, 52 RNA-Seq transcriptomic data detected elevated aggrecan and versican gene expression in red Duroc DPT wounds at POD 60 relative to respective burns in the Yorkshire (Supplementary Figure S15). Quantitative real-time PCR analyses verified an upregulation of aggrecan and versican approximately 2- and 5-fold change higher in red Duroc DPT wounds relative to Yorkshire DPT wounds at POD 60, respectively (Supplementary Figure S16). These data suggest that the increased dermal thickness in red Duroc DPT wounds is contributed by elevated high molecular weight proteoglycan levels and their affinity to water.
The red Duroc DPT burn wounds showed a noticeable decrease in collagen concentration, which was lower than the control uninjured tissue and superficial burn wounds at POD 30 and significantly lower at POD 15 and 60 (Figure 3a). Similar to our findings, lower collagen concentrations have been reported in HSs relative to uninjured skin or mature scars isolated from patient samples as determined by hydroxyproline concentrations in lyophilized tissue.48 Interestingly, upregulation of COL1A1, COL1A2, and COL3A1 gene expression was detected in red Duroc superficial and DPT burn wounds at POD 30 and 60 (Supplementary Figure S4). Comparatively, red Duroc DPT burn wound COL1A2 gene expression at POD 60 was significantly higher than the respective wounds in Yorkshire (Supplementary Figure S14). An important note to emphasize is that collagen concentrations of the wound tissues were calculated based on dry weight by lyophilizing the harvested tissue to remove any confounding effects from moisture. Furthermore, the decreased collagen concentrations might be caused by the elevated expression of collagenases MMP-9 (Supplementary Figure S14) and MMP-13 (data not shown) in red Duroc DPT wounds. Using a mouse full-thickness wound model, Hattori et al demonstrated that MMP-9/MMP-13 double-knockout mice elicited significantly lower dermis height and higher wound dermis width in response to wounding, compared with wild-type mice.53 Similarly, the lower collagen concentrations and increased dermal thickness of our red Duroc DPT wounds may be contributed by elevated MMP-9 and MMP-13 expression.
The difference in collagen orientation among healthy and scarred skin is reported with HSs exhibiting a higher degree of collagen orientation paralleled to the epidermis.54 GSEA utilizing GO indicated that collagen fibril organization (GO:0030199) enrichment was positively scored for Yorkshire and red Duroc porcine models at POD 60 DPT burns, suggesting that collagen fibril morphology arrangement remained active (Figure 2f). Positive enrichment scoring of our burn wound transcriptomes occurred as early as POD 15 in the Yorkshire and at POD 7 in the red Duroc pigs, indicating that the red Duroc DPT burn wounds have a more prolonged collagen fibril arrangement duration than the Yorkshire. Similar functional trends were detected for genes associated with extracellular structure organization (GO:0043062) with a positive NES observed at POD 60 in Duroc DPT burn wounds, indicative of active ECM rearrangement (Figure 2g).
Correlated to HS formation,55 the expression profiles of platelet-derived growth factor subunit A (PDGFA), platelet-derived growth factor subunit B (PDGFB), and connective tissue growth factor (CTGF) genes, which are involved in the regulation of collagen deposition in fibroblast55, 56 (Supplementary Figure S14), were generally higher in Duroc than in the Yorkshire DPT bun wounds (Supplementary Figure S14). CTGF expression profiles in both red Duroc and Yorkshire DPT burn wounds follow a pattern similar to previously reported in Yorkshire full-thickness excisional wounds.57
Comparison of DPT burn wound areas in both breeds showed that the red Duroc had a higher degree of wound contraction. When comparing DPT burn wounds of both breeds, the red Duroc had significantly higher MMP-3 gene expression than Yorkshire at POD 7 and was noticeably higher at POD 15 (Supplementary Figure S14). MMP-3 is involved in ECM modulation and wound contraction.58 The increased MMP-3 gene expression suggests that the red Duroc DPT wounds were more conducive for HS formation.59 The HS phenotype of Duroc DPT wounds is further strengthened by prolonged upregulation of the positive regulation of fibroblast proliferation gene set (GO:0048146)60 from POD 0 to 15 (Supplementary Figure S11). This is in contrast to Yorkshire DPT burns, which showed a positive NES only at POD 7. Intriguingly, similar trends were detected in the superficial burn wounds in the red Duroc showing a prolonged upregulation of the positive regulation of fibroblast proliferation gene set spanning from POD 0 to 15, while Yorkshire SUP burn wounds showed an upregulation at POD 3.
Additionally, GSEA of DPT burn wounds in both breeds identified different trends in the positive enrichment of the Regulation of Fibroblast Migration gene set (GO:0010574). Red Duroc DPT burn wounds demonstrated a prolonged upregulation of this gene set throughout the duration of the study (Supplementary Figure S11). In contrast, this gene set was downregulated in the Yorkshire DPT at POD 60 burn wound. These differences in red Duroc and Yorkshire fibroblasts support previous porcine animal studies, highlighting differences in injury responses between dermal fibroblasts of each breed.18, 19 Compared with Yorkshire fibroblasts, earlier studies showed that Duroc fibroblasts elicit a higher baseline contractile state with inherent myofibroblast-like characteristics with less responsiveness to TGF-β1.19, 43 Similar to our findings, one of the earlier works reported higher α-SMA gene expression levels in Duroc than Yorkshire fibroblast.43
Additional molecular insight into the relative gene expression profiles of both burn types in both breeds was gained using a PCA plot of all DPT wounds for both breeds (Supplementary Figure S9). Yorkshire DPT wound samples demonstrated tighter clustering, dependent of time points. Conversely, Duroc DPT wounds were more dissimilar with maximum variations occurring at D15 and D30 postburn, independent of time point. Due to limited study subjects and lack of prior studies, we cannot conclude if this differential transcriptome heterogeneity we observed between Yorkshire and Duroc pigs in our study is breed specific. When both breeds were considered, the POD 60 DPT burn wounds were proximal and do not strongly distinguish from one another; however, the Yorkshire D60 DPT burn wounds appeared closer to their respective control uninjured tissue than the POD 60 Duroc wounds. When SUP burn samples were analyzed, the two breeds were more distinguished from one another on the PCA plot (Supplementary Figure S10). Greater heterogeneity of samples from SUP burn wounds suggests that the wound healing process of superficial burn wounds differed more than DPT burn wounds between these two breeds.
In conclusion, our study is the first to compare the cellular and physiological responses of the red Duroc and Yorkshire porcine burn wound models to DPT and superficial burn wounds at the transcriptomic levels. Compared with the Yorkshire pigs, the red Duroc pigs DPT wounds seemingly showed a higher propensity to forming hypertrophic scarring along with more prolonged and elevated immune responses, fibroblast migration and proliferation, as well as heightened levels of ECM modulation and wound contraction. Using our findings of the differences in burn wound, microenvironments allow better design for molecular targets for testing treatment strategies of hypertrophic and normotrophic scarring studies in these porcine models.
Supplementary Material
Contributor Information
Jesse Q Nguyen, Division of Combat Wound Repair, US Army Institute of Surgical Research, JBSA Fort Sam Houston, TX, USA.
Fatemeh Sanjar, Division of Combat Wound Repair, US Army Institute of Surgical Research, JBSA Fort Sam Houston, TX, USA.
S L Rajasekhar Karna, Division of Combat Wound Repair, US Army Institute of Surgical Research, JBSA Fort Sam Houston, TX, USA.
Andrea B Fourcaudot, Division of Combat Wound Repair, US Army Institute of Surgical Research, JBSA Fort Sam Houston, TX, USA.
Li-Ju Wang, Greehey Children’s Cancer Research Institute, University of Texas – Health San Antonio, TX, USA.
David T Silliman, Division of Combat Wound Repair, US Army Institute of Surgical Research, JBSA Fort Sam Houston, TX, USA.
Zhao Lai, Greehey Children’s Cancer Research Institute, University of Texas – Health San Antonio, TX, USA; Department of Molecular Medicine, University of Texas – Health San Antonio, TX, USA.
Yidong Chen, Greehey Children’s Cancer Research Institute, University of Texas – Health San Antonio, TX, USA; Department of Epidemiology and Biostatistics, University of Texas – Health San Antonio, TX, USA.
Kai P Leung, Division of Combat Wound Repair, US Army Institute of Surgical Research, JBSA Fort Sam Houston, TX, USA.
This work was supported in part through the Congressionally Directed Medical Research Programs, U.S. Army Medical Research and Development Command W81XWH-15-Z-0083, and the Naval Medical Research Center’s Advanced Medical Development Program (MIPR N3239815MHX040). Transcriptomic data were generated in the Genome Sequencing Facility, which is supported by UT Health San Antonio, NIH-NCI P30 CA054174 (Cancer Center at UT Health San Antonio), NIH Shared Instrument grant 1S10OD021805-01 (S10 grant), and CPRIT Core Facility Award (RP160732). This work was also supported by the Cancer Prevention and Research Institute of Texas Core Facility Award (RP160732 to Y.C.); the National Center for Advancing Translational Sciences; National Institutes of Health, through the Clinical and Translational Science Award (CTSA) UL1 TR002645; Mays Cancer Center P30 Cancer Center Support Grant from the National Cancer Institute (NCI P30 CA054174); and NIH Shared Instrument S10 grant 1S10OD021805-01 to the GCCRI Genome Sequencing Facility.
Data Availability
Sequenced reads were submitted to the NCBI Sequence Read Archive (SRA) and are accessible under BioProject PRJNA706694.
Conflict of interest statement. The authors declare no conflict of interest. DOD disclaimer. The views expressed in this article are those of the authors and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, Depart of Defense, or the U.S. Government. One of the authors (K.P.L.) is an employee of the U.S. Government. The work presented is part of his official duties.
References
- 1. Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers 2020;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol 2010;19:777–83. [DOI] [PubMed] [Google Scholar]
- 3. Bombaro KM, Engrav LH, Carrougher GJet al. What is the prevalence of hypertrophic scarring following burns? Burns 2003;29:299–302. [DOI] [PubMed] [Google Scholar]
- 4. Zacharevskij E, Baranauskas G, Varkalys K, Rimdeika R, Kubilius D. Comparison of non-surgical methods for the treatment of deep partial thickness skin burns of the hand. Burns 2018;44:445–52. [DOI] [PubMed] [Google Scholar]
- 5. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–21. [DOI] [PubMed] [Google Scholar]
- 6. Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 2016;388:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez N, Goldberg DJ. Update on the treatment of scars. J Drugs Dermatol 2019;18:550. [PubMed] [Google Scholar]
- 8. Tredget EE, Levi B, Donelan MB. Biology and principles of scar management and burn reconstruction. Surg Clin North Am 2014;94:793–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qian LW, Fourcaudot AB, Yamane K, You T, Chan RK, Leung KP. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen 2016;24:26–34. [DOI] [PubMed] [Google Scholar]
- 10. Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109–22. [DOI] [PubMed] [Google Scholar]
- 11. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 2010;10:415–24. [DOI] [PubMed] [Google Scholar]
- 13. Johnson A, DiPietro LA. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J 2013;27:3893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med 2007;4:e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol 1978;7:39–52. [DOI] [PubMed] [Google Scholar]
- 16. Xie Y, Zhu KQ, Deubner Het al. The microvasculature in cutaneous wound healing in the female red Duroc pig is similar to that in human hypertrophic scars and different from that in the female Yorkshire pig. J Burn Care Res 2007;28:500–6. [DOI] [PubMed] [Google Scholar]
- 17. Cuttle L, Kempf M, Phillips GEet al. A porcine deep dermal partial thickness burn model with hypertrophic scarring. Burns 2006;32:806–20. [DOI] [PubMed] [Google Scholar]
- 18. Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen 2007;15:S32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Hemptinne I, Gallant-Behm CL, Noack CL, Parreno J, Hart DA. Dermal fibroblasts from red Duroc and Yorkshire pigs exhibit intrinsic differences in the contraction of collagen gels. Wound Repair Regen 2008;16:132–42. [DOI] [PubMed] [Google Scholar]
- 20. Brandenburg KS, WeaverAJ, Jr., Qian Let al. Development of Pseudomonas aeruginosa biofilms in partial-thickness burn wounds using a Sprague-Dawley Rat model. J Burn Care Res 2019;40:44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gravante G, Palmieri MB, Esposito Get al. Apoptotic death in deep partial thickness burns vs. normal skin of burned patients. J Surg Res 2007;141:141–5. [DOI] [PubMed] [Google Scholar]
- 22. Medina JL, Fourcaudot AB, Sebastian EA, Shankar R, Brown AW, Leung KP. Standardization of deep partial-thickness scald burns in C57BL/6 mice. Int J Burns Trauma 2018;8:26–33. [PMC free article] [PubMed] [Google Scholar]
- 23. Wang XQ, Kravchuk O, Winterford C, Kimble RM. The correlation of in vivo burn scar contraction with the level of alpha-smooth muscle actin expression. Burns 2011;37:1367–77. [DOI] [PubMed] [Google Scholar]
- 24. Karna SL, D′Arpa P, Chen Tet al. RNA-Seq transcriptomic responses of full-thickness dermal excision wounds to Pseudomonas aeruginosa acute and biofilm infection. PLoS One 2016;11:e0165312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 2013;14:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31:166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subramanian A, Tamayo P, Mootha VKet al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov MN, Sergushichev A. Fast gene set enrichment analysis, bioRxiv, doi.org/10.1101/060012, 2021, preprint: not peer reviewed. [Google Scholar]
- 30. The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res2019;47:D330–D8. [DOI] [PMC free article] [PubMed]
- 31. Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG. Burns: pathophysiology of systemic complications and current management. J Burn Care Res 2017;38:e469–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeschke MG. Postburn hypermetabolism: past, present, and future. J Burn Care Res 2016;37:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greenhalgh DG. Sepsis in the burn patient: a different problem than sepsis in the general population. Burns Trauma 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Godleski M, Lee AF, Goverman Jet al. Quantifying contracture severity at hospital discharge in adults: a Burn Model System National Database Study. J Burn Care Res 2018;39:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gangemi EN, Gregori D, Berchialla Pet al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg 2008;10:93–102. [DOI] [PubMed] [Google Scholar]
- 36. Dedovic Z, Koupilova I, Brychta P. Time trends in incidence of hypertrophic scarring in children treated for burns. Acta Chir Plast 1999;41:87–90. [PubMed] [Google Scholar]
- 37. Gaines C, Poranki D, Du W, Clark RA, Van Dyke M. Development of a porcine deep partial thickness burn model. Burns 2013;39:311–9. [DOI] [PubMed] [Google Scholar]
- 38. Singh M, Nuutila K, Minasian R, Kruse C, Eriksson E. Development of a precise experimental burn model. Burns 2016;42:1507–12. [DOI] [PubMed] [Google Scholar]
- 39. Blackstone BN, Kim JY, McFarland KLet al. Scar formation following excisional and burn injuries in a red Duroc pig model. Wound Repair Regen 2017;25:618–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci 2014;71:3241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dahiya P. Burns as a model of SIRS. Front Biosci (Landmark Ed) 2009;14:4962–7. [DOI] [PubMed] [Google Scholar]
- 42. Gallant-Behm CL, Tsao H, Reno C, Olson ME, Hart DA. Skin wound healing in the first generation (F1) offspring of Yorkshire and red Duroc pigs: evidence for genetic inheritance of wound phenotype. Burns 2006;32:180–93. [DOI] [PubMed] [Google Scholar]
- 43. Sood RF, Muffley LA, Seaton MEet al. Dermal fibroblasts from the Red Duroc Pig have an inherently fibrogenic phenotype: an in vitro model of fibroproliferative scarring. Plast Reconstr Surg 2015;136: 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu KQ, Carrougher GJ, Couture OP, Tuggle CK, Gibran NS, Engrav LH. Expression of collagen genes in the cones of skin in the Duroc/Yorkshire porcine model of fibroproliferative scarring. J Burn Care Res 2008;29:815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilgus TA, Wulff BC. The importance of mast cells in dermal scarring. Adv Wound Care (New Rochelle) 2014;3:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gallant-Behm CL, Hildebrand KA, Hart DA. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regen 2008;16:226–33. [DOI] [PubMed] [Google Scholar]
- 47. LeBert DC, Squirrell JM, Rindy Jet al. Matrix metalloproteinase 9 modulates collagen matrices and wound repair. Development 2015;142:2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Chemical characterization and quantification of proteoglycans in human post-burn hypertrophic and mature scars. Clin Sci (Lond) 1996;90:417–25. [DOI] [PubMed] [Google Scholar]
- 49. Eremenko E, Ding J, Kwan P, Tredget EE. The biology of extracellular matrix proteins in hypertrophic scarring. Adv Wound Care (New Rochelle) 2022;11:234–54. [DOI] [PubMed] [Google Scholar]
- 50. Hasegawa K, Yoneda M, Kuwabara Het al. Versican, a major hyaluronan-binding component in the dermis, loses its hyaluronan-binding ability in solar elastosis. J Invest Dermatol 2007;127:1657–63. [DOI] [PubMed] [Google Scholar]
- 51. Ghatak S, Maytin EV, Mack JAet al. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol 2015;2015:834893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol 2010;29:248–53. [DOI] [PubMed] [Google Scholar]
- 53. Hattori N, Mochizuki S, Kishi Ket al. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol 2009;175:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verhaegen PD, van Zuijlen PP, Pennings NMet al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: an objective histopathological analysis. Wound Repair Regen 2009;17:649–56. [DOI] [PubMed] [Google Scholar]
- 55. Zhu Z, Ding J, Tredget EE. The molecular basis of hypertrophic scars. Burns Trauma 2016;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 1993;4:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang JF, Olson ME, Reno CR, Wright JB, Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med 2001;51:341–8. [PubMed] [Google Scholar]
- 58. Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008;40:1334–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle) 2015;4:119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith CL, Eppig JT. The mammalian phenotype ontology: enabling robust annotation and comparative analysis. Wiley Interdiscip Rev Syst Biol Med 2009;1:390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequenced reads were submitted to the NCBI Sequence Read Archive (SRA) and are accessible under BioProject PRJNA706694.