Abstract
Background
Selumetinib was recently approved for the treatment of inoperable symptomatic plexiform neurofibromas (PNs) in children with neurofibromatosis type 1 (NF1). This parallel phase II study determined the response rate to selumetinib in children with NF1 PN without clinically significant morbidity.
Methods
Children with NF1 and inoperable PNs, which were not yet causing clinically significant morbidity but had the potential to cause symptoms, received selumetinib at 25 mg/m2 orally twice daily (1 cycle = 28 days). Volumetric magnetic resonance imaging analysis and outcome assessments, including patient-reported (PRO), observer-reported, and functional outcome measures were performed every 4 cycles for 2 years, with changes assessed over time. A confirmed partial response (cPR) was defined as PN volume decrease of ≥20% on at least 2 consecutive scans ≥3 months apart.
Results
72% of subjects experienced a cPR on selumetinib. Participants received selumetinib for a median of 41 cycles (min 2, max 67) at data cutoff. Approximately half of the children rated having some target tumor pain at baseline, which significantly decreased by pre-cycle 13. Most objectively measured baseline functions, including visual, motor, bowel/bladder, or airway function were within normal limits and did not clinically or statistically worsen during treatment.
Conclusions
Selumetinib resulted in PN shrinkage in most subjects with NF1 PN without clinically significant morbidity. No new PN-related symptoms developed while on selumetinib, and PRO measures indicated declines in tumor-related pain intensity. This supports that selumetinib treatment may prevent the development of PN-related morbidities, though future prospective studies are needed to confirm these results.
Clinical Trial registration
ClinicalTrials.gov NCT01362803.
Keywords: MEK inhibitor, neurofibromatosis type 1, patient-reported outcome measures, plexiform neurofibroma, selumetinib
Key Points.
Selumetinib resulted in plexiform neurofibroma shrinkage in 72% of children with plexiform neurofibromas that were not causing significant tumor-related symptoms at baseline.
No new plexiform neurofibroma-related symptoms developed while on selumetinib.
Importance of the Study.
Selumetinib has received regulatory approval for use in children with inoperable symptomatic plexiform neurofibromas (PNs) and neurofibromatosis type 1 (NF1). In this phase II study, we used selumetinib to treat children with inoperable PN that were not causing significant tumor-related symptoms at baseline but based on their location had the potential to lead to significant PN-related complications in the future. Selumetinib resulted in PN shrinkage in 18 of the 25 (72%) subjects and no new PN-related symptoms developed while on selumetinib. These findings support that selumetinib treatment may prevent the development of PN-related symptoms, though further prospective studies are needed.
Neurofibromatosis type 1 (NF1) is a genetic disorder characterized by RAS pathway activation1 and multiple progressive tumor and non-tumor manifestations with limited treatment options. In a phase I trial of the oral selective MEK inhibitor selumetinib (AZD6244 or ARRY-142886) for children with inoperable NF1-related plexiform neurofibromas (PNs), tumor shrinkage and anecdotal clinical improvement were demonstrated in 17 of the 24 (71%) children.2 A subsequent phase II trial of selumetinib in 50 children with symptomatic PN-related morbidity (stratum 1) demonstrated confirmed PN shrinkage in 34 (68%) subjects, as well as clinical improvement, indicated by improvements in child-reported pain intensity scores, overall quality of life (QOL), functional outcomes of strength and range of motion after 1 year on treatment.3 These results led to the US Food and Drug Administration (FDA) approval of selumetinib (Koselugo) in April 2020 for children ≥2 years old with inoperable, symptomatic PNs.
Another population of patients for whom treatment with MEK inhibitors may be beneficial are children with PNs that are at risk of causing tumor-related complications. The phase II study therefore also included a stratum (stratum 2) for children with inoperable PNs not causing clinically significant morbidity but deemed to be at risk for developing serious PN-related complications to confirm the response rate of PNs to selumetinib (primary objective) and to assess if patients on treatment developed any new or worsening PN-related symptoms or functional impairments (key secondary objectives). The results of stratum 2 from the phase II study are reported here.
Methods
Trial Oversight
This National Cancer Institute (NCI)-coordinated, Cancer Therapy Evaluation Program (CTEP)-sponsored trial had four participating sites (Children’s Hospital of Philadelphia [CHOP], Cincinnati Children’s Hospital Medical Center [CCHMC], Children’s National Hospital [CNH], NCI). AstraZeneca supplied selumetinib. Trial oversight was nearly identical to the previously reported phase I and II studies.2,3 The protocol was approved by the institutional review board at each participating site. All patients or their legal guardians provided written informed consent.
Patients
Eligibility criteria were similar to the previously reported phase I and II trials2,3 and included children with a clinical diagnosis of NF1, 2-18 years of age, able to swallow intact capsules, and with inoperable measurable PN. In the phase II trial, we enrolled subjects on two strata: stratum 1 for children with at least one PN-related morbidity and stratum 2 for those with no clinically significant PN-related morbidity but potential for development of significant PN-related morbidity, such as (but not limited to) head and neck lesions that could compromise the airway or great vessels, paraspinal lesions that could cause myelopathy, brachial or lumbar plexus lesions that could cause nerve compression and loss of function, lesions that could result in major deformity (eg, orbital lesions) or become significantly disfiguring, lesions of the extremity that could cause limb hypertrophy or loss of function, and lesions that could become painful (complete eligibility criteria and other details are provided in the previously published trial protocol3). Determination of the presence or absence of clinically significant tumor-related morbidity was made by the study team, using baseline history and physical examinations at the time of enrollment. For stratum 2, determination of the absence of clinically significant morbidity and potential morbidities was made by investigators at participating institutions and solely based on clinical and imaging evaluations at baseline. For PN with potential for disfigurement, representative patient photography was reviewed by the study team including all participating site principal investigators. Patients taking scheduled pain medication(s) directed at symptoms associated with their PN were not eligible to be enrolled on stratum 2; however, those taking infrequent or intermittent non-prescription pain medication(s) directed at mild symptoms associated with their PN were eligible. Results of the prospectively administered patient-reported and functional evaluations were not used to determine eligibility but for research purposes only.
Evaluations
All subjects underwent scheduled clinical and laboratory safety evaluations, as well as echocardiogram and ophthalmology examinations. Magnetic resonance imaging (MRI), patient-reported (PRO) and observer-reported outcomes (ObsRO), and functional response evaluations were performed after every 4 cycles for the first year and then as described in the study protocol of the phase II trial.3
Drug Administration and Safety Assessments
Selumetinib was administered at the recommended phase II dose (25 mg/m2/dose)2,4 approximately every 12 hours in 28-day cycles on a continuous dosing schedule. Patients with progressive disease (PD) at trial entry (≥20% increase in PN volume within 15 months prior to enrollment) could remain on selumetinib as long as they did not have disease progression on treatment. Patients who did not have disease progression at trial entry could continue treatment for a maximum of 2 years unless a partial response (PR) was observed, in which case treatment could continue until meeting off-treatment criteria, as per the phase II protocol.3 Adverse events were graded using the NCI Common Terminology Criteria for Adverse Events, version 4.0. Definition of dose-modifying toxicities was nearly identical to that of dose-limiting toxicities in our phase I trial.2 Up to two dose reductions were allowed for selumetinib-related toxicities.
Tumor Response Evaluations
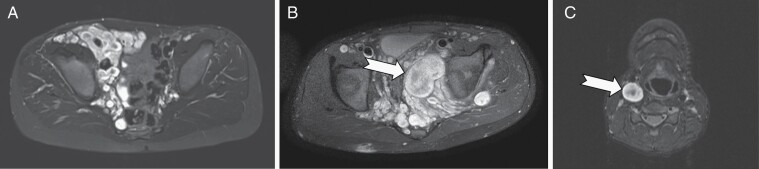
Tumor response evaluation was performed centrally at the NCI by volumetric analysis of the MRI of the target PN,5,6 which was defined as the most clinically relevant and measurable PN. The target PN was defined as typical appearing PN, nodular PN (includes distinct nodular lesion(s) (each ≥3 cm longest diameter) that account for ≥30% of total PN volume), or a solitary nodular lesion (entire tumor is one distinct nodular lesion) (Figure 1). Subjects were considered evaluable for response after receiving at least one dose of study drug. A PR was defined as target PN volume decrease from baseline of ≥20%; confirmed PR (cPR) = PR confirmed on consecutive restaging examinations at least 3 months apart; durable PR (dPR) was a PR lasting for ≥12 cycles (approximately 1 year). PD = PN volume increase from baseline of ≥20% or, if a patient had achieved a PR, an increase of ≥20% from the best response.
Fig. 1.
Examples of types of target plexiform neurofibromas (PN). PN are shown on axial STIR (short T1 inversion recovery) MRI sequences. (A) Typical appearing PN of the right pelvic wall displaying the characteristic central dot sign (dark center surrounded by bright rim) within individual small nodules. (B) Nodular pelvic PN consists of typical appearing and large distinct nodular components that account for ≥30% of total PN volume. (C) A solitary distinct nodular lesion (DNL) of the right carotid sheath. Arrows highlight DNLs defined as a single nodule ≥3 cm longest diameter, often with loss of the central dot sign.
Assessment of Potential PN-Related Symptoms
Comprehensive prospective, standardized clinical evaluations of PRO/ObsRO were conducted for all subjects, and functional outcomes were tailored to the location of the child’s target PN. Based on the target PN location and clinical presentation, each participant was assigned to one or more PN-related potential morbidity categories (motor dysfunction, airway impairment, vision impairment, disfigurement, bowel/bladder dysfunction, and other), which determined their functional evaluations.
PRO/ObsRO measures.
—Children ≥8 years old completed self-report PRO measures including the Numeric Rating Scale-11 (NRS-11)7,8 assessing tumor pain intensity, the Pain Interference Index (PII),9 the Pediatric Quality of Life Inventory-Generic Core scales (PedsQL),10 and the Global Impression of Change (GIC) scale.7,11 Parents of children ≥5 years old completed the parent-report PII and GIC scale7,11 and parents of children ≥2 years old completed the parent-report PedsQL.10 Qualitative content analysis of the GIC comments was used to document any perceived changes in disfigurement, as there is no standardized methodology to assess PN-specific disfigurement.
Functional evaluations.
—Each participant underwent standardized functional evaluations based on their potential PN-related morbidity categories. Children with the potential for motor morbidity underwent range of motion and strength assessments, specifically in the body quadrant where the target PN was located. Children with potential for airway impairment underwent pulmonary function testing (spirometry, oscillometry) and sleep study evaluations. Children with the potential for visual impairment underwent exopthalmometry and visual acuity testing. Those with the potential for bowel or bladder dysfunction completed a modified dysfunctional elimination syndrome questionnaire.12 Key measurements were chosen from each morbidity category to assess for change over time.
Response Criteria and Statistical Considerations
There are no validated thresholds for clinically meaningful change in the pediatric NF1 population for most functional measures obtained. Therefore, as previously published for stratum 1, we described the changes in each measurement over time, primarily between baseline and pre-cycle 13, using descriptive statistics and Wilcoxon signed-rank tests. Where available, clinically meaningful change was defined using criteria published by the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) collaboration.13–15 Detailed descriptions of the functional and PRO/ObsRO measures used were described in the previously published stratum 1 data.3 For comparing categorical parameters between two response categories, a Fisher’s exact test was used. For comparing the difference between two groups of subjects a Mann-Whitney U test was used. A Pearson correlation coefficient was used to assess the relationship between continuous variables. The joint effect of enrollment age and PN type on response was first assessed by individual univariate logistic regression models for each parameter followed by a multivariable logistic regression model. For the PRO/ObsRO and functional outcome measures, subjects were considered evaluable if they had measurements completed at baseline and pre-cycle 13 and no missing data at either of these time points. All evaluable subjects were included in the analyses, including those with scores indicating no pain (rating of 0) or baseline dysfunction because it was possible that pain or morbidities could develop or worsen while on treatment. To evaluate changes in PRO/ObsRO and functional measures over time, and where there were a sufficient number of subjects to compare (n ≥ 5), we used the Wilcoxon matched-pairs signed-rank tests. To evaluate individual change in target tumor pain ratings on the NRS-11, a decrease of ≥2 points was considered clinically meaningful improvement.16,17 Progression-free survival (PFS) was determined using the Kaplan-Meier method, based on the number of cycles received. All P values are two-tailed and reported without adjustment for multiple comparisons.
Results
Subject Characteristics
Twenty-five children (median age 12.3 years [range 4.5, 18.1]) enrolled on stratum 2 between November 12, 2015 and September 6, 2018 (Table 1). Data as of February 27, 2021 are reported here. Median target PN volume at baseline was 381 mL (interquartile range [IQR]: 140-740 mL) with the most common target PN location being the trunk. Eleven subjects (44%) had progressive PN at study enrollment, 9 (36%) had non-progressive PN and 5 (20%) did not have sufficient data prior to study entry to determine progression status. Twenty (80%) of the target PN had a typical appearance, 4 (16%) had distinct nodular components and 1 (4%) was a solitary nodular PN (Figure 1). There was a median of 3 (range 1, 5) potential PN-related morbidities per subject, the most common being the potential for disfigurement (n = 17), motor dysfunction (n = 17), pain/sensory deficit (n = 15), and bowel or bladder dysfunction (n = 12).
Table 1.
Subject and Plexiform Neurofibroma (PN) Baseline Characteristics
| Subjects enrolled (n) | 25 |
| Median age at enrollment, years (min, max) | 12.3 (4.5, 18.1) |
| Sex: male, female (n) | 16, 9 |
| Race | |
| White | 17 (68%) |
| Black or African American | 4 (16%) |
| Asian | 3 (12%) |
| Unknown | 1 (4%) |
| Target PN location (n) | |
| Head only | 4 (16%) |
| Head/neck | 4 (16%) |
| Neck/trunk | 4 (16%) |
| Trunk only | 9 (36%) |
| Trunk/extremity | 4 (16%) |
| Extremity only | 0 (0%) |
| Target PN progression status | |
| Progressive | 11 |
| Non-progressive | 9 |
| Unknown | 5 |
| Target PN baseline volume, median (IQR) | 381 mL (140, 740) |
| Target PN type | |
| Typical PN | 20 (80%) |
| Nodular PN | 4 (16%) |
| Solitary nodular PN | 1 (4%) |
| Number of potential PN morbidities per subject, median (range) | 3 (1, 5) |
| Type of potential PN-related morbidity, n (%) | |
| Motor | 17 (68%) |
| Disfigurement | 17 (68%) |
| Pain/sensory deficit | 15 (60%) |
| Bowel/bladder | 12 (48%) |
| Airway | 7 (28%) |
| Vision | 3 (12%) |
| Other (eg, facial muscle dysfunction, hearing loss, abnormal speech or swallowing) | 7 (28%) |
Tumor Volumetric Response
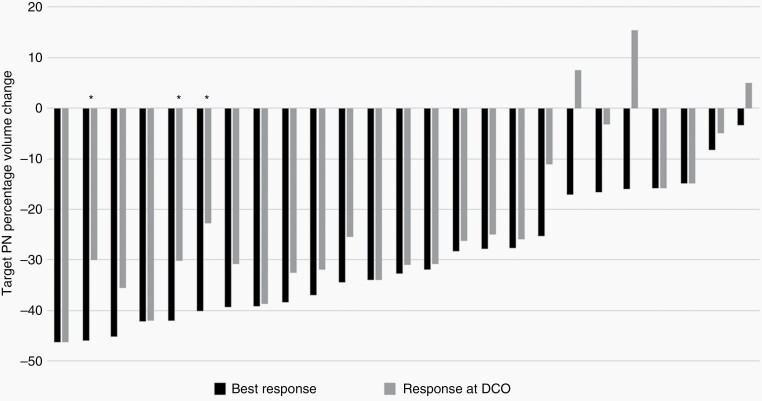
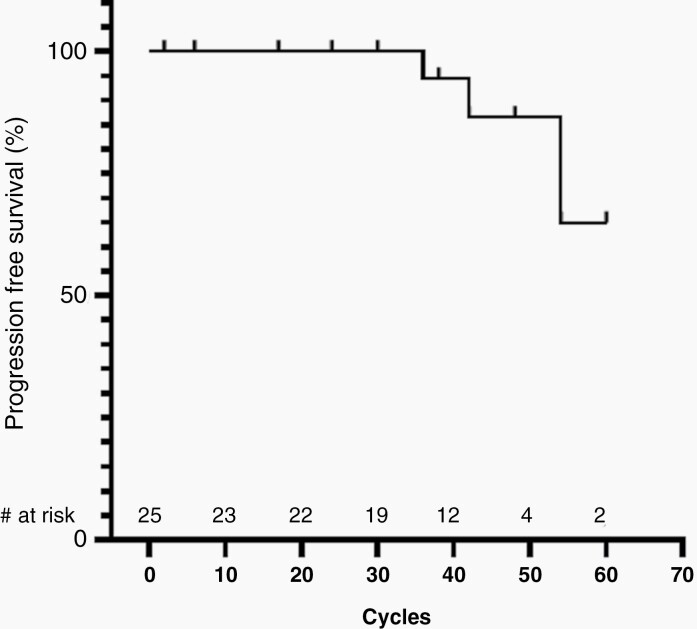
Eighteen of the 25 participants (72%) had a cPR. Seventeen children (68%) had a dPR (≥12 cycles). Median time to initial PR was 8 cycles (IQR 4-8) and median time to best response was 24 cycles (IQR 14-27). Median PN volume change from baseline to best response was −32.7% (IQR −39.7 to −16.8%) (Figure 2). The median number of treatment cycles was 41 (IQR 34-49). Median PFS has not been reached. PFS at 48 cycles was 86.6% (95% confidence interval [CI]: 55.2%-96.6%) (Figure 3). The median age at enrollment of those with a PR (9.7 years, IQR 7.3-12.8) was lower than those who did not achieve a PR (16.0 years, IQR 14.0-17.1) (P = .0018), and there was a moderately strong correlation between age at enrollment and percentage of tumor volume change at best response (r = 0.64, P = .0005). Nodular or solitary nodular PN (Figure 1) (n = 5) were less likely than typical PN to achieve a cPR (1/5 vs 17/20 responders; P = .012). While the associations of PN type and enrollment age with response were each individually confirmed in a univariate logistic model, when evaluated jointly in a multivariable model, only enrollment age remained well associated with response by having P < .05 (in joint model, odds ratio for PN nodular type relative to typical 0.035 (95% CI: <0.001 to 2.638; P = .13); odds ratio for each year of age: 0.655 (95% CI: 0.444-0.968; P = .034)). There was no difference in cPR rate based on baseline PN volume (P = .84), PN progression status prior to study entry (P = .32), PN location (P = .66), or subject sex (P = .35).
Fig. 2.
Tumor volumetric response through February 27, 2021 data cutoff (DCO). Black bars indicate the best response of tumor and gray bars indicate the response at the last restaging evaluation subject had prior to DCO. Asterisks (*) indicate progressive disease (≥20% increase from best response after a partial response).
Fig. 3.
Progression-free survival (PFS) curve. Median PFS has not been reached. PFS at 48 cycles was 86.6% (95% CI: 55.2%-96.6%). Of note, though the maximum duration of treatment was 67 cycles, due to limitations from the COVID-19 pandemic the last evaluable restaging evaluation for that subject was performed after 60 cycles. Number at risk (# at risk) indicates the number of subjects remaining in the study at each time point.
At data cutoff, 12 subjects (48%) had PR, 2 (8%) continued to have stable disease, 3 (12%) subjects had PD and 8 (32%) were off-treatment. No subjects had tumor growth ≥20% above baseline while on treatment. Off-treatment reasons were refused further treatment (n = 3), adverse event (asymptomatic lipase increase) (n = 1), deemed in best interest of the patient (n = 1), developed intercurrent illness (n = 1), and initiated commercial selumetinib (n = 2).
PRO/ObsRO and Functional Outcome Measures
Overall, there was no evidence of worsening in any of the PRO or functional measures obtained in the trial after 12 cycles of treatment.
Pain intensity.
—Eighteen of the 25 children enrolled were ≥8 years and completed self-report pain measures at baseline. Prior to starting treatment, 56% (n = 10) of these 18 children rated having no pain (rating of 0) in their physician-selected target tumor on the NRS-11 while 44% (n = 8) rated having some degree of target tumor pain; 28% (n = 5) rated it as mild (rating of 1-3) and 17% (n = 3) as moderate (rating of 4-6). Self-reported target tumor pain intensity significantly decreased from baseline to pre-cycle 13 in the group of children with both baseline and pre-cycle 13 target tumor pain ratings including 0 (see Statistical Considerations; n = 16, mean difference = −1.00, P = .016) with much of the decline occurring as early as pre-cycle 3 (n = 17, mean difference = −0.76; P = .047) (Table 2). Of the 5 participants with a baseline target tumor pain rating of ≥2 points and a pre-cycle 13 rating, 4 (80%) had a decrease of ≥2 points in their pre-cycle 13 NRS-11 score, which is considered clinically meaningful improvement,16,17 and one participant had a decrease of 1 point.
Table 2.
Patient-Reported Outcome (PRO)/Observer-Reported Outcome (ObsRO) Measure Results
| Baseline | Pre-Cycle 13 | Pre-Cycle 13-Baseline | |||||
|---|---|---|---|---|---|---|---|
| PRO/ObsRO Measures | N Mean (Min, Max) |
n Mean (Min, Max) |
n Mean Diffa P-value (95% Confidence Interval)* |
||||
| Pain intensity | |||||||
| Numeric Rating Scale-11 (NRS-11) (self-report) | Selfb n = 18 1.33 (0, 6) |
Selfb n = 16 0.38 (0, 3) |
Selfc n = 16 −1.00 P = .016 (−1.87 to −0.13) |
||||
| Pain interference | |||||||
| Pain Interference Index (PII) (self- and parent-report) | Selfd n = 18 0.68 (0, 2.33) |
Parente n = 24 0.47 (0, 3.5) |
Selfd n = 15 0.48 (0, 1.67) |
Parente n = 20 0.21 (0, 1.67) |
Selff n = 15 −0.32 P = .12 (−10.76 to 0.11) |
Parentf n = 20 −0.30 P = .28 (−0.77 to 0.17) |
|
| Physical function | |||||||
| PROMIS Physical Function (self- and parent-report) | Domain | Selfg | Parenth | Selfg | Parenth | Selff | Parentf |
| Mobilityj | n = 12 47.0 (35.6, 58.5) |
n = 16 43.9 (32.7, 56.5) |
n = 12 49.1 (38.6, 58.5) |
n = 16 44.4 (28.6, 56.5) |
n = 10 3.2 P = .20 (−1.5 to 7.9) |
n = 14 2.5 P = .29 (−1.0 to 6.0) |
|
| Upper extremityj | n = 12 49.3 (35.9, 56.7) |
n = 15 42.0 (28.2, 54.8) |
n = 13 52.5 (36.9, 56.7) |
n = 16 44.3 (27.5, 54.8) |
n = 11 3.1 P = .062 (0.0 to 6.1) |
n = 13 3.6 P = .074 (0.04 to 7.1) |
|
| Quality of life | |||||||
| PedsQL Generic QOL Scale (self-and parent-report) | Domain | Selfg n = 18 |
Parenth n = 25 |
Selfg n = 16 |
Parenth n = 21 |
Selff n = 16 |
Parentf n = 21 |
| Total | 82.0 (58.7, 100.0) |
80.2 (54.2, 100.0) |
87.5 (62.0, 100.0) |
83.3 (52.2, 100.0) |
5.6 P = .029 (1.0 to 10.2) |
4.1 P = .087 (−1.4 to 9.5) |
|
| Physical | 83.2 (43.8, 100.0) |
82.7 (40.6, 100.0) |
85.7 (59.4, 100.0) |
84.8 (31.3, 100.0) |
3.3 P = .43 (−3.9 to 10.6) |
4.8 P = .13 (−3.3 to 12.8) |
|
| Emotional | 85.6 (40.0, 100.0) |
82.6 (30.0, 100.0) |
91.9 (60.0, 100.0) |
85.7 (50.0, 100.0) |
6.2 P = .055 (0.4 to 12.1) |
2.9 P = .33 (−3.0 to 8.7) |
|
| Social | 80.8 (40.0, 100.0) |
79.9 (31.3, 100.0) |
87.2 (50.0, 100.0) |
85.5 (45.0, 100.0) |
5.9 P = .043 (−0.2 to 12.0) |
5.7 P = .15 (−3.2 to 14.7) |
|
| Schooli | n = 14 78.6 (45.0, 100.0) |
n = 20 73.6 (45.0, 100.0) |
n = 16 86.3 (55.0, 100.0) |
n = 21 76.4 (25.0, 100.0) |
n = 12 9.6 P = .0039 (3.5 to 15.7) |
n = 16 7.1 P = .13 (−5.7 to 19.9) |
*The 95% confidence intervals listed here have not been adjusted for multiplicity and therefore inferences drawn from the intervals may not be reproducible.
aUsed the Wilcoxon matched-pairs signed-rank test to compare baseline and pre-cycle 13 scores.
bIncludes all children ≥8 years old in stratum 2 with a self-reported NRS-11 target PN pain intensity score at baseline and all those with this score at the pre-cycle 13 evaluation, including baseline scores of 0 indicating no pain.
cCompares the target PN pain intensity scores of patients ≥8 years old completing the NRS-11 at both baseline and pre-cycle 13.
dIncludes all children ≥8 years old in stratum 2 with self-reported PII total scores at baseline and all those with this score at the pre-cycle 13 visit evaluation, including baseline total scores of 0 indicating no pain interference.
eIncludes all parents of children >5 years old in stratum 2 with parent-reported PII total scores at baseline and all those with this score at the pre-cycle 13 evaluation, including baseline total scores of 0 indicating no pain interference.
fCompares the scores of the patients ≥8 years old in stratum 2 completing the scales at both baseline and pre-cycle 13 and the scores of parents of children >5 years completing the scales (or parents of children >2 years for the PedsQL) at both baseline and pre-cycle 13.
gIncludes all children ≥8 years old in stratum 2 with self-reported data from the scales at baseline and all those completing the scales at pre-cycle 13.
hIncludes parents of children >5 years old in stratum 2 with parent-reported scales (or ≥2 years old for the PedsQL) at baseline and all those completing the scales at pre-cycle 13.
iThe school domain was not completed if a child was not in school the week before the evaluation because the response period was in the past 7 days; therefore, the number of children and parents completing the school domain is slightly lower than the other domains.
jThe PROMIS physical functioning forms assessing mobility and upper extremity function were administered only to the children >8 years of age in stratum 2 with a potential motor morbidity and the parents of children >5 years of age with a potential motor morbidity.
Pain interference.
—On the PII, self-reported (n = 15) and parent-reported (n = 20) pain interference did not show a significant change from baseline to the pre-cycle 13 evaluation. There was a decrease in pain interference rated by children at pre-cycle 5 (n = 18; mean difference = −0.33, P = .039) and by parents at pre-cycle 9 (n = 22; mean difference = −0.35, P = .047), but at pre-cycle 13, these mean levels of pain interference remained lower than baseline but the variability was much greater (self-report P = .12; parent report P = .28) (Table 2).
Physical functioning.
—At baseline, the parent’s rating of their child’s mobility and upper extremity (UE) function generated T-scores that were both below the normative mean of 50 (n = 16; mobility mean = 43.9, P = .012; n = 15; UE mean = 42.0, P = .020). These results indicate that this group of children demonstrate mild functional motor impairment and poorer physical function than their same-aged peers at the beginning of the study (albeit still within one standard deviation of the normative mean). However, the children’s self-report T-scores were much closer to the normative mean of 50 in these domains at baseline (n = 12; mobility mean = 47.0, P = .17; n = 12; UE mean = 49.3, P = .67), suggesting that these children do not see their physical functioning as being different than their peers. In evaluating changes with selumetinib, the children’s and parent’s ratings of mobility and UE scores were only slightly higher at pre-cycle 13 compared to baseline. Although there was some variability in the child and parent PROMIS T-scores across evaluation points, there were no consistent changes in these mean scores that would indicate meaningful improvements or declines in these specific domains of physical functioning for the total sample (Table 2).
Health-related quality of life.
—On the PedsQL, the mean total QOL score improved from baseline to pre-cycle 3 through pre-cycle 9 based on both the child and parent measures (all P values <.031) with better QOL continuing through pre-cycle 13 according to child self-report (n = 16; mean difference = 5.6, P = .029) but less so by parent report (n = 21; mean difference = 4.1, P = .087). When examining individual change, 62% of the child ratings and 52% of the parent ratings indicated clinically meaningful improvement in overall QOL based on the distribution-based minimally clinically important difference (MCID) for child self-report (4.4 points) and parent report (4.5 points).18 In the separate physical, emotional, social, and school domains, there were some upward trends but no clear improvements in the parents’ or children’s mean scores from baseline to pre-cycle 13 except for in the social (n = 16; mean difference = 5.9, P = .043) and school (n = 12; mean difference = 9.6, P = .0039) domains as rated by children (Table 2).
Global impression of change.
—At pre-cycle 13, 69% (n = 11 out of 16) of children and 68% (n = 15 out of 22) of parents rated that the child’s “tumor-related problems other than pain” were “minimally improved” to “much improved” (GIC rating of 1-3) compared to baseline with the breakdown of ratings as follows: 1 (very much improved; n = 5 children, n = 4 parents), 2 (much improved; n = 4 children, n = 6 parents), and 3 (minimally improved; n = 2 children, n = 5 parents). Five children and six parents rated having “no change.” No children and only one parent reported any tumor-related problems as being “minimally worse,” while no participants reported any changes as being “much worse” or “very much worse.” Qualitative content analysis of the reported changes from baseline revealed that the top three positive comments (range from 1 to 3 comments per participant) were improved appearance (n = 10 parents, n = 3 children), decreased pain (n = 7 parents, n = 7 children), and improved function (n = 3 parents, n = 3 children). The one neutral comment was lighter hair (n = 1 parent, n = 4 children). The few negative comments included nausea (n = 1 parent, n = 2 children), dry skin (n = 1 child), a toe infection (n = 1 parent), and decreased function (n = 1 parent).
Potential Motor Dysfunction (n = 17)
Strength.
—Of the cohort with potential motor morbidity, there were 8 subjects with evaluable strength data at baseline and pre-cycle 13. They had a median strength in the target PN quadrant (measured on the Kendall 10-point scale19) of 9.62 (IQR: 8.95-9.75) at baseline when compared with 9.63 (IQR: 9.0-10.0) at pre-cycle 13, which was essentially unchanged (P > .05) (Table 3).
Table 3.
Functional Evaluation Results
| Target PN-Related Potential Morbidity and Outcome Measures | Baseline | Pre-Cycle 13 | Median Differencea P-value (95% Confidence Interval)b |
|---|---|---|---|
| Potential motor morbidity (n = 17) | Median (IQR) | Median (IQR) | |
| Strength of muscles in target PN body quadrant on 0-10 scale (n = 8) | 9.62 (8.95 to 9.75) | 9.63 (9.0-10) | 0.11 P = .69 (−0.63 to 0.83) |
| Sum of degrees of motion in joints of target PN body quadrant (n = 12) | 827.5 (500.3 to 945.3) | 851 (517.5-982.5) | 4 P = .47 (−27.89 to 51.22) |
| Z-scores of degrees of motion in joints of target PN body quadrant (n = 12) | −0.91 (−1.29 to 0.01) | −0.37 (−1.73 to 0.44) | 0.30 P = .11 (−0.32 to 0.77) |
| Potential airway morbidity (n = 7) | Median (IQR) | Median (IQR) | |
| FEV1 (liters) (n = 4)c | 1.58 (1.11 to3.16) | 1.96 (1.43 to 3.28) | 0.31 P = N/Ad (−0.06 to 0.61) |
| FEV1 % predicted (n = 4)c | 95.5 (87 to 107) | 104.5 (93.5 to 132) | 11.5 P = N/Ad (−8.6 to 35.64) |
| Impulse oscillometry (cmH2O)c R5 (n = 4) R20 (n = 4) |
8.84 (6.67 to 9.45) 4.51 (4.23 to 7.22) |
4.32 (3.66 to 6.93) 3.69 (2.72 to 4.78) |
R5:−3.6 P = N/Ad (−6.9 to 0.20) R20:−1.57 P = N/Ad (−3.7 to 0.52) |
| Impulse oscillometry % predicted (%)c R5 (n = 4) R20 (n = 4) |
156.5 (100.3 to 213.5) 100.5 (75.0 to 129.8) |
86.5 (66.75 to 124.3) 74.5 (57.75 to 91.25) |
R5:−79.5 P = N/Ad (−132.8 to 4.31) R20:−36.5 P = N/Ad (−62.9 to 8.41) |
| Polysomnography: apnea-hypopnea index (events/hr)c (n = 4) | 0.1 (0.03-1.0) | 0.7 (0.1-145) | 0.3 P = N/Ad (−0.35 to 1.1) |
| Potential visual morbidity (n = 3) | Median (IQR) | Median (IQR) | |
| Visual acuity affected eye (LogMar) (n = 2) | 0 | −0.05 (−0.1 to 0) | Not evaluable |
| Exophthalmometry affected eye (n = 2) | 14.17 (13.33-15.0) | 14.67 (13.0-16.33) | Not evaluable |
Abbreviations: FEV1: forced expiratory volume in 1 second; PN: plexiform neurofibroma.
aUsed the Wilcoxon matched-pairs signed-rank test to compare baseline and pre-cycle 13 scores.
bThe 95% confidence intervals listed here have not been adjusted for multiplicity and therefore inferences drawn from the intervals may not be reproducible.
cDoes not include two subjects with severe kyphoscoliosis as these subjects were considered to have respiratory limitations unrelated to the target PN.
dUnable to calculate a meaningful P-value with Wilcoxon signed-rank test with ≤4 subjects.
Range of motion.
—Of 12 children with potential motor morbidity and evaluable range of motion testing, sum of range of motion scores in the target PN quadrant joints did not change appreciably after 12 cycles of treatment (P > .05). When compared with the range of motion in the general population, the median range of motion z-score at baseline was −0.91 (IQR: −1.29 to 0.01), with a slight increase to −0.37 at pre-cycle 13 (IQR: −1.73 to 0.44, P = .11) (Table 3).
Potential Airway Impairment (n = 7)
Spirometry.
—No participant had a tracheostomy or other assisted breathing devices at baseline. Six subjects with potential for airway morbidity from their target PN completed spirometry testing at baseline and after 12 cycles of treatment. Before treatment, the median percentage of predicted forced expiratory volume in one second (FEV1) (based on age, gender, and height) for all six subjects was 90%. Two of these children had abnormally low FEV1 relative to the percentage predicted at baseline (56% and 72%, respectively), but this was likely due to the presence of severe scoliosis in these subjects rather than a PN-related morbidity. Excluding these two children, the median measured percentage of predicted FEV1 at baseline was 95.5% (IQR: 87-107) and at pre-cycle 13 was 104.5% (IQR: 93.5-132, P = .25), which was essentially unchanged. After 12 cycles of treatment, the FEV1 absolute and percentage predicted had remained the same or increased (improved) slightly in all subjects (Table 3). As none of these subjects had baseline FEV1 between 40% and 80% of predicted for age, sex, and height, the REiNS criteria for clinically significant improvement or worsening could not be meaningfully applied.13
Oscillometry.
—The percentage predicted R5 measurement, which is a measure of total airway resistance, was abnormal at baseline (>120%) in 3 of the 4 subjects. In all 4 subjects, the R5 percentage predicted decreased (improved) from baseline to pre-cycle 13, including 3 subjects where it improved by ≥20%, and 2 subjects which normalized to <120% of predicted value. The baseline percentage predicted R20, a measure of proximal airway resistance, was abnormal in only 1 of the 4 subjects at baseline, and this subject had improvement of >20% after 12 cycles on treatment. None of the subjects had worsening of >20% in absolute or percentage predicted R5 or R20. Notably, the REiNS recommendation for a clinically meaningful change in impulse oscillometry is a >20% change in the R10, a measure of central airway resistance, rather than R5 or R20; however, this value was not consistently obtained on all subjects and therefore could not be evaluated.
Polysomnography.
—Of 4 subjects with polysomnography, there was no meaningful change in median apnea-hypopnea index (AHI) from baseline (median 0.1, IQR 0.03-1) to pre-cycle 13 (median 0.7, IQR 0.1-1.45). However, none of the children had a baseline AHI >5, which is considered to be the lower limit necessary to see a meaningful effect of treatment.13
Potential Visual Impairment (n = 3)
Visual acuity and exophthalmometry were measured for 2 subjects with potential orbital impairment. Baseline visual acuity and exophthalmometry were within normal limits and no clinically significant changes were observed (Table 3).
Potential Bladder and Bowel Function (n = 12)
No subjects had baseline daytime bladder or bowel incontinence, and no one developed daytime incontinence after 12 cycles of selumetinib treatment. One patient, a 15-year-old boy, had nighttime bladder incontinence at baseline which became less frequent while on treatment.
Potential Disfigurement (n = 17)
The children’s and parents’ ratings and associated comments on the GIC did not indicate any worsening of PN-related appearance or disfigurement. Although no participants were considered to have clinically significant disfigurement by the study team at baseline, 17 were classified as having potential disfigurement based on tumor location. Of these, 5 parents and 1 child reported some qualitative improvement in tumor-related size or appearance (eg, tumors have reduced in size, the small tumors are no longer visible) at pre-cycle 13.
Safety and Tolerability
All 25 participants experienced at least one toxicity that was possibly drug-related, but most toxicities were either grade 1 or 2 (97.7%) (Supplementary Table 1). Similar to prior studies, common toxicities were rash (acneiform, maculopapular, dry skin), headache, gastrointestinal symptoms (nausea, vomiting), asymptomatic elevation of creatine phosphokinase (CPK), and paronychia. Five subjects (20%) required dose reductions for toxicities including asymptomatic CPK increase (grade 4), asymptomatic left ventricular ejection fraction decrease (grade 2), elevated lipase (grade 4), and paronychia (grade 2). One subject required discontinuation of treatment due to toxicity (persistent asymptomatic lipase elevation). No participant developed central serous retinopathy or other vision-threatening ocular toxicities which have been attributed to this drug in other settings.
Discussion
In 2020, selumetinib received FDA approval for children with NF1 and symptomatic inoperable PNs. However, in the phase II study that led to approval in this population (stratum 1), no complete responses were observed, and PN-related symptoms were not completely reversible. In this study, we aimed to evaluate if selumetinib is tolerable and has the potential to prevent the development of symptoms in inoperable asymptomatic PN at risk for development of morbidity.
In this study of children with inoperable PNs without clinically significant morbidity (stratum 2), the majority (72%) demonstrated a cPR to selumetinib, and 68% had a PR lasting at least 1 year. This is comparable to the previously reported 68% of patients with symptomatic, inoperable PNs that experienced cPR.3 Notably, the subjects in this stratum who achieved a cPR were younger than those who did not, in contrast to the results from stratum 1 where there was no difference in age between responders and non-responders. PNs are known to grow more rapidly in young children20 and therefore may respond better to targeted therapy; however, it is unclear why a similar trend was not identified in stratum 1. Future studies including larger datasets of patients treated with MEK inhibitors are likely needed to determine whether this trend holds. While three subjects in stratum 2 ultimately developed PD, none of whom had prior dose reductions, no participants in this group had tumor growth ≥20% above their baseline volume, despite the fact that the majority of subjects had growing, progressive PN at baseline. This lack of progression above baseline suggests that MEK inhibition may have delayed the natural growth trajectory of these tumors.
This study highlights the potential clinical implications of PN shrinkage in a group of relatively asymptomatic children. As required per eligibility, subjects enrolled on this stratum did not have clinically significant morbidity at baseline as determined by the study team. However, PRO/ObsRO measures indicated that some participants still experienced PN-related pain and concerns about appearance. Although these children were not on scheduled pain medications, notably, there was self-reported tumor-related pain at baseline in nearly half the subjects (44%), as well as poorer parent-reported physical functioning compared to same-aged peers. These findings highlight the importance of obtaining prospective patient- and parent-reported data on clinical trials and reinforce the need for PRO/ObsRO measures that specifically assess PN-related morbidities in this population.
As expected, the mean scores at baseline of these stratum 2 participants suggest less pain and better physical functioning than those in stratum 1 who had symptomatic PN. After 1 year of treatment with selumetinib, self-reported tumor pain intensity ratings significantly declined, with changes noted as early as 3 months post-baseline that were sustained to pre-cycle 13; and most (4/5) of the children who had tumor pain at baseline demonstrated declines of ≥2 points, indicating clinically meaningful change. This pattern of improvement in tumor-related pain intensity mirrors that seen in stratum 1 except with lower initial pain ratings.3 In contrast, although there was some decline in pain interference mean scores over the longitudinal assessments, these were not substantially lower at pre-cycle 13 compared to baseline, suggesting that the degree of tumor-related pain did not interfere to a high degree with their daily functioning at baseline, resulting in less room for improvement with treatment. Overall, these children also showed no worsening of pain or pain interference over time with treatment.
Total QOL scores also significantly improved over 1 year of selumetinib treatment, which was deemed clinically meaningful change in over half of the children, as rated by both children and parents. In addition, some participants reported an improvement in the appearance of their PN with treatment. These subjective changes could be related to a placebo effect but were noted consistently across several PRO measures. As mentioned, while subjects enrolled on this stratum did not have clinically significant morbidity, some noted pain, concerns about appearance, and poorer physical functioning at baseline; thus, the potential for clinically meaningful improvements in these less significant morbidities should not be overlooked.
Most of the objectively measured baseline functional evaluations, including airway, visual, motor, and bowel/bladder function were within normal limits for this group of patients. This highlights the ability of the study clinicians to correctly stratify patients by the presence or absence of objectively measurable PN-related functional deficits using only history and physical examination findings, prior to formal functional evaluation. Though 3 subjects did have abnormal measure of total airway resistance (R20), the functional implications of this abnormality are not clear. Of note, several subjects without clinically meaningful symptoms identified by the study team rated having some PN-related symptoms on the baseline PRO measures, suggesting that these measures may help identify subtle, variable, or underreported symptoms in some patients.
Importantly, there was no apparent change in clinical status in any functional evaluation, though the number of children tested was small. Previous studies of the natural history of NF1 have demonstrated a significant correlation between PN growth and development of clinical morbidity.1,21 Therefore, it is possible that subjects did not develop morbidity because of tumor growth inhibition by selumetinib. However, as this was not a randomized controlled trial, it is not possible to determine whether treatment truly prevented the development of morbidity.
Similar to stratum 1, selumetinib was safe and tolerable in the majority of subjects on stratum 2.2,3 However, as with prior experience, we found that all participants experienced at least one selumetinib-related toxicity, such as acneiform rash or gastrointestinal toxicity. This must be considered when weighing the potential risks and benefits of treatment in this population. Importantly, clinicians must continue to clinically assess patients on selumetinib therapy for changes in PN-related symptoms as there is no evidence that treatment with selumetinib monotherapy will prevent the transformation of a PN to a malignant peripheral nerve sheath tumor.
A limitation of this study is the relatively small sample size, which makes it difficult to generalize results at a population level. In addition, this was not a randomized controlled trial, and therefore it is not possible to conclude whether the patients experienced clinical benefit by preventing morbidity beyond tumor shrinkage. A randomized controlled trial of treatment vs observation to compare the growth and development of PN-related morbidity in children with asymptomatic tumors in high-risk locations is currently in development and should help more definitively answer this important question. It is further notable that despite a 72% PR rate at best response, at the time of data cutoff 52% of patients continued to have a PR, with 3 patients experiencing disease progression in later cycles. This highlights the importance of delineating an appropriate timeline for MEK inhibition therapy in this asymptomatic population. Furthermore, patients enrolled on clinical trials are usually higher risk phenotypes than those with NF1 at large.20 While this limits generalizability of the results, it is notable that even the more high-risk patients have tumor response and may have clinical benefit from MEK inhibition therapy.
The findings from this study will assist the development of guidelines for the management of patients with asymptomatic, NF1-related PNs at risk for causing morbidity. Future randomized controlled studies are planned to further evaluate if treatment of these patients can prevent PN-related symptoms from developing. In addition, future studies are needed to define the appropriate time for starting MEK inhibition therapy in this asymptomatic population, as well as the appropriate duration of treatment.
Supplementary Material
Acknowledgments
We thank the collaborators and teams at the participating sites who provided guidance and advice and helped make this study possible, including Joseph Andress, Melissa Baker, Alessandra Brofferio, Ashura Buckley, Victoria Collier, Derrick Delgado, Kathy Farrell, Joseph Fontana, Andrea Gillespie, Ann Harrington, Marielle Holmblad, Jason Levine, Michaele Smith, Mary Anne Tamula, Ratnakar Patti, Scott Plotkin, Katie Smith, and Megan Westendorf. We also thank CTEP, AstraZeneca, and NTAP for their support. The authors would also like to sincerely thank the patients and families who participated in this study.
Contributor Information
Andrea M Gross, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Brittany Glassberg, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Pamela L Wolters, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Eva Dombi, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Andrea Baldwin, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Michael J Fisher, Division of Oncology, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
AeRang Kim, Center for Cancer and Blood Disorders, Children’s National Hospital, Washington, DC, USA.
Miriam Bornhorst, Center for Cancer and Blood Disorders, Children’s National Hospital, Washington, DC, USA.
Brian D Weiss, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Jaishri O Blakeley, Department of Neurology, Johns Hopkins University, Baltimore, Maryland, USA.
Patricia Whitcomb, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Scott M Paul, Rehabilitation Medicine Department, NIH Clinical Center, Baltimore, Maryland, USA.
Seth M Steinberg, Biostatistics and Data Management Section, Center for Cancer Research, National Cancer Institute, Baltimore, Maryland, USA.
David J Venzon, Biostatistics and Data Management Section, Center for Cancer Research, National Cancer Institute, Baltimore, Maryland, USA.
Staci Martin, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Amanda Carbonell, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Kara Heisey, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Janet Therrien, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Oxana Kapustina, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Anne Dufek, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Joanne Derdak, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Malcolm A Smith, Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, Maryland, USA (M.A.S.).
Brigitte C Widemann, Pediatric Oncology Branch, Center for Cancer research, National Cancer Institute, Bethesda, Maryland, USA.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health; the Center for Cancer Research, National Cancer Institute (NCI); the NCI Cancer Therapy Evaluation Program (CTEP); AstraZeneca (provision of selumetinib and funding for the pharmacokinetic analysis); the Developmental and Hyperactive Ras Tumor (DHART) Spore (U54 CA196519-04) for supporting the pharmacodynamic studies; and the Neurofibromatosis Therapeutic Acceleration Program (NTAP) [funding for trial conduct at the Children’s Hospital of Philadelphia (CHOP) and Cincinnati Children’s Hospital Medical Center (CCHMC)]. B.G. was funded by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors.
Conflict of interest statement. Dr. Miriam Bornhorst was a paid consultant for AstraZeneca from July 2018 to February 2020. The other authors report no financial conflicts of interest.
Authorship statement. B.C.W., E.D., M.J.F., A.K., B.D.W., and J.O.B. conceived of and designed the trial. A.M.G., B.G., and P.L.W. drafted, and subsequently all authors revised, the manuscript. All authors approved the documents for submission.
References
- 1. Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123(1):124–133. [DOI] [PubMed] [Google Scholar]
- 2. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382(15):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banerjee A, Jakacki RI, Onar-Thomas A, et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2017;19(8):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mautner VF, Asuagbor FA, Dombi E, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10(4):593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81:S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- 8. Wolters PL, Martin S, Merker VL, et al. Patient-reported outcomes in neurofibromatosis and schwannomatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S6–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin S, Nelson Schmitt S, Wolters PL, et al. Development and validation of the English Pain Interference Index and Pain Interference Index-Parent report. Pain Med. 2015;16(2):367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 11. McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9(9):771–783. [DOI] [PubMed] [Google Scholar]
- 12. Afshar K, Mirbagheri A, Scott H, MacNeily AE. Development of a symptom score for dysfunctional elimination syndrome. J Urol. 2009;182(4 Suppl):1939–1943. [DOI] [PubMed] [Google Scholar]
- 13. Plotkin SR, Davis SD, Robertson KA, et al. Sleep and pulmonary outcomes for clinical trials of airway plexiform neurofibromas in NF1. Neurology. 2016;87(7 Suppl 1):S13–S20. (In Eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolters PL, Martin S, Merker VL, et al. Patient-reported outcomes of pain and physical functioning in neurofibromatosis clinical trials. Neurology. 2016;87(Suppl 1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81(21 Suppl 1):S15–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. [DOI] [PubMed] [Google Scholar]
- 17. Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88(3):287–294. [DOI] [PubMed] [Google Scholar]
- 18. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329–341. [DOI] [PubMed] [Google Scholar]
- 19. Kendall FP, McCreary EK, Provance PG, Rodgers M, Romani W.. Muscles: Testing and Function, with Posture and Pain. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 20. Akshintala S, Baldwin A, Liewehr DJ, et al. Longitudinal evaluation of peripheral nerve sheath tumors in neurofibromatosis type 1: growth analysis of plexiform neurofibromas and distinct nodular lesions. Neuro Oncol. 2020;22(9):1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gross AM, Singh G, Akshintala S, et al. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018;20(12):1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.