Abstract
Background
Nearly all patients with newly diagnosed glioblastoma experience recurrence following standard-of-care radiotherapy (RT) + temozolomide (TMZ). The purpose of the phase III randomized CheckMate 548 study was to evaluate RT + TMZ combined with the immune checkpoint inhibitor nivolumab (NIVO) or placebo (PBO) in patients with newly diagnosed glioblastoma with methylated MGMT promoter (NCT02667587).
Methods
Patients (N = 716) were randomized 1:1 to NIVO [(240 mg every 2 weeks × 8, then 480 mg every 4 weeks) + RT (60 Gy over 6 weeks) + TMZ (75 mg/m2 once daily during RT, then 150-200 mg/m2 once daily on days 1-5 of every 28-day cycle × 6)] or PBO + RT + TMZ following the same regimen. The primary endpoints were progression-free survival (PFS) and overall survival (OS) in patients without baseline corticosteroids and in all randomized patients.
Results
As of December 22, 2020, median (m)PFS (blinded independent central review) was 10.6 months (95% CI, 8.9-11.8) with NIVO + RT + TMZ vs 10.3 months (95% CI, 9.7-12.5) with PBO + RT + TMZ (HR, 1.1; 95% CI, 0.9-1.3) and mOS was 28.9 months (95% CI, 24.4-31.6) vs 32.1 months (95% CI, 29.4-33.8), respectively (HR, 1.1; 95% CI, 0.9-1.3). In patients without baseline corticosteroids, mOS was 31.3 months (95% CI, 28.6-34.8) with NIVO + RT + TMZ vs 33.0 months (95% CI, 31.0-35.1) with PBO + RT + TMZ (HR, 1.1; 95% CI, 0.9-1.4). Grade 3/4 treatment-related adverse event rates were 52.4% vs 33.6%, respectively.
Conclusions
NIVO added to RT + TMZ did not improve survival in patients with newly diagnosed glioblastoma with methylated or indeterminate MGMT promoter. No new safety signals were observed.
Keywords: glioblastoma, MGMT promoter, nivolumab, PD-L1, temozolomide
Key Points.
NIVO did not improve survival in newly diagnosed glioblastoma with methylated MGMT promoter.
No new safety signals were detected with NIVO + standard of care in this study.
Nivolumab could be considered within future combination strategies.
Importance of the Study.
The continued urgent need for novel treatment mechanisms to improve clinical outcomes in patients with glioblastoma and the demonstrated benefit of immune checkpoint inhibitors (ICIs) in various tumor types have led to the investigation of ICI efficacy in glioblastoma. Nearly all patients with newly diagnosed glioblastoma have recurrence after standard-of-care surgical resection followed by radiotherapy (RT) and temozolomide (TMZ). Methylation of the MGMT promoter is a positive prognostic factor and predictor of TMZ benefit in this patient population. Here we report data from the largest phase III study in patients with glioblastoma and methylated MGMT promoter. Nivolumab vs placebo added to RT + TMZ did not improve survival. However, compared with previous trials, a higher median OS was observed in both treatment arms, potentially resulting from advances in patient care. Additionally, as no new safety signals were observed with nivolumab, this regimen can safely be considered for the basis of additional combination strategies.
Glioblastoma is the most common and aggressive primary malignant brain tumor in adults.1,2 Standard-of-care treatment for patients with newly diagnosed disease usually involves surgical resection followed by radiotherapy (RT) with concomitant and adjuvant temozolomide (TMZ).3,4 Approval of TMZ was based on a phase III study that showed overall survival (OS) improved from 12.1 months with RT alone to 14.6 months with TMZ-based chemoradiotherapy (hazard ratio [HR], 0.63; P < .001).3,5 More recently, the use of tumor-treating fields was also approved by the FDA for use in combination with adjuvant TMZ after standard-of-care surgery and RT + TMZ.6 However, no other therapies have been approved in patients with newly diagnosed glioblastoma, and nearly all treated patients experience recurrence, highlighting the need for novel therapies.2
O6-methylguanine DNA methyltransferase (MGMT) promoter status is a key prognostic factor in glioblastoma.7–11 Epigenetic silencing of the MGMT gene via promoter methylation increases sensitivity to alkylating agents such as TMZ, and patients with tumors with a methylated MGMT promoter treated with TMZ achieve longer OS than those who have tumors with an unmethylated MGMT promoter.7,9,11–13 Observed rates of MGMT promoter methylation are variable and depend on the assay used; however, approximately 35% of patients with newly diagnosed glioblastoma are reported to have tumors with a methylated MGMT promoter.11
Nivolumab (NIVO), a fully human immunoglobulin G4 monoclonal antibody that targets the programmed cell death-1 (PD-1) receptor, is approved for the treatment of multiple advanced cancers and has demonstrated anti-tumor activity in patients with melanoma with brain metastases.14 The immune checkpoint transmembrane protein programmed death-1 ligand 1 (PD-L1) is frequently expressed in primary glioblastomas, and high expression levels have been associated with shorter survival.15 Preclinical data have suggested that RT induces cell death and the release of tumor antigens, which promote tumor-specific immune responses that could be amplified with immune-stimulating agents, such as immune checkpoint pathway inhibitors.16 Moreover, in murine glioma models, combination of a PD-1 inhibitor with RT improved OS compared with either treatment alone.17
Results from the randomized phase III CheckMate 143 study (NCT02017717) demonstrated that OS was comparable between NIVO and bevacizumab (9.77 vs 10.02 months) in patients with recurrent glioblastoma; however, a trend toward longer median OS was observed with NIVO vs bevacizumab (16.95 vs 10.12 months) in a subgroup of patients with a methylated MGMT promoter and no baseline corticosteroid use.18 A preliminary signal was also observed in the phase I cohorts 1c and 1d of the CheckMate 143 study in patients with newly diagnosed glioblastoma with a methylated or indeterminate MGMT promoter who were treated with NIVO in combination with RT + TMZ.19 Median progression-free survival (PFS) was 15.47 months (95% CI, 7.10 months to not estimable) in 15 patients with tumors with a methylated or indeterminate MGMT promoter compared with 6.47 months (95% CI, 4.14-10.18 months) in 16 patients with tumors with an unmethylated MGMT promoter; respective median OS was 33.38 months (95% CI, 16.20 months to not estimable) compared with 16.49 months (95% CI, 12.94-22.08 months).19 These findings supported further evaluation of NIVO in combination with standard-of-care RT + TMZ in patients with a methylated MGMT promoter.
Here we report the final analysis of the phase III CheckMate 548 trial (NCT02667587), which investigated the efficacy and safety of RT + TMZ in combination with NIVO or placebo (PBO) in patients with newly diagnosed glioblastoma with a methylated or indeterminate MGMT promoter.
Methods
Patients
Eligible patients had newly diagnosed, histologically confirmed supratentorial glioblastoma (WHO grade IV malignant glioma) and had not received treatment for glioblastoma other than surgery. Postoperative baseline MRI obtained either <72 hours or >14 days after surgery was required prior to randomization. A surgical resection of ≥20% of enhancing tumor was required, and patients must have fully recovered from surgery with no major ongoing safety issues.
Patients had to be ≥18 years of age, have a KPS (Karnofsky performance status) of ≥70, and be eligible to receive RT + concomitant TMZ. Screening for MGMT methylation status was performed in parallel with the phase III CheckMate 498 study (NCT02617589).20,21 Patients initially provided informed consent to participate and undergo screening in CheckMate 498 and then had MGMT status determined by an independent central laboratory. Patients with unmethylated MGMT promoter tumors proceeded with participation and randomization in CheckMate 498. Patients with methylated or indeterminate MGMT promoter status were removed from CheckMate 49820,21 and became eligible to participate in this study. A total of 1002 prerandomized patients were therefore screened in CheckMate 498. Patients without a tumor sample were not eligible for participation in either study.
Patients receiving corticosteroids to manage glioblastoma symptoms at the time of screening were required to discontinue or taper use so that dose at randomization was ≤20 mg of prednisone or ≤3 mg of dexamethasone daily (or equivalent); inhaled or topical corticosteroids and adrenal replacement corticosteroid doses of >10 mg of prednisone daily (or equivalent) to manage other conditions were permitted in the absence of active autoimmune disease.
Other exclusion criteria included recurrent or secondary glioblastoma; metastatic extracranial or leptomeningeal disease; active, known, or suspected autoimmune disease; concomitant use of a carmustine wafer; use of any noninvasive anticancer medical device (eg, NovoTTF); and unresolved CNS hemorrhage.
Study Oversight
The study was conducted in accordance with Good Clinical Practice guidelines per the International Conference on Harmonisation and ethical principles of the European Union Directive and US Code of Federal Regulations and registered at ClinicalTrials.gov (NCT02667587). The protocol was approved by an institutional review board or independent ethics committee at each site before study activation. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Study Design and Treatment
This study followed a single-blind, or “site-subject blinded,” design. Investigators, patients, and site staff were blinded to the therapy administered. Each investigative site had an unblinded pharmacist or designee, and designated BMS Research and Development staff were unblinded to facilitate drug supply and safety monitoring. Patients remained blinded to treatment conditions except in the event of a medical emergency or pregnancy in which knowledge of the treatment was critical for patient management and safety. Patients were randomly assigned, 1:1, to 2 treatment arms. The patients in the first arm received NIVO (240 mg every 2 weeks] for 8 doses and then 480 mg every 4 weeks) + RT (60 Gy over 6 weeks) + TMZ (75 mg/m2 once daily during RT followed by a 4-week treatment break, and then 150-200 mg/m2 once daily on days 1-5 of every 28-day cycle). The patients in the second arm received PBO (every 2 weeks for 8 doses and then every 4 weeks) + RT + TMZ following the same schedule and dosing regimen. Treatment continued until the occurrence of unacceptable toxicity or disease progression. However, NIVO treatment could be continued beyond suspected progression until confirmation of progression by follow-up MRI if evidence of investigator-assessed clinical benefit and tolerance of study drug were observed. Randomization was stratified according to the degree of surgical resection (complete vs partial) at baseline. Complete resection was defined as visible-total removal of an MRI-detectable tumor; however, invasive glioblastoma still remained. Partial resection was defined as <90% of macroscopic removal of the tumor mass, or >10 mm residual.
The primary endpoints were PFS by blinded independent central review (BICR) in all randomized patients and OS in all randomized patients and in those without baseline corticosteroid use. Secondary endpoints included OS rate at 12 and 24 months and PFS per investigator assessment. Key exploratory endpoints included safety and tolerability and efficacy outcomes by tumor PD-L1 expression category.
Assessments
Tumor samples were assessed for MGMT promoter methylation status using a methylation-specific polymerase chain reaction assay from a central laboratory. A sample was determined to be MGMT methylated when the ratio of MGMT to β-actin control was ≥2 (calculated as [methylated MGMT/β-actin] × 1000),13 and β-actin and MGMT were within the reportable range (β-actin ≥10 copies and MGMT ≥10 copies). A sample was determined to be MGMT unmethylated when the ratio of MGMT to β-actin control was <2 and as MGMT indeterminate when results were unable to be determined.
Disease status was assessed by investigators using contrast-enhanced MRI at baseline, approximately 4 weeks after completion of RT, every 8 weeks up to 24 months after randomization, and then every 12 weeks until progression according to Radiologic Assessment in Neuro-Oncology (RANO) criteria.22 RANO criteria recommend that within the first 12 weeks after completion of RT, when pseudoprogression is the most prevalent, progression can only be determined if the majority of the new enhancement was outside of the radiation field or if there was pathological confirmation of progressive disease (PD). Evidence suggests that patients treated with immunotherapy may derive clinical benefit despite initial evidence of disease progression; therefore, patients in the NIVO + RT + TMZ arm may have continued NIVO in the setting of suspected progression at investigator discretion until progression was confirmed.
PFS was defined as the time from randomization to documented progression or death from any cause, and OS was defined as the time from randomization to death.
An exploratory retrospective analysis of existing progression data from BICR per RANO criteria22 was used to estimate the rate of pseudoprogression as defined by immunotherapy RANO (iRANO) criteria in the NIVO + RT + TMZ arm.23 Pseudoprogression was evaluated in patients treated with NIVO + RT + TMZ who had PFS of ≤6 months from the first NIVO dose. Patients with follow-up scans ≥3-months post-PD and unconfirmed PD (no confirmation of PD worsening) while remaining on treatment were considered as having pseudoprogression.
Tumor PD-L1 expression was determined using a validated immunohistochemistry assay (PD-L1 IHC 28-8 pharmDx). PD-L1 positivity was defined as the percentage of tumor cells with membranous staining using 1% and 5% cutoff values. Patients were randomized to treatment regardless of tumor PD-L1 expression.
Adverse events were assessed continuously during the study per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.24
Statistical Analysis
PFS and OS comparisons were based on a 2-sided log-rank test stratified by surgical resection at baseline (complete vs partial). OS analysis was conducted in the randomized population without baseline corticosteroids use and was planned approximately 20 months after completion of accrual or when 236 deaths were reported. OS and PFS curves, medians with 95% CIs, and OS rates at 12 and 24 months with 95% CIs were estimated using Kaplan-Meier methodology; HRs and corresponding 2-sided (95%) CIs were estimated using a Cox proportional hazards model, with treatment arm as a single covariate stratified by surgical resection (complete vs partial) at baseline. Baseline patient characteristics in all randomized patients and safety in all treated patients were characterized using descriptive statistics.
Results
Patients and Treatment
From May 11, 2016, through December 9, 2019, 716 patients with tumors with methylated or indeterminate MGMT promoter methylation status were randomized; 709 received study treatment with either NIVO + RT + TMZ (n = 355) or PBO + RT + TMZ (n = 354) (see Supplementary Figure S1). Patients were enrolled at 118 sites across 19 countries.
No marked imbalances in baseline characteristics or demographics were observed between the arms (Table 1; Supplementary Table S1). Among all patients, 353 (98.6%) and 349 (97.5%) had a methylated MGMT promoter status, 4 (1.1%) and 7 (2.0%) had an indeterminate MGMT promoter status, and 1 (0.3%) and 2 (0.6%) had nonreported MGMT promoter status in the NIVO + RT + TMZ and PBO + RT + TMZ arms, respectively. Among patients with evaluable PD-L1 expression (n = 356 in each arm), baseline tumor PD-L1 expression was ≥1% in 126 patients (35.4%) in the NIVO + RT + TMZ arm and in 118 patients (33.1%) in the PBO + RT + TMZ arm; baseline tumor PD-L1 expression was <1% in 230 patients (64.6%) and 238 patients (66.9%), respectively. Complete surgical resection had been performed in 199 patients (55.6%) in the NIVO + RT + TMZ arm and in 200 patients (55.9%) in the PBO + RT + TMZ arm. Most patients were not receiving corticosteroids at baseline (NIVO + RT + TMZ = 246/358 [68.7%]; PBO + RT + TMZ = 261/358 [72.9%]).
Table 1.
Patient Demographics and Baseline Characteristics
| Variable | Nivolumab + RT + TMZ n = 358 No. (%) |
Placebo + RT + TMZ n = 358 No. (%) |
|---|---|---|
| Age | ||
| Median (range), years | 60.0 (24-79) | 60.0 (18-81) |
| Age, years | ||
| <65 | 245 (68.4) | 237 (66.2) |
| ≥65 to <75 | 90 (25.1) | 104 (29.1) |
| ≥75 | 23 (6.4) | 17 (4.7) |
| Sex | ||
| Male | 205 (57.3) | 197 (55.0) |
| Female | 153 (42.7) | 161 (45.0) |
| Histopathologic diagnosis | ||
| Glioblastoma | 353 (98.6) | 353 (98.6) |
| Gliosarcoma | 4 (1.1) | 5 (1.4) |
| Not reported | 1 (<1) | 0 |
| RPA classa | ||
| III | 32 (8.9) | 23 (6.4) |
| IV | 287 (80.2) | 306 (85.5) |
| V | 38 (10.6) | 29 (8.1) |
| Not reported | 1 (<1) | 0 |
| Extent of surgeryb | ||
| Complete resection | 199 (55.6) | 200 (55.9) |
| Partial resection | 158 (44.1) | 158 (44.1) |
| Not reported | 1 (0.3) | 0 |
| Karnofsky performance status | ||
| 100 | 82 (22.9) | 89 (24.9) |
| 90 | 160 (44.7) | 162 (45.3) |
| 80 | 78 (21.8) | 78 (21.8) |
| 70 | 34 (9.5) | 28 (7.8) |
| 60 | 1 (<1) | 0 |
| Not reported | 3 (<1) | 1 (<1) |
| Time from initial diagnosis to randomization | ||
| Median (range), weeks | 5.29 (3.0-40.1c) | 5.36 (2.7-13.4) |
| MGMT promoter methylation status | ||
| Methylated | 353 (98.6) | 349 (97.5) |
| Indeterminate | 4 (1.1) | 7 (2.0) |
| Not reported | 1 (0.3) | 2 (0.6) |
| Patients with evaluable PD-L1 expression | ||
| PD-L1 expression level | 356 (99.4) | 356 (99.4) |
| <1% | 230 (64.6) | 238 (66.9) |
| ≥1% | 126 (35.4) | 118 (33.1) |
| Corticosteroid used | ||
| Yes | 112 (31.3) | 97 (27.1) |
| ≤3 mg/day | 89 (24.9) | 73 (20.4) |
| >3 mg/day | 23 (6.4) | 24 (6.7) |
| No | 246 (68.7) | 261 (72.9) |
Abbreviations: MGMT, O6-methylguanine DNA methyltransferase; NIVO + RT + TMZ, nivolumab + radiotherapy + temozolomide; PD-L1, programmed cell death-1 ligand 1; PBO + RT + TMZ, placebo + radiotherapy + temozolomide; RPA, recursive partitioning analysis.
aThe RPA classes were as follows: class III: age <50 years and Karnofsky performance status ≥90 (on a scale of 0-100, with higher scores indicating better function); class IV, <50 years and Karnofsky performance status <90 (or ≥50 years, Karnofsky performance status ≥70, complete or partial tumor resection, and ability to work); class V, ≥50 years, Karnofsky performance status ≥70, complete or partial tumor resection, and inability to work (or ≥50 years, Karnofsky performance status ≥70, and tumor-biopsy specimen only; or ≥50 years and Karnofsky performance status <70).8
bThis characteristic was used as a stratification factor.
cThe patient with 40.1 weeks from the initial diagnosis to the start of RT had two partial resections prior to randomization, with no RT or systemic cancer therapies in between.
dBased on average corticosteroid use 5 days prior to the start of dosing or randomization date for patients not treated.
Median duration of NIVO treatment was 10.4 months (range, <0.1-52.5 months); the median duration of TMZ was 7.3 months (range, 0.2-43.5 months) in the NIVO + RT + TMZ arm and 7.4 months (range, <0.1-46.2 months) in the PBO + RT + TMZ arm. A median of 15.0 (range, 1-62) NIVO doses was received.
At data cutoff (December 22, 2020), most patients had discontinued treatment (318 patients [89.6%] in the NIVO + RT + TMZ arm and 310 patients [87.6%] in the PBO + RT + TMZ arm). The most common reasons for treatment discontinuation were disease progression (NIVO + RT + TMZ, n = 177 [49.9%]; PBO + RT + TMZ, n = 222 [62.7%]) and study drug toxicity (NIVO + RT + TMZ, n = 74 [20.8%]; PBO + RT + TMZ, n = 18 [5.1%]) (Supplementary Figure S1).
Efficacy
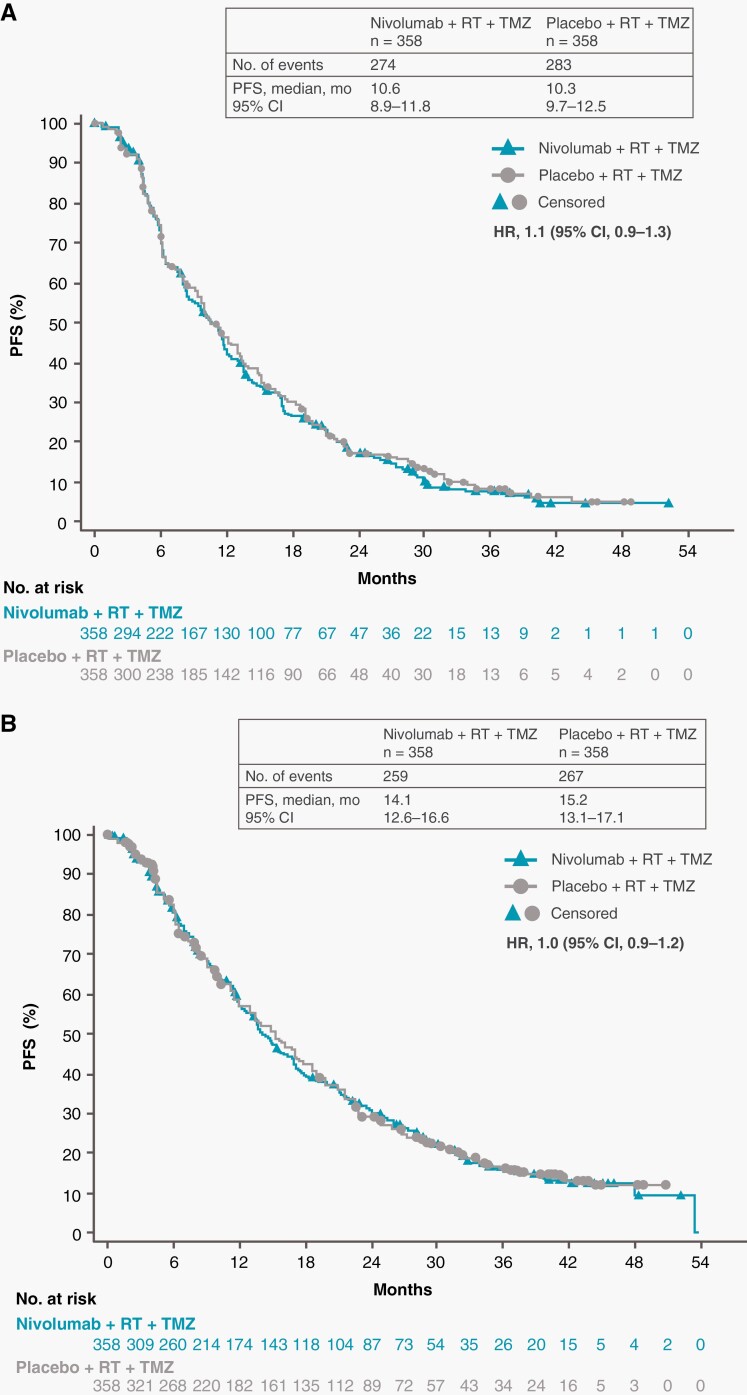
At data cutoff, the minimum potential follow-up was 12.5 months in the NIVO + RT + TMZ arm and 19.5 months in the PBO + RT + TMZ arm. Median PFS per BICR was 10.6 months (95% CI, 8.9-11.8 months) with NIVO + RT + TMZ vs 10.3 months (95% CI, 9.7-12.5) with PBO + RT + TMZ (HR, 1.1; 95% CI, 0.9-1.3) (Figure 1A). Median PFS per investigator assessment was 14.1 months (95% CI, 12.6-16.6 months) and 15.2 months (95% CI, 13.1-17.1 months), respectively (HR, 1.0; 95% CI, 0.9-1.2) (Figure 1B).
Fig. 1.
Progression-free survival in all patients. Number of events, median PFS, and the Kaplan-Meier curve for PFS per blinded independent central review (A) and investigator (B) assessment. Symbols indicate censored observations. Abbreviations: HR, hazard ratio; PFS, progression-free survival; RT, radiotherapy; TMZ, temozolomide.
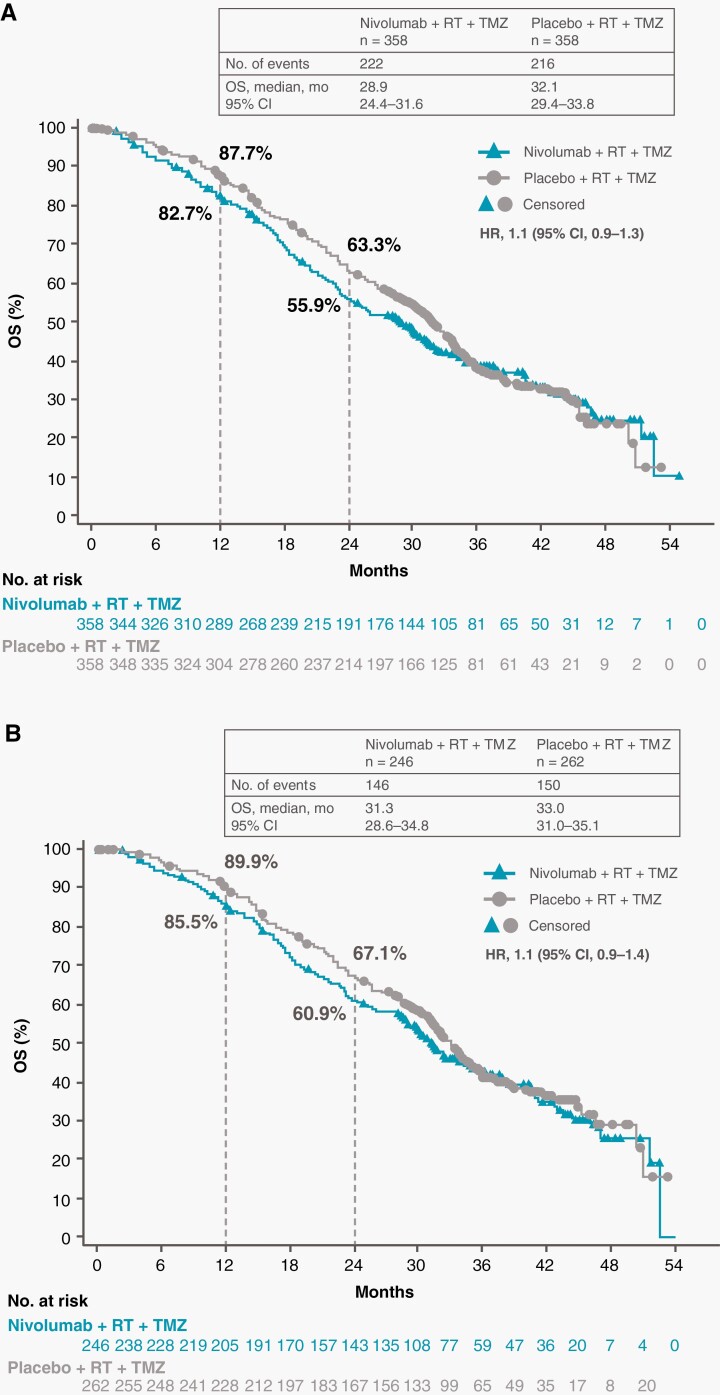
Among all patients, the median OS was 28.9 months (95% CI, 24.4-31.6 months) in the NIVO + RT + TMZ arm and 32.1 months (95% CI, 29.4-33.8 months) in the PBO + RT + TMZ arm (HR, 1.1; 95% CI, 0.9-1.3) (Figure 2A). The median OS was 31.3 months (95% CI, 28.6-34.8 months) and 33.0 months (95% CI, 31.0-35.1 months), respectively, in patients without baseline corticosteroid use (HR, 1.1; 95% CI, 0.9-1.4) (Figure 2B).
Fig. 2.
Overall survival in all patients and patients without baseline corticosteroids. Number of events, median OS, and the Kaplan-Meier curve for OS in all patients (A) and in patients without baseline corticosteroid use (B). Symbols indicate censored observations. Abbreviations: OS, overall survival; RT, radiotherapy; TMZ, temozolomide.
The 12-month OS rates in all patients were 82.7% (95% CI, 78.3%-86.3%) in the NIVO + RT + TMZ arm and 87.7% (95% CI, 83.8%-90.8%) in the PBO + RT + TMZ arm. The 24-month OS rates were 55.9% (95% CI, 50.5%-61.0%) in the NIVO + RT + TMZ arm and 63.3% (95% CI, 58.0%-68.2%) in the PBO + RT + TMZ arm. Among patients without baseline corticosteroid use, the 12-month OS rates were 85.5% (95% CI, 80.4%-89.4%) in the NIVO + RT + TMZ arm and 89.9% (95% CI, 85.5%-93.0%) in the PBO + RT + TMZ arm. The 24-month OS rates were 60.9% (95% CI, 54.4%-66.8%) in the NIVO + RT + TMZ arm and 67.1% (95% CI, 61.0%-72.6%) in the PBO + RT + TMZ arm.
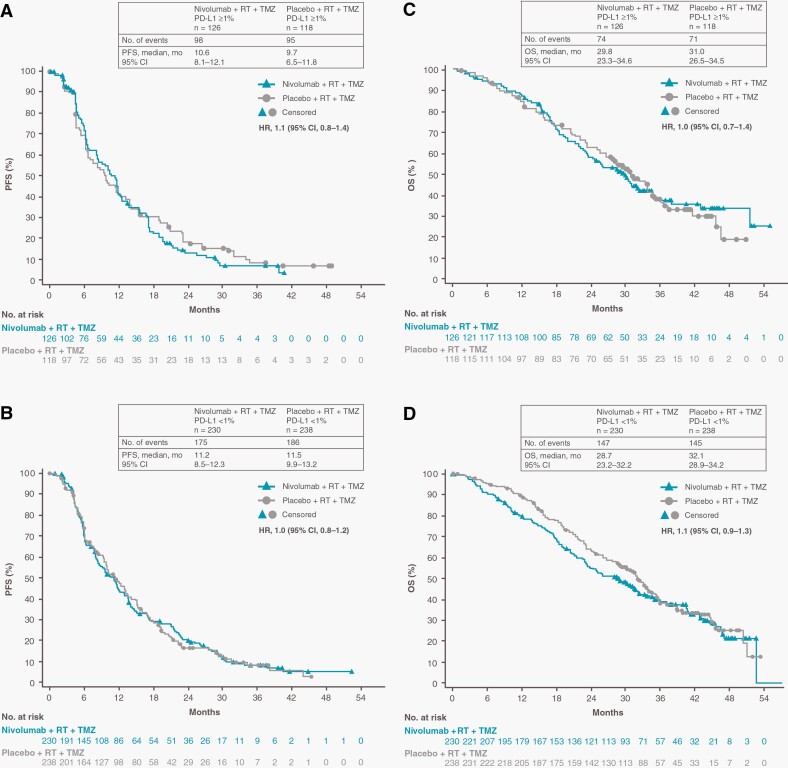
Among patients with baseline PD-L1 expression ≥1%, median PFS was 10.6 months (95% CI, 8.1-12.1 months) in the NIVO + RT + TMZ arm and 9.7 months (95% CI, 6.5-11.8 months) in the PBO + RT + TMZ arm (HR, 1.1; 95% CI, 0.8-1.4) (Figure 3A). The median PFS in patients with PD-L1 <1% was 11.2 months (95% CI, 8.5-12.3 months) in the NIVO + RT + TMZ arm and 11.5 months (95% CI, 9.9-13.2 months) in the PBO + RT + TMZ arm (HR, 1.0; 95% CI, 0.8-1.2) (Figure 3B). Median OS among patients with baseline PD-L1 expression ≥1% was 29.8 months (95% CI, 23.3-34.6 months) in the NIVO + RT + TMZ arm and 31.0 months (95% CI, 26.5-34.5 months) in the PBO + RT + TMZ arm (HR, 1.0; 95% CI, 0.7-1.4) (Figure 3C). The median OS in patients with PD-L1 <1% was 28.7 months (95% CI, 23.2-32.2 months) in the NIVO + RT + TMZ arm and 32.1 months (95% CI, 28.9-34.2 months) in the PBO + RT + TMZ arm (HR, 1.1; 95% CI, 0.9-1.4) (Figure 3D).
Fig. 3.
Progression-free survival and overall survival by PD-L1 expression. Number of events, median PFS, and Kaplan-Meier curves for PFS in all patients with baseline PD-L1 expression ≥1% (A) and <1% (B). Number of events, median OS, and Kaplan-Meier curves for OS in all patients with baseline PD-L1 expression ≥1% (C) and <1% (D). Symbols indicate censored observations. Abbreviations: BICR, blinded independent central review; OS, overall survival; PD-L1, programmed death-1 ligand 1; PFS, progression-free survival; RT, radiotherapy; TMZ, temozolomide.
PFS and OS data by baseline PD-L1 expression ≥5% are shown in Supplementary Figure S2. Among patients with baseline PD-L1 expression ≥5%, median PFS was 8.4 months (95% CI, 6.2-12.3 months) in the NIVO + RT + TMZ arm and 9.9 months (95% CI, 6.5-13.1 months) in the PBO + RT + TMZ arm (HR, 1.1; 95% CI, 0.8-1.6). Median PFS in patients with PD-L1 <5% was 11.5 months (95% CI, 9.7-12.1 months) in the NIVO + RT + TMZ arm and 11.3 months (95% CI, 9.8-13.1 months) in the PBO + RT + TMZ arm (HR, 1.0; 95% CI, 0.8-1.2). Median OS was 29.2 months (95% CI, 21.8-42.9 months) and 28.9 (95% CI, 23.7-31.6 months) in the NIVO + RT + TMZ arm (HR, 1.0; 95% CI, 0.6-1.4) and 31.3 months (95% CI, 23.2-36.0 months) and 31.8 months (95% CI, 28.8-33.8 months) in the PBO + RT + TMZ arm (HR, 1.1; 95% CI, 0.9-1.4) in patients with ≥5% and <5% PD-L1 expression, respectively.
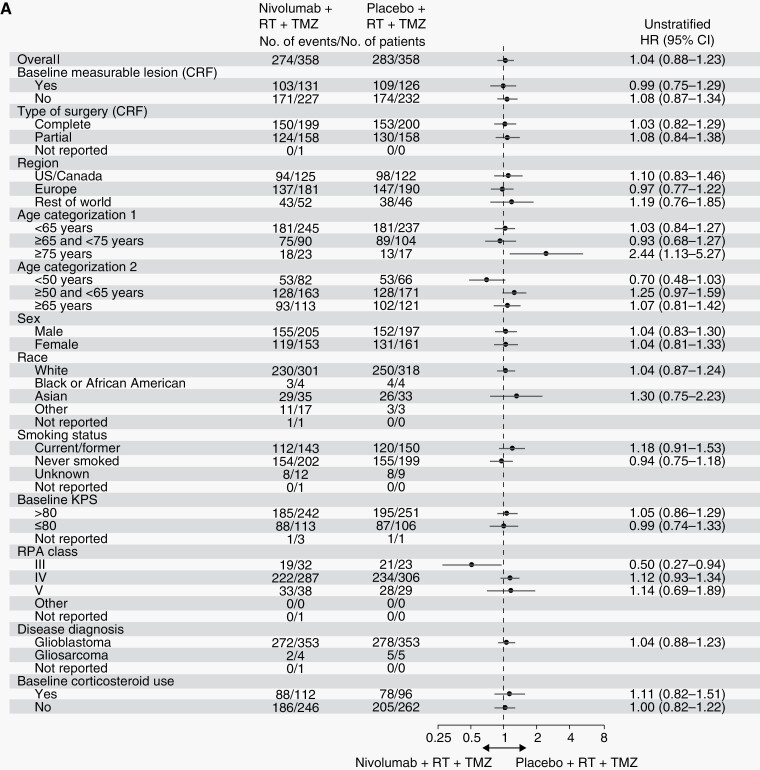
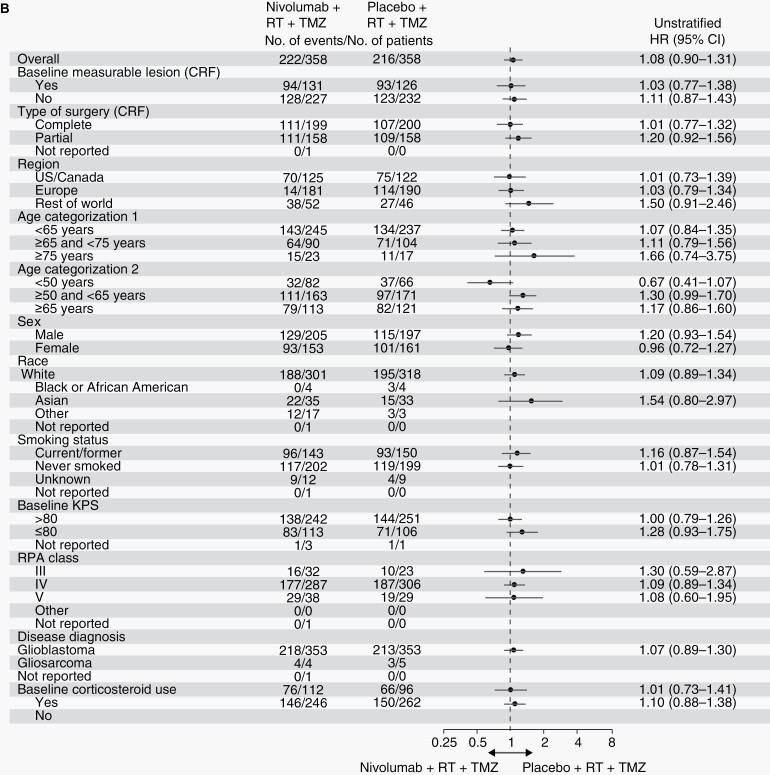
These results were consistent across several subgroup analyses (Figure 4; Supplementary Figure S3).
Fig. 4a.
Progression-free survival and overall survival in prespecified patient subgroups defined by baseline clinical characteristics. Forest plots of unstratified hazard ratios for progression per blinded independent central review (A) or death (B) in the analysis of treatment effect in prespecified patient subgroups according to baseline characteristics. Abbreviations: CRF, case report form; HR, hazard ratio; RPA, recursive partitioning analysis; RT, radiotherapy; TMZ, temozolomide.
Safety
Any-grade treatment-related adverse events (TRAEs) were reported in 92.4% of patients treated with NIVO + RT + TMZ and 83.6% of patients treated with PBO + RT + TMZ (Table 2). The most frequent TRAE was nausea (34.1%) in the NIVO + RT + TMZ arm and fatigue (32.8%) in the PBO + RT + TMZ arm. Rates of grade 3/4 TRAEs were 52.4% with NIVO + RT + TMZ and 33.6% with PBO + RT + TMZ. Neurological TRAEs occurred in 23.1% of patients treated with NIVO + RT + TMZ (grade 3/4, 5.1%) and 16.7% of patients treated with PBO + RT + TMZ (grade 3/4, 0.6%) (Table 2). The most frequent neurological TRAEs in both arms were headache (NIVO + RT + TMZ, 9.3%; PBO + RT + TMZ, 5.9%) and dysgeusia (NIVO + RT + TMZ, 5.6%; PBO + RT + TMZ, 4.2%). Any-grade serious TRAEs occurred in 105 patients (29.6%) treated with NIVO + RT + TMZ and 36 patients (10.2%) treated with PBO + RT + TMZ (Table 2). The most frequent serious TRAEs in both arms (NIVO + RT + TMZ/PBO + RT + TMZ) were tumor flare (2.5%/1.4%), pancytopenia (2.3%/0.6%), and thrombocytopenia (2.0%/1.7%).
Table 2.
Treatment-Related Adverse Events
| Event | Nivolumab + RT + TMZ, n = 355 No. (%) |
Placebo + RT + TMZ, n = 354 No. (%) |
||
|---|---|---|---|---|
| Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | |
| Any TRAE | 328 (92.4)a | 186 (52.4) | 296 (83.6) | 119 (33.6) |
| TRAEs in ≥10% of patients in either arm | ||||
| Nausea | 121 (34.1) | 7 (2.0) | 110 (31.1) | 2 (0.6) |
| Fatigue | 112 (31.5) | 13 (3.7) | 116 (32.8) | 7 (2.0) |
| Constipation | 72 (20.3) | 3 (0.8) | 62 (17.5) | 0 |
| Alopecia | 67 (18.9) | 0 | 52 (14.7) | 1 (0.3) |
| Platelet count decreased | 66 (18.6) | 23 (6.5) | 61 (17.2) | 17 (4.8) |
| Lymphocyte count decreased | 60 (16.9) | 40 (11.3) | 54 (15.3) | 35 (9.9) |
| Thrombocytopenia | 58 (16.3) | 25 (7.0) | 55 (15.5) | 15 (4.2) |
| Vomiting | 58 (16.3) | 4 (1.1) | 46 (13.0) | 0 |
| Decreased appetite | 55 (15.5) | 2 (0.6) | 58 (16.4) | 2 (0.6) |
| Pruritus | 52 (14.6) | 2 (0.6) | 47 (13.3) | 1 (0.3) |
| ALT increased | 43 (12.1) | 15 (4.2) | 22 (6.2) | 2 (0.6) |
| Rash | 42 (11.8) | 2 (0.6) | 33 (9.3) | 4 (1.1) |
| Lymphopenia | 38 (10.7) | 19 (5.4) | 30 (8.5) | 19 (5.4) |
| WBC count decreased | 31 (8.7) | 1 (0.3) | 36 (10.2) | 13 (3.7) |
| Neurological TRAEs | 82 (23.1) | 18 (5.1) | 59 (16.7) | 2 (0.6) |
| Neurological TRAEs in ≥2% of patients in either arm | ||||
| Headache | 33 (9.3) | 2 (0.6) | 21 (5.9) | 0 |
| Dysgeusia | 20 (5.6) | 0 | 15 (4.2) | 0 |
| Dizziness | 10 (2.8) | 0 | 11 (3.1) | 0 |
| Cognitive disorder | 8 (2.3) | 0 (0) | 2 (0.6) | 0 |
| Hemiparesis | 7 (2.0) | 3 (0.8) | 3 (0.8) | 0 |
| Memory impairment | 7 (2.0) | 0 (0) | 6 (1.7) | 0 |
| Serious TRAEs | 105 (29.6)a | 81 (22.8) | 36 (10.2) | 26 (7.3) |
| Serious TRAEs in ≥2% of patients in either arm | ||||
| Pancytopenia | 8 (2.3) | 8 (2.3) | 2 (0.6) | 2 (0.6) |
| Thrombocytopenia | 7 (2.0) | 7 (2.0) | 6 (1.7) | 6 (1.7) |
| Tumor flare | 9 (2.5) | 5 (1.4) | 5 (1.4) | 3 (0.8) |
| TRAEs leading to discontinuation | 81 (22.8)a | 60 (16.9) | 32 (9.0) | 20 (5.6) |
Abbreviations: ALT, alanine aminotransferase; RT, radiotherapy; TMZ, temozolomide; TRAE, treatment-related adverse events; WBC, white blood cells.
aOne grade 5 event (respiratory distress) occurred with NIVO + RT + TMZ.
Four treatment-related deaths were reported in the NIVO + RT + TMZ arm: respiratory failure, respiratory distress, pancytopenia, and pneumocystis pneumonia (1 each). No treatment-related deaths were reported in the PBO + RT + TMZ arm.
Any-grade TRAEs leading to discontinuation occurred in 81 patients (22.8%) in the NIVO + RT + TMZ arm and 32 patients (9.0%) in the PBO + RT + TMZ arm. Treatment-related immune-mediated AEs reported by category are shown in Supplementary Table S2.
Pseudoprogression was evaluated in patients treated with NIVO + RT + TMZ who had PFS of ≤6 months from the first NIVO dose. Patients with follow-up scans ≥3-months post-PD and unconfirmed PD (no confirmation of PD worsening) while remaining on treatment were considered as having pseudoprogression. Sixty-five patients in the NIVO + RT + TMZ arm were determined to be at risk for pseudoprogression among those with PD (n = 237). Forty of these patients had follow-up scans ≥3-months post-PD, and of those, 20 were confirmed as having pseudoprogression and the other 20 had PD without pseudoprogression, per iRANO criteria.23 Therefore, the rate of pseudoprogression among treated patients who had PD was 8.4%. Pseudoprogression could not be determined in the remaining 25 patients who had follow-up scans <3 months (10.5% of patients with PD).
Discussion
CheckMate 548 was a randomized PBO-controlled phase III study investigating the efficacy and safety of NIVO added to RT + TMZ in patients with newly diagnosed glioblastoma with a methylated or indeterminate MGMT promoter. The study did not meet its primary endpoints of improved PFS by BICR and OS in the overall population and OS in the population without baseline corticosteroid use. There were no differences in PFS or OS according to PD-L1 expression ≥1% or ≥5% or within prespecified patient subgroups defined by baseline clinical characteristics. Subgroup analyses for age and recursive partitioning analysis (RPA) class suggest potential trends for individuals aged >75 years and in RPA class III for PFS, although the small number of patients in these categories precludes definitive conclusions.
Although NIVO has shown efficacy in several other cancer types, no survival benefit over that with standard of care was observed in patients with newly diagnosed glioblastoma with a methylated or indeterminate MGMT promoter. Median OS was 28.9 months (95% CI, 24.4-31.6 months) with NIVO + RT + TMZ vs 32.1 months (95% CI, 29.4-33.8 months) with PBO + RT + TMZ in this study. Prior studies of RT + TMZ in patients with newly diagnosed glioblastoma with methylated MGMT promoter reported median OS of 21.7 months (95% CI, 17.4-30.4 months),9 21.4 months (95% CI, 17.6-29.0 months),8 and 26.3 months (95% CI, 23.9-34.7 months).25 However, eligibility criteria were slightly different, with this study excluding patients who had biopsy-only. Additional research is therefore needed to determine if the higher OS in this study compared with older studies could represent ongoing advances in surgery, monitoring, supportive care, or other clinical management aspects. Interestingly, some long-term survivors in the NIVO arm had baseline PD-L1 of >5% (see Supplementary Figure S2); this observation may warrant further investigation.
No new safety signals were observed with the addition of NIVO to RT + TMZ. Increased rates of TRAEs, including serious TRAEs and TRAEs leading to discontinuation, were observed, but this finding most likely reflects toxicities due to the addition of NIVO to standard-of-care therapy. NIVO monotherapy has been associated with rare instances of life-threatening and/or fatal serious adverse reactions, including respiratory failure, respiratory distress, myocarditis, and pneumocystis pneumonia. Radiation and TMZ are independently associated with pancytopenia26,27 and secondary infections, including respiratory infections.13,28–33 Neurological toxicities as a whole seemed more prevalent in the NIVO + RT + TMZ arm, particularly headaches, although other events were relatively rare. Of note, lymphopenia rates were 10.7% and 8.5% in the NIVO + RT + TMZ and PBO + RT + TMZ arms, respectively, which may contribute to the immunosuppressive glioblastoma tumor microenvironment and impact outcomes of immunotherapy.34,35
Pseudoprogression is a condition in which changes induced by immunotherapy, chemoradiation, or both produce a transient increase in apparent tumor burden followed by tumor regression.36,37 This phenomenon is also known to happen after RT + TMZ therapy, occurring in 10% to 30% of patients with newly diagnosed glioblastoma, and introduces challenges with interpreting imaging changes.23,38–40 In an exploratory analysis, we evaluated radiographic findings with iRANO criteria23 retrospectively, as these criteria were not available at the time of study design. Among the 237 patients with PD in the NIVO + RT + TMZ arm, 65 were considered at risk. Of those, 20 were confirmed as having pseudoprogression. Use of the iRANO guidelines23 may allow for improvements in immunotherapy trial design and patient management in the future. However, given the overlap in the PFS curves across both arms and similar duration of TMZ treatment, it is unlikely that an excess in pseudoprogression and potential early discontinuation of treatment would account for the lack of efficacy in the NIVO arm compared with the PBO arm.
Recent clinical trials have suggested that immune checkpoint inhibitors may affect the tumor microenvironment of glioblastomas, including enhanced expression of cytokine and chemokine transcripts, higher immune-cell infiltration, and augmented T-cell receptor clonal diversity among tumor-infiltrating T lymphocytes.41–43 Results of a small study suggest that the addition of neoadjuvant pembrolizumab prior to salvage surgery followed by continued adjuvant therapy may extend survival.43 In contrast, and in line with the results of our trial, recent results from the phase III CheckMate 498 study (NCT02617589) in patients with newly diagnosed glioblastoma with an unmethylated MGMT promoter demonstrated that immunotherapy with NIVO did not improve survival.20,21
As the addition of a single immunotherapy agent was not detrimental for outcomes in this study, additional considerations for treatment failure that could be further investigated include differences in the glioblastoma tumor microenvironment, T-cell exhaustion, and the potential for rapid tumor growth to leave insufficient time for immune system function. Additionally, novel combination checkpoint strategies, or combinations with other agents, such as a myeloid modulator, may be next considerations.
Limitations of the study include the lack of immune-predictive biomarkers and comprehensive genomic characterization due to the limited availability of tumor samples; therefore, novel biomarkers associated with this tumor type remain to be further explored.
In conclusion, CheckMate 548 was the largest phase III study conducted to date in patients with glioblastoma with a methylated or indeterminate MGMT promoter. Although results indicated that immunotherapy with NIVO did not add clinical benefit to standard-of-care RT + TMZ, it could be considered within new combination treatment strategies. Glioblastoma remains a difficult disease to treat, with few effective treatment options, and the role of immunotherapy in this treatment landscape remains an area for further investigation.
Supplementary Material
Acknowledgments
We thank the patients and their families who made this study possible; Ono Pharmaceutical Company Ltd, Osaka, Japan; and the staff of Dako, an Agilent Technologies company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay. Medical writing and editorial assistance was provided by Larra Yuelling, PhD, of SciMentum, Inc., a Nucleus Holding Ltd company, and was funded by Bristol Myers Squibb. Results from this study were presented at the 26th Annual Meeting of the Society for Neuro-Oncology, November 18-21, 2021, Boston, MA, USA.
Contributor Information
Michael Lim, Department of Neurosurgery, Stanford University School of Medicine, Palo Alto, California, USA.
Michael Weller, Department of Neurology and Brain Tumor Center, University Hospital and University of Zurich, Zurich, Switzerland.
Ahmed Idbaih, Sorbonne Université, Institut du Cerveau—Paris Brain Institute—ICM, Inserm, CNRS, AP-HP, Hôpital Universitaire La Pitié Salpêtrière, Paris, France.
Joachim Steinbach, Frankfurt Cancer Institute, Goethe University, Frankfurt, Germany; Institute of Neurooncology, Goethe University Hospital, Frankfurt, Germany.
Gaetano Finocchiaro, Unit of Molecular Neuro-Oncology, Neurological Institute C. Besta, Milan, Italy.
Raju R Raval, Translational Therapeutics Program, The Ohio State University Comprehensive Cancer Center, Columbus, Ohio, USA.
George Ansstas, Department of Medicine, Oncology Division, Washington University Medical School, St. Louis, Missouri, USA.
Joachim Baehring, Department of Neurology, Yale University School of Medicine, New Haven, Connecticut, USA.
Jennie W Taylor, Departments of Neurology and Neurological Surgery, University of California San Francisco, San Francisco, California, USA.
Jerome Honnorat, Neuro-Oncology Department, Hospices Civils de Lyon, SynatAc Team, Institute MeLis, INSERM U1314/CNRS UMR 5284, Université de Lyon, Université Claude Bernard Lyon 1, Lyon, France.
Kevin Petrecca, Department of Neurology and Neurosurgery, Brain Tumour Research Centre, Montreal Neurological Institute-Hospital, McGill University, Montreal, Quebec, Canada.
Filip De Vos, Medical Oncology, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands.
Antje Wick, Neurology Clinic, University of Heidelberg, National Center for Tumor Diseases, Heidelberg, Germany.
Ashley Sumrall, Neuro-Oncology Department, Levine Cancer Institute, Charlotte, North Carolina, USA.
Solmaz Sahebjam, Moffitt Cancer Center, University of South Florida, Tampa, Florida, USA.
Ingo K Mellinghoff, Department of Neurology and Human Oncology & Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Masashi Kinoshita, Department of Neurosurgery, Kanazawa University, Ishikawa, Japan.
Mustimbo Roberts, Bristol Myers Squibb, Princeton, New Jersey, USA.
Ruta Slepetis, Bristol Myers Squibb, Princeton, New Jersey, USA.
Deepti Warad, Bristol Myers Squibb, Princeton, New Jersey, USA.
David Leung, Bristol Myers Squibb, Princeton, New Jersey, USA.
Michelle Lee, Syneos Health, Morrisville, North Carolina, USA.
David A Reardon, Center for Neuro-Oncology, Dana-Farber/Harvard Cancer Center, Boston, Massachusetts, USA.
Antonio Omuro, Department of Neurology, Yale University School of Medicine, New Haven, Connecticut, USA; Department of Neurology and Human Oncology & Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Funding
This study was supported by Bristol Myers Squibb, Inc., Princeton, NJ, USA.
Conflict of interest statement. M. Lim received research support from Accuray, Arbor Pharmaceuticals, Biohaven, Bristol Myers Squibb, Kirin-Kyowa, Tocagen, and UroGen; received personal and consultant fees from Biohaven, Black Diamond Therapeutics, Century Therapeutics, Hemispherian, InCando, InCephalo Therapeutics, Insightec, Novocure, Noxxon, Pyramid Bio, Sanianoia, Stryker, VBI; and has shares in Egret Theraeutics. M.W. received other funding from Bristol Myers Squibb; grants from Merck (EMD), MSD, Novocure, and Quercis; and personal fees from AbbVie, Bristol Myers Squibb, Merck (EMD), MSD, Orbus, and Y-mAbs. A.I. received research grants from Carthera, Transgene, Sanofi, Air Liquide, Servier Pharmaceuticals, Nutritheragene; travel funding from Novocure, Carthera, and Leo Pharma; served on advisory boards for Leo Pharma and Novocure. J.S. received other funding from AbbVie; received personal fees from Medac, Med-Update, Novocure, Roche, and Seagen; and served on advisory boards for Novocure and Seagen. G.F. received other funding from Genenta. R.R.R. has nothing to disclose. G.A. has nothing to disclose. J.B. has served as a consultant for Bristol Myers Squibb. J.W.T. received grants from AbbVie, Agios, and Navio and personal fees from Medlink. J.H. has nothing to disclose. K.P. has nothing to disclose. F.D.V. received funding from Agios, Bristol Myers Squibb, Novartis, and Roche. A.W. has nothing to disclose. A.S. received grant funding from Kura Oncology and Exelixis; other funding from Bristol Myers Squibb, Novocure, Merck, Bayer, AbbVie, Oncoceutics, and Caris Life Sciences. S.S. received grant funding from Bristol Myers Squibb, Brooklyn Immunotherapeutics, and Merck; other funding from Boehringer Ingelheim, Eli Lilly, Merck; and personal fees from AbbVie. I.K.M. received research funding from Amgen, General Electric, Lilly, Kazia Therapeutics, and Servier Pharmaceuticals; other funding from Agios, Black Diamond Therapeutics, Debiopharm Group, Puma Biotechnology, Servier Pharmaceuticals, Voyager Therapeutics, DC Europa Ltd, Kazia Therapeutics, Novartis, Cardinal Health, Roche, Vigeo Therapeutics, Samus Therapeutics, Prelude Therapeutics, and AstraZeneca. M.K. has nothing to disclose. M.R. is an employee and received stocks from Bristol Myers Squibb. R.S. is an employee of Bristol Myers Squibb. D.W. is an employee of Bristol Myers Squibb. D.L. was an employee and received stocks from Bristol Myers Squibb. M. Lee is an employee of Bristol Myers Squibb. D.A.R. received other funding from Acerta Pharma, Agenus, Celldex, EMD Serono, Incyte, Inovio, Midatech, Omniox, and Tragara and personal fees from AbbVie, Advantagene, Agenus, Amgen, Bayer, Bristol Myers Squibb, Celldex, DelMar, EMD Serono, Genentech/Roche, Inovio, Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, and Taiho Oncology, Inc. A.O. has served as a consultant on ad hoc advisory boards for KIYATEC, Merck, Pyramid, and Ono Pharmaceutical Company Ltd, and has received grant funding from Agios, Arcus Biosciences, and Denovo.
Authorship statement. Study conception and design: M. Lim., M.W., G.F., R.R.R., A.S., S.S., D.A.R., and A.O. Data acquisition: M. Lim, M.W., A.I., J.S., G.F., G.A., J.B., J.T., J.H., K.P., F.D.V., A.W., A.S., S.S., I.M., M.K., D.A.R., and A.O. Data analyses: M.R., R.S., D.W., and M. Lee. Data interpretation: all authors. Contribution to and approval of manuscript: all authors.
Data Availability
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wen PY, Weller M, Lee EQ, et al. . Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Weller M, van den Bent M, Preusser M, et al. . EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TEMODAR (temozolomide) US Prescribing Information: Merck Sharp & Dohme Corp, November 2019.
- 6. US Food and Drug Administration. Premarket Approval (PMA): Optune. 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100034S013
- 7. Esteller M, Garcia-Foncillas J, Andion E, et al. . Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 8. Gilbert MR, Wang M, Aldape KD, et al. . Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 10. Kim DC, Kim KU, Kim YZ. Prognostic role of methylation status of the MGMT promoter determined quantitatively by pyrosequencing in glioblastoma patients. J Korean Neurosurg Soc. 2016;59(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weller M, Stupp R, Reifenberger G, et al. . MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39–51. [DOI] [PubMed] [Google Scholar]
- 12. Wick W, Platten M, Meisner C, et al. . Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 13. Malmström A, Grønberg BH, Marosi C, et al. . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 14.Opdivo (nivolumab) US Prescribing Information: Bristol-Myers Squibb, September 2021.
- 15. Nduom EK, Wei J, Yaghi NK, et al. . PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang C, Wang X, Soh H, et al. . Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng J, See AP, Phallen J, et al. . Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reardon DA, Brandes AA, Omuro A, et al. . Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Omuro A, Reardon DA, Sampson JH, et al. . Nivolumab plus radiotherapy with or without temozolomide in newly diagnosed glioblastoma: results from exploratory phase 1 cohorts of CheckMate 143. Neuro Oncol Adv. 2022;4(1):vdac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bristol Myers Squibb. Bristol Myers Squibb announces phase 3 CheckMate-498 study did not meet primary endpoint of overall survival with Opdivo (nivolumab) plus radiation in patients with newly diagnosed MGMT-unmethylated glioblastoma multiforme. https://news.bms.com/news/corporate-financial/2019/Bristol-Myers-Squibb-Announces-Phase-3-CheckMate--498-Study-Did-Not-Meet-Primary-Endpoint-of-Overall-Survival-with-Opdivo-nivolumab-Plus-Radiation-in-Patients-with-Newly-Diagnosed-MGMT-Unmethylated-Glioblastoma-Multiforme/default.aspx. Accessed September 1, 2021.
- 21. Omuro A, Brandes AA, Carpentier AF, et al. . Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase 3 trial. Neuro Oncol. Published online April 14, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 23. Okada H, Weller M, Huang R, et al. . Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Bethesda, MD: US Department of Health and Human Services; 2010. [Google Scholar]
- 25. Stupp R, Hegi ME, Gorlia T, et al. . Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 26. Vredenburgh JJ, Desjardins A, Kirkpatrick JP, et al. . Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;82(1):58–66. [DOI] [PubMed] [Google Scholar]
- 27. Gilbar PJ, Pokharel K, Mangos HM. Temozolomide-induced aplastic anaemia: case report and review of the literature. J Oncol Pharm Pract. 2021;27(5):1275–1280. [DOI] [PubMed] [Google Scholar]
- 28. Grossman SA, Ye X, Lesser G, et al. . Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stupp R, Dietrich P-Y, Kraljevic SO, et al. . Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20(5):1375–1382. [DOI] [PubMed] [Google Scholar]
- 30. Sengupta S, Marrinan J, Frishman C, Sampath P. Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol. 2012;2012:831090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kizilarslanoglu MC, Aksoy S, Yildirim NO, et al. . Temozolomide-related infections: review of the literature. J BUON. 2011;16(3):547–550. [PubMed] [Google Scholar]
- 32. Brault C, Zerbib Y, Chouaki T, Maizel J, Nyga R. Temozolomide is a risk factor for invasive pulmonary aspergillosis: a case report and literature review. Infect Dis Now. 2021;51(7):630–632. [DOI] [PubMed] [Google Scholar]
- 33. Jbeli AH, Yu J. Blastomycosis and histoplasmosis in a patient with glioblastoma receiving temozolomide. S D Med. 2016;69(10):447–450. [PubMed] [Google Scholar]
- 34. Kleinberg L, Sloan L, Grossman S, Lim M. Radiotherapy, lymphopenia, and host immune capacity in glioblastoma: a potentially actionable toxicity associated with reduced efficacy of radiotherapy. Neurosurgery. 2019;85(4):441–453. [DOI] [PubMed] [Google Scholar]
- 35. Mathios D, Kim JE, Mangraviti A, et al. . Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8(370):370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aquino D, Gioppo A, Finocchiaro G, Bruzzone MG, Cuccarini V. MRI in glioma immunotherapy: evidence, pitfalls, and perspectives. J Immunol Res. 2017;2017:5813951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abdalla G, Hammam A, Anjari M, D’Arco DF, Bisdas DS. Glioma surveillance imaging: current strategies, shortcomings, challenges and outlook. BJR Open. 2020;2(1):20200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 39. Radbruch A, Fladt J, Kickingereder P, et al. . Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol. 2015;17(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brandes AA, Franceschi E, Tosoni A, et al. . MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 41. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. . Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 42. Zhao J, Chen AX, Gartrell RD, et al. . Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019;25(3):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. . Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.