Abstract
Background
Large real-world-evidence studies are required to confirm the durability of response, effectiveness, and safety of ustekinumab in Crohn’s disease (CD) patients in real-world clinical practice.
Methods
A retrospective, multicentre study was conducted in Spain in patients with active CD who had received ≥1 intravenous dose of ustekinumab for ≥6 months. Primary outcome was ustekinumab retention rate; secondary outcomes were to identify predictive factors for drug retention, short-term remission (week 16), loss of response and predictive factors for short-term efficacy and loss of response, and ustekinumab safety.
Results
A total of 463 patients were included. Mean baseline Harvey-Bradshaw Index was 8.4. A total of 447 (96.5%) patients had received prior biologic therapy, 141 (30.5%) of whom had received ≥3 agents. In addition, 35.2% received concomitant immunosuppressants, and 47.1% had ≥1 abdominal surgery. At week 16, 56% had remission, 70% had response, and 26.1% required dose escalation or intensification; of these, 24.8% did not subsequently reduce dose. After a median follow-up of 15 months, 356 (77%) patients continued treatment. The incidence rate of ustekinumab discontinuation was 18% per patient-year of follow-up. Previous intestinal surgery and concomitant steroid treatment were associated with higher risk of ustekinumab discontinuation, while a maintenance schedule every 12 weeks had a lower risk; neither concomitant immunosuppressants nor the number of previous biologics were associated with ustekinumab discontinuation risk. Fifty adverse events were reported in 39 (8.4%) patients; 4 of them were severe (2 infections, 1 malignancy, and 1 fever).
Conclusions
Ustekinumab is effective and safe as short- and long-term treatment in a refractory cohort of CD patients in real-world clinical practice.
Keywords: Crohn’s disease, effectiveness, real-world evidence, safety, ustekinumab
Introduction
Crohn’s disease (CD) is a chronic immune-mediated inflammatory disease affecting the gastrointestinal tract.1-3 It is a relapsing disease characterized by recurrent, destructive pathological inflammation1,3,4 that causes significant morbidity and impact on quality of life.5-7 While it is currently an incurable disease,8 there are a number of treatments that target clinical symptoms, including biologic therapies, such as tumor necrosis factor (TNF) inhibitors, integrin inhibitors, and interleukin (IL)-12/23 inhibitors.9 However, approximately 30% of patients receiving biologics do not respond to induction, 30% to 50% eventually have a loss of response (LoR), and some develop adverse events (AEs).9-12
Ustekinumab is a fully human IgG1κ monoclonal antibody that binds with specificity to the shared p40 protein subunit of human cytokines IL-12 and IL-23.13 Ustekinumab is used to treat adult patients with moderately to severely active CD who have had an inadequate response, lost response, or are intolerant either to conventional therapy or to a TNF-α inhibitor, or who have medical contraindications to such therapies.13 The efficacy and safety of induction therapy with ustekinumab in patients with moderately to severely active CD was demonstrated in the IM-UNITI trials, in which ustekinumab was shown to be effective and safe in the long-term (up to 5 years in preliminary data).14-16
Real-world data are needed to provide supporting evidence for the efficacy and safety observed in randomized clinical trials. Some studies have been published evaluating the efficacy of ustekinumab in clinical practice.17-33 The sample size in these studies was small and many of the studies did not investigate the durability of response (ie, the time from reaching remission to LoR). LoR to biologic therapies is common in patients with CD,34-37 and is a significant challenge for physicians managing these patients in clinical practice. Hence, it is important to gather long-term postmarketing data on the durability of response of agents used to treat patients with CD and to confirm the long-term benefit and safety of this drug in the clinical practice setting.
We therefore designed a nationwide cohort study in patients with CD treated with ustekinumab in order to systematically assess its real-world effectiveness and safety. The aim of our study was to evaluate the durability of ustekinumab treatment and reasons for ustekinumab discontinuation in a real-world setting. We also aimed to assess the short-term effectiveness and to identify predictive factors for the short- and long-term benefit and the safety of ustekinumab during follow-up in clinical practice.
Methods
Study Design and Patient Population
A retrospective, multicenter, noninterventional study was carried out in 61 Spanish hospitals. Patients included were ≥18 years of age, had active CD (Harvey-Bradshaw Index [HBI] >4), had received ≥1 initial dose of intravenous (IV) ustekinumab ≥6 months prior to the start of the study, and were followed up by gastroenterologists in the hospital setting. Patients who had received ustekinumab for a different indication, who received subcutaneous (SC) ustekinumab as induction therapy, who had been or were involved in clinical trials of ustekinumab, who had a stoma (as in these patients, the number of daily stools is unknown, and therefore disease activity and HBI cannot be calculated), or those with CD in remission (HBI ≤4) were excluded. The study was conducted according to the ethical principles of the Declaration of Helsinki. The study protocol was approved by the Drug Research Ethics Committee (Comité de Ética de la Investigación con Medicamentos) at the Hospital Universitario de La Princesa in Madrid, Spain, and patients gave written or verbal informed consent to participate in the study.
Ustekinumab Treatment
Eligible patients received SC ustekinumab 90 mg at week 8, and were then scheduled to receive SC ustekinumab 90 mg at regular intervals every 8 to 12 weeks (maintenance therapy), as per the approved dosage in the Summary of Product Characteristics (SPC).13 Patients underwent dose optimization during maintenance therapy (escalation/intensification or de-escalation/de-intensification) at the judgment of the treating physician.
Study Outcomes
The primary outcome of interest was the ustekinumab retention rate from the start of ustekinumab treatment (first ustekinumab dose received by the patient) to the last recorded dose. Secondary outcomes were the retention rate of ustekinumab with and without immunosuppressive treatment and according to previous lines of treatment; evaluation of the short-term effectiveness at 8 and 16 weeks, and to identify predictive factors of remission; evaluation of ustekinumab long-term effectiveness; evaluation of prognostic factors related to the cumulative retention rate of ustekinumab; proportion of patients who underwent dose escalation or intensification during the follow-up period and response to dose adjustments; and proportion of patients who underwent de-escalation or de-intensification of the dose during the follow-up period. To evaluate the tolerability of ustekinumab, safety endpoints, including AEs, serious AEs, and the relationship of the AEs to ustekinumab were assessed.
Data Collection
During the study period, there was 1 study visit scheduled for each patient in which the available clinical practice data were collected in an electronic case report form. Data collected included patient demographics and disease characteristics, treatment history for CD and reasons for discontinuation, concomitant use of immunomodulators or steroids, and biologic treatments for diseases other than CD. Ustekinumab treatment data collected included treatment start date, response to ustekinumab, maintenance dosing regimen, treatment after LoR to ustekinumab, ustekinumab dose intensification or de-intensification, response after dose de-intensification, and AEs. Endoscopic evaluation and biologic markers (C-reactive protein [CRP] or fecal calprotectin) were recorded when available. Patients were followed up until last administration of ustekinumab or last visit, whichever came first. Data were remotely monitored to assess data quality.
Definitions
Dosing-related definitions
Dose escalation was defined as a shortening of the administration interval from every 12 weeks to every 8 weeks; dose intensification was defined as a shortening of the ustekinumab administration interval to <8 weeks (every 4 or 6 weeks) or receipt of a reinduction IV dose of ustekinumab.
Dose de-escalation was defined as an increase in the interval between administration of SC ustekinumab 90 mg from every 8 weeks to every 12 weeks; dose de-intensification was defined as an increase in the ustekinumab administration interval to less often than every 12 weeks.
As the present study was retrospective and observational, there was no specific protocol for dose adjustments; therefore, the indications for dosage adjustments were based on the clinicians’ criteria.
Response-related definitions
Retention rate was defined as the proportion of patients maintained under ustekinumab treatment at a certain time point. Remission was defined as an HBI value of ≤4 and clinical response as a decrease in HBI of ≥3 points from baseline. LoR was defined as the reappearance of symptoms along with endoscopic, radiographic, or biochemical evidence (eg, CRP levels ≥5 mg/L, fecal calprotectin levels >150 µg/g), which led to treatment intensification, dose escalation, addition of other drugs, switching to other treatment, or surgery.
Statistical Analysis
For categorical variables, percent values and their 95% confidence intervals (CIs) were calculated. For continuous variables, the mean ± SD or the median (interquartile range [IQR]) were calculated, depending on whether or not they were normally distributed.
With respect to the retention on ustekinumab treatment, the Kaplan-Meier method was used, in which patients were censored at the time of treatment discontinuation for any reason. Any differences between survival curves were evaluated using the log-rank test. Stepwise multivariate analysis using the Cox regression model was used to investigate factors potentially associated with ustekinumab discontinuation. In the log-rank test and in the multivariate analysis, P values of <.05 were considered to be statistically significant. The same method was used to estimate the incidence of LoR and to identify predictive factors of LoR. Only patients in remission at week 16 were included in the LoR analysis.
For the short-term effectiveness endpoint, the dependent variables were clinical remission and response at week 16; the nonresponse imputation method was used for missing values. In the univariate analysis, categorical variables were compared using the chi-square test, and quantitative variables were compared using the appropriate test. A logistic regression model was used to evaluate variables associated with the likelihood of achieving remission after ustekinumab induction.
The primary analysis was conducted using data from all patients included in the study. In addition, a subanalysis of patients who received only the approved ustekinumab dosage (SPC subgroup) was performed.
Results
Patient Population
A total of 526 patients were screened for inclusion, and 463 patients met the inclusion criteria and were included in the study population; 293 (63.3%) received the treatment as per the approved dosage and were considered as the SPC subgroup (Supplementary Tables 1-4 and Supplementary Figure 1). Patients were followed up for a median of 15.5 (IQR, 12.6-18.5) months.
Patient demographics and baseline characteristics are summarized in Table 1. Mean age was 47 years, mean age at diagnosis was 33 years, and approximately 50% of patients were female. Mean disease duration was 14.1 years, and mean time until ustekinumab initiation was 12.6 years. Most patients had ileocolonic or ileal CD, and inflammatory disease was the most common disease type at ustekinumab initiation, followed by stricturing disease. Active perianal disease at baseline was seen in 14% of patients.
Table 1.
Patient baseline demographics and disease characteristics (N = 463)
| Demographics | |
|---|---|
| Age, y | 47.1 ± 13.4 |
| Age at diagnosis, y | 33.4 ± 14.5 |
| Female | 232 (50.1) |
| Smokers | 118 (25.5) |
| Comorbidities | 230 (49.7) |
| Disease characteristics at baseline | |
| Disease duration, y | 14.1 ± 9 |
| Time from diagnosis to UST initiation, y | 12.6 ± 9 |
| Age at UST initiation, y | 45.6 ± 13.4 |
| Extraintestinal manifestations | 181 (39.1) |
| CD location | |
| Ileocolonic | 218 (47.1) |
| Ileal | 190 (41) |
| Colonic | 55 (11.9) |
| Upper gastrointestinal tract | 37 (8) |
| CD behavior | |
| Inflammatory | 245 (52.9) |
| Stricturing | 132 (28.5) |
| Penetrating | 86 (18.6) |
| Active perianal disease | 65 (14.0) |
| Harvey-Bradshaw Index score | 8.4 ± 3.5 |
| Prior use of biologics for CD treatment | 447 (96.5) |
| Previous anti-TNF | |
| Adalimumaba | 374 (83.7) |
| Infliximaba | 348 (77.9) |
| Previous vedolizumaba | 109 (24.4) |
| Previous surgery for CDb | 281 (60.7) |
| Abdominal | 218 (47.1) |
| Perianal | 106 (22.9) |
| ≥1 concomitant immunosuppressant | 163 (35.2) |
| Azathioprine | 106 (65) |
| Methotrexate | 47 (28.8) |
| Mercaptopurine | 13 (8) |
| Number of biologics for CD treatment | |
| 1 | 138 (30.9) |
| 2 | 168 (37.6) |
| ≥3 | 141 (31.5) |
Values are mean ± SD or n (%).
Abbreviations: CD, Crohn’s disease; TNF, tumor necrosis factor; UST, ustekinumab.
Percentages are based on the number of patients who received previous biologics for CD treatment.
Total number of surgeries (patients could have had more than 1).
Prior use of biologics was reported by >96% of patients, most commonly adalimumab and infliximab (Table 1). Approximately one-third of patients were receiving concomitant immunosuppressants at baseline (Table 1), and approximately 40% of patients were receiving steroids. At least 1 previous abdominal surgery for CD was reported in 47.1% of patients.
At the time of the first ustekinumab dose, endoscopic assessment was available in 174 (37.6%) patients, of whom 128 showed disease activity: 56.3% had moderate and 35.2% had severe disease activity. With regard to other indicators of disease activity, mean HBI was 8.4, mean CRP was 18.3 mg/L, and mean fecal calprotectin was 892.5 mg/kg.
Ustekinumab Retention Rate
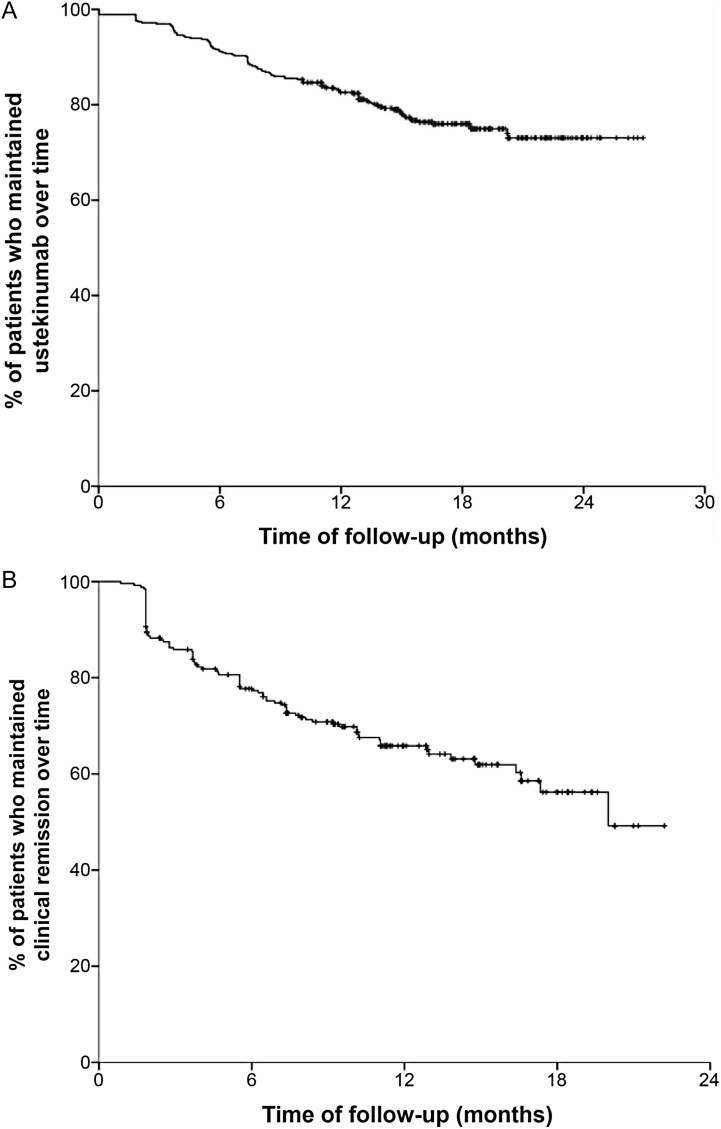
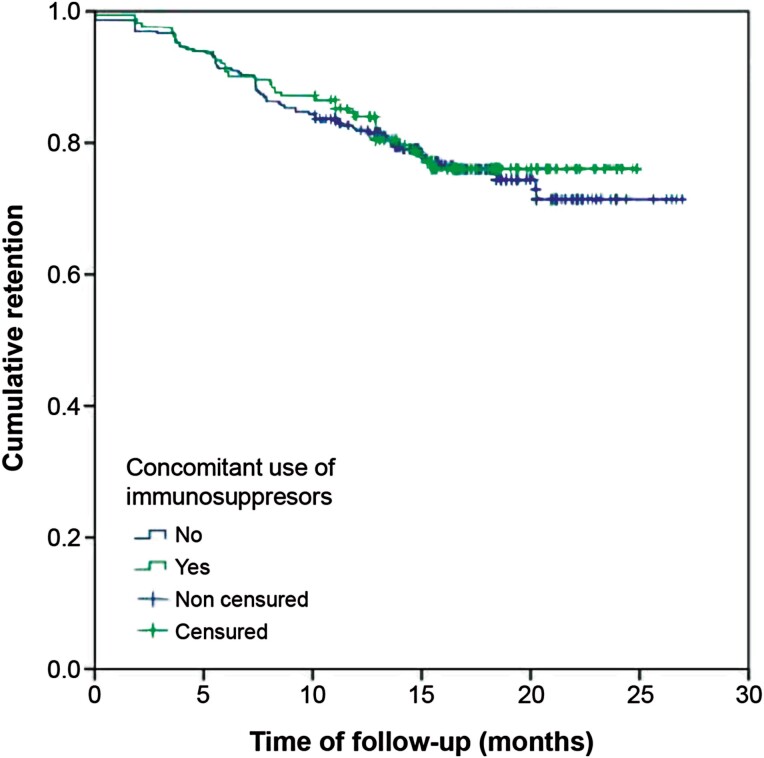
Primary and secondary outcomes are summarized in Table 2. After a median follow-up of 15.5 months, 356 patients (76.9%; 95% CI, 72.8%-80.7%) were still receiving ustekinumab and 107 (23.1%) had discontinued the treatment. The incidence of ustekinumab discontinuation was 18.4% per patient-year of follow-up (Figure 1). The incidence was similar between patients receiving ustekinumab monotherapy or combination therapy with immunosuppressants (17.5% vs 18.9%, P = .71) (Figure 2). The reasons for discontinuation were primary failure (27.1%), LoR (26.2%), partial response (25.2%), AE (5.6%), patient’s decision (4.7%), and other reasons (11.2%).
Table 2.
Summary of primary and secondary outcomes
| Result | |
|---|---|
| Primary outcome | |
| Ustekinumab retention rate | 76.9% (95% CI, 72.8%-80.7%) Median follow-up: 15.5 mo |
| Discontinuation incidence | 18.4% per patient-year of follow-up |
| Predictive factors of discontinuation | Previous intestinal surgery, concomitant steroid treatment |
| Secondary outcomes | |
| Short-term effectiveness | |
| • Clinical remission at 8 wk | 44.0% |
| • Clinical remission at 16 wk | 56.1% |
| • Predictive factor of lower probability of remission at 16 wk | Older age, higher HBI at baseline, previous abdominal surgery |
| • Clinical response at 8 wk | 57.6% |
| • Clinical response at 16 wk | 70.2% |
| • Predictive factors of lower probability of response at 16 wk | Previous surgery |
| Long-term effectiveness | |
| • Loss of response incidence | 29.7% per patient-year of follow-up |
| • Predictive factors of higher risk of loss of response | Number of previous biologics, higher HBI at baseline, severe vs mild |
| • Probability of sustaining remission | |
| ◦ 6 mo | 84% |
| ◦ 12 mo | 74% |
| ◦ 18 mo | 66% |
| • Clinical remission at 6 mo | 57.9% |
| • Clinical remission at 18 mo | 57.2% |
| Dose optimization | |
| • Dose escalation | 4.5% |
| • Dose intensification | 21.6% |
| • Dose de-escalation | 5.8% |
Abbreviations: CI, confidence interval; HBI, Harvey-Bradshaw Index.
Figure 1.
A and B, Survival curve of patients who maintained ustekinumab treatment over time and maintained clinical remission over time.
Figure 2.
Ustekinumab retention based on the concomitant use of immunosuppressive therapy.
Regarding maintenance treatment, 396 (85.5%) patients started SC ustekinumab with a schedule of once every 8 weeks, 48 (10.4%) every 12 weeks, and 2 (0.4%) every 4 weeks. Five patients received only 1 ustekinumab dose and 12 patients received only 2 doses, and therefore did not start the maintenance phase.
In the multivariate analysis (Table 3), previous intestinal surgery and concomitant treatment with steroids were associated with a higher risk of ustekinumab discontinuation, while a maintenance schedule of once every 12 weeks was associated with a lower risk. Other factors, such as the severity of the disease at the start of treatment, combination therapy with immunomodulators, or previous exposure to biologics (irrespective of the line of treatment) had no impact on treatment retention.
Table 3.
Multivariate analysis of factors associated with ustekinumab discontinuation
| Factor | HR | 95% CI |
|---|---|---|
| Previous abdominal surgery (yes vs no) | 2.14 | 1.47-3.18 |
| Concomitant steroid treatment (yes vs no) | 1.82 | 1.24-2.67 |
| Maintenance schedule (every 8 wk vs every 12 wk) | 0.26 | 0.08-0.81 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Short-term effectiveness
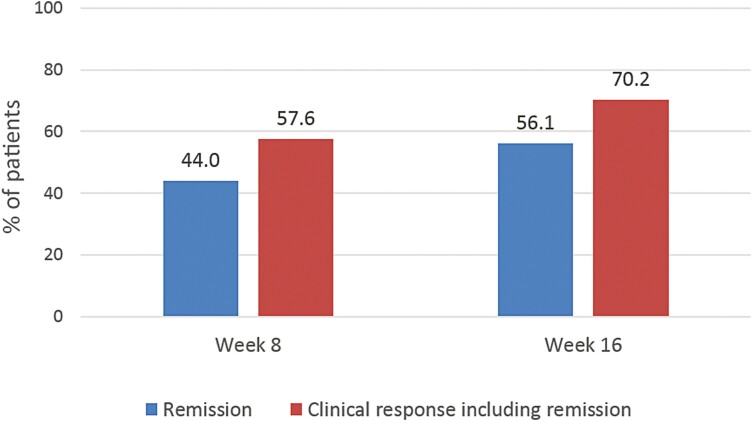
Overall, 44.0% of patients reached clinical remission at week 8 and 56.1% at week 16 (Figure 3). With respect to clinical response (including both patients with response and with remission), 57.6% of patients achieved this endpoint at week 8 and 70.2% at week 16 (Figure 3).
Figure 3.
Short-term effectiveness of ustekinumab at weeks 8 and 16.
Patients who were in remission at week 16, compared with those who were not, were younger (mean age 44.5 ± 13.9 years vs 47.3 ± 12.5 years; P = .01) at baseline, had a lower weight at baseline (67.0 ± 14.6 kg vs 71.4 ± 15.6 kg; P < .01), had a lower HBI score at first dose (7.6 ± 3.0 vs 9.6 ± 3.8; P < .01), and had a higher CRP at first dose (19.1 ± 30.6 mg/L vs 17.2 ± 38.1 mg/L; P = .02).
The multivariate analysis showed that older age (odds ratio [OR], 0.6; 95% CI, 0.4-0.9), previous abdominal surgery (OR, 0.6; 95% CI, 0.4-0.9), and higher HBI at baseline (OR, 0.8; 95% CI, 0.8-0.9) were associated with a lower probability of achieving remission at week 16. With regard to response to ustekinumab, the only factor associated with a lower probability of having a response at week 16 was previous surgery (OR, 0.7; 95% CI, 0.4-1.0).
Long-term effectiveness
Long-term effectiveness was analyzed in patients achieving clinical remission at week 16. The probability of maintaining clinical remission over time is shown in Figure 2. In the patients who achieved clinical remission at week 16 (n = 256), the median time of follow-up was 15.7 (IQR, 12.9-19.0) months and the median time to LoR was 11.1 (IQR, 7.1-14.7) months. The incidence of LoR was 29.7% per patient-year of follow-up. The probability of sustaining remission was 84%, 74%, and 66% at 6, 12, and 18 months, respectively. In addition, no difference was observed in the probability of sustaining remission between patients receiving a maintenance schedule of ustekinumab once every 12 weeks compared with once every 8 weeks (P = .36).
The number of previous biologics (hazard ratio [HR], 1.2; 95% CI, 1.0-1.5) and higher HBI score at baseline (moderate vs mild [HR, 1.5; 95% CI, 1.0-2.3] and severe vs mild [HR, 4.0; 95% CI, 1.0-17.0]) were associated with a higher risk of LoR. Neither concomitant treatment with immunomodulators nor the number of previous biologics was associated with the likelihood of maintaining remission during follow-up.
More than half of patients achieved clinical remission at 6 months, the majority of whom had steroid-free remission. Specifically, at 6 months, 57.9% (n = 268 of 463) of patients had clinical remission and 53.3% (n = 247 of 463) had steroid-free remission. At 12 months, 57.2% (n = 250 of 437) and 50.8% (n = 222 of 437) of patients had clinical remission and steroid-free remission, respectively. At 18 months, the percentages of patients who had clinical remission and steroid-free remission were 41.5% (n = 113 of 272) and 35.7% (n = 97 of 272), respectively.
Dose Optimization
Of the 463 patients analyzed, the dose was escalated in 21 (4.5%) due to LoR (n = 14 [66.7%]) or primary failure (n = 5 [23.8%]). Other reasons for dose escalation were persistently high fecal calprotectin levels and capsule endoscopy showing involvement of the proximal jejunum (n = 1 [4.8%]) and partial response (n = 1 [4.8%]).
Patient characteristics based on ustekinumab dose optimization are summarized in Table 4. The proportion of patients with previous exposure to vedolizumab and median CRP levels at baseline were significantly higher among patients who received escalated or intensified treatment than in those who did not. The distribution of other variables did not significantly differ between the subgroups (Table 4).
Table 4.
Baseline demographics and disease characteristics by treatment modification
| Treatment Not Escalated or Intensified (n = 342) | Treatment Escalated or Intensified (n = 121) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 47.1 ± 12.8 | 47.0 ± 15.1 | .97 |
| Age at diagnosis, y | 33.1 ± 14.4 | 34.3 ± 14.8 | .45 |
| Female | 179 (52.3) | 53 (43.8) | .11 |
| Smokers | 87 (25.4) | 31 (25.6) | .30 |
| Comorbidities at entry | 171 (50.0) | 59 (48.8) | .83 |
| Disease characteristics at baseline | |||
| Disease duration, years | 14.4 ± 8.9 | 13.1 ± 9.4 | .07 |
| Time from diagnosis to UST initiation, y | 13.0 ± 8.9 | 11.6 ± 9.3 | .05 |
| Age at UST initiation, y | 45.7 ± 12.8 | 45.4 ± 15.1 | .98 |
| Extraintestinal manifestations | 130 (38.0) | 51 (42.1) | .45 |
| CD location | |||
| Ileocolonic | 166 (48.5) | 52 (43.0) | .51 |
| Ileal | 135 (39.5) | 55 (45.5) | |
| Colonic | 41 (12.0) | 14 (11.6) | |
| Upper gastrointestinal tract | 27 (7.9) | 10 (8.3) | .85 |
| CD behavior | |||
| Inflammatory | 181 (52.9) | 64 (52.9) | .74 |
| Stricturing | 100 (29.2) | 32 (26.4) | |
| Penetrating | 61 (17.8) | 25 (20.7) | |
| Active perianal disease | 48 (14.0) | 17 (14.0) | .91 |
| Harvey-Bradshaw Index score | 7.5 (6.0-10.0) | 8.0 (6.0-10.0) | .64 |
| CRP, mg/L | 5.3 (1.4-15.0) | 10 (3.2-22.2) | <.01 |
| FC, µg/g | 459.0 (127.5-1256.0) | 639.0 (305.0-1430.0) | .29 |
| Prior use of biologics for CD treatment | 331 (96.8) | 116 (95.9) | .576 |
| Previous anti-TNF | |||
| Adalimumaba | 276 (83.4) | 98 (84.5) | .884 |
| Infliximaba | 254 (76.7) | 94 (81.0) | .366 |
| Previous vedolizumaba | 71 (21.5) | 38 (32.8) | .017 |
| Previous surgery for CDb | 217 (63.5) | 64 (52.9) | .051 |
| Abdominal | 169 (49.4) | 49 (40.5) | .112 |
| Perianal | 77 (22.5) | 29 (24.0) | .801 |
| ≥1 concomitant immunosuppressant | 125 (36.5) | 38 (31.4) | .321 |
| Azathioprine | 82 (65.6) | 24 (63.2) | .847 |
| Methotrexate | 34 (27.2) | 13 (34.2) | .419 |
| Mercaptopurine | 11 (8.8) | 2 (5.3) | .734 |
| Number of biologics for CD treatment | |||
| 1 | 105 (31.7) | 33 (28.4) | .15 |
| 2 | 130 (39.3) | 38 (32.8) | |
| ≥3 | 96 (29.0) | 45 (38.8) | |
Values are mean ± SD, n (%), or median (interquartile range).
Abbreviations: CD, Crohn’s disease; CRP, C-reactive protein; FC, fecal calprotectin; TNF, tumor necrosis factor; UST, ustekinumab.
Percentages are based on the number of patients who received previous biologics for CD treatment.
Total number of surgeries (patients could have had more than 1).
One hundred (21.6%) patients intensified treatment: 61 (13.2%) increased dose frequency from every 8 weeks to every 4 weeks, 33 (7.1%) increased dose frequency from every 8 weeks to every 6 weeks, and 6 patients received reinduction with IV ustekinumab. The main reasons for treatment intensification were LoR in 71 (71.0%) patients and primary failure in 17 (17.0%) patients (Supplementary Table 5). Treatment intensification resulted in remission in 42 (77.8%) of 54 patients who increased administration from every 8 weeks to every 4 weeks, 16 (80.0%) of 20 patients who increased from every 12 weeks to every 8 weeks, and 5 (83.3%) of 6 patients who underwent reinduction. Overall, 63 (78.8%) of 80 patients regained remission following treatment intensification.
Ustekinumab administration was de-escalated from every 8 weeks to every 12 weeks in 27 (5.8%) patients: 24 (88.9%) due to sustained remission, 1 (3.7%) due to sustained response, 1 (3.7%) due to herpes simplex infection, and 1 (3.7%) for clinician choice. Among patients who de-escalated treatment due to sustained remission, 15 (62.5%) maintained remission over time and 9 (37.5%) subsequently needed to reescalate treatment.
Thirty-one of the 121 patients who increased the dose as the first treatment change required a second change: 19 intensified, 1 escalated, 4 received reinduction, 5 returned to the initial dose, and 2 received IV ustekinumab (260-520 mg, depending on the patient’s weight) maintenance therapy on regular basis. None of these patients had de-escalation of treatment.
Safety
Fifty AEs (30.3%) were reported in 39 (8.4%) patients, of which only 4 were severe (2 infections, 1 malignancy, and 1 fever). The association with ustekinumab was considered possible in 36 (72%) patients, probable in 13 (26%) patients, and very likely in 1 (2%) patient (Table 5). These AEs led to temporary interruption of treatment in 19.6% of patients and suspension of treatment in 17.9%.
Table 5.
Most frequent adverse events reported during ustekinumab treatment
| Adverse Event | Patients (N = 463) |
|---|---|
| Infusion reaction | 29 (6.3) |
| Allergic reaction | 9 (1.9) |
| Arthralgias | 5 (1.1) |
| Dyspnea | 5 (1.1) |
| Headache | 7 (1.5) |
| Psoriasis | 5 (1.1) |
| Severe and opportunistic infections | 5 (1.1) |
| Pruritus | 4 (0.9) |
| Asthenia | 3 (0.6) |
| Herpes zoster | 3 (0.6) |
| Lupus | 2 (0.4) |
| Blurred vision | 1 (0.2) |
| Dizziness | 1 (0.2) |
| Fever | 1 (0.2) |
| Granuloma annulare | 1 (0.2) |
| Neoplasm | 1 (0.2) |
Values are n (%).
Discussion
To our knowledge, our study has the largest cohort and one of the longest follow-up periods among the published real-world studies of refractory CD patients treated with ustekinumab, including a total of 463 patients and a follow-up of up to 24 months. Our results provide new data regarding the effectiveness and safety of ustekinumab in clinical practice.
Although the patients in our study had refractory CD, after a median follow-up of 15.5 months, 76.9% were still receiving ustekinumab, and only 23.1% had discontinued treatment, with an ustekinumab discontinuation rate of 18.4% per patient-year. In addition, our results showed that previous exposure to biologics (irrespective of the line of treatment) and concomitant use of immunomodulators had no effect on ustekinumab discontinuation. Previous studies conducted in patients with refractory CD reported that treatment persistence on ustekinumab was 83% at the end of follow-up (~1 year),23 and only 6.7% of patients discontinued ustekinumab within the first year.27
In our study, ustekinumab was associated with an overall clinical response rate of 57.6% at week 8 and 70.2% at week 16, and clinical remission rates of 44.0% and 56.1% at weeks 8 and 16, respectively. These findings are consistent with those of other real-world studies of ustekinumab, which reported early response (≤16 weeks) and remission rates of 46.0% to 73.9% and 16.0% to 55.6%, respectively.17,18,22,24-27,29-32 Long-term remission rates (≥1 year) in other real-world studies ranged from 14.0% to 49.0%,20,24,28,32 showing some variation between studies.
In addition, older age, previous surgery, and higher HBI at baseline were associated with a lower probability of achieving remission at week 16. Other studies have identified the same negative prognostic factors.21 Although we demonstrated that previous exposure to biologics had no impact on the effectiveness of ustekinumab, in previous studies, prior use of anti-TNF agents was associated with the absence of clinical remission or response.26,29,32 Moreover, we have demonstrated that concomitant immunomodulator use does not affect the effectiveness of ustekinumab, while in the study conducted by Engel et al,24 prior administration or concomitant use of immunomodulators were associated with better outcomes. Other predictive factors identified in previous studies included body mass index, which was associated with lower rates of steroid-free remission21; age and smoking, which were associated with poor response22; and stricturing phenotype, which was associated with poor outcomes.24
The rate of LoR was 29.7% per patient-year of follow-up. This rate was similar among patients receiving SC ustekinumab 90 mg every 12 weeks and those who were treated every 8 weeks. In our study, the characteristics of patients who started receiving ustekinumab every 12 weeks were similar to those who started receiving it every 8 weeks, except that the prevalence of perianal disease was higher in the group receiving ustekinumab every 12 weeks. In patients with CD, LoR to all biologic drugs is expected.17,21,32 However, LoR to ustekinumab is probably not due to an immunogenic mechanism, because as the results of our study show, immunomodulators that decrease antibody formation do not affect drug response or treatment duration or retention, likely due to the activation of an alternative inflammatory pathway. Dose escalation or intensification may be an option to counteract LoR. In our study, 4.5% of patients required dose escalation and 21.6% required treatment intensification (ie, increased dose frequency or reinduction with IV ustekinumab); approximately 79% of these patients regained response following these changes; however, 24.8% of patients who needed a dose increase required a second change and did not reduce the dosage again during follow-up. In patients who had increased administration frequency from every 8 weeks to every 4 weeks, remission was regained in 77.8%. The intensification rates observed in the current study are substantially lower in comparison with those of a previous retrospective cohort study that included 238 patients with CD, which found that over half of the patients required dose intensification after almost 1 year of treatment.38 It is possible that this difference was due to the disease state at the time of intensification, as in Dalal et al,38 some of the patients who underwent intensification had a longer CD duration and greater proportions of patients used ≥2 prior TNF antagonists, and had penetrating disease. In the study presented here, almost all patients (96.5%) had received a prior biologic treatment, with approximately one-third receiving 1 biologic treatment, including TNF antagonists, and two-thirds receiving ≥2 biologic treatments. Similar to Dalal et al, more patients receiving ≥2 biologic treatments, including TNF antagonists, prior to study initiation required treatment intensification or dose escalation (25.1%) in comparison with patients who received only 1 biologic treatment (10.0%). Thus, it is possible that the low number of patients who required a second dose increase was due to the disease state and prior treatment of our population sample.
The effectiveness of increasing ustekinumab dose frequency to once every 4 weeks is becoming increasingly relevant. A recent study reported similar results to our study, with two-thirds of patients regaining response following intensification of ustekinumab to 90 mg every 4 weeks.25 Similarly, another study reported a response rate of 65.8% at week 16 after treatment intensification to every 4 weeks.30 In our study, reinduction with IV ustekinumab was also effective, resulting in 83% of patients regaining response. Although there are limited data for this outcome, a recent study has shown endoscopic improvement and remission in patients who underwent ustekinumab reinduction.19
Regarding treatment de-escalation, currently there are no criteria for deciding whether patients should receive ustekinumab every 8 weeks or every 12 weeks. In our study, the only significant difference in clinical characteristics between patients who started treatment on a regimen of once every 8 weeks or once every 12 weeks was a history of perianal surgery. Therefore, we cannot conclude that there was a pattern for the selection of a treatment schedule. However, the ustekinumab schedule was de-escalated from every 8 weeks to every 12 weeks in 27 patients due to sustained remission or sustained response, and among these patients, 65.2% maintained remission over time. Thus, dose de-escalation may be an option in patients with adequate response to maintenance treatment administered once every 8 weeks. In this context, it is worth noting that different maintenance dosing is recommended by the U.S. Food and Drug Administration and the European Medicines Agency; the European Medicines Agency recommends maintenance therapy every 12 or 8 weeks,13 whereas the Food and Drug Administration recommends an SC dose of 90 mg every 8 weeks.39
The safety of ustekinumab has been recently evaluated in 2 systematic reviews and meta-analyses comparing the rates of AEs in patients treated with ustekinumab vs placebo in clinical trials, showing no differences between these groups.40,41 This was also true when comparing high vs low doses of ustekinumab.40,41 In our cohort, AEs were infrequent (8.4% of patients), with a similar rate to that reported in some real-world studies30 and lower than in others.17,18,24,26 The AE rate reported in our study is also lower than that reported in clinical trials of ustekinumab,14,42 maybe because AEs are underreported in real-life studies.43
The limitations of the present study are mainly those associated with its retrospective design, including the potential for missing data in clinical records. To overcome the potential heterogeneity in clinical assessment, clinicians were asked to provide HBI score values at every visit. In addition, we could not evaluate mucosal healing. This reflects real-world clinical practice, in which endoscopy studies are generally not carried out if patients show good response after induction. Concomitant use of steroids can be difficult to evaluate in real-world studies, in which follow-up is often not defined in the study protocol (follow-up time was different for each patient). Finally, as almost 100% of our patients had previously failed treatment with biologic agents, we could not properly assess the effect of exposure to these drugs on the effectiveness of ustekinumab.
Nevertheless, our study has several strengths. First, this is the largest and longest real-world study to evaluate the effectiveness of ustekinumab in CD published to date. Second, clinical activity was categorized based on HBI, and several other objective parameters were also reported, including CRP and fecal calprotectin levels. We also investigated the effect of the concomitant immunomodulator use, LoR, and the evolution of the disease after ustekinumab dose adjustment.
Conclusions
Ustekinumab was effective in patients with CD in real-world clinical practice, including those with refractory disease. Concomitant immunomodulator use did not appear to provide an additional benefit either in the short term or in the long term. Approximately 20% of patients discontinued ustekinumab per patient-year of follow-up, mainly owing to primary failure and LoR. A relevant proportion of patients who had achieved remission later relapsed, but treatment intensification was able to regain remission in approximately 79% of patients. Finally, safety was consistent with the known profile of ustekinumab.
Supplementary Material
Acknowledgments
We thank Eliana Mesa, MD, Sheridan Henness, PhD, and Fernando Sánchez Barbero, PhD, who provided assistance with manuscript development on behalf of Springer Healthcare Communications. This medical writing assistance was funded by Janssen-Cilag Spain.
Contributor Information
María Chaparro, Gastroenterology Department, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa, Universidad Autónoma de Madrid, and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Iria Baston-Rey, Gastroenterology Department, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela, Spain.
Estela Fernández-Salgado, Gastroenterology Department, Complejo Hospitalario de Pontevedra, Pontevedra, Spain.
Javier González García, Gastroenterology Department, Hospital Público Comarcal la Inmaculada, Almería, Spain.
Laura Ramos, Gastroenterology Department, Hospital Universitario de Canarias, Tenerife, Spain.
María Teresa Diz-Lois Palomares, Gastroenterology Department, Hospital Universitario A Coruña, A Coruña, Spain.
Federico Argüelles-Arias, Gastroenterology Department, Hospital Universitario Virgen Macarena, Facultad de Medicina, Universidad de Sevilla, Seville, Spain.
Eva Iglesias Flores, Gastroenterology Department, Hospital Universitario Reina Sofía, Universidad de Córdoba, Instituto Maimónides de Investigación Biomédica de Córdoba, Córdoba, Spain.
Mercedes Cabello, Gastroenterology Department, Hospital Universitario Virgen de Valme, Seville, Spain.
Saioa Rubio Iturria, Gastroenterology Department, Complejo Hospitalario de Navarra, Pamplona, Spain.
Andrea Núñez Ortiz, Gastroenterology Department, Hospital Universitario Virgen del Rocío, Seville, Spain.
Mara Charro, Gastroenterology Department, Hospital de Barbastro, Barbastro, Spain.
Daniel Ginard, Gastroenterology Department, Hospital Universitario Son Espases, Palma, Spain.
Carmen Dueñas Sadornil, Gastroenterology Department, Hospital San Pedro de Alcántara, Cáceres, Spain.
Olga Merino Ochoa, Gastroenterology Department, Hospital Universitario Cruces, Barakaldo, Spain.
David Busquets, Gastroenterology Department, Hospital Universitari de Girona Doctor Josep Trueta, Girona, Spain.
Eduardo Iyo, Gastroenterology Department, Hospital Comarcal de Inca, Inca, Spain.
Ana Gutiérrez Casbas, Gastroenterology Department, Hospital General Universitario de Alicante, Instituto de Investigación Sanitaria y Biomédica de Alicante, Alicante, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Patricia Ramírez de la Piscina, Gastroenterology Department, Hospital Universitario de Araba-Txagorritxu, Araba-Txagorritxu, Spain.
Marta Maia Boscá-Watts, Gastroenterology Department, Hospital Clínico Universitario de Valencia, Valencia, Spain.
Maite Arroyo, Gastroenterology Department, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain.
María José García, Gastroenterology Department, Hospital Universitario Marqués de Valdecilla, Instituto de Investigación Sanitario Valdecilla (IDIVAL), Santander, Spain.
Esther Hinojosa, Gastroenterology Department, Hospital de Manises, Manises, Spain.
Jordi Gordillo, Gastroenterology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Pilar Martínez Montiel, Gastroenterology Department, Hospital Universitario 12 de Octubre, Madrid, Spain.
Benito Velayos Jiménez, Gastroenterology Department, Hospital Clínico Universitario de Valladolid, Valladolid, Spain.
Cristina Quílez Ivorra, Gastroenterology Department, Hospital Marina Baixa, Villajoyosa, Spain.
Juan María Vázquez Morón, Gastroenterology Department, Hospital Universitario Juan Ramón Jiménez, Huelva, Spain.
José María Huguet, Gastroenterology Department, Hospital General Universitario de Valencia, Valencia, Spain.
Yago González-Lama, Gastroenterology Department, Hospital Universitario Puerta de Hierro, Majadahonda, Spain.
Ana Isabel Muñagorri Santos, Gastroenterology Department, Hospital Universitario de Donostia, Donostia-San Sebastián, Spain.
Víctor Manuel Amo, Gastroenterology Department, Hospital Regional de Málaga, Málaga, Spain.
María Dolores Martín-Arranz, Gastroenterology Department, Hospital Universitario de La Paz, Institute for Health Research La Paz, Universidad Autónoma de Madrid, Madrid, Spain.
Fernando Bermejo, Gastroenterology Department, Instituto de Investigación Sanitaria del Hospital La Paz, Hospital Universitario de Fuenlabrada, Madrid, Spain.
Jesús Martínez Cadilla, Gastroenterology Department, Hospital Alvaro Cunqueiro, Vigo, Spain.
Cristina Rubín de Célix, Gastroenterology Department, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa, Universidad Autónoma de Madrid, and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Paola Fradejas Salazar, Gastroenterology Department, Hospital Virgen de la Concha, Zamora, Spain.
Antonio López San Román, Gastroenterology Department, Hospital Universitario Ramón y Cajal, Madrid, Spain.
Nuria Jiménez, Gastroenterology Department, Hospital General Universitario de Elche, Elche, Spain.
Santiago García López, Gastroenterology Department, Hospital Universitario Miguel Servet, Zaragoza, Spain.
Anna Figuerola, Gastroenterology Department, Hospital General Universitario de Castellón, Castellón de la Plana, Spain.
Itxaso Jiménez, Gastroenterology Department, Hospital Universitario de Galdakao-Usansolo, Galdakao, Spain.
Francisco José Martínez Cerezo, Gastroenterology Department, Hospital Universitario Sant Joan de Reus, Reus, Spain.
Carlos Taxonera, Gastroenterology Department, Instituto de Investigación del Hospital Clínico San Carlos, Hospital Clínico Universitario San Carlos, Madrid, Spain.
Pilar Varela, Gastroenterology Department, Hospital Universitario de Cabueñes, Gijón, Spain.
Ruth de Francisco, Gastroenterology Department, Instituto de Investigación Biosanitaria del Principado de Asturias, Hospital Universitario Central de Asturias, Oviedo, Spain.
David Monfort, Gastroenterology Department, Consorci Sanitari de Terrassa, Terrassa, Spain.
Gema Molina Arriero, Gastroenterology Department, Complejo Hospitalario Universitario de Ferrol, Ferrol, Spain.
Alejandro Hernández Camba, Gastroenterology Department, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain.
Francisco Javier García-Alonso, Gastroenterology Department, Hospital Universitario Rio Hortega, Valladolid, Spain.
Manuel Van Domselaar, Gastroenterology Department, Hospital Universitario de Torrejón, Torrejón de Ardoz, Madrid, Spain.
Ramón Pajares Villarroya, Gastroenterology Department, Hospital Universitario Infanta Sofía, Madrid, Spain.
Alejandro Núñez, Gastroenterology Department, Hospital Clínico Universitario de Salamanca, Salamanca, Spain.
Francisco Rodríguez Moranta, Gastroenterology Department, Hospital Universitario Bellvitge, Barcelona, Spain.
Ignacio Marín-Jiménez, Servicio de Aparato Digestivo, IiSGM, Hospital General Universitario Gregorio Marañón, Madrid, Spain; Facultad de Medicina, Universidad Complutense de Madrid, Madrid, Spain.
Virginia Robles Alonso, Gastroenterology Department, Hospital Universitario Vall d´Hebrón, Barcelona, Spain.
María del Mar Martín Rodríguez, Gastroenterology Department, Hospital Universitario Virgen de las Nieves, Granada, Spain.
Patricia Camo-Monterde, Gastroenterology Department, Hospital Universitario San Jorge, Huesca, Spain.
Iván García Tercero, Gastroenterology Department, Hospital General Universitario Santa Lucía, Cartagena, Spain.
Mercedes Navarro Llavat, Gastroenterology Department, Hospital de Sant Joan Despí Moisès Broggi, Sant Joan Despí, Spain.
Lara Arias García, Gastroenterology Department, Hospital Universitario de Burgos, Burgos, Spain.
Daniel Hervías Cruz, Gastroenterology Department, Hospital General Universitario de Ciudad Real, Ciudad Real, Spain.
Sara Sulleiro, Janssen Medical Department, Madrid, Spain.
Cynthia Novella, Janssen Medical Department, Madrid, Spain.
Eugenia Vispo, Janssen Medical Department, Madrid, Spain.
Manuel Barreiro-de Acosta, Gastroenterology Department, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela, Spain.
Javier P Gisbert, Gastroenterology Department, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa, Universidad Autónoma de Madrid, and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Author Contributions
M.Ch., M.B.-d.A. and J.P.G. contributed to the study design, data collection, data analysis, data interpretation, and writing of the manuscript. The remaining authors were responsible for identifying patients for inclusion in the study and extracting data from their medical records. All authors approved the final version of the manuscript, including the authorship list.
Funding
This work was supported by Janssen-Cilag Spain. This sponsor had a partial role in study design, analysis, and interpretation of data. Medical writing and editorial assistance for the preparation of this article was funded by Janssen-Cilag Spain. This assistance was provided by the following individuals, who are either employees of Springer Healthcare Communications (S.H.C.), or were contracted by S.H.C. to undertake editorial work in connection with the preparation of the article: Eliana Mesa, MD, Sheridan Henness, PhD, Fernando Sánchez Barbero, PhD.
Conflicts of Interest
M.Ch. has served as a speaker, or has received research or education funding from MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, and Tillotts Pharma. I.B.-R. has served as a speaker for MSD, Janssen, Pfizer, and Takeda. E.F.-S. has served as a speaker for Janssen, AbbVie, Tillotts Pharma, Ferring, MSD, Shire, Takeda, Falk, and Faes Farma. L.R. has served as a speaker or has received education funding from MSD, AbbVie, Takeda, Janssen, and Ferring. F.A.-A. has served as a speaker, consultant, and advisory member for or has received research funding from Janssen, MSD, AbbVie, Pfizer, Kern Pharma, Biogen, Sandoz, Takeda, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Gebro Pharma, Amgen, and Vifor Pharma. E.I.F. has served as a consultant or advisory member or received research and education funding from AbbVie, MSD, Pfizer, Takeda, Janssen, Ferring, and Dr. Falk Pharma. S.R.I. has served as a speaker or received research or education funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals, and Tillotts Pharma. D.B. has served as a speaker for AbbVie, Pfizer, Takeda, Janssen, Ferring, and Tillots Pharma. A.G.C. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Tillotts Pharma, Chiesi, and Otsuka Pharmaceutical. M.M.B.-W. has served as speaker or advisory board member or has received research and education funding from MSD, Ferring, AbbVie, Janssen, Biogen, and Takeda. M.A. has served as speaker for Takeda, Janssen, and AbbVie. M.J.G. has received financial support for travelling and educational activities from MSD, Janssen, AbbVie, Takeda, and Ferring. P.M.M. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Roche, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, and Otsuka Pharmaceutical. J.M.V.M. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Takeda, Janssen, Kern, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, and Gebro Pharma. J.M.H. has served as a speaker or received research or education funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Sandoz, Ferring, and Faes Farma. Y.G.-L. has served as speaker, consultant, and advisory member for or has received unrestricted grants from MSD, AbbVie, Takeda, Janssen, Ferring, Shire Pharmaceuticals, and GebroPharma. A.I.M.S. has received fees from Jansen and Takeda. M.D.M.-A. has received fees as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Shire Pharmaceuticals, Tillotts Pharma, and Faes Pharma. F.B. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Takeda, Janssen, Pfizer, Biogen, Amgen, Ferring, Faes Farma, Tillotts Pharma, Dr. Falk Pharma, Chiesi, and Gebro Pharma. N.J. has received fees from Janssen, Takeda, AbbVie and Tillots. C.T. has served as a speaker, consultant, and advisory board member for MSD, AbbVie, Pfizer, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, and Tillots. A.H.C. has served as speaker for or received research or education funding from AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Adacyte Therapeutics, and Ferring. F.R.M. has served as advisory member or received financial support for traveling and educational activities from Pfizer, Jansen, MSD, AbbVie, and Takeda. M.M.M.R. has served as speaker or received financial support for educational activities from MSD, Takeda, Janssen, AbbVie, Tillotts Pharma and Ferring. I.G.T. has served as a speaker from MSD, AbbVie, Pfizer, Takeda, Kern, Shire Pharmaceuticals, and Janssen. M.N.L. has served as a speaker or received financial support for traveling or received research funding from AbbVie, Janssen, Takeda, MSD, Pfizer, Ferring, Dr. Falk Pharma, Tillots Pharma, and Biogen. M.B.-d.A. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Hospira, Takeda, Janssen, Kern, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals, and Tillotts Pharma. J.P.G. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Roche, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, and Vifor Pharma. S.S., C.N. and E.V. are employees of Janssen. All other authors have nothing to declare.
References
- 1. Baumgart DC, Sandborn WJ.. Crohn’s disease. Lancet. 2012;380:1590–1605. [DOI] [PubMed] [Google Scholar]
- 2. Veauthier B, Hornecker JR.. Crohn’s disease: diagnosis and management. Am Fam Physician. 2018;98:661–669. [PubMed] [Google Scholar]
- 3. Wilson JC, Furlano RI, Jick SS, Meier CR.. Inflammatory bowel disease and the risk of autoimmune diseases. J Crohns Colitis. 2016;10:186–193. [DOI] [PubMed] [Google Scholar]
- 4. Xavier RJ, Podolsky DK.. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. [DOI] [PubMed] [Google Scholar]
- 5. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ.. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. [DOI] [PubMed] [Google Scholar]
- 6. Knowles SR, Keefer L, Wilding H, et al. . Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part II. Inflamm Bowel Dis. 2018;24:966–976. [DOI] [PubMed] [Google Scholar]
- 7. Tomazoni EI, Benvegnú DM.. Symptoms of anxiety and depression, and quality of life of patients with Crohn’s disease. Arq Gastroenterol. 2018;55:148–153. [DOI] [PubMed] [Google Scholar]
- 8. Gomollón F, Dignass A, Annese V, et al. ECCO. . 3rd European evidence-based consensus on the diagnosis and management of crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 9. Torres J, Bonovas S, Doherty G, et al. . ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22. [DOI] [PubMed] [Google Scholar]
- 10. Chaparro M, Gisbert JP.. New molecules in the treatment of inflammatory bowel disease. Gastroenterol Hepatol. 2016;39:411–423. [DOI] [PubMed] [Google Scholar]
- 11. Samaan M, Campbell S, Cunningham G, et al. . Biologic therapies for Crohn’s disease: optimising the old and maximising the new. F1000Res. 2019;8:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atreya R, Neurath MF.. Mechanisms of molecular resistance and predictors of response to biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2018;3:790–802. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency. STELARA (ustekinumab). Summary of product characteristics. 2013. Accessed July 21, 2021.https://www.ema.europa.eu/en/documents/product-information/stelara-epar-product-information_en.pd
- 14. Feagan BG, Sandborn WJ, Gasink C, et al. UNITI-IM-UNITI Study Group. . Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 15. Hanauer SB, Sandborn WJ, Feagan BG, et al. . IM-UNITI: three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis. 2020;14:23–32. [DOI] [PubMed] [Google Scholar]
- 16. Sandborn WJ, Rutgeerts P, Gasink C, et al. . OP010 Long term efficacy and safety of ustekinumab for Crohn’s disease: results from IM-UNITI long-term extension through 2 years. J Crohns Colitis. 2017;11:S6. [Google Scholar]
- 17. Alric H, Amiot A, Kirchgesner J, et al. . The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther. 2020;51:948–957. [DOI] [PubMed] [Google Scholar]
- 18. Battat R, Kopylov U, Bessissow T, et al. . Association between Ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2017;15:1427–1434.e2. [DOI] [PubMed] [Google Scholar]
- 19. Bennett A, Evers Carlini L, Duley C, et al. . A single center experience with long-term ustekinumab use and reinduction in patients with refractory Crohn disease. Crohns Colitis 360. 2020;2:otaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. . Ustekinumab for Crohn’s disease: results of the ICC registry, a nationwide prospective observational cohort study. J Crohns Colitis. 2020;14:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biemans VBC, van der Woude CJ, Dijkstra G, et al. Dutch Initiative on Crohn and Colitis (ICC). . Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn’s disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther. 2020;52:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casas Deza D, García López S, Lafuente Blasco M, et al. . Efficacy and safety of ustekinumab in real clinical practice. Retrospective multicentre study. ARAINF cohort. Gastroenterol Hepatol. 2020;43:126–132. [DOI] [PubMed] [Google Scholar]
- 23. Eberl A, Hallinen T, Af Björkesten CG, et al. . Ustekinumab for Crohn’s disease: a nationwide real-life cohort study from Finland (FINUSTE). Scand J Gastroenterol. 2019;54:718–725. [DOI] [PubMed] [Google Scholar]
- 24. Engel T, Yung DE, Ma C, et al. . Effectiveness and safety of Ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig Liver Dis. 2019;51:1232–1240. [DOI] [PubMed] [Google Scholar]
- 25. Fumery M, Peyrin-Biroulet L, Nancey S, et al. . Effectiveness and safety of ustekinumab intensification at 90 mg every four weeks in Crohn’s disease: a multicenter study. J Crohns Colitis. 2020;15:222–227. [DOI] [PubMed] [Google Scholar]
- 26. Greenup AJ, Rosenfeld G, Bressler B.. Ustekinumab use in Crohn’s disease: a Canadian tertiary care centre experience. Scand J Gastroenterol. 2017;52:1354–1359. [DOI] [PubMed] [Google Scholar]
- 27. Harris KA, Horst S, Gadani A, et al. . Patients with refractory Crohn’s disease successfully treated with Ustekinumab. Inflamm Bowel Dis. 2016;22:397–401. [DOI] [PubMed] [Google Scholar]
- 28. Harris RJ, McDonnell M, Young D, et al. . Early real-world effectiveness of ustekinumab for Crohn’s disease. Frontline Gastroenterol. 2020;11:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iborra M, Beltrán B, Fernández-Clotet A, et al. GETECCU Group (Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa). . Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn’s disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2019;50:278–288. [DOI] [PubMed] [Google Scholar]
- 30. Kopylov U, Hanzel J, Liefferinckx C, et al. . Effectiveness of ustekinumab dose escalation in Crohn’s disease patients with insufficient response to standard-dose subcutaneous maintenance therapy. Aliment Pharmacol Ther. 2020;52:135–142. [DOI] [PubMed] [Google Scholar]
- 31. Macaluso FS, Maida M, Ventimiglia M, et al. . Effectiveness and safety of Ustekinumab for the treatment of Crohn’s disease in real-life experiences: a meta-analysis of observational studies. Expert Opin Biol Ther. 2020;20:193–203. [DOI] [PubMed] [Google Scholar]
- 32. Townsend T, Razanskaite V, Dodd S, et al. . Comparative effectiveness of ustekinumab or vedolizumab after one year in 130 patients with anti-TNF-refractory Crohn’s disease. Aliment Pharmacol Ther. 2020;52:1341–1352. [DOI] [PubMed] [Google Scholar]
- 33. Wils P, Bouhnik Y, Michetti P, et al. Groupe d’Etude Thérapeutique des Affections Inflammatoires du Tube Digestif (GETAID). . Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: a multicentre experience. Aliment Pharmacol Ther. 2018;47:588–595. [DOI] [PubMed] [Google Scholar]
- 34. Qiu Y, Chen BL, Mao R, et al. . Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol. 2017;52:535–554. [DOI] [PubMed] [Google Scholar]
- 35. Billioud V, Sandborn WJ, Peyrin-Biroulet L.. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106:674–684. [DOI] [PubMed] [Google Scholar]
- 36. Zhang QW, Shen J, Zheng Q, Ran ZH.. Loss of response to scheduled infliximab therapy for Crohn’s disease in adults: a systematic review and meta-analysis. J Dig Dis. 2019;20:65–72. [DOI] [PubMed] [Google Scholar]
- 37. Shmidt E, Kochhar G, Hartke J, et al. . Predictors and management of loss of response to vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:2461–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dalal RS, Njie C, Marcus J, et al. . Predictors of Ustekinumab failure in Crohn’s disease after dose intensification. Inflamm Bowel Dis. 2021;27:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Food and Drug Administration. STELARA® (ustekinumab) prescribing information. Accessed July 21, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf
- 40. Rolston VS, Kimmel J, Popov V, et al. . Ustekinumab does not increase risk of adverse events: a meta-analysis of randomized controlled trials. Dig Dis Sci. 2021;66:1631–1638. [DOI] [PubMed] [Google Scholar]
- 41. Sandborn WJ, Feagan BG, Danese S, et al. . Safety of Ustekinumab in inflammatory bowel disease: pooled safety analysis of results from phase 2/3 studies. Inflamm Bowel Dis. 2021;27:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandborn WJ, Gasink C, Gao LL, et al. CERTIFI Study Group. . Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. [DOI] [PubMed] [Google Scholar]
- 43. Queiroz NSF, Regueiro M.. Safety considerations with biologics and new inflammatory bowel disease therapies. Curr Opin Gastroenterol. 2020;36:257–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.