Abstract
Many studies in patients with brain tumors evaluating innovative PET tracers have been published in recent years, and the initial results are promising. Here, the Response Assessment in Neuro-Oncology (RANO) PET working group provides an overview of the literature on novel investigational PET tracers for brain tumor patients. Furthermore, newer indications of more established PET tracers for the evaluation of glucose metabolism, amino acid transport, hypoxia, cell proliferation, and others are also discussed. Based on the preliminary findings, these novel investigational PET tracers should be further evaluated considering their promising potential. In particular, novel PET probes for imaging of translocator protein and somatostatin receptor overexpression as well as for immune system reactions appear to be of additional clinical value for tumor delineation and therapy monitoring. Progress in developing these radiotracers may contribute to improving brain tumor diagnostics and advancing clinical translational research.
Keywords: fluciclovine, Immuno-PET, PSMA, somatostatin, TSPO
In the last few years, there has been a significant increase in the use of PET in the field of neuro-oncology, also highlighted by the substantial increase of published studies. On this basis, the RANO (Response Assessment in Neuro-Oncology) PET working group provided clinical practice guidelines and recommendations for the use of PET imaging in patients with the most common brain tumor entities such as gliomas, brain metastases, and meningiomas.1–3 Recently, the PET/RANO group also provided evidence-based recommendations for PET-based radiotherapy planning and monitoring in glioma patients.4 In addition, the RANO group has published in close collaboration with major American and European societies for Neuro-Oncology (EANO) and Nuclear Medicine - the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) - joint practice guidelines for PET procedure standards in patients with brain tumors.5 Notably, these technical guidelines are of utmost importance to ensure comparability of study results and to reach consensus across studies and institutions regarding acquisition parameters.
PET imaging enables the noninvasive evaluation of molecular and metabolic processes in numerous neurological diseases including brain tumors. The continuously growing landscape of PET tracers enables the evaluation of many biochemical processes in this group of patients. Currently, radiolabeled amino acids and somatostatin receptor ligands are the PET tracers of choice to supplement conventional MR imaging for brain tumor diagnostics and frequently used in many neurooncological centers (especially in Europe).6 Radiolabeled amino acids have the main advantage that tracer uptake is independent of a blood–brain barrier (BBB) disruption, which is highly helpful to delineate the tumor extent, particularly the nonenhancing parts in gliomas (eg, for biopsy guidance). Further important indications for amino acid PET in glioma patients are the differential diagnosis of treatment-related changes (eg, pseudoprogression, radionecrosis), and the assessment of response to brain cancer treatment (eg., radiotherapy, alkylating chemotherapy, immune checkpoint inhibitors, targeted therapies). The latter two indications are also of great clinical value for patients with brain metastases.7 In patients with meningioma, one of the most important indications for PET using somatostatin receptor ligands is the assessment of tumor extent, especially at the skull base with bony involvement and in meningiomas with complex geometry.3
With the advent of newer treatment options in neuro-oncology such various immunotherapy options, the needs for additional information derived from neuroimaging in terms of characterization of the tumor environment, the evaluation of tumoral drug accumulation, immune cell infiltration, and the diagnosis of treatment-related changes are steadily increasing. Some of these requirements may be met by already existing PET tracers, while others can be addressed by novel ones. Currently, several other promising and innovative radiopharmaceuticals are under investigation, and this review aims to discuss the clinical value of these new PET probes in patients with brain tumors. Furthermore, newer indications of more established “standard” PET tracers for the evaluation of the glucose metabolism, amino acid transport, hypoxia, cell proliferation, and others are also addressed in this article. In addition, the role of PET tracers in evaluating the ability of novel agents to cross the blood–brain barrier and produce adequate pharmacodynamic effects will be discussed.
Search Strategy, Selection Criteria
A PubMed search of the published literature with the combination of the search terms “glioma”, “glioblastoma”, “astrocytoma”, “brain metastases”, “meningioma”, “PET”, “positron”, “immunotherapy”, “checkpoint inhibitor”, “pseudoprogression”, “treatment-related changes”, “treatment monitoring”, “treatment response”, and combinations thereof prior to and inclusive of December 2021 was performed. Additionally, articles identified through searches of the authors’ own files were included in the search.
Metabolic PET Imaging
Amino Acid Metabolism and Transport
Today, radiolabeled amino acids are the preferred PET tracers in neuro-oncology.8 These tracers are helpful in clinical decision-making regarding differential diagnosis, prognostication, delineation of brain tumor extent for diagnostic and treatment planning (ie, stereotactic biopsy-guidance, resection, and radiotherapy), the assessment of response to radiotherapy, alkylating chemotherapy, and other antitumoral agents), and the differentiation of tumor relapse from treatment-related changes such as pseudoprogression or radiation necrosis.6,8 For the diagnosis of treatment-related changes in patients either with glioma or brain metastases, the diagnostic performance was described as high (range of sensitivity and specificity, 80–90%), and allows therefore meaningful clinical decision-making.9–11 In clinical routine, the most established amino acid tracers are [11C]-methyl-L-methionine (MET), O-(2-[18F]fluoroethyl)-L-tyrosine (FET), and 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (FDOPA) whose benefits have been documented in previous publications of the PET/RANO group.1,2 It is assumed that uptake of these tracers is mainly based on the increased expression and functionality of large neutral amino acid transporters of the L-type (LAT) in gliomas and brain metastases (ie, subtypes LAT1 and LAT2).8 Beside these tracers, a wide range of natural and nonnatural amino acids have been labeled with positron emitters and investigated for PET in humans in the last two decades.12,13 Intensive research in this field is further stimulated by recent insights into the role of amino acid transporters and amino acid metabolism in brain tumors.12,13
It is beyond the scope of this chapter to provide a complete overview of all amino acid tracers developed. This review focus on a few selected tracers that are currently of particular interest. For a more detailed overview, we refer to a recent review article.13
Fluciclovine (FACBC).—
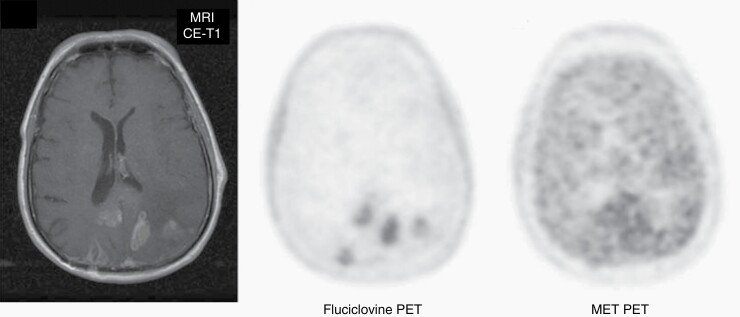
The synthetic amino acid analog anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (FACBC or Fluciclovine) was first used for brain tumor imaging in 1999.14 This tracer initially gained clinical interest particularly for the diagnosis of recurrence of prostate cancer.15 Fluciclovine has been approved in the USA and Europe for evaluation of recurrent prostate cancer16 and has also orphan drug status for glioma imaging in the USA. Transport of Fluciclovine is mediated to some extent by LAT1 but predominantly by another neutral amino acid transporter, ASCT2, which is not expressed at the luminal side of the BBB.17 In general, a significantly higher tumor-to-brain contrast is observed with Fluciclovine compared to the established amino acid tracers,18 which is primarily due to the low transport of Fluciclovine through the intact BBB compared with MET, FET, and FDOPA (Figure 1). In relation to these tracers, initial studies suggest that Fluciclovine PET is of clinical value for the differentiation of tumor relapse from treatment-related changes,16,19 and is also currently evaluated a in phase III clinical trial for this indication in patients with brain metastases after radiotherapy (NCT04410133; RELEVATE trial). Other studies suggest that Fluciclovine accumulates in nonenhancing gliomas and identified infiltrating tumor areas without contrast enhancement on MRI.20,21 These observations are promising and warrant a further clinical evaluation of Fluciclovine especially in comparison with the clinically established amino acid tracers.
Fig. 1.
Patient with multifocal relapse of a glioblastoma after surgery and temozolomide chemoradiation on contrast-enhanced (CE) MRI. A significantly higher tumor-to-brain contrast is observed with PET using the tracer anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (Fluciclovine) compared to [11C]-methyl-L-methionine (MET). Modified, with permission, from Michaud and coworkers.19.
Tryptophan derivates.—
Another amino acid tracer of considerable interest is the L-tryptophan analogue [11C]-methyl-L-tryptophan (AMT). AMT is transported via the LAT1 system and not incorporated intracellularly into proteins but metabolized via different pathways.22 In particular, AMT is converted to α-methyl-serotonin in serotonin synthesizing neurons and trapped in serotoninergic terminals.23 The potential role of AMT for tumor diagnosis was stimulated by a report on high expression of indoleamine 2,3-dioxygenase, the initial and rate-limiting enzyme of the kynurenine pathway, which is upregulated in various cancers including gliomas.24 The first clinical results using AMT PET to delineate the extent of nonenhancing gliomas25 are comparable to the results with established amino acid tracers.26 Furthermore, AMT kinetics allowed differentiation between glioblastomas and metastatic brain tumors,27 which has not yet been reported using MET, FET, or FDOPA PET. One study reported a poor prognosis in pretreated gliomas with high AMT uptake,28 which is line with other amino acid tracers. In newly diagnosed glioblastoma, however, an inverse relationship was observed, that is, a longer survival in contrast-enhancing tumors with increased AMT uptake,29 which is an unusual finding.
Nevertheless, the clinical applicability of AMT is hampered by labeling with the short half-life of C-11 (ie, 20 min), which limits is application to centers with an on-site cyclotron. To solve this problem, several 18F-labeled tryptophan derivatives are currently under development and may facilitate the clinical use of tryptophan analogues.30
Glutamine derivates.—
The development of the PET tracer 4-(2S,4R)-[18F]fluoroglutamine (FGln) was driven by the fact that glutamine is one of the major nutrients for tumor cells. Experimental studies have shown that FGln is predominantly transported through the ASC system, particularly subtype ASCT2.31 First studies in animals and humans have demonstrated a high tumor-to-brain contrast with FGln similar to that observed with FACBC but it remains unclear whether FGln detects tumor parts in nonenhancing glioma subregions.32,33
Cell Proliferation
The most widely used PET tracer for tumor proliferation assessment is 3′-deoxy-3′-[18F]fluorothymidine (FLT).13 FLT enters the cells by both active transport through nucleoside transporters and by passive diffusion.34 The tracer is not incorporated into DNA but is trapped after monophosphorylation by thymidine kinase-1, which is exclusively expressed in the cytoplasm during S-phase of DNA synthesis.35 Controversially, other studies suggested that FLT uptake in brain tumors depends predominantly on increased permeability and increased influx via the disrupted BBB while intracellular trapping appears to be less important.36 Correspondingly, increased FLT uptake has also been reported in nontumoral lesions with BBB disruption such as subacute infarction, multiple sclerosis, and radionecrosis.37
The relationship between FLT uptake and prognosis in glioma patients has been demonstrated in several studies.38,39 In contrast, the value of FLT PET in differentiating between glioma relapse and treatment-related changes remains controversial. A meta-analysis yielded a sensitivity and specificity of 82% and 76%, which is lower than that of radiolabeled amino acids.40 In addition, a study in glioblastoma patients reported that serial FLT PET imaging was not helpful to discriminate between progression and pseudoprogression.41
Regarding the delineation of glioma extent, the value of FLT PET remains controversial. Despite a reported strong relationship between FLT uptake and contrast enhancement on MRI (ie, tumor portions with intact BBB are not detected),42 several studies observed that FLT uptake volumes may be larger than gadolinium-enhanced volumes on MRI35,43 suggesting that an increased transport of FLT into glioma tissue occurs even before BBB disruption. This finding is also supported by the observation that only a small number of glioma cells may damage the integrity of the BBB.44 Despite efforts to improve FLT tracer accumulation in the brain,45 it appears that FLT PET has limited capacity to accurately define the gliomas extent although tracer uptake beyond contrast enhancement is possible under certain circumstances. Furthermore, several studies have reported a considerable value of FLT PET for the assessment of response to antiangiogenic therapy in patients with recurrent malignant glioma.46,47 In patients with brain metastases, a pilot study suggested that FLT PET is also of value for the evaluation response to checkpoint inhibitors and targeted therapy.48 Nevertheless, the number of studies is small and further studies are warranted.
The blood supply to meningioma is predominantly from the external carotid circulation and tracer permeability is not limited by the BBB making these tumors more available for imaging with a larger variety of PET tracers. FLT uptake in meningioma correlates with biomarkers of proliferation, such as the Ki-67 index, that is used to assess tumor grade and growth potential.49 Initial clinical evaluation has also suggested that FLT uptake could be a promising surrogate imaging biomarker for predicting subsequent tumor progression in treatment-naive and asymptomatic patients with residual meningioma.50
Cell Membrane Biosynthesis
Choline is an essential nutrient needed for the biosynthesis of phosphatidylcholine and an important component of the cell membrane.13 The choline derivatives [11C]choline (Cho), [18F]methylcholine, and [18F]fluoroethyl-choline exhibit similar properties and have been evaluated for brain tumor diagnostics.13 However, in newly diagnosed brain tumors, choline tracers play only a minor role as the tracer accumulation has only low specificity.51
More meaningful results were achieved for the differentiation of tumor relapse and treatment-related changes in patients with gliomas or brain metastases.13,51 Of note, the accuracy of this group of tracers appears to be lower than that of radiolabeled amino acids52 suggesting that these tracers are not the first choice for this indication.51
PET Imaging of Receptor Expression and Other Targets
Somatostatin Receptor Expression
Meningiomas of all WHO grades abundantly express somatostatin receptor subtype 2 (SSTR2).53 The somatostatin receptor analogs [68Ga]DOTA-Tyr3-octreotide (DOTATOC) and [68Ga]DOTA-D-Phe1-Tyr3-octreotate (DOTATATE) have a high affinity for SSTR2.54 Labeled with the positron-emitting nuclide Ga-68, DOTATATE has been increasingly used for PET imaging of meningioma patients with a high tumor-to-background ratio, particularly for differential diagnosis and target volume definition for treatment planning.55,56 It also proved helpful in the differentiation of meningiomas from other pathologies and to specifically delineate tumor remnants.57–59
SSTR overexpression in meningiomas has also been proposed as a therapeutic target for systemic treatment.3 DOTATOC and DOTATATE are agents with a chelator site (DOTA) and a binding site for somatostatin receptors (eg, octreotide, octreotate). It is feasible to label the chelator site with β +-emitting Ga-68 for PET diagnostic purposes, or with β −-emitting radioisotopes such as Y-90 and Lu-177 for therapeutic purposes.60,61 These therapeutic radionuclides deliver SSTR-targeted β −-radiation within a few millimeters distance of the binding site, in contrast to biochemical receptor interference as proposed for somatostatin analogs (eg, octreotide, pasireotide). Radionuclides with SSTR-targeted β −-radiation have shown efficacy in small series of patients with recurrent, heavily pretreated meningiomas.60,62
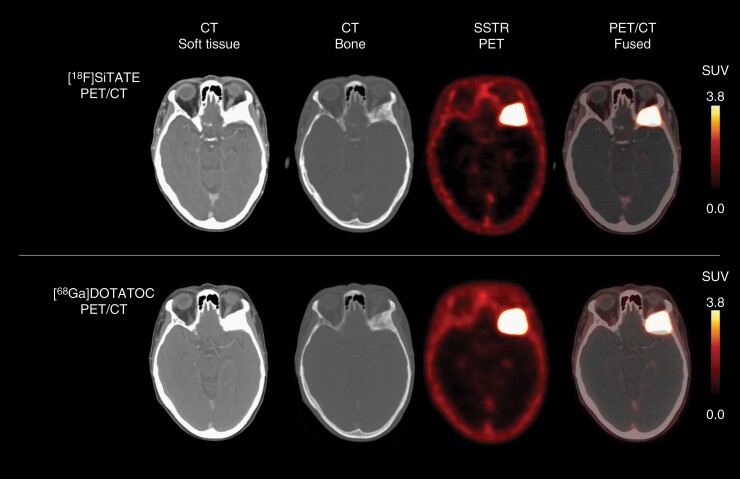
[18F]SiTATE (formerly known as [18F]SiFAlin-TATE) is a novel 18F-labeled SSTR targeting peptide and provides remarkable tumor-to-background ratio at a higher resolution. Of note, due to the labeling with F-18, a cost-intensive Ge-68/Ga-68 generator is no longer required for tracer synthesis. In addition, the lower spatial resolution, and the shorter half-life of 68Ga-labeled peptides (ie, 68 min) are further shortcomings compared with F-18 (Figure 2). Thus, [18F]SiTATE might be a promising PET tracer for meningioma imaging.63
Fig. 2.
18F-SiTATE (top row) and 68Ga-DOTATATE PET/CT (bottom row) of a patient with a newly diagnosed WHO grade I sphenoid wing meningioma. Visually, somatostatin receptor (SSTR) expression on 18F-SiTATE PET was highly comparable to 68Ga-DOTATATE PET. Note the lower spatial resolution of PET using the 68Ga-labeled peptide DOTATATE compared to 18F-SiTATE PET.
EGFR Expression
A number of PET tracers have been synthesized to noninvasively detect the mutational status of the tyrosine kinase epidermal growth factor receptor (EGFR) some of which are based on radiolabeling of existing tyrosine kinase inhibitors (TKI) such as [11C]erlotinib64 or [11C]osimertinib.65 This would allow a “precision medicine” approach identifying molecular heterogeneity between or within tumours in the same patient over space and time useful for the selection, therapy prediction, and response monitoring before and during TKI treatment. There is some preliminary clinical evidence to support this use in systemic NSCLC using [11C]erlotinib,6411C-labeled 4-N-(3-bromoanilino)-6,7-dimethoxyquinazoline ([11C]PD153035),66 and N-(3-chloro-4-fluorophenyl)-7-(2-(2-(2-(2-18F-fluoroethoxy) ethoxy) ethoxy) ethoxy)-6-methoxyquinazolin-4-amine ([18F]MPG).67 In patients with NSCLC brain metastases68 and glioblastoma,69 PET tracer accumulation of EGFR-targeted ligands has been shown. There are ongoing clinical studies addressing the pharmacokinetic properties of [11C]osimertinib in brain metastases (NCT03463525), and future clinical studies will determine whether this tracer may play a role in patient selection as a trial enrichment tool or in treatment prediction. Nevertheless, it should be considered the heterogeneous expression of EGFR poses a substantial challenge for the effective use of EGFR-targeted therapies.70
Chemokine Receptor Expression for Lymphoma Imaging
The PET tracer [68Ga]pentixafor binds to the C-X-C chemokine receptor type 4 (CXCR4) which is overexpressed in several human cancer types, including glioma, and may be involved in glioma initiation and renewal.71 However, a pilot study using [68Ga]pentixafor in glioblastoma showed highly variant inter- and intra-tumor accumulation that was high in only a subset of patients72 possibly owing to limited BBB penetration of this relatively high molecular weight tracer. For primary and secondary CNS lymphoma, the results with [68Ga]pentixafor are more encouraging with several studies showing high lesion uptake and diagnostic accuracy73,74 outperforming 2-deoxy-2-[18F]fluoro-D-glucose (FDG) owing to the superior tumor-to-background contrast due to the lack of uptake in healthy brain. Initial results suggested that lower [68Ga]pentixafor uptake at baseline is associated with a better response to treatment.74 Furthermore, [68Ga]pentixafor can potentially be used as a companion diagnostics for targeted radionuclide therapy using [177Lu]pentixather.75
Translocator Protein (TSPO)
The translocator protein (TSPO) is a mitochondrial membrane protein which is overexpressed in gliomas and represents an interesting target for glioma imaging. TSPO expression levels have been reported to correlate with malignancy and inversely with patient outcome, but the exact role and function of TSPO in glioma genesis, progression, and resistance to treatment is not yet fully clarified.76 There is evidence that TSPO is critically involved in mitochondrial energy metabolism and indirectly in angiogenesis and glioma growth.77 As TSPO is not only overexpressed by tumor cells but also by tumor-associated microglia and macrophages, TSPO-directed PET tracers are considered to image gliomas including their inflammatory microenvironment.78 Although the first TSPO PET scans in glioma patients have been performed more than 30 years ago using the first-generation TSPO ligand [11C]PK11195,79 the interest remained low over decades due to high levels of nonspecific tracer binding. With the advent of new generation high-affinity TSPO radioligands such as [18F]DPA-714 or [18F]GE-180, improved signal-to-noise ratios were reported and led to first TSPO PET studies with encouraging results for the preclinical and clinical glioma setting.80–82
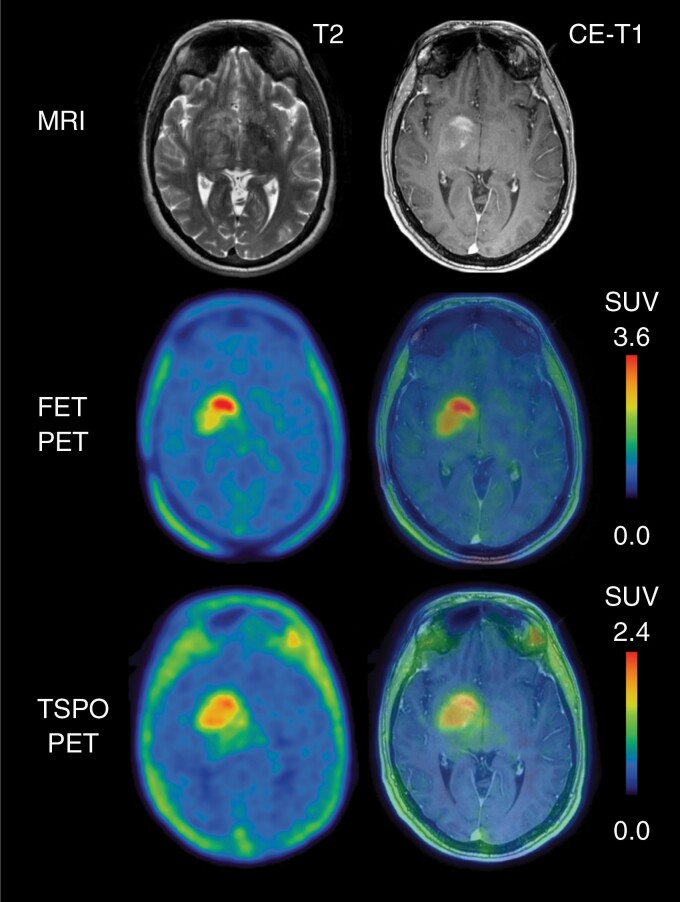
In glioblastoma patients, TSPO PET showed high tumor-to background contrast with intense tracer signal even in areas without contrast enhancement on MRI.82,83 Interestingly, when spatially compared to conventional amino acid PET using FET, TSPO-directed imaging showed only partial overlap,82,83 pointing to the complementary character of both imaging modalities (Figure 3).
Fig. 3.
Multimodal imaging including contrast-enhanced (CE) MRI, O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET, and translocator protein (TSPO) PET using the tracer [18F]GE-180 of patient with a newly diagnosed WHO CNS grade 4 glioblastoma (IDH-wildtype, TERT promoter mutant, MGMT promoter not methylated). In comparison to FET PET, the spatial distribution of [18F]GE-180 uptake is moderate, additionally showing [18F]GE-180 uptake in tumor regions which are without increased FET uptake.
The relative contribution of each cell type to the TSPO PET signal and the influence of glioblastoma subtypes on TSPO expression is still under investigation.84 While preclinical studies reported a high range of contributing TSPO-positive myeloid cells, a first pilot study in glioma patients suggested that TSPO PET may assess the individual profile of immunosuppressive myeloid cell infiltration in gliomas.82 More data is needed to elucidate the cellular source of tracer signal in glioma patients, which may depend on additional factors such as tumor subtype, previous treatments, or individual state of the immune system.
Prostate-specific Membrane Antigen (PSMA)
Due to its strong expression in prostate cancer, the prostate-specific membrane antigen (PSMA) has been the most intensively studied target in Nuclear Medicine during the past 5 years. However, PSMA is not only overexpressed in prostate cancer cells, but also in the neovasculature of nonprostatic, highly vascularized tumor entities such as breast cancer or gastrointestinal cancer.85,86 In gliomas, studies with histological validation investigating the level of PSMA expression observed a positive correlation with WHO tumor grade.87,88
Recently, first case reports and pilot studies have reported the general feasibility of PSMA PET imaging in gliomas, showing absent uptake in healthy brain tissue and variable tracer signal in tumor tissue, with highest uptake in WHO grade IV tumors.89,90 On the other hand, an experimental study reported increased uptake of 68Ga- and 18F-labeled PSMA ligands in the peritumoral area of different glioma models, indicating uptake of these ligands in activated astrocytes.91 This may represent a limitation for the clinical application of these tracers in glioma patients.
Furthermore, 177Lu-labeled PSMA is considered as a theranostic agent with considerable response and low toxicity in prostate cancer patients. In view of the short range of β − particles and the lack of evidence on PSMA penetration beyond the contrast enhancement, this approach seems to be more promising for brain metastases than for infiltrating gliomas. Of note, theranostic data on glioma patients are still comparatively low. So far, only two case reports provided data in terms of dosimetry92 and response assessment.93
Integrins
Integrins are a family of cell–cell and cell–extracellular matrix adhesion molecules contributing to migration, invasion, cell survival, proliferation, and angiogenesis.94 In particular, αvβ3 and αvβ5 integrins are overexpressed in glioblastoma cells, and vasculature hereby being involved in tumor–host interaction.95,96 Targeting integrins and the tumor microenvironment has been considered as therapeutic strategy in malignant gliomas.97 PET using the 18F-labeled glycosylated arginine-glycine- aspartic acid tripeptide (Galacto-RGD) has been shown to be suitable for noninvasive assessment of αvβ3 expression in both murine tumor models and in patients with extracranial cancer.98,99 In glioblastoma patients, a correlation between Galacto-RGD uptake and immunohistochemical αvβ3 integrin expression in corresponding tumor samples could be demonstrated.100 New RGD peptide analogs have been developed for PET imaging of αvβ3 integrins which might gain further importance once integrin-related treatment decisions or therapies will be relevant for glioma patients.101
PET Imaging of Hypoxia
Hypoxia of the tumor and tumor microenvironment has long been investigated in brain tumors as a potential mediator of treatment resistance, whose presence has been associated with worse tumor control and survival.102 Its noninvasive characterization with advanced PET tracers coupled with technologic advances offers a possible avenue to direct therapy to putative treatment-resistant niches in a biology-driven manner.
The most extensively investigated radiotracer for the assessment of hypoxia is [18F]fluoromisonidazole (FMISO), initially by Spence et al. and by others.103 In a recent study, FMISO PET and oxygen-saturation-mapping MRI were carried out in the lung cancer brain metastases models to further characterize tumor hypoxia and evaluate the potential of hypoxia image-guided radiotherapy.104 The effect of radiotherapy on tumor volume, survival, and proliferation evaluated by FLT PET was determined. Control of both nonhypoxic and hypoxic brain metastases and a significant decrease in tumor cell proliferation as measured by FLT PET could be achieved, whereas tumor control of hypoxic cortical brain metastases with standard radiation was suboptimal.104
In glioblastoma, hypoxic, low oxygen tension niches harbor glioma stem cells, and stimulate cellular plasticity towards a stem-like phenotype associated with treatment resistance.105 Moreover, the hypoxic microenvironment particularly in glioblastoma patients evaluated using FMISO PET seems to stimulate aberrant neo-angiogenesis and was associated with worse prognosis.106 Additional studies also demonstrate the association of FMISO PET uptake and earlier tumor progression.107
While several factors (eg, slow clearance of unbound tracer from normoxic tissue, low tumor-to-background ratio) have limited the clinical translation of FMISO, additional radiotracers such as [18F]flouroazomycin arabinoside (FAZA) may provide enhanced tumor-to-background ratio and offer an avenue for tailoring radiotherapy to hypoxic tumor regions in glioma patients.108 Novel hypoxia tracers such as 2-(2-Nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-[18F]pentafluoropropyl)-acetamide ([18F]EF5) and 1-(2-[18F]fluoro-1[hydroxymethyl]ethoxy)methyl-2-nitroimidazole ([18F]FRP170), evaluated in preclinical and limited clinical studies to date,108 should be incorporated in studies of hypoxia modulators or biologically tailored radiotherapy approaches with the potential to improve therapeutic outcomes in patients with brain tumors.
PET Imaging of the Immune System
There is increasing interest in understanding the effects of novel therapies on the immune response in brain tumors to identify promising agents for further development. Various strategies are being evaluated including in vivo imaging of T-cells by targeting indicators of metabolic reprogramming (eg, deoxycytidine kinase, deoxyguanosine kinase, thymidine kinase 1), cell-surface activation markers (eg, interleukin-2, CXCR4), cell surface lineage markers (eg, CD3, CD4, CD8, T-cell receptor), and immune checkpoints (eg, PD-L1, PD1).109–111
So-called ImmunoPET probes are being developed to image the immune response as they have the advantages of high specificity and affinity towards their target, generating high signal-to-noise ratios, and high-contrast images.111 However, the large size of intact antibodies limit tissue penetration and are slowly cleared. In addition, the Fc regions can bind to other cells such as macrophages. To overcome these issues, smaller minibodies, diabodies, and nanobodies which can penetrate tissue better and are cleared faster are being evaluated for radiolabeling with PET isotopes.109,111
In the following paragraphs, the most relevant approaches of PET imaging of immune system reactions for patients with brain tumors are summarized.
PET Imaging of T-Cell Metabolic Reprogramming
Since activated T-cells undergo metabolic reprogramming (ie, engaging distinct metabolic pathways such as glycolysis, oxidative phosphorylation, and fatty acid synthesis to meet their bioenergetic needs), adaptive metabolic pathways may serve as potential targets of PET tracers to distinguish active from nonactive T-cells.110 In particular, deoxycytidine kinase, the key enzyme in the cytosolic deoxyribonucleoside salvage pathway, can be targeted by 18F-labeled clofarabine. This probe has been used in glioblastoma patients vaccinated with tumor lysate-pulsed dendritic cells and anti-PD-1 therapy to show increased immune response in both the tumors and secondary lymphoid organs.112
Deoxyguanosine kinase is a rate-limiting enzyme in the deoxyribonucleoside salvage pathway, which is being targeted for PET imaging in cancer. A PET tracer targeting deoxyguanosine kinase, 2′-deoxy-2′-[18F]fluoro-9-β-D-arabinofuranosylguanine ([18F]F-AraG), showed preferential accumulation in activated T-cells,110,111 and its utility in monitoring T-cell responses is being evaluated in some glioblastoma trials.
PET Imaging of Cell–Surface Lineage Markers
There is particular interest in imaging specific T-cell populations by targeting cell–surface lineage markers such as CD3, CD4, and CD8. A recent report showed that a humanized CD8-targeted antibody fragment, IAB22M2C, radiolabeled with Zr-89 as a PET probe was able to successfully target CD8+ T-cells in tumors.113 This probe has the potential to image T-cell infiltration and is being evaluated in glioblastoma patients.
PET Imaging of Immune Checkpoints
The advent of immune checkpoint inhibitors for cancer treatment has prompted the development of PET tracers to image PD-1 or PD-L1 immune checkpoint expression.114 A first-in-human study115 suggests that Immuno-PET using nivolumab labeled with Zr-89 may be valuable for response assessment. In that study, patients exhibited increased uptake in nonsmall cell lung cancers and in brain metastases.115 A subsequent study confirmed these initial results using [89Zr]pembrolizumab.116
In contrast to PET with a Zr-89-linked antibody tracer, Nienhuis et al. used an adnectin-based PD-L1 ligand labeled with F-18 (BMS986192).117 An adnectin is an engineered protein, designed to bind with high affinity and specificity to therapeutically relevant targets such as PD-L1. In comparison to a Zr-89-linked antibody tracer, adnectin-based PET labeled with F-18 exposes the patients to a much lower radiation dose, which allows the acquisition of multiple follow-up PET scans in the same patient within a short time frame. A pilot study suggested that baseline BMS986192 uptake was able to predict an immune checkpoint inhibitor-induced reduction in tumor volume in patients with melanoma brain metastases.117
PET Imaging of Adoptively Transferred T-Cell Therapy
There is increasing interest in adoptively transferred T-cell therapies (including CAR-T therapy). Development of these therapies for brain tumors would be significantly enhanced by an improved understanding of the distribution and fate of these cells. This can be achieved either by in vivo imaging of directly labeled T-cell therapeutics or by using reporter genes. An example of the latter is a pilot study using the herpes simplex virus type 1 thymidine kinase (HSV1-tk) system to track engineered immune cells in patients with gliomas. CD8+ cytotoxic T lymphocytes were engineered to express both HSV1-tk and interleukin-13 zetakine chimeric antigen receptor, and then adoptively transferred into recurrent high-grade glioma patients. PET imaging with 9-[4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine (FHBG) was used to successfully track HSV1-tk reporter gene expression present in CAR-T-cells118 (Figure 4). This type of approach, if optimized, provides the potential for monitoring in vivo cell trafficking, and help optimize cell-based therapies, including CAR-T-cell therapies, for brain tumors.
Fig. 4.
Serial contrast-enhanced (CE) MRI and 9-[4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine (FHBG) PET imaging of a glioblastoma patient at relapse. The patient was treated with engineered cytotoxic T lymphocytes (CTL), which express both HSV1-tymidine kinase and interleukin-13 zetakine chimeric antigen receptor. These CTLs specifically targeted tumor cells in an IL-13 zetakine-dependent manner and allowed molecular PET imaging because these T-cells expressed HSV1-tymidine kinase which mediated increased FHBG uptake one week after intratumoral CTL injection compared to baseline. In addition, the volume of contrast enhancement decreased after injection. Modified, with permission, from Keu and colleagues.118.
Special Considerations for FDG PET
FDG is the most utilized PET agent in oncology. This widely available and commonly used radiotracer has a couple critical limitations to its application in neuro-oncology. First, since glucose is the main energy source of the brain, there is marked background uptake hindering delineation of the lesion of interest. Efforts to address low lesion-to-background ratios such as delayed timepoint have shown to be useful but may be less appealing in routine clinical practice due to the added patient wait time. Second, the lack of specificity for distinguishing brain tumors from nonneoplastic lesions remains a major limitation to the widespread adoption of FDG PET in neuro-oncology.1
Recent work suggests a niche application of FDG PET in settings where glucose metabolism of brain tumors is the specific target of therapy. For example, Mai et al. leveraged inhibition of EGFR-driven glucose metabolism with the erlotinib, to prime glioblastoma for apoptosis via p53 signaling.119 Following erlotinib administration, the MDM2 inhibitor Idasanutlin, a p53 stabilizer, was used to promote synthetic lethality in preclinical models. Interestingly, FDG PET showed differences in glioblastoma uptake shortly after erlotinib administration between metabolic responders and nonresponders to Idasanutlin. Another special consideration for FDG PET is the evaluation of drugs targeting the glycolytic Pi3K/mTOR pathway (eg, GDC-0084) in terms of brain pharmacokinetics and -dynamics.120 In that study, FDG PET and concentration data from brain tumor tissue suggested that these agents are able to penetrate the BBB. The ability to use FDG PET to noninvasively predict sensitivity to combination therapy is promising for future clinical trials with agents targeting these glycolytic pathways due to the benefit of availability and technical expertise using this radiotracer.
Conclusions and Limitations
Advanced PET imaging for brain tumors is a rapidly emerging field. Yet, the implementation of PET imaging needs to address major challenges including half-life and availability of tracers and general access for brain tumor patients to these modalities. Many of these challenges are currently still driven by cost and reimbursement issues.
On a scientific level, it is important that the value of new PET imaging approaches is accompanied by appropriate preclinical research, correlative studies with MR imaging, and ideally pathological confirmation of target expression. This all needs to be done in a tumor-specific manner, that hopefully should allow to implement PET imaging into future clinical trials for validation of benefits for patients affected by brain tumors. Moreover, it would be beneficial if groups working with new tracers could try to harmonize the technical procedure of tracer application, imaging, and read-outs at an early stage of clinical testing. This has already been done for amino acid tracers and would help to obtain meaningful results for new tracer systems quicker and would also ease to establish validated tracer in the field of neuro-oncology.
Outlook
Currently, PET imaging is being integrated into clinical neuro-oncology for diagnosis, depicting tumor volume, and for follow-up. In addition, new tracers might identify different biological components within the tumor reflecting either cellular diversity or even molecular signatures. This could serve as a potent tool to study tumor–host-interaction and the microenvironment (eg, relation of tumor and tumor-associated macrophages) and help to identify potentially druggable targets (eg, integrins in glioma, other targets in brain metastases). Radiolabeled drugs can be imaged within their target tissue helping to perform “in situ pharmacokinetics”, even in a more personalized way.
With the concept of theranostics, the same ligand can be used either for imaging or for therapeutic short-range irradiation. This radiopeptide therapy has been successfully performed in meningiomas (using SSTR2 ligands) and is currently considered for gliomas expressing PSMA. With more ligands being explored and validated in brain tumors, this could pave the way to an additional treatment concept in the future. Thus, the spectrum for personalized precision neuro-oncology could be enlarged.
Consortia with shared expertise and mutually stipulated imaging protocols are warranted to validate these concepts and to introduce into the field what has proven to be useful.
Contributor Information
Norbert Galldiks, Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Kerpener St. 62, 50937 Cologne, Germany; Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany; Center of Integrated Oncology (CIO), Universities of Aachen, Bonn, Cologne, and Düsseldorf, Germany.
Karl-Josef Langen, Institute of Neuroscience and Medicine (INM-3, -4), Research Center Juelich, Juelich, Germany; Center of Integrated Oncology (CIO), Universities of Aachen, Bonn, Cologne, and Düsseldorf, Germany; Department of Nuclear Medicine, University Hospital RWTH Aachen, Aachen, Germany.
Nathalie L Albert, Department of Nuclear Medicine, Ludwig Maximilians-University of Munich, Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Ian Law, Department of Clinical Physiology, Nuclear Medicine and PET, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Michelle M Kim, Department of Radiation Oncology, University of Michigan, Ann Arbor, Michigan, USA.
Javier E Villanueva-Meyer, Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.
Riccardo Soffietti, Department of Neuro-Oncology, University and City of Health and Science Hospital, Turin, Italy.
Patrick Y Wen, Center for Neuro-Oncology, Dana-Farber/Brigham and Women’s Cancer Center, Boston, Massachusetts, USA.
Michael Weller, Department of Neurology, Clinical Neuroscience Center University Hospital and University of Zurich, Zurich, Switzerland.
Joerg C Tonn, Department of Neurosurgery, University Hospital of Munich (LMU), Munich, Germany; German Cancer Consortium (DKTK), Partner Site Munich, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Funding
None declared.
Conflict of interest statement. Related to the present work, the authors disclosed no potential conflicts of interest.
References
- 1. Albert NL, Weller M, Suchorska B, et al. Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016; 18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galldiks N, Langen KJ, Albert NL, et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 2019; 21(5):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galldiks N, Albert NL, Sommerauer M, et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017; 19(12):1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galldiks N, Niyazi M, Grosu AL, et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients - a report of the PET/RANO group. Neuro Oncol. 2021; 23(6):881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019; 46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galldiks N, Lohmann P, Albert NL, Tonn JC, Langen KJ. Current status of PET imaging in neuro-oncology. Neurooncol Adv. 2019; 1(1):vdz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galldiks N, Abdulla DSY, Scheffler M, et al. Treatment monitoring of immunotherapy and targeted therapy using (18)F-FET PET in patients with melanoma and lung cancer brain metastases: initial experiences. J Nucl Med. 2021; 62(4):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017; 13(5):279–289. [DOI] [PubMed] [Google Scholar]
- 9. Bashir A, Mathilde Jacobsen S, Molby Henriksen O, et al. Recurrent glioblastoma versus late posttreatment changes: diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. 2019; 21(12):1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Werner JM, Weller J, Ceccon G, et al. Diagnosis of pseudoprogression following lomustine-temozolomide chemoradiation in newly diagnosed glioblastoma patients using FET-PET. Clin Cancer Res. 2021; 27(13):3704–3713. [DOI] [PubMed] [Google Scholar]
- 11. Ceccon G, Lohmann P, Stoffels G, et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2017; 19(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, McConathy J. Radiolabeled amino acids for oncologic imaging. J Nucl Med. 2013; 54(7):1007–1010. [DOI] [PubMed] [Google Scholar]
- 13. Choudhary G, Langen KJ, Galldiks N, McConathy J. Investigational PET tracers for high-grade gliomas. Q J Nucl Med Mol Imaging. 2018; 62(3):281–294. [DOI] [PubMed] [Google Scholar]
- 14. Shoup TM, Olson J, Hoffman JM, et al. Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med. 1999; 40(2):331–338. [PubMed] [Google Scholar]
- 15. Laudicella R, Albano D, Alongi P, et al. (18)F-Facbc in prostate cancer: a systematic review and meta-analysis. Cancers. 2019; 11(9):1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bogsrud TV, Londalen A, Brandal P, et al. 18F-Fluciclovine PET/CT in suspected residual or recurrent high-grade glioma. Clin Nucl Med. 2019; 44(8):605–611. [DOI] [PubMed] [Google Scholar]
- 17. Ono M, Oka S, Okudaira H, et al. Comparative evaluation of transport mechanisms of trans-1-amino-3-[(1)(8)F]fluorocyclobutanecarboxylic acid and L-[methyl-(1)(1)C]methionine in human glioma cell lines. Brain Res. 2013; 1535:24–37. [DOI] [PubMed] [Google Scholar]
- 18. Albano D, Tomasini D, Bonu M, Giubbini R, Bertagna F. (18)F-Fluciclovine ((18)F-FACBC) PET/CT or PET/MRI in gliomas/glioblastomas. Ann Nucl Med. 2020; 34(2):81–86. [DOI] [PubMed] [Google Scholar]
- 19. Michaud L, Beattie BJ, Akhurst T, et al. (18)F-Fluciclovine ((18)F-FACBC) PET imaging of recurrent brain tumors. Eur J Nucl Med Mol Imaging. 2020; 47(6):1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsuyuguchi N, Terakawa Y, Uda T, Nakajo K, Kanemura Y. Diagnosis of brain tumors using amino acid transport PET imaging with (18)F-fluciclovine: a comparative study with L-methyl-(11)C-methionine PET imaging. Asia Ocean J Nucl Med Biol. 2017; 5(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wakabayashi T, Iuchi T, Tsuyuguchi N, et al. Diagnostic performance and safety of positron emission tomography using (18)F-fluciclovine in patients with clinically suspected high- or low-grade gliomas: a multicenter phase IIb trial. Asia Ocean J Nucl Med Biol. 2017; 5(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juhasz C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging. 2014; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL. A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. J Cereb Blood Flow Metab. 1990; 10(1):1–12. [DOI] [PubMed] [Google Scholar]
- 24. Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003; 9(10):1269–1274. [DOI] [PubMed] [Google Scholar]
- 25. Kamson DO, Juhasz C, Buth A, et al. Tryptophan PET in pretreatment delineation of newly-diagnosed gliomas: MRI and histopathologic correlates. J Neurooncol. 2013; 112(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alkonyi B, Barger GR, Mittal S, et al. Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of alpha-11C-methyl-L-tryptophan PET. J Nucl Med. 2012; 53(7):1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamson DO, Mittal S, Buth A, et al. Differentiation of glioblastomas from metastatic brain tumors by tryptophan uptake and kinetic analysis: a positron emission tomographic study with magnetic resonance imaging comparison. Mol Imaging. 2013; 12(5):327–337. [PMC free article] [PubMed] [Google Scholar]
- 28. Kamson DO, Mittal S, Robinette NL, et al. Increased tryptophan uptake on PET has strong independent prognostic value in patients with a previously treated high-grade glioma. Neuro Oncol. 2014; 16(10):1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. John F, Bosnyak E, Robinette NL, et al. Multimodal imaging-defined subregions in newly diagnosed glioblastoma: impact on overall survival. Neuro Oncol. 2019; 21(2):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. John F, Muzik O, Mittal S, Juhasz C. Fluorine-18-labeled PET radiotracers for imaging tryptophan uptake and metabolism: a systematic review. Mol Imaging Biol. 2020; 22(4):805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lieberman BP, Ploessl K, Wang L, et al. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J Nucl Med. 2011; 52(12):1947–1955. [DOI] [PubMed] [Google Scholar]
- 32. Venneti S, Dunphy MP, Zhang H, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015; 7(274):274ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dunphy MPS, Harding JJ, Venneti S, et al. In vivo PET assay of tumor glutamine flux and metabolism: in-human trial of (18)F-(2S,4R)-4-fluoroglutamine. Radiology. 2018; 287(2):667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nikaki A, Angelidis G, Efthimiadou R, et al. (18)F-fluorothymidine PET imaging in gliomas: an update. Ann Nucl Med. 2017; 31(7):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005; 46(12):1948–1958. [PubMed] [Google Scholar]
- 36. Shinomiya A, Kawai N, Okada M, et al. Evaluation of 3’-deoxy-3’-[18F]-fluorothymidine (18F-FLT) kinetics correlated with thymidine kinase-1 expression and cell proliferation in newly diagnosed gliomas. Eur J Nucl Med Mol Imaging. 2013; 40(2):175–185. [DOI] [PubMed] [Google Scholar]
- 37. Choi SJ, Kim JS, Kim JH, et al. [18F]3’-deoxy-3’-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging. 2005; 32(6):653–659. [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto Y, Ono Y, Aga F, et al. Correlation of 18F-FLT uptake with tumor grade and Ki-67 immunohistochemistry in patients with newly diagnosed and recurrent gliomas. J Nucl Med. 2012; 53(12):1911–1915. [DOI] [PubMed] [Google Scholar]
- 39. Idema AJ, Hoffmann AL, Boogaarts HD, et al. 3’-Deoxy-3’-18F-fluorothymidine PET-derived proliferative volume predicts overall survival in high-grade glioma patients. J Nucl Med. 2012; 53(12):1904–1910. [DOI] [PubMed] [Google Scholar]
- 40. Treglia G, Muoio B, Trevisi G, et al. Diagnostic performance and prognostic value of PET/CT with different tracers for brain tumors: a systematic review of published meta-analyses. Int J Mol Sci. 2019; 20(19):4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brahm CG, den Hollander MW, Enting RH, et al. Serial FLT PET imaging to discriminate between true progression and pseudoprogression in patients with newly diagnosed glioblastoma: a long-term follow-up study. Eur J Nucl Med Mol Imaging. 2018; 45(13):2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nowosielski M, DiFranco MD, Putzer D, et al. An intra-individual comparison of MRI, [18F]-FET and [18F]-FLT PET in patients with high-grade gliomas. PLoS One. 2014; 9(4):e95830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Collet S, Guillamo JS, Berro DH, et al. Simultaneous mapping of vasculature, hypoxia and proliferation using DSC-MRI, (18)F-FMISO PET, and (18)F-FLT PET in relation to contrast enhancement in newly diagnosed glioblastoma. J Nucl Med. 2021; 62(10):1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Watkins S, Robel S, Kimbrough IF, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014; 5:4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tamura Y, Takahashi K, Takata K, et al. Noninvasive evaluation of cellular proliferative activity in brain neurogenic regions in rats under depression and treatment by enhanced [18F]FLT-PET imaging. J Neurosci. 2016; 36(31):8123–8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007; 25(30):4714–4721. [DOI] [PubMed] [Google Scholar]
- 47. Schwarzenberg J, Czernin J, Cloughesy TF, et al. 3’-deoxy-3’-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med. 2012; 53(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nguyen NC, Yee MK, Tuchayi AM, et al. Targeted therapy and immunotherapy response assessment with F-18 fluorothymidine positron-emission tomography/magnetic resonance imaging in melanoma brain metastasis: a pilot study. Front Oncol. 2018; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bashir A, Binderup T, Vestergaard MB, et al. In vivo imaging of cell proliferation in meningioma using 3’-deoxy-3’-[(18)F]fluorothymidine PET/MRI. Eur J Nucl Med Mol Imaging. 2020; 47(6):1496–1509. [DOI] [PubMed] [Google Scholar]
- 50. Bashir A, Vestergaard MB, Marner L, et al. PET imaging of meningioma with 18F-FLT: a predictor of tumour progression. Brain. 2020; 143(11):3308–3317. [DOI] [PubMed] [Google Scholar]
- 51. Calabria FF, Barbarisi M, Gangemi V, Grillea G, Cascini GL. Molecular imaging of brain tumors with radiolabeled choline PET. Neurosurg Rev. 2018; 41(1):67–76. [DOI] [PubMed] [Google Scholar]
- 52. Verburg N, Koopman T, Yaqub M, et al. Direct comparison of [(11)C] choline and [(18)F] FET PET to detect glioma infiltration: a diagnostic accuracy study in eight patients. EJNMMI Res. 2019; 9(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dutour A, Kumar U, Panetta R, et al. Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer. 1998; 76(5):620–627. [DOI] [PubMed] [Google Scholar]
- 54. Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000; 27(3):273–282. [DOI] [PubMed] [Google Scholar]
- 55. Afshar-Oromieh A, Wolf MB, Kratochwil C, et al. Comparison of 68Ga-DOTATOC-PET/CT and PET/MRI hybrid systems in patients with cranial meningioma: initial results. Neuro Oncol. 2015; 17(2):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hadi I, Biczok A, Terpolilli N, et al. Multimodal therapy of cavernous sinus meningioma: impact of surgery and (68)Ga-DOTATATE PET-guided radiation therapy on tumor control and functional outcome. Neurooncol Adv. 2021; 3(1):vdab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rachinger W, Stoecklein VM, Terpolilli NA, et al. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015; 56(3):347–353. [DOI] [PubMed] [Google Scholar]
- 58. Ueberschaer M, Vettermann FJ, Forbrig R, et al. Simpson grade revisited - intraoperative estimation of the extent of resection in meningiomas versus postoperative somatostatin receptor positron emission tomography/computed tomography and magnetic resonance imaging. Neurosurgery. 2020; 88(1):140–146. [DOI] [PubMed] [Google Scholar]
- 59. Vay SU, Werner JM, Kabbasch C, et al. Uncovering an optic nerve sheath meningioma using 68Ga-DOTATATE PET/CT. Clin Nucl Med. 2021; 46(9):e464–e465. [DOI] [PubMed] [Google Scholar]
- 60. Seystahl K, Stoecklein V, Schüller U, et al. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016; 18(11):1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gerster-Gillieron K, Forrer F, Maecke H, et al. 90Y-DOTATOC as a therapeutic option for complex recurrent or progressive meningiomas. J Nucl Med. 2015; 56(11):1748–1751. [DOI] [PubMed] [Google Scholar]
- 62. Mirian C, Duun-Henriksen AK, Maier A, et al. Somatostatin receptor-targeted radiopeptide therapy in treatment-refractory meningioma: individual patient data meta-analysis. J Nucl Med. 2021; 62(4):507–513. [DOI] [PubMed] [Google Scholar]
- 63. Unterrainer M, Lindner S, Beyer L, et al. PET imaging of meningioma using the novel SSTR-targeting peptide 18F-SiTATE. Clin Nucl Med. 2021; 46(8):667–668. [DOI] [PubMed] [Google Scholar]
- 64. Memon AA, Weber B, Winterdahl M, et al. PET imaging of patients with non-small cell lung cancer employing an EGF receptor targeting drug as tracer. Br J Cancer. 2011; 105(12):1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Varrone A, Varnas K, Jucaite A, et al. A PET study in healthy subjects of brain exposure of (11)C-labelled osimertinib - a drug intended for treatment of brain metastases in non-small cell lung cancer. J Cereb Blood Flow Metab. 2020; 40(4):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meng X, LooBW, Jr., Ma L, et al. Molecular imaging with 11C-PD153035 PET/CT predicts survival in non-small cell lung cancer treated with EGFR-TKI: a pilot study. J Nucl Med. 2011; 52(10):1573–1579. [DOI] [PubMed] [Google Scholar]
- 67. Sun X, Xiao Z, Chen G, et al. A PET imaging approach for determining EGFR mutation status for improved lung cancer patient management. Sci Transl Med. 2018; 10(431):eaan8840. [DOI] [PubMed] [Google Scholar]
- 68. Weber B, Winterdahl M, Memon A, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol. 2011; 6(7):1287–1289. [DOI] [PubMed] [Google Scholar]
- 69. Sun J, Cai L, Zhang K, et al. A pilot study on EGFR-targeted molecular imaging of PET/CT With 11C-PD153035 in human gliomas. Clin Nucl Med. 2014; 39(1):e20–e26. [DOI] [PubMed] [Google Scholar]
- 70. Furnari FB, Cloughesy TF, Cavenee WK, Mischel PS. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat Rev Cancer. 2015; 15(5):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ehtesham M, Min E, Issar NM, et al. The role of the CXCR4 cell surface chemokine receptor in glioma biology. J Neurooncol. 2013; 113(2):153–162. [DOI] [PubMed] [Google Scholar]
- 72. Jacobs SM, Wesseling P, de Keizer B, et al. CXCR4 expression in glioblastoma tissue and the potential for PET imaging and treatment with [(68)Ga]Ga-pentixafor/[(177)Lu]Lu-pentixather. Eur J Nucl Med Mol Imaging. 2022; 49(2):481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Starzer AM, Berghoff AS, Traub-Weidinger T, et al. Assessment of central nervous system lymphoma based on CXCR4 expression in vivo using 68Ga-pentixafor PET/MRI. Clin Nucl Med. 2021; 46(1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Herhaus P, Lipkova J, Lammer F, et al. CXCR4-targeted PET imaging of central nervous system B-cell lymphoma. J Nucl Med. 2020; 61(12):1765–1771. [DOI] [PubMed] [Google Scholar]
- 75. Herrmann K, Schottelius M, Lapa C, et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu- and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J Nucl Med. 2016; 57(2):248–251. [DOI] [PubMed] [Google Scholar]
- 76. Ammer LM, Vollmann-Zwerenz A, Ruf V, et al. The role of translocator protein TSPO in hallmarks of glioblastoma. Cancers (Basel). 2020; 12(10):2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fu Y, Wang D, Wang H, et al. TSPO deficiency induces mitochondrial dysfunction, leading to hypoxia, angiogenesis, and a growth-promoting metabolic shift toward glycolysis in glioblastoma. Neuro Oncol. 2020; 22(2):240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zinnhardt B, Roncaroli F, Foray C, et al. Imaging of the glioma microenvironment by TSPO PET. Eur J Nucl Med Mol Imaging. 2021; 49(1):174–185. [DOI] [PubMed] [Google Scholar]
- 79. Junck L, Olson JM, Ciliax BJ, et al. PET imaging of human gliomas with ligands for the peripheral benzodiazepine binding site. Ann Neurol. 1989; 26(6):752–758. [DOI] [PubMed] [Google Scholar]
- 80. Winkeler A, Boisgard R, Awde AR, et al. The translocator protein ligand [18F]DPA-714 images glioma and activated microglia in vivo. Eur J Nucl Med Mol Imaging. 2012; 39(5):811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Albert NL, Unterrainer M, Fleischmann DF, et al. TSPO PET for glioma imaging using the novel ligand (18)F-GE-180: first results in patients with glioblastoma. Eur J Nucl Med Mol Imaging. 2017; 44(13):2230–2238. [DOI] [PubMed] [Google Scholar]
- 82. Zinnhardt B, Muther M, Roll W, et al. TSPO imaging-guided characterization of the immunosuppressive myeloid tumor microenvironment in patients with malignant glioma. Neuro Oncol. 2020; 22(7):1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Unterrainer M, Fleischmann DF, Diekmann C, et al. Comparison of (18)F-GE-180 and dynamic (18)F-FET PET in high grade glioma: a double-tracer pilot study. Eur J Nucl Med Mol Imaging. 2019; 46(3):580–590. [DOI] [PubMed] [Google Scholar]
- 84. Cai L, Kirchleitner SV, Zhao D, et al. Glioblastoma exhibits inter-individual heterogeneity of TSPO and LAT1 expression in neoplastic and parenchymal cells. Int J Mol Sci. 2020; 21(2):612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nomura N, Pastorino S, Jiang P, et al. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014; 14(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Haffner MC, Kronberger IE, Ross JS, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009; 40(12):1754–1761. [DOI] [PubMed] [Google Scholar]
- 87. Traub-Weidinger T, Poetsch N, Woehrer A, et al. PSMA expression in 122 treatment naive glioma patients related to tumor metabolism in (11)C-methionine PET and survival. J Pers Med. 2021; 11(7):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Holzgreve A, Biczok A, Ruf VC, et al. PSMA expression in glioblastoma as a basis for theranostic approaches: a retrospective, correlational panel study including immunohistochemistry, clinical parameters and PET imaging. Front Oncol. 2021; 11:646387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kunikowska J, Bartosz K, Leszek K. Glioblastoma multiforme: another potential application for (68)Ga-PSMA PET/CT as a guide for targeted therapy. Eur J Nucl Med Mol Imaging. 2018; 45(5):886–887. [DOI] [PubMed] [Google Scholar]
- 90. Unterrainer M, Niyazi M, Ruf V, Bartenstein P, Albert NL. The endothelial prostate-specific membrane antigen is highly expressed in gliosarcoma and visualized by [68Ga]-PSMA-11 PET: a theranostic outlook for brain tumor patients? Neuro Oncol. 2017; 19(12):1698–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oliveira D, Stegmayr C, Heinzel A, et al. High uptake of (68)Ga-PSMA and (18)F-DCFPyL in the peritumoral area of rat gliomas due to activated astrocytes. EJNMMI Res. 2020; 10(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kunikowska J, Charzynska I, Kulinski R, et al. Tumor uptake in glioblastoma multiforme after IV injection of [(177)Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2020; 47(6):1605–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kumar A, Ballal S, Yadav MP, et al. 177Lu-/68Ga-PSMA theranostics in recurrent glioblastoma multiforme: proof of concept. Clin Nucl Med. 2020; 45(12):e512–e513. [DOI] [PubMed] [Google Scholar]
- 94. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010; 10(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Burgett ME, Lathia JD, Roth P, et al. Direct contact with perivascular tumor cells enhances integrin alphavbeta3 signaling and migration of endothelial cells. Oncotarget. 2016; 7(28):43852–43867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schnell O, Krebs B, Wagner E, et al. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008; 18(3):378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 98. Beer AJ, Grosu AL, Carlsen J, et al. [18F]galacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007; 13(22 Pt 1):6610–6616. [DOI] [PubMed] [Google Scholar]
- 99. Beer AJ, Haubner R, Wolf I, et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging alpha v beta3 expression. J Nucl Med. 2006; 47(5):763–769. [PubMed] [Google Scholar]
- 100. Schnell O, Krebs B, Carlsen J, et al. Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 2009; 11(6):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liolios C, Sachpekidis C, Kolocouris A, Dimitrakopoulou-Strauss A, Bouziotis P. PET diagnostic molecules utilizing multimeric cyclic RGD peptide analogs for imaging integrin alphavbeta3 receptors. Molecules. 2021; 26(6):1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Berghoff AS, Ilhan-Mutlu A, Dinhof C, et al. Differential role of angiogenesis and tumour cell proliferation in brain metastases according to primary tumour type: analysis of 639 cases. Neuropathol Appl Neurobiol. 2015; 41(2):e41–e55. [DOI] [PubMed] [Google Scholar]
- 103. Spence AM, Muzi M, Swanson KR, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008; 14(9):2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Corroyer-Dulmont A, Valable S, Fantin J, et al. Multimodal evaluation of hypoxia in brain metastases of lung cancer and interest of hypoxia image-guided radiotherapy. Sci Rep. 2021; 11(1):11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Boyd NH, Tran AN, Bernstock JD, et al. Glioma stem cells and their roles within the hypoxic tumor microenvironment. Theranostics. 2021; 11(2):665–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bekaert L, Valable S, Lechapt-Zalcman E, et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging. 2017; 44(8):1383–1392. [DOI] [PubMed] [Google Scholar]
- 107. Hirata K, Yamaguchi S, Shiga T, Kuge Y, Tamaki N. The roles of hypoxia imaging using (18)F-fluoromisonidazole positron emission tomography in glioma treatment. J Clin Med. 2019; 8(8):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stieb S, Eleftheriou A, Warnock G, Guckenberger M, Riesterer O. Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy. Eur J Nucl Med Mol Imaging. 2018; 45(12):2201–2217. [DOI] [PubMed] [Google Scholar]
- 109. Wei W, Jiang D, Ehlerding EB, Luo Q, Cai W. Noninvasive PET imaging of T cells. Trends Cancer. 2018; 4(5):359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xiao Z, Pure E. Imaging of T-cell responses in the context of cancer immunotherapy. Cancer Immunol Res. 2021; 9(5):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li C, Han C, Duan S, et al. Visualizing T cell responses: the T cell PET imaging toolbox. J Nucl Med. 2022; 63(2):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Antonios JP, Soto H, Everson RG, et al. Detection of immune responses after immunotherapy in glioblastoma using PET and MRI. Proc Natl Acad Sci USA. 2017; 114(38):10220–10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pandit-Taskar N, Postow MA, Hellmann MD, et al. First-in-humans imaging with (89)Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med. 2020; 61(4):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kasten BB, Udayakumar N, Leavenworth JW, et al. Current and future imaging methods for evaluating response to immunotherapy in neuro-oncology. Theranostics. 2019; 9(17):5085–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Niemeijer AN, Leung D, Huisman MC, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018; 9(1):4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Niemeijer AN, Oprea Lager DE, Huisman MC, et al. First-in-human study of (89)Zr-pembrolizumab PET/CT in patients with advanced stage non-small-cell lung cancer. J Nucl Med. 2022; 63(3):362–367. [DOI] [PubMed] [Google Scholar]
- 117. Nienhuis PH, Antunes IF, Glaudemans A, et al. (18)F-BMS986192 PET imaging of PD-L1 in metastatic melanoma patients with brain metastases treated with immune checkpoint inhibitors. A pilot study. J Nucl Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Keu KV, Witney TH, Yaghoubi S, et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med. 2017; 9(373):eaag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mai WX, Gosa L, Daniels VW, et al. Cytoplasmic p53 couples oncogene-driven glucose metabolism to apoptosis and is a therapeutic target in glioblastoma. Nat Med. 2017; 23(11):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wen PY, Cloughesy TF, Olivero AG, et al. First-in-human phase I study to evaluate the brain-penetrant PI3K/mTOR inhibitor GDC-0084 in patients with progressive or recurrent high-grade glioma. Clin Cancer Res. 2020; 26(8):1820–1828. [DOI] [PubMed] [Google Scholar]